Abstract
Background
Elevated fibrosis has been found in patients with intrauterine adhesion, which indicates that fibrotic factors may play a critical role in formation of intrauterine adhesion. The aim of this study was to identify the effect of hyaluronic acid (HA) at high and low molecular weight on fibrosis of the endometrium in a mouse model of Asherman’s syndrome.
Material/Methods
Endometrial fibrosis in a mouse model of Asherman’s syndrome was confirmed. Then HA at high and low molecular weight was injected into the uterine cavity. Endometrial fibrosis was compared among the control group, LMW-HA, and HMW-HA group. The extent of endometrial fibrosis was calculated using Masson stain. The fibrosis markers (TGFβ1, CTGF, collagen I, and collagen III) in endometrial tissue were detected using immunohistochemistry and Western blotting.
Results
The ratio of the area with endometrial fibrosis to total endometrial area in the HMW-HA group was significantly decreased compared to the control group (P<0.05). The expression of fibrosis markers (TGFβ1, CTGF, collagen I, and collagen III) in the endometrium was attenuated in the HMW-HA group compared to the control group, but the LMW-HA group had no similar effect.
Conclusions
Hyaluronic acid at high molecular weight may attenuate the degree of endometrial fibrosis after endometrial damage, which may contribute to preventing formation of intrauterine adhesions.
MeSH Keywords: Fibrosis, Gynatresia, Hyaluronic Acid
Background
Asherman’s syndrome (AS) [1], also known as intrauterine adhesion (IUA), is defined as a wide range of partial to complete adhesions within the uterine cavity due to scars usually occurring after curettage and various forms of hysteroscopic surgery, including resection of septa, and hysteroscopic resection of solitary and multiple fibroids. Trauma to the basal layer of the endometrium is widely accepted to be the main cause of IUA [2]. The human endometrium is capable of extensive self-renewal during the menstrual cycle. Endometrial stem cells (ESC) are believed to be involved in endometrial regeneration [3]. Bone marrow-derived mesenchymal stem cells (MSC) are reported to be the possible original source of ESC [4]. MSC can also differentiate into other cellular lineages, such as adipogenic and myogenic lineages, in response to different environments [5], which suggests that the differentiation niche of MSC is the critical factor for determining differentiation direction. Our previous research showed that the increased level of fibrosis and expression of fibrosis markers in IUA altered the endometrial stem cell differentiation niche in human endometrium [6], indicating that aberrant activation of fibrosis might be involved in the pathology of IUA and interfere in MSC differentiation.
Several researchers have reported use of hyaluronic acid (HA) to prevent adhesion of the uterine cavity after hysteroscopic surgery in humans [7–9] and peritoneal adhesion in some animal models as a mechanical barrier gel [10,11]. However, the mechanism by which HA prevents uterine adhesion is undefined; it is only known that it functions as a mechanical barrier. HA is a component of the extracellular matrix and was reported to have a reverse function in anti-inflammation at different molecular weights [12,13]. In this study, we examined the effect of HA at high and low molecular weight on the fibrotic degree and the expression of fibrosis markers in the endometrium in a mouse model of IUA.
Material and Methods
Animals maintenance and treatment
This study was approved by the Chongqing Medical University Subcommittee on Research Animal Care. Kunming mice were purchased from the Animal Center of Chongqing Medical University. Six-month-old virgin female Kunming mice were maintained in an appropriate room with controlled temperature and a 12-h light cycle, and were fed standard mouse chow and water. We randomly divided 120 uterine horns from 60 female mice into 2 groups: the sham operation group and the endometrial damage group. Kunming mice were used in the experimental induction of postoperative uterine horn adhesion, as defined by Khrouf [14] and Hu [6], with slight modification. The mice were cycle-synchronized according to their vaginal smear analysis. Ten minutes before the creation of the model, the mice in the damaged group inhaled diethyl for anesthesia. The abdomen was shaved and prepared with iodophors solution. The uterine horns were exposed by an abdominal midline incision of 1 cm longitudinally and a piece of gauze was used to prevent abdominal evisceration. Then, the endometrial lining of the middle and upper two-thirds of the uterus was scraped using a 27-gauge needle. The curette was rotated and withdrawn 4 times. The uterine horn was injected with a 6-mg/L solution of lipopolysaccharide (LPS, Sigma) after curettage. In the sham operation group, the mice only underwent abdominal surgical incision without uterine curettage. Ten mice (n=20 uterine horns) were killed in each group at 1, 2, and 4 estrous cycles after surgery. One horn of each mouse was used for protein detection by immunohistochemistry. The other horn was stored at −80°C. Another 30 mice were randomly divided into 3 groups: control, LMW, and HMW groups. Uterine horns were curetted and injected with LPS, followed by uterine perfusion with 0.5 ml of high molecular weight hyaluronic acid (HMW-HA) (1.2×106 KD, Hangzhou Singclean Medical Product Co., Ltd.), low molecular weight hyaluronic acid (LMW-HA) (0.3~0.5×106 KD, Sigma-Aldrich, USA), or normal saline as control.
Histologic examination
The uterine tissues were fixed in paraffin and routinely stained with Masson stains. On each Masson-stained slice, 4 high-power fields were selected, and the extent of endometrial fibrosis was calculated as the ratio between the fibrotic area and total endometrial area with NIS Element AR 4.0. The average of 4 fields was calculated. The procedure in detail was as follows. A threshold of fibrotic extent was identified before measurement by picking values of 3 points located in fibrotic areas and was applied to every sample uniformly. The region of fibrosis in each high-power field with intensity of greater than or equal to the threshold was marked. The total area of the marked region was calculated automatically. Total endometrial area per high-power field was measured by drawing the edge of the endometrium.
Immunohistochemistry
Immunohistochemistry was performed according to the SP kit instructions (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China). After dewaxing and hydration, the sections were heated in citrate buffer (AR0024, Boster, Wuhan, China) in a microwave oven for 20 min for antigen retrieval. The sections were then cooled naturally to room temperature. The sections were washed in PBS for 5 min ×3 cycles. The sections were then incubated in 3% H202 for 15 min at room temperature and washed with PBS for 5 min ×3 cycles. The sections were blocked in 5% goat serum for 30 min at 37°C. Anti-transforming growth factor beta 1 (TGF β1) rabbit polyclonal antibody (1:100, bs-0086R, Biosynthesis Biotechnology Co., LTD. Beijing, China), anti-connective tissue growth factor (CTGF) rabbit polyclonal antibody (1:400, ab6992, Abcam Company), and anti-collagen I and III rabbit polyclonal antibody (1:100, bs-10423R, bs-0549R, Biosynthesis Biotechnology Co., Ltd. Beijing, China) were incubated with the sections overnight at 4°C. Negative controls included omission of primary antibody and use of irrelevant primary antibodies. The corresponding secondary antibodies, which were conjugated to horseradish peroxidase (Zhongshan Golden Bridge Biotechnology Co. Ltd. Beijing, China), were incubated with the sections for 15 min at room temperature. The sections were washed in PBS for 5 min ×3 cycles. The sections were incubated in horseradish enzyme-labeled chain avidin solution (Zhongshan Golden Bridge Biotechnology Co. Ltd. Beijing, China) for 15 min at room temperature and washed in PBS for 5 min ×3 cycles. The proteins were visualized by diaminobenzidine (DAB).
Western blot
Tissues were lysed with a RIPA buffer containing protease inhibitors. Aliquots of the lysates containing 25 μg of total protein were run on a SDS-polyacrylamide gel. The proteins were transferred to a PVDF membrane. The membrane was incubated overnight at 4°C in TBST containing 5% non-fat milk powder. Anti-collagen I and III rabbit polyclonal antibody (1:500), anti-TGF beta 1 rabbit polyclonal antibody (1:500), anti-CTGF rabbit polyclonal antibody (1:5000), and anti-GAPDH (AB10016, 1:1000, Sangon Biotech, China) were incubated with the membrane overnight at 4°C. Secondary antibodies that were conjugated to horseradish peroxidase were incubated with the membrane for 1 h at room temperature. The proteins that were revealed by Western blotting were visualized by chemiluminescence (Beyotime Institute of Biotechnology). The densities of bands were analyzed by a gel imaging system and calculated compared to the internal control.
Statistical analyses
Data obtained from this study are expressed as the mean ±SEM and analyzed using the t test between 2 groups. Comparisons among multiple groups were made using one-way ANOVA followed by t test using SPSS software 19.0. Statistical significance was defined as P<0.05.
Results
The degree of endometrial fibrosis in mice
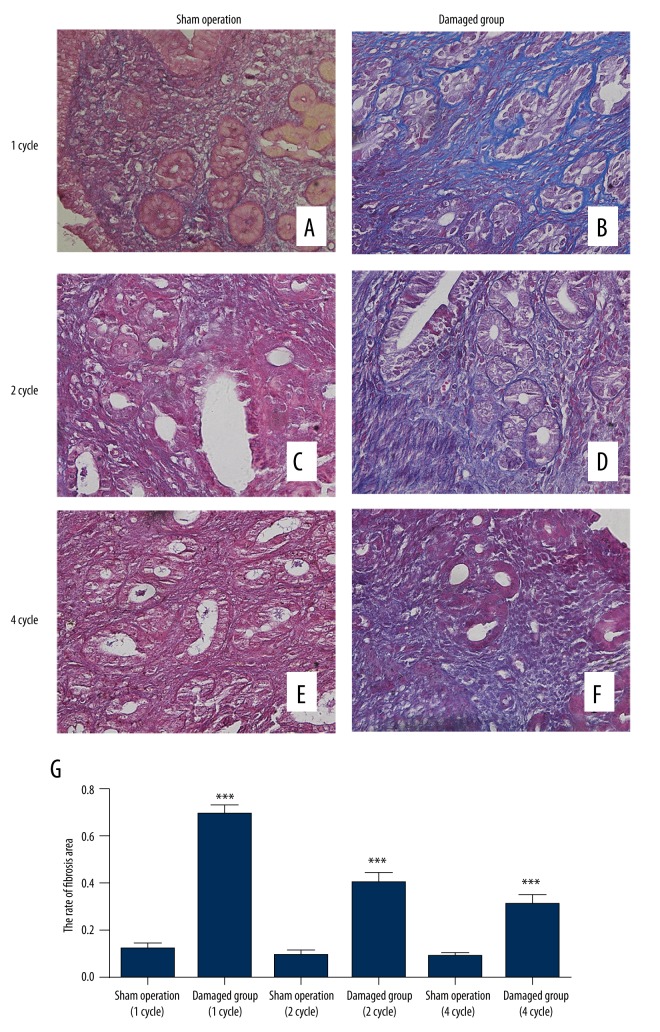
The ratios of the area with endometrial fibrosis to total endometrial area were calculated at 1, 2, and 4 estrous cycles after surgery. The extent of endometrial fibrosis in the damaged group was significantly higher than that in the sham operation group at 1, 2, and 4 estrous cycles after surgery, and the extent of endometrial fibrosis had the highest ratio at the first estrous cycle in the damaged group (Figure 1).
Figure 1.
(A, C, E) Masson stains for mouse endometrial tissues (400×) in sham operation at 1, 2, and 4 estrous cycles after the operation. (B, D, F) Masson stains for mouse endometrial tissues in the damaged group at 1, 2, and 4 estrous cycles after the operation. (G) The rate of fibrosis area. *** P<0.001 (versus sham operation); error bars, SEM.
Expression of TGFβ1, CTGF, collagen I, and collagen III in endometrium of mice between the sham operation group and damaged group
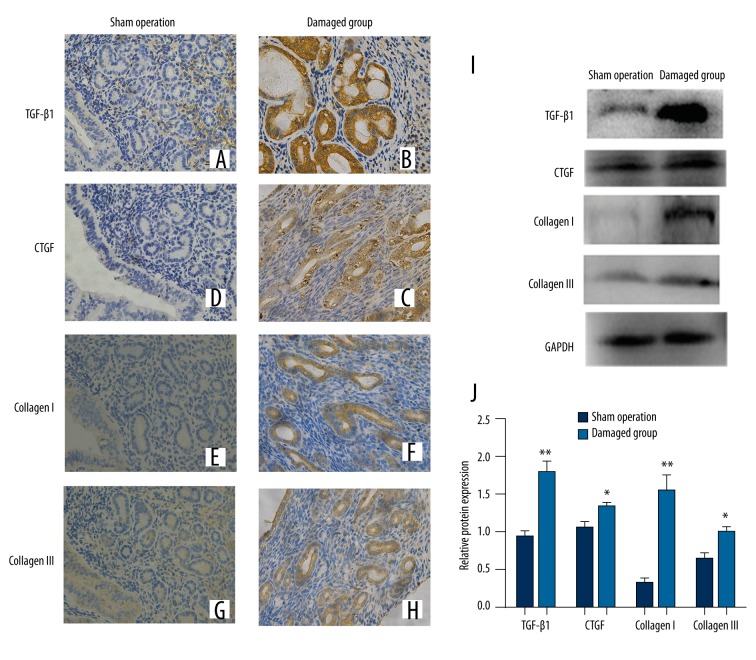
We examined expression of TGFβ1, CTGF, collagen I, and collagen III in endometrium of mice in the sham operation group and the damaged group by immunohistochemistry and Western blot. TGFβ1, CTGF, collagen I, and collagen III were expressed in the cytoplasm of epithelial and stromal cells. The expressions of these proteins in the damaged group were significantly higher than in the sham operation group at 1 estrous cycle after surgery (Figure 2).
Figure 2.
The expressions of fibrosis markers in the mouse endometrial tissues were detected by immunohistochemistry (400×) (A, C, E, G) TGFβ1, CTGF, collagen I, collagen III expressed in endometrial tissue of the sham operation group respectively. (B, D, F, H) TGFβ1, CTGF, collagen I, collagen III expressed in the damaged group respectively. (I). The protein expression levels of TGFβ1, CTGF, collagen I, and collagen III detected by Western blotting. (J) Ratios of TGFβ1, CTGF, collagen I, and collagen III/GAPDH in the sham operation group and damaged group. * P<0.05, **P<0.01 (versus sham operation); error bars, SEM.
The degree of endometrial fibrosis in mice treated with HA
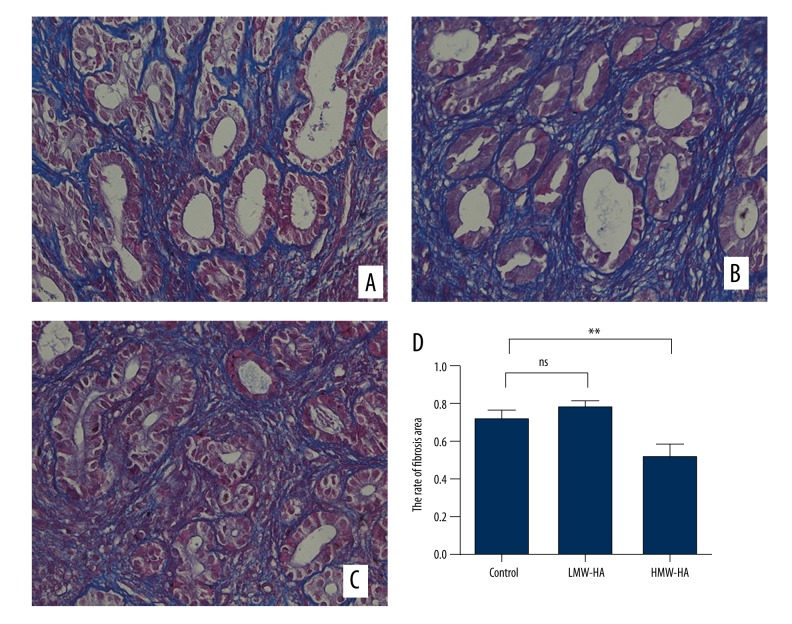
The ratios of the area with endometrial fibrosis to total endometrial area were calculated between the control group and the HA group with 2 different molecular weights. The extent of endometrial fibrosis was significantly reduced in the HMW-HA group compared to the control group. There was no significant difference in extent of endometrial fibrosis between the LMW-HA group and the control group (Figure 3).
Figure 3.
Masson stains for mouse endometrial tissue (400×). (A) Endometrium of the control group. (B) Endometrium of the LMW-HA group. (C) Endometrium of the HMW-HA group. (D) The rate of fibrosis areas. ** P<0.01 (HMW-HA versus control group); error bars, SEM.
Expression of TGFβ1, CTGF, collagen I, and collagen III in endometrium of mice treated with HA (HMW or LMW)
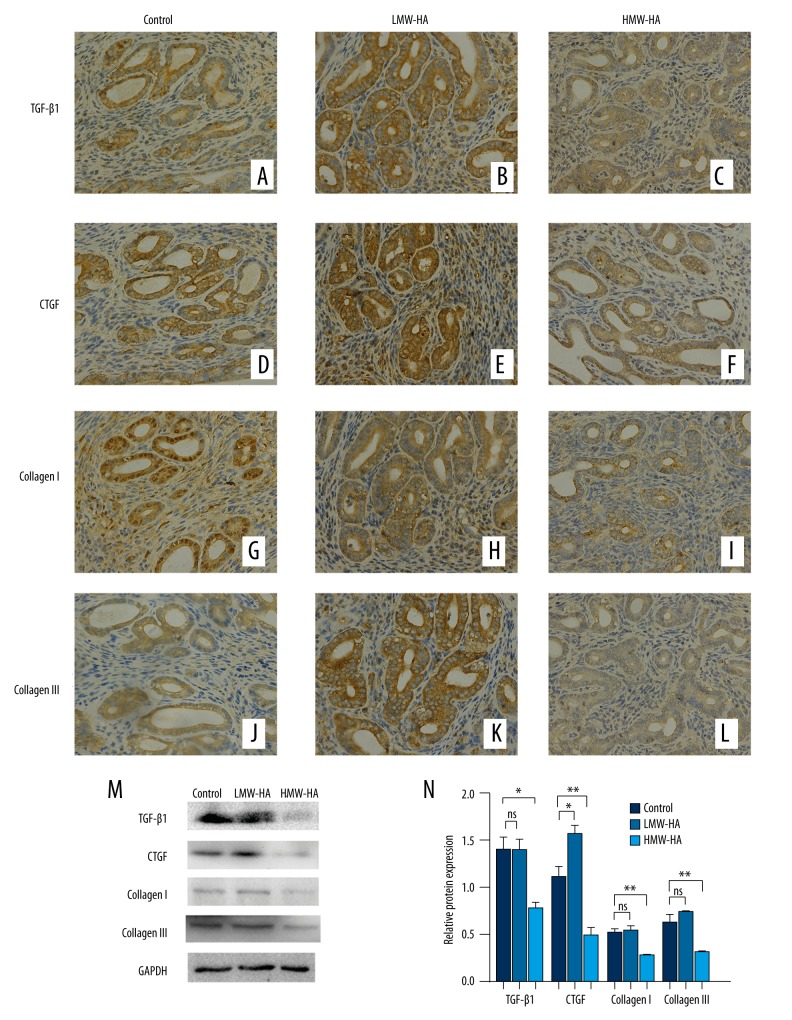
We examined expressions of TGFβ1, CTGF, collagen I, and collagen III in endometrium of uterus of mice by immunohistochemistry and Western blot, as shown in Figure 4. The expressions of TGFβ1, CTGF, collagen I, and collagen III detected by immunohistochemistry decreased in the HMW-HA group (Figure 4C, 4F, 4I, 4L, respectively) compared to the control group (Figure 4A, 4D, 4G, 4J, respectively). The LMW-HA group (Figure 4B, 4E, 4H, 4K, respectively) had no similar effect. The expression levels of TGFβ1, CTGF, collagen I, and collagen III detected by Western blot were significantly decreased in the HMW-HA group compared to the control group. Expression levels of TGFβ1, collagen I, and collagen III were not significantly different between the LMW-HA group and the control group (Figure 4M, 4N).
Figure 4.
Expression levels of fibrosis markers in the mouse endometrial tissues were detected by immunohistochemistry (400×). (A–C) TGFβ1 expressed in endometrium of the control, LMW-HA, and HMW-HA group, respectively. (D–F) CTGF expressed in the 3 groups. (G–I) Collagen I expressed in the 3 groups. (J–L) Collagen III expressed in the 3 groups. (M) The expression levels of TGFβ1, CTGF, collagen I, and collagen III detected by Western blotting in control, LMW-HA, and HMW-HA groups. (N) Ratios of TGFβ1, CTGF, collagen I, and collagen III/GAPDH in the control, LMW-HA, and HMW-HA groups. * P<0.05, ** P<0.01 (versus control group); error bars, SEM.
Discussion
Trauma to a gravid uterine cavity is known to be the main cause of IUA. Such trauma could be induced by uterine curettage in the postpartum period after spontaneous miscarriage or during termination of pregnancy. Trauma to a non-gravid uterine cavity could also result in IUA [1]. Adhesions between the opposing surfaces of the endometrium are composed of fibrotic tissues. Our previous research found that the expressions of fibrosis markers and ESC markers were higher in the endometrial tissue with IUA compared to the control group [6], both in humans and in animal models, suggesting that the number of ESC increases in IUA, and that fibrotic factors plays an important role in the pathogenesis of IUA.
In the present study, the ratio of the area with endometrial fibrosis to total endometrial area was reduced after injection of HMW-HA in an animal model of Asherman’s syndrome. The expressions of fibrosis makers (TGF-β, CTGF, collagen III, and collagen I) were also decreased. These data suggest that HMW-HA, but not LMW-HA, can decrease the degree of fibrosis when the endometrium is treated with curettage and perfusion of LPS. These results are in agreement with other published reports that suggest HMW-HA can be anti-fibrotic in renal fibrosis [15], as well as being anti-inflammatory [16,17].
HA clearly has many function in the body, interacting with a number of cell types, primarily by the cell surface receptor CD44 [18]. HA may also interact with cells via other receptors as well, including TLR-2, TLR-4, RHAMM, and LYVE1 [16,19]. HA was reported to have an anti-fibrotic effect in the kidneys through interacting with CD44 [15]. The combination of CD44 and HA can inhibit the function of TGF-β1, which plays a very important role in organic fibrosis. Endometrium stromal and epithelial cells are known to have some of these receptors on their surface, such as CD44 [20], which might be involved in the mechanism of the anti-fibrotic effect of HA in uterine endometrium. Admittedly, the mechanism by which HMW-HA reduced the expression of fibrosis markers needs to be further studied.
Approximately 6–8 million induced abortions per year were carried out between 2003 to 2007 in China, in which repeat abortions accounted for up to 50% [21], which can lead to repeated damage to the endometrium and uterine adhesion. Although the most important way to prevent repeated artificial abortion is to increase the population’s awareness of contraception, preventing formation of IUA after uterine surgery is also important. The HMW-HA can be used as a physical barrier and also as a potential anti-fibrotic agent that may improve the outcome of endometrial trauma.
Conclusions
HA at high molecular weight may attenuate the fibrotic degree of endometrium after endometrial damage. Injection of HA of HMW into the uterine cavity after endometrial damage, such as that caused by curettage or endoscopic surgery, may prevent Asherman’s syndrome.
Footnotes
Source of support: Departmental sources
References
- 1.Yu D, Wong YM, Cheong Y, et al. Asherman syndrome – one century later. Fertil Steril. 2008;89:759–79. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadi F, Siahbazi S, Akhbari F, et al. Hysterosalpingography finding in intra uterine adhesion (Asherman’ s syndrome): A pictorial essay. Int J Fertil Steril. 2013;7:155–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124–30. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanova L, Horcajadas JA, Esteban FJ, et al. The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod. 2010;82:1076–87. doi: 10.1095/biolreprod.109.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Zeng B, Jiang X, et al. The expression of marker for endometrial stem cell and fibrosis was increased in intrauterine adhesious. Int J Clin Exp Pathol. 2015;8:1525–34. [PMC free article] [PubMed] [Google Scholar]
- 7.Lin X, Wei M, Li TC, et al. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: A cohort study. Eur J Obstet Gynecol Reprod Biol. 2013;170:512–16. doi: 10.1016/j.ejogrb.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, et al. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Mater Res. 2002;63:10–14. doi: 10.1002/jbm.10040. [DOI] [PubMed] [Google Scholar]
- 9.Guida M, Acunzo G, Di Spiezio Sardo A, et al. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: A prospective, randomized, controlled study. Hum Reprod. 2004;19:1461–64. doi: 10.1093/humrep/deh238. [DOI] [PubMed] [Google Scholar]
- 10.Kelekci S, Uygur D, Yilmaz B, et al. Comparison of human amniotic membrane and hyaluronate/carboxymethylcellulose membrane for prevention of adhesion formation in rats. Arch Gynecol Obstet. 2007;276:355–59. doi: 10.1007/s00404-007-0376-7. [DOI] [PubMed] [Google Scholar]
- 11.Attar R, Yildirim G, Kumbak B, et al. Efficacy of melatonin and hyaluronate/carboxymethylcellulose membrane in preventing adhesion reformation following adhesiolysis in a rat uterine model. J Obstet Gynaecol Res. 2011;37:125–31. doi: 10.1111/j.1447-0756.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang PM, Syrkina O, Yu L, et al. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology. 2010;15:1131–39. doi: 10.1111/j.1440-1843.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 13.Baeva LF, Lyle DB, Rios M, et al. Different molecular weight hyaluronic acid effects on human macrophage interleukin 1beta production. J Biomed Mater Res Part A. 2014;102:305–14. doi: 10.1002/jbm.a.34704. [DOI] [PubMed] [Google Scholar]
- 14.Khrouf M, Morel O, Hafiz A, et al. Evaluation of the rabbit as an experimental model for human uterine synechia. J Hum Reprod Sci. 2012;5:175–80. doi: 10.4103/0974-1208.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito T1, Williams JD, Fraser D, Phillips AO. Hyaluronan attenuates transforming growth factor-β1-mediated signaling in renal proximal tubular epithelial cells. Am J Pathol. 2004;164:1979–88. doi: 10.1016/s0002-9440(10)63758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Ann Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 17.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: Are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10–37. doi: 10.1053/sarh.2002.33720. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan KM, DeLott LB, West RA, et al. Hyaluronic acid of high molecular weight inhibits proliferation and induces cell death in U937 macrophage cells. Life Sci. 2004;75:3087–102. doi: 10.1016/j.lfs.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Poncelet C, Leblanc M, Walker-Combrouze F, et al. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet Gynecol Scand. 2002;81:195–203. doi: 10.1034/j.1600-0412.2002.810302.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu SC, Qiu HY. [Induced abortion in China: Problems and interventions]. Zhongguo yi xue ke xue yuan xue bao Acta Academiae Medicinae Sinicae. 2010;32:479–82. doi: 10.3881/j.issn.1000-503X.2010.05.001. [in Chinese] [DOI] [PubMed] [Google Scholar]