Abstract
Assessment of influenza virus disease progression and efficacy of antiviral therapy in the widely used mouse models relies mostly on body weight loss and lung virus titers as markers of disease. However, both parameters have their shortcomings. Therefore, the aim of our study was to find non-invasive markers in the murine model of severe influenza that could detect disease early and predict disease outcome. BALB/c mice were lethally infected with influenza A(H1N1)pdm09 virus and serum samples were collected at various time points. Enzyme-linked immunosorbent assays were performed to quantify amounts of serum amyloid A (SAA), C-reactive protein, complement 3, transferrin, corticosterone, prostaglandin E2, H2O2, and alpha-2,6-sialyltransferase. We found that SAA was the most promising candidate with levels acutely and temporarily elevated by several hundred-fold 3 days post virus inoculation. Upon treatment with oseltamivir phosphate, levels of SAA were significantly decreased. High levels of SAA were associated with poor disease prognosis, whereas body weight loss was not as a reliable predictor of disease outcome. SAA levels were also transiently increased in BALB/c mice infected with influenza A(H3N2) and influenza B virus, as well as in C57BL/2, Swiss-Webster, and DBA.2 mice infected with influenza A(H1N1)pdm09 virus. High levels of SAA often, but not always, were associated with disease outcome in these other influenza virus mouse models. Therefore, SAA represents a valid biomarker for influenza disease detection in all tested mouse strains but its prognostic value is limited to BALB/c mice infected with influenza A(H1N1)pdm09 virus.
Keywords: Influenza, mouse model, biomarker, antiviral
1. Introduction
Considered by some as merely a nuisance, influenza is a highly contagious acute respiratory infection caused by influenza A and B viruses. The disease is often self-limiting and typically presents with sudden onset of fever, arthralgia, some respiratory symptoms, headache, and general malaise (Taubenberger and Morens, 2008). Occasionally influenza cases escalate into hemorrhagic bronchitis, viral pneumonia, and acute respiratory distress syndrome (ARDS) with sometimes fatal outcome. Certain risk factors (age, pregnancy, obesity, and other co-morbidities) have been associated with a higher likelihood of these complications, but even otherwise healthy adults have been seen with severe cases of influenza requiring intensive care (Bautista et al., 2010). It was also noted that a large proportion of children that died from seasonal influenza in the winter of 2003-2004 in the UK had no apparent risk factors or underlying illness (Galiano et al., 2012). Thus, predicting which patients will be more prone to developing severe influenza is a challenge, as disease outcome is a function of highly complex host-virus interactions.
To study these and many other aspects of influenza virus biology, as well as to assess the efficacy of antiviral therapy and vaccines, animal models that mimic the disease progression in humans have been used extensively. Ferrets are regarded by many as the ideal small animal host for influenza as they are naturally susceptible to human influenza viruses, and they display disease symptoms as seen in humans (Matsuoka et al., 2009). However, the large size of the animals coupled with the fact that infected ferrets can potentially be also contagious to humans make special caging and handling necessary. Furthermore, some of the observed disease parameters, such as elevated temperature, can vary considerably among infected ferrets (Smith et al., 1933; Stark et al., 2013), thus making statistical inferences difficult. Although mice do not develop a fever or display many other typical symptoms associated with influenza in humans (coughing, sneezing), they offer several advantages over ferrets. Cost, ease of genetic manipulation (knock-out mice), ethical considerations, availability of serological assays and reagents, and a massive body of literature compiled over 80 years make mice the most widely used animal model, particularly mice of the BALB/c strain. Mice are not naturally susceptible to most seasonal human influenza viruses, however, requiring the virus to be adapted by serial lung passaging. By the same token, most mice strains (including BALB/c mice) were found not to transmit the disease to humans or other mice, which simplifies handling procedures (Bouvier and Lowen, 2010).
Parameters in mice used to monitor disease progression and evaluate antiviral treatment and vaccine efficacy depend on the severity of influenza virus infection (Barnard, 2009). If mice are infected with influenza viruses of low pathogenicity, only body weight loss and lung virus titers can be reliably utilized as a measure of morbidity. In severe infections in mice, the effects can further be gauged by weight and histological changes of the lung. Although body weight loss generally indicates disease progression, it may not reliably predict disease outcome in lethal infections of mice. Lung virus titers are commonly measured as they correlate well with infection status, but they do not always reflect antiviral treatment effects (Wong et al., 2011). In this case, mice have to be sacrificed to obtain lung tissue. The use of plethysmography to assess the physiological impact on lung function has been reported (Julander et al., 2011). Arterial oxygen saturation (SaO2) has also been utilized to monitor moderate to severe respiratory disease based on the inverse correlation of oxygen saturation in blood with viral inoculum (Sidwell et al., 1992). This procedure has not garnered widespread acceptance since non-invasive measurements can only be performed on white mice. Measurement of cytokine levels on the other hand has become very popular. Cytokines are primary mediators of the inflammatory response, which is often elicited by infectious agents. As such, they affect a multitude of physiological processes related to self-defense (Sladkova and Kostolansky, 2006). One important function of cytokines is the regulation of acute-phase proteins and their synthesis. As part of the first line of defense, acute-phase proteins are a group of proteins whose plasma concentrations change in response to inflammation to restore homeostasis (Baumann and Gauldie, 1994).
Serological markers as disease indicators are widely used in human medicine, but their application in the mouse model of influenza is less established. Only alpha-1-acid glycoprotein (AGP), an acute-phase protein, has been described to correlate with disease status in mice infected with influenza A or B virus and to respond to antiviral treatment in a dose-dependent manner (Sidwell et al., 2001). Although results from this study suggested AGP could be used as an additional parameter for the evaluation of antiviral therapy, it has been later noted that the long-term availability of the commercial kit used to determine AGP serum levels in mice was uncertain (Sidwell and Smee, 2004).
The purpose of our study was to evaluate other non-invasive serological parameters as early disease markers and indictors of disease severity in the mouse model of severe influenza virus infection. In this investigation, the kinetics of several acute-phase proteins (serum amyloid A, SAA, C-reactive protein, complement factor 3, transferrin) and molecules associated with the host immune response (corticosterone, prostaglandin E2, hydrogen peroxide, alpha-2,6-sialylstransferase) were assessed by enzyme-linked immunosorbent assay (ELISA) in several mouse strains using a variety of influenza strains as inocula. SAA was the most promising candidate and was further evaluated in relation to antiviral therapy with oseltamivir phosphate (Tamiflu®), an established, FDA-approved anti-influenza drug.
2. Materials and methods
2.1. Animals
Specific pathogen-free, female BALB/c, C57BL/6, Swiss-Webster, and DBA.2 mice (6-8 weeks-old, approximately 17-19 g) were obtained from Charles River Laboratories (Wilmington, MA). The animals were maintained on Teklad Rodent Diet (Harlan Laboratories Inc., Indianapolis, IN) and tap water ad libitum. Mice were quarantined for three days prior to the initiation of each experiment. Studies were completed in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University. The experiments were conducted in the AAALAC-accredited Laboratory Animal Research Center of Utah State University.
2.2. Viruses
Pandemic and now seasonal influenza A/California/04/2009 (H1N1) virus (referred to as influenza A(H1N1)pdm09 virus in this article) was originally received from Dr. Elena Govorkova, St. Jude Children's Research Hospital, Memphis, TN. The virus was adapted to mice by Natalia Ilyushina and colleagues (Ilyushina et al., 2010) at the same institution. The plaque-purified virus was amplified at Utah State University in Madin-Darby canine kidney (MDCK) cells to prepare stocks for use in mouse studies. Virus pools were pre-titrated in BALB/c mice to determine approximate 90% and 100% lethal doses (LD90 and LD100). The virus stock was diluted in Minimum Essential Medium with Earle's Balanced Salts and L-glutamine (MEM, HyClone, GE Healthcare Life Sciences, Logan, UT) at 1:150 and 1:200, respectively. Mice were infected with 90 or 100 μl of the diluted stock virus. The viral challenge dose per animal was approximately 103.8 and 103.9 50% cell culture infectious doses (CCID50), which corresponded to approximately 1LD90 and 1LD100, respectively.
Influenza A/Victoria/3/75 (H3N2) was obtained from the American Type Culture Collection (Manassas, VA). It was passaged once through embryonated chicken eggs, once in MDCK cells, and 7 times in BALB/c mice. A pool was then prepared in MDCK cells and pre-titrated in BALB/c mice prior to any experiment. Mice were infected with 100 μl of the diluted stock virus (1:20 in MEM). The viral challenge dose per animal was approximately 104.4 CCID50, which corresponded to approximately 1LD100.
Influenza B/Sichuan/379/99 (Yamagata lineage) virus was obtained from the Centers for Disease Control and Prevention (Atlanta, GA). The virus was propagated twice in MDCK cells and then further passaged 10 times in BALB/c mice to create a virus pool for animal studies. The virus stock was previously tested for titer and infectivity in BALB/c mice. The titer was determined to be 107.2 CCID50/ml, and an inoculum of 100 μl with a 1:30 dilution of virus stock in MEM solution equaled approximately 2LD90 in BALB/c mice.
2.3. Antiviral compound
Oseltamivir phosphate (Tamiflu®) was purchased from a local pharmacy. A 75-mg capsule was dissolved in sterile phosphate buffered saline (PBS) to final concentrations of 1, 10, and 20 mg/ml. All dilutions were prepared immediately prior to the start of the experiment and stored in injection bottles at 4°C for the duration of the treatment period.
2.4. Experimental Design
Animals were weighed, ear-tagged, and randomly assigned to treatment groups. Mice were anesthetized by intraperitoneal injection of ketamine/xylene (50/5 mg/kg) and then infected intranasally with a 90- or 100-μl suspension of diluted stock virus as mentioned above to achieve the desired viral challenge. Control animals were sham-infected with MEM only. Mice were observed daily for weight loss and mortality. In the first experiment, pre-assigned mice were sacrificed at specific time points for blood collection. In all other experiments, blood (∼25-50 μl) was repeatedly drawn from the mandibular vein (cheek bleed) of the same animals and collected in uncoated, conical microcentrifuge tubes. Blood was allowed to clot at 4°C for an hour before the serum (∼10-15 μl per animal, less for mice showing progressive disease symptoms) was carefully removed from the top with a syringe without disturbing the pellet containing red blood cells. Serum samples were stored at -80°C until further analysis. For the antiviral therapy experiment, oseltamivir phosphate was used as a therapeutic treatment and given orally twice a day, 12 hours apart, for 5 days, starting at 4 hours post infection (+4 hpi, bid × 5). Sterile saline (placebo) was administered to infected control animals in parallel. The endpoint of the mouse experiments was 30% weight loss or mortality unless justification of extension of not using this as an endpoint as approved by the ICUUC.
2.5. Biomarker quantification
Biomarker candidate levels from mouse serum samples were quantified with commercially available kits. Mouse C-Reactive Protein ELISA Test Kit, Mouse Serum Amyloid A Test Kit, and Mouse Transferrin ELISA Kit were purchased from Life Diagnostics (West Chester, PA). Mouse Complement Factor 3 Immunoperoxidase Assay Kit was obtained from GenWay Biotech (San Diego, CA). DetectX® Hydrogen Peroxide Colorimetric Detection Kit, DetectX® Prostaglandin E2 Enzyme Immunoassay Kit and DetectX® Corticosterone Enzyme Immuno Kit were ordered from Arbor Assays (Ann Arbor, MI). Alpha-2,6-Sialyltransferase Assay Kit was obtained from Immuno-Biological Laboratories Co. (Minneapolis, MN). All assays were performed as suggested by the manufacturer with the exception that wash steps were increased from 6 to 8 with smaller volumes of wash buffer (250 μl instead of the suggested 400 μl). Initially, mouse serum samples were tested in serial half log or log10 dilutions. Optimum dilutions for SAA levels ranged between 1:1000 and 1:10,000. Absorbance measurements were taken using a computerized microplate reader (SPECTRAmax Plus, Molecular Devices Corporation, Sunnyvale, CA). A standard curve was generated with the most appropriate fit (four-parameter fit for most ELISAs including SAA) and unknown concentrations were interpolated and corrected for sample dilution. Samples were run in at least duplicates. SAA levels were determined from 3 independent assays in Experiment 1, from 2 independent assays in Experiments 2 and 3, and from 1 independent assay in all other experiments. The SAA ELISA kit used in this study preferentially detects SAA2. For simplicity, we will refer in this article to the protein as SAA without the isoform designation.
2.6. Lung score, weight, histopathology, and virus titer
Lung hemorrhage score (lung score) measurements were made by visual inspection of discoloration of the lung after necropsy and were estimated on a scale of 0-4 in 0.5-U increments, with a score of 4 being 100% hemorrhaged. Lung weight was measured after scoring and recorded in milligram. Lung histopathology assessment was performed at the Veterinary Diagnostics Laboratory, Utah State University, Logan, UT. Samples were formalin fixed and sectioned material was stained with hematoxylin and eosin and observed for pathological changes and evidence of virus infection. All observations were made blinded. Lung virus titers were determined as described in Smee and Barnard (2013).
2.7. Cytokine and chemokine determination
Cytokines and chemokines were determined from supernatant fluid of lung homogenates (local cytokines/chemokines) and from serum (systemic cytokines/chemokines) using commercially available test kits as described by the manufacturer (Quansys Inc. Logan, UT).
2.8. Statistical analysis
In Experiment 1, serum samples were pooled from five mice per group. This precluded statistical assessment. In all other experiments, SAA levels from individual animals were analyzed either by unpaired, two-tailed t-test or by analysis of variance (ANOVA) followed by appropriate multiple comparison tests. Mean body weights were analyzed by two-way ANOVA followed by appropriate multiple comparison tests. Kaplan-Meier survival curves were generated and compared by the log-rank (Mantel-Cox) test followed by pairwise comparison using the Gehan-Breslow-Wilcoxon test. Hazard ratios and their confidence intervals were computed using the Mantel-Haenszel method. Lung hemorrhage scores, lung weight, lung virus titers, cytokines and chemokines were analyzed with nonparametric one-way ANOVA followed by appropriate multiple comparison tests. All statistical analyses were performed in GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA).
3. Results
3.1. Initial screening of eight biomarker candidates for disease detection in the mouse model of severe influenza
To assess the propensity of eight biomarkers as early indicators of influenza disease, BALB/c mice were experimentally infected with a lethal dose of influenza A(H1N1)pdm09 virus. Cohorts of 5 animals were sacrificed for serum collection at early time points during the infection (0, 4, 8, 12, 16, 20, 24, 36, 48, and 72 hours post-virus inoculation, hpi). The amount of each potential biomarker was determined with commercially available ELISA kits and the kinetics are presented in Figure 1. While the majority of tested candidates did not show meaningful differences in sera from infected and sham-infected animals, serum amyloid A (SAA) and corticosterone levels increased at 72 hpi (Fig. 1A, E). SAA levels were more than 2000-fold higher in infected animals, whereas the increase in corticosterone was only 2-fold. A 2-fold increase was also seen in transferrin levels in infected animals at 16 hpi (Fig. 1D). A suppression of an early peak of alpha-2,6-sialyltransferase was detected in sera collected from infected animals at 8 hpi (Fig. 1H). In summary, SAA was the biomarker candidate with the highest fold-change compared to sham-infected animals. Therefore, we focused on this particular protein for further evaluation.
Fig. 1.
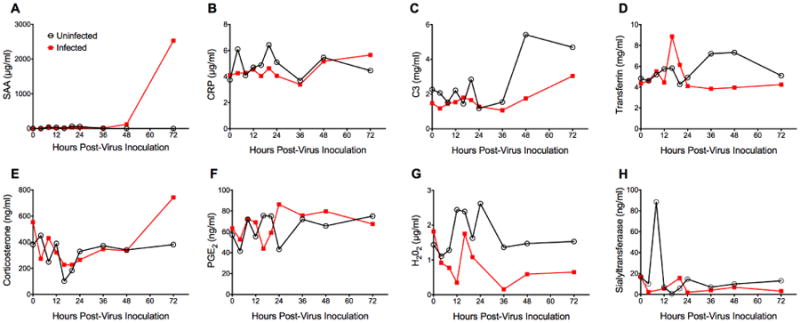
Screening of serological biomarker candidates for early detection of influenza virus disease in BALB/c mice. Mice (n=50) were intranasally infected with mouse-adapted influenza A/California/04/2009 (H1N1)pdm09 virus (1LD100). Control animals (uninfected) received dilution vehicle (MEM) only. At specific time points (0, 4, 8, 12, 16, 20, 24, 36, 48, 72 hours post-virus inoculation), 5 animals per cohort were sacrificed for serum collection. Survival and body weight were monitored daily (n=5, data not shown). The experiment was terminated 3 days post-virus inoculation. Serum was analyzed for levels of (A) Serum amyloid A (SAA), (B) C-reactive protein (CRP), (C) Complement factor 3 (C3), (D) Transferrin, (E) Corticosterone, (F) Prostaglandin E2 (PGE2), (G) H2O2, and (H) alpha-2,6-Sialyltransferase. SAA levels were quantified in three independent ELISA assays and CRP levels were determined in two independent ELISA assays. All other candidates were tested only once. Mean and SD values could not be calculated as serum samples were pooled per cohort to assure sufficient testing material for all markers. SAA showed the greatest potential as a biomarker candidate.
3.2. Mouse serum amyloid A (SAA) as an early influenza disease marker
Since the first animal experiment was terminated at 72 hpi, a time point that coincided with a dramatic increase in SAA levels in infected mice, a second animal experiment was designed to determine whether SAA levels would peak at a later point or possibly stayed elevated during the entire course of infection. Results from this experiment are presented in Figure 2. As expected, infected animals started to lose weight shortly after virus exposure indicative of a successful infection (Fig. 2A). At the end of the experiment (7 dpi), infected animals had lost almost 30% of their initial body weight. However, none of the infected animals had died before termination of the experiment (data not shown). In sham-infected control mice SAA levels increased slightly over the first 2 days, after which they were found to be reduced to or even below the limit of detection (Fig. 2B). This minor change (statistically not significant), which was noticed in the first experiment as well (Fig. 1A), was most likely elicited by stress and potential trauma to the animals during the procedure of sham-infection. Whereas control animals recovered, as indicated by a reduction of SAA to baseline levels, SAA levels in infected animals spiked at 3 dpi to various degrees followed by a rapid decrease over the next days (Fig. 2B). At 3 dpi, mean SAA levels from infected animals were significantly higher than mean SAA levels from sham-infected animals (471 ± 255 vs. 14 ± 11 μg/ml, P = 0.0003, Fig. 2C). The broad range of SAA concentrations in infected animals at 3 dpi might be indicative of natural mouse-to-mouse variability. Slight elevations of SAA concentrations were observed at 7 dpi in a few infected and uninfected mice (Fig. 2B). While we do not have a plausible explanation for this, we find it not to be of practical importance to our study, since this elevation was observed in animals regardless of infection status and statistical analysis confirmed lack of significance (data not shown). Although this experiment clearly demonstrated that SAA was an early (72 hpi) indicator of severe influenza virus infection, no correlations between absolute SAA levels and experimental outcome (prediction of death) could be made since all infected animals were still alive at the end of the experiment. A third animal experiment was conducted to address this point.
Fig. 2.
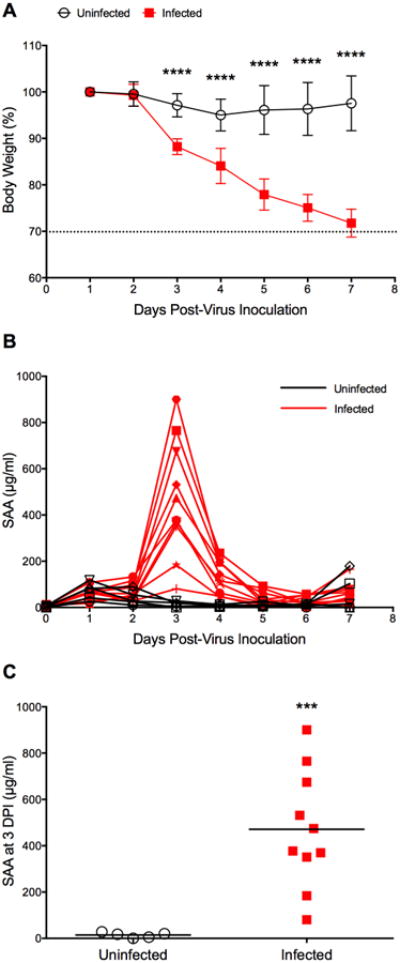
Time course of SAA in BALB/c mice infected with influenza A/California/04/2009 (H1N1)pdm09 virus. Mice (n=10) were intranasally infected with virus (1LD90). Control animals (uninfected, n=5) received dilution vehicle (MEM) only. At specific time points (0-7 dpi), animals were cheek-bled for serum collection. The experiment was terminated 7 days post-virus inoculation. (A) Survival and body weight were monitored daily (survival data not shown since all animals were still alive at the end of the experiment). Body weight data are presented as the mean ± SD. The dashed line indicates 30% body weight loss. ****P < 0.0001 for days 3-7 dpi, compared with the uninfected control group; two-way ANOVA with Sidak's multiple comparison test. (B) Time course of SAA as determined in three independent ELISA assays of which one representative data set is shown here. (C) SAA levels in serum collected at 3 dpi. ***P = 0.0003, compared to uninfected control; unpaired t-test with Welch's correction.
3.3. Effect of antiviral treatment on SAA levels and SAA as an early predictor of influenza disease outcome
Generally, mice are anticipated to succumb to the consequences of a lethal infection with influenza virus between days 6 and 14 (Smee and Barnard, 2013). Accordingly, infected BALB/c mice were monitored for 21 days. Animals were challenged with a slightly lower concentration of viral inoculum of influenza A(H1N1)pdm09 virus to achieve an approximate 1LD90. This lower dose (as compared to 1LD100 in the first experiment) was used to examine the possibility that SAA levels could be predictive of the outcome of influenza virus infections in mice in which some survivors would be expected. Furthermore, we wanted to test the hypothesis that antiviral treatment would reduce SAA levels in infected animals while also protecting from death. Thus, we included two additional groups in the experiment in which infected animals were treated with either a low dose (1 mg/kg/d) or a high dose (10 mg/kg/d) of the neuraminidase inhibitor oseltamivir phosphate. Treatments started 4 hpi and were administered orally twice a day for five days (+4 hpi, bid × 5). Kaplan-Meier survival curves indicated that placebo-treated animals died between 7 and 12 dpi (Fig. 3A). At the end of the experiment only one animal survived in that group, which confirmed the infective dose (1LD90). Treatment with oseltamivir at both concentrations was 100% effective and protected against death. Mice were ∼ 18 times less likely to die as rapidly compared to infected mice receiving only saline (hazard ratio of 18.29). However, antiviral treatment did not protect from weight loss although this was less severe in oseltamivir-treated mice (Fig. 3B). Surprisingly, weight loss was more pronounced in this experiment compared with the weight loss results from the previous experiment even though the viral challenge was lower (1LD90 vs. 1LD100). This could be due to differences in the innate immune response based on genetic mouse-to-mouse variability. Also, weight and age of mice affect virus infectivity (Smee and Barnard, 2013). We can never obtain mice of the exact same ages as our commercial supplier always sends us a range of ages.
Fig. 3.
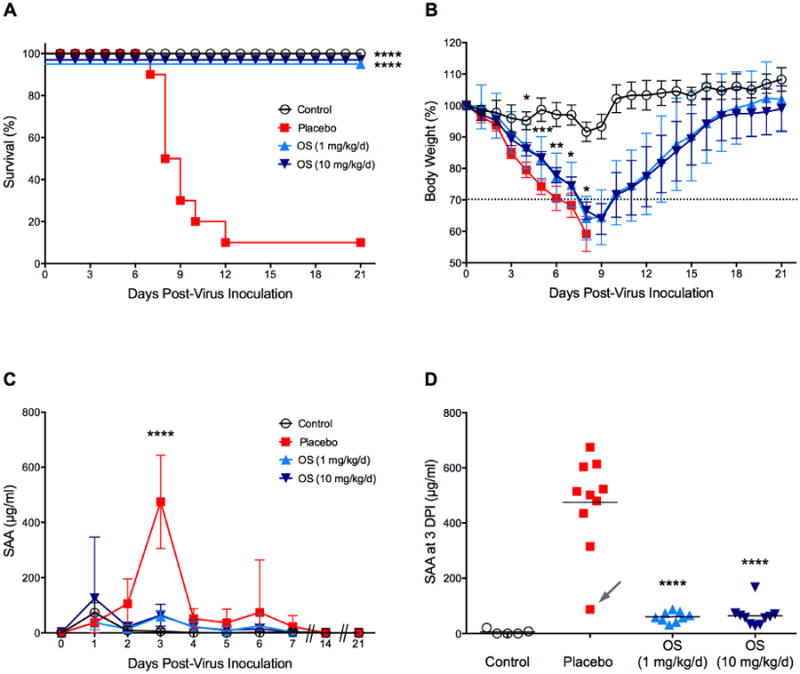
Extended SAA time course and effect of antiviral treatment on SAA levels in BALB/c mice. Mice (n=30) were intranasally infected with mouse-adapted influenza A/California/04/2009 (H1N1)pdm09 virus (1LD90). Control animals (uninfected, n=10) received dilution vehicle (MEM) only. Four hours later, ten mice per group received placebo (saline), oseltamivir at 1 mg/kg/d and 10 mg/kg/d, respectively, twice daily for 5 successive days by oral gavage (+4 hpi, bid × 5). At specific time points (0-7, 14, and 21 dpi), animals were cheek-bled for serum collection. The experiment was terminated 21 days post-virus inoculation. Survival and body weight were monitored daily. (A) Kaplan-Meier survival curves were computed and compared with the log-rank (Mantel-Cox) and Gehan-Wilcoxon test. The hazard ratio was 18.29. (B) Percent body weight change was calculated by dividing the daily body weight by the weight of the same mouse on Day 0. Oseltamivir-treated animals lost significantly less weight at 4-8 dpi compared to placebo animals. (C) Time course of SAA as determined from a single ELISA assay. SAA levels sharply increased at 3 dpi in the placebo group. (D) SAA amounts in serum samples from individual animals taken 3 dpi. Samples were tested in quadruplicates in two independent ELISA assays. Both oseltamivir treatments significantly reduced SAA levels to uninfected control levels. The dashed line in (B) indicates 30% body weight loss. Arrow in (D) points to the only surviving animal from the placebo group with low SAA levels (87 μg/ml). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
The time course of SAA was comparable to the previous experiment and showed an acute increase at 3 dpi compared to the uninfected control group (Fig. 3C; P < 0.0001). SAA levels ranged from 315 to 675 μg/ml with a mean of 475 μg/ml. One notable outlier can be seen at the low end (87 μg/ml, Fig. 3D, arrow). Treatment with oseltamivir reduced SAA levels significantly (P < 0.0001) with a mean of 61 μg/ml for mice treated with a low dose, and a mean of 64 μg/ml for mice treated with the high dose. Importantly, both means were not significantly different from sham-infected control mice (6 μg/ml).
To summarize the results from this experiment, high SAA levels at 3 dpi (> 300 μg/ml) were linked to future death in BALB/c mice lethally challenged with influenza A(H1N1)pdm09 virus and low levels of SAA were associated with the efficacy of oseltamivir treatment and thus survival.
3.4. SAA levels and the systemic cytokine/chemokine response in BALB/c mice infected with influenza A(H1N1)pdm09 virus and other influenza disease parameters
To further support our hypothesis that serum levels of SAA are a prognostic factor for the outcome of severe influenza infection in BALB/c mice, we evaluated other influenza disease parameters, such as the local and systematic immune and inflammatory response and virus-associated pathology in the lung along with the SAA and weight loss measurements in the same animals. Results are presented in Figure 4. Kaplan-Meier survival curves indicate that oseltamivir-treated mice were 100% protected, whereas all placebo animals died between 5 and 7 dpi (Fig. 4A). This surprisingly early death could have been related to a new virus pool with unexpectedly high infectivity. Oseltamivir-treated animals lost weight during the infection but significantly less than placebo animals between 2 and 4 days dpi (Fig. 4B). Mean serum SAA levels were significantly elevated in placebo animals (1808 μg/ml; P < 0.001) compared to control animals, and oseltamivir significantly reduced SAA levels to control levels (Fig. 4C; P = 0.0007). It is interesting to note that mean SAA levels in this experiment (1808 μg/ml) were much higher than in the previous two experiments (471 and 475 μg/ml, respectively), but comparable to those in the first experiment (2529 μg/ml). Histopathological findings in lungs of oseltamivir-treated mice included generally mild to moderate epithelial injury and inflammatory reaction in the bronchi, bronchioles and alveolar parenchyma, which were diagnosed as bronchointerstitial pneumonia with diffuse bronchial and bronchiolar epithelial cell regeneration. No significant microscopic lesions were found in the lungs of control animals. Lung virus titers were determined as 5.58 log10 CCID50/ml and below detection limit (0.67 log10 CCID50/ml) in control animals. Placebo animals did not survive the infection and therefore no histopathological findings, lung virus titers, and cytokine data at 7 dpi were available for these animals. To show the utility of the SAA response as a good indicator of the severity of the infection, mouse lung cytokine responses to an infection with the influenza A(H1N1)pdm09 were also evaluated at day 3 post virus exposure when all mice were still alive. At day 3 dpi, there was a general trend for a modest proinflammatory cytokine response in the lungs of infected placebo animals compared to the response for infected, treated with oseltamivir or untreated, uninfected mice (Fig. 4D). Five out of 12 tested cytokines were without significant changes in placebo animals compared to oseltamivir-treated animals and only MCP-1 and RANTES were significantly increased. The cytokine response was somewhat muted for an influenza virus infection of mice compared to infections using other influenza virus strains, which is in agreement with the findings of Belser et al. (2010) who had reported a similar phenomenon previously when evaluating the lung cytokine response to H1N1 2009 pandemic virus infection of mice.
Fig. 4.
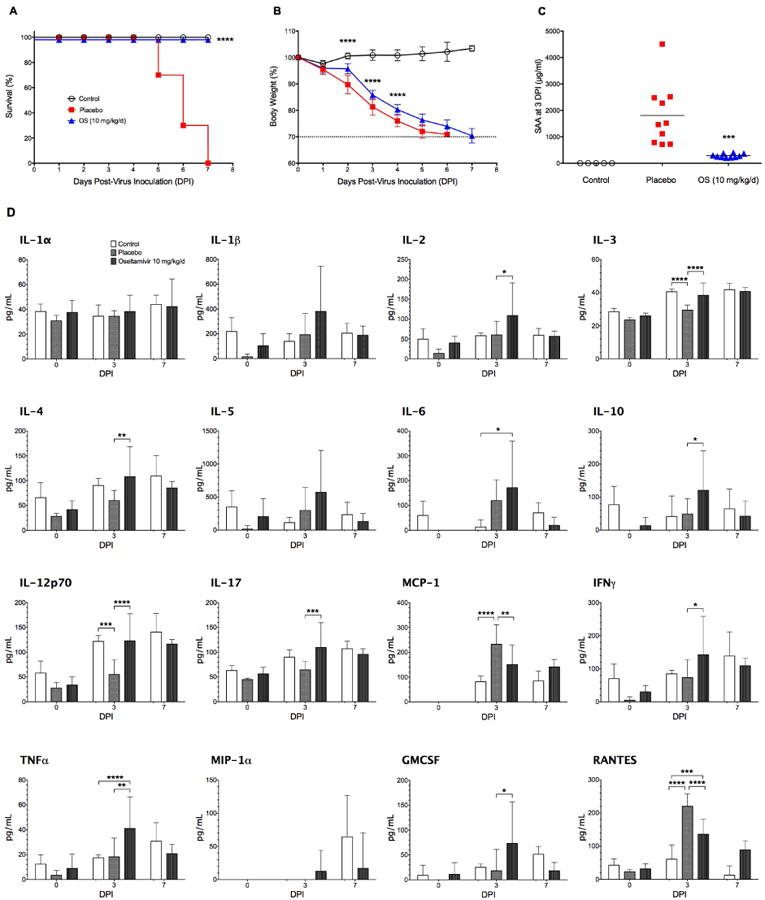
SAA levels and systemic cytokine/chemokine profile in BALB/c mice infected with mouse-adapted influenza A/California/04/2009 (H1N1)pdm09 virus (1LD100) and treated with oseltamivir (+4 hpi, 1mg/kg/d, bid × 5). (A) Kaplan-Meier survival curves. The hazard ratio was 14.08. (B) Percent body weight. Oseltamivir-treated animals lost significantly less weight at 2-4 dpi compared to placebo animals. (C) SAA amounts in serum samples at 3 dpi. Oseltamivir treatment significantly reduced SAA levels to uninfected control levels. (D) Proinflammatory cytokines/chemokines in serum samples at 0, 3, and 7 dpi. Note that all placebo animals had died before the end of the experiment. The dashed line in (B) indicates 30% body weight loss. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
3.5. SAA levels in BALB/c mice infected with influenza A(H3N2) and influenza B virus
We now wanted to address the question whether SAA was a universal biomarker of influenza virus infection in BALB/c mice. We therefore challenged BALB/c mice with other influenza viruses that cause less pathogenesis in the same amount of time. SAA levels in mice experimentally infected with influenza A/Victoria/3/75 (H3N2) showed a similar overall profile but peaked slightly earlier at 2 dpi (Fig. 5A). They were also generally lower than SAA levels in BALB/c mice challenged with influenza A(H1N1)pdm09 virus, often a more virulent virus in mice (Song et al., 2011). Although the challenge dose with influenza A(H3N2) was calculated as lethal, survival data and lung weight (as an indicator for edema and thus presumably for lung pathology) showed no difference between infected and non-infected animals (Supplement Fig. S1). Yet even with such a mild infection, SAA levels were significantly elevated in placebo animals at 2 and 3 dpi (P < 0.0001). Oseltamivir treatment reduced SAA levels significantly (P = 0.0195) from 43 to 20 μg/ml (Fig. 5B). While animals with the highest concentration of SAA at 3 dpi later succumbed to the infection (69 and 61 μg/ml), other animals with similar values in that same treatment group survived. When BALB/c mice were challenged with influenza B/Sichuan/379/99 virus, SAA levels peaked at 3 dpi (Fig. 5C), and oseltamivir treatment led to a significant decrease from 125 to 37 μg/ml (P = 0.0036, Fig. 5D). In both experiments, SAA was an early biomarker of influenza virus disease. However, SAA levels were not always predictive of disease outcome as several animals in the oseltamivir-treated group had low SAA levels, yet did not survive.
Fig. 5.
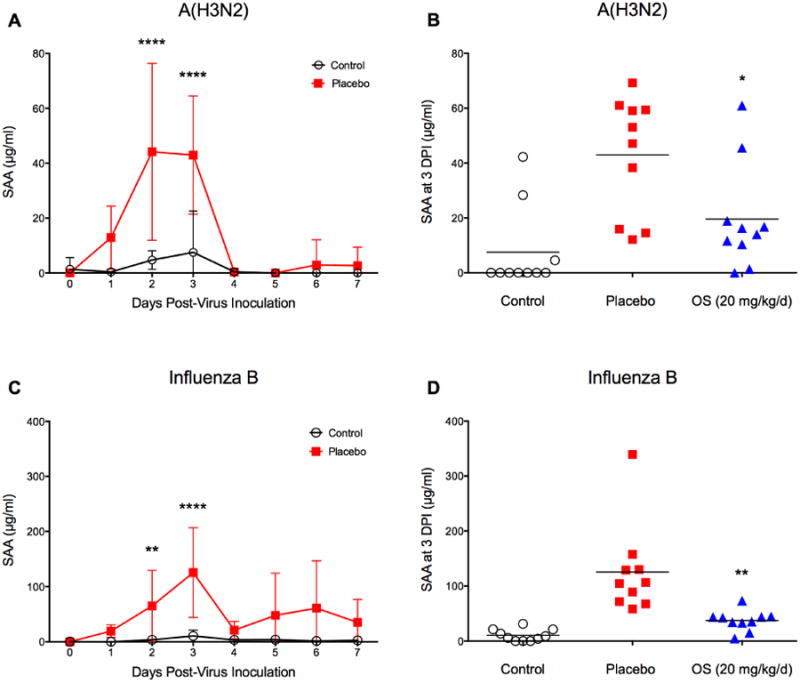
SAA time course in BALB/c mice infected with influenza A(H3N2) and influenza B virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected with (A, B) mouse-adapted influenza A/Victoria/3/75 (H3N2) virus (1LD100) and (C, D) mouse-adapted influenza B/Sichuan/379/99 virus (2LD90), respectively, and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. The experiment was terminated 14 days post-virus inoculation. Survival and body weight were monitored daily (data shown in Supplement Figures S1 and S2). SAA was significantly elevated at 2 and 3 dpi. Data are presented as mean ± SD. **P < 0.01, ****P < 0.0001 compared to placebo; two-way ANOVA with Sidak's multiple comparison test. Oseltamivir treatment reduced mean SAA levels at 3 dpi significantly in (B) P = 0.0195 and (D) P = 0.0036. Unpaired t-test with equal SD.
3.6. SAA levels in other mouse strains infected with influenza A(H1N1)pdm09 virus
Mice strains differ in their response to influenza virus infections (Pica et al., 2011). We therefore expanded our experiments to include influenza virus infections in C57BL/6 (Fig. 6A and 6B), Swiss-Webster (Fig. 6C and 6D), and DBA.2 mice (Fig. 6E and 6F). Animals were infected with a lethal dose of influenza A(H1N1)pdm09 virus and treated 4 hours post infection with a higher dose of oseltamivir (20 mg/kg/d) than used in the previous experiment. We knew that 10 mg/kg/d of oseltamivir protected 100% of BALB/c mice using the inoculum as indicated. For the other mouse strains the dose was increased by two-fold, since the sensitivities of these other virus strains used in the experiments shown were only generally known. In all tested mice strains, the SAA time course was similar: SAA was significantly increased 2 to 3 days after viral challenge. Mean values varied though greatly between strains with 49, 202, and 1096 μg/ml for C57BL/6, Swiss-Webster, and DBA.2 mice, respectively. Treatment with oseltamivir resulted in a significant decrease of SAA levels at 3 dpi only in DBA.2 mice (P = 0.0056).
Fig. 6.
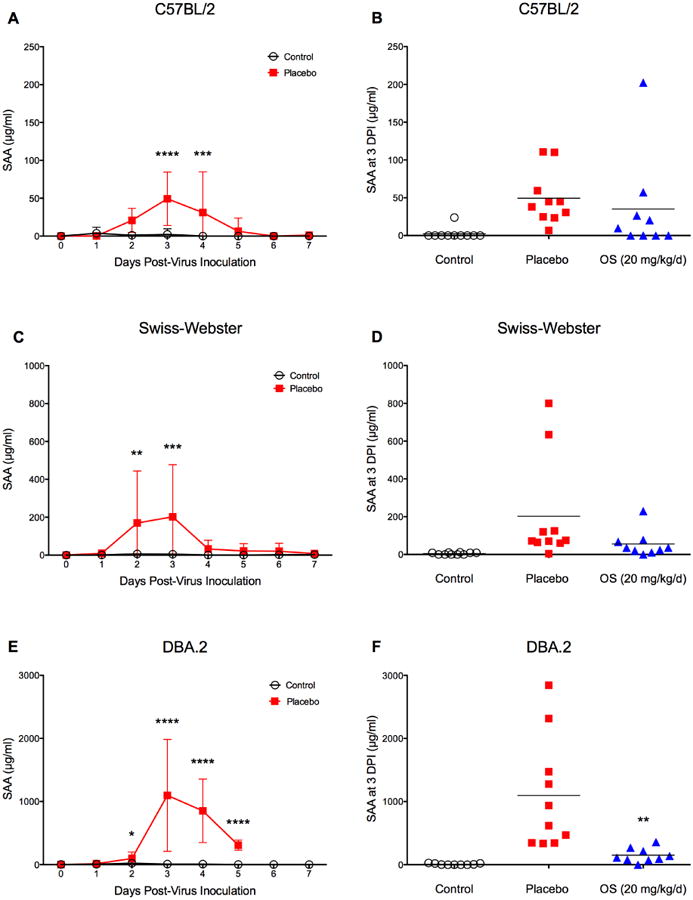
SAA time course in C57/BL2, Swiss-Webster, and DBA.2 mice infected with influenza A/California/04/09 (H1N1)pdm09 virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected (1LD100) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. The experiment was terminated 14 days post-virus inoculation. Survival and body weight were monitored daily (data shown in Supplement Figures S3-5). SAA peaked at 3dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo; two-way ANOVA with Sidak's multiple comparison test. Oseltamivir treatment reduced mean SAA levels at 3 dpi, but only significantly in (F) P = 0.0056. Unpaired t-test with equal SD.
Body weight, survival curves, and hazard ratios, lung scores and lung weights for other influenza virus infections in the BALB/c mouse can be found in the Supplementary Figures 1-2 (S1, S2). The same parameters for influenza A(H1N1)pdm09 virus infections in C57BL/6 (S3), Swiss-Webster (S4), and DBA.2 (S5) mice are shown in the Supplementary Figures 3-5.
4. Discussion
Eight serological biomarkers were examined for their diagnostic (ability to detect disease early) and prognostic potential (ability to predict disease outcome, i.e. death) in the mouse model of severe influenza virus infection. Minor differences were only found for transferrin, corticosterone, and alpha-2,6-sialyltransferase. Serum levels of transferrin at 16 hpi and corticosterone at 72 hpi in influenza virus-infected mice were two-fold elevated compared to sham-infected control animals. Transferrin is an iron-binding glycoprotein. In humans, it is known to be a negative acute phase reactant (Gabay and Kushner, 1999), but it was demonstrated to be a positive acute phase reactant in mice (Gordeuk et al., 1988). Corticosterone, the main glucocorticoid in amphibians, birds, and rodents is involved in immune reactions and stress responses. When BALB/c mice were challenged with influenza A/Puerto Rico/8/1934 (H1N1) virus, plasma concentrations of corticosterone were significantly different from healthy control animals and peaked at 96 hpi (Dunn et al., 1989). A similar trend was seen in our experiments. Serum levels of alpha-2,6-sialyltransferase were about five-fold higher in influenza virus-infected mice compared to sham-infected control mice. This difference was only seen very early during the disease progression at 8 hpi. The lack of a biological explanation for this observed acute change in our study justifies further investigation. Serum levels of complement 3, prostaglandin E2, H2O2, and C-reactive protein (CRP) did not indicate a potential as a biomarker for influenza virus disease progression in BALB/c mice. These results are surprising as particularly CRP is a major acute-phase protein in humans and is used as an inflammatory marker of virus infections (Nakayama et al., 1993; Zimmerman et al., 2010) and other disease states (Kushner et al., 1978). Its usefulness in the mouse model is still debated though (Torzewski et al., 2014).
Mouse serum amyloid A (SAA) on the other hand was the most promising and robust early biomarker candidate tested so far. SAA belongs to a family of small, evolutionary conserved apolipoproteins with 4 members in man and 5 members in mice (Uhlar and Whitehead, 1999). SAA's physiological functions have been intensely investigated since its isolation and identification in the early 1970s (Isersky et al., 1971; Linke et al., 1975) but without reaching a clear consensus. SAA's pathological relevance has been undoubtedly demonstrated though. It is the serum precursor of the amyloid A protein that is found deposited in reactive (secondary) amyloid A amyloidosis (Husebekk et al., 1985; Tape et al., 1988), a progressive and often fatal complication of certain rheumatic and chronic inflammatory diseases (Kindy and de Beer, 1999). SAA and its isoforms are proposed to be differentially involved in cholesterol transport and metabolism. Based on the responsiveness to inflammation, SAA isoforms are divided into acute-phase SAA or constitutive-phase SAA. Both, SAA1 and SAA2 have been shown to be the major vertebrate acute-phase reactants modulating various immunological responses during the inflammation process (Uhlar and Whitehead, 1999). The ELISA kit used in this study preferentially detects SAA2 and thus, our following discussion will focus on the role of SAA as an acute-phase protein.
In our experiments, lethal infections with influenza A(H1N1)pdm09, influenza A(H3N2) and influenza B virus in BALB/c mice, and influenza A(H1N1)pdm09 virus infections in C57BL/2, Swiss-Webster, and DBA.2 mice resulted in acute increases in mouse SAA levels predominantly at three days post-virus inoculation with a subsequent decrease to pre-virus exposure levels. Similar transient changes in mouse SAA levels have been observed after subcutaneous injection with lipopolysaccharide (Foyn Bruun et al., 1998; Ikeda et al., 1999; Tkalcevic et al., 2011) or silver nitrate (Glojnaric et al., 2007). The first simulated the initial response to endotoxic shock and the latter was used as a model of sterile inflammation. Mouse SAA levels have also been found transiently elevated after injection with the tocolytic agent ritodrine, which was found to cause liver damage (Tsuchiya et al., 2013), and after exposure to silica nanoparticles (Higashisaka et al., 2011). In both studies, SAA was proposed as a sensitive and early biomarker for the detection of hepatic injury, and for assessing the risk of exposure to silica nanoparticles, respectively. Various virus infections (cytomegalovirus, varicella-zoster, herpes simplex, rubella, and measles virus) have been reported to raise SAA concentrations in humans (Sarov et al., 1982; Shainkin-Kestenbaum et al., 1982), but to our understanding only one study published a time course of changes in SAA concentrations after challenge with human rhinovirus HRV9 and human influenza A/Eng/40/83 (H3N2) virus (Whicher et al., 1985). Few studies have examined the effect of influenza virus exposure on SAA levels in other species. Hulten and colleagues (1999) reported higher SAA levels in horses suffering from equine influenza virus infection during the acute stage compared to convalescent samples. And Pomorska-Mol and colleagues observed significantly higher concentrations of SAA in pigs after infection with H3N2 and H1N1 swine influenza virus (Pomorska-Mol et al., 2014; Pomorska-Mol et al., 2012). To our knowledge, our study is the first to systematically assess serological biomarker candidates in different mouse strains (BALB/c, C57BL/2, Swiss-Webster, DBA.2) lethally infected with influenza A(H1N1)pdm09 virus, influenza A(H3N2) virus and influenza B virus and to examine the effect of antiviral treatment on SAA levels.
Lethal infections with influenza A(H1N1)pdm09 elicited a very strong but transient SAA response in BALB/c mice at 3 dpi. SAA levels above 300 μg/ml (Experiment 3) and 700 μg/ml (Experiment 4), respectively, were indicative of disease progression as 9/10 animals with high SAA levels died of influenza-associated pneumonia in Experiment 3 and 10/10 in Experiment 4. The only surviving animal in the placebo group had very low SAA levels (< 100 μg/ml). Oseltamivir treatment significantly reduced the SAA response but not in a dose-dependent manner. Therefore, SAA is not well suited for the efficacy assessment of the anti-influenza drug oseltamivir phosphate, at least not in the here tested concentrations.
The proinflammatory cytokine response was also evaluated at day 3 and the findings for mice infected with the pdm09H1N1 virus did not usefully elucidate the potential severity of the infection or the potential outcome death due the virus infection, in agreement with other studies using similar influenza A(H1N1)pdm09 viruses (Belser et al., 2010). On the other hand, if SAA levels were high, such as at day 3 post virus exposure, then the mice with high SAA levels (i.e., > 300 μg/ml in Experiment 3 and 700 μg/ml in Experiment 4) were less likely to survive the infection. This confirms the value of SAA as an additional tool to analyze the probable experimental influenza disease outcome in mice early in the infection process, which is likely very possible for BALB/c mice infected with influenza A(H1N1)pdm09 virus.
Although body weight loss has been considered the gold standard for monitoring severe influenza disease progression (van der Laan et al., 2008), it is not the best reliable predictor of infection outcome or survival. Only in 2 out of our 7 animal experiments with complete survival data was weight loss significantly lower in oseltamivir-treated animals compared to placebo at 3 dpi, and these animals survived the infection. In the majority of our experiments, body weight loss was an indicator of disease progression but not a predictor of the true seriousness of disease progression in a particular mouse.
When BALB/c mice were challenged with other influenza virus strains, results, however, imply a more complex situation. Although SAA levels indicated infection status, it often, but not always did predict survivability of influenza-infected mice. Similar results were obtained with C57BL/2, Swiss-Webster, and DBA.2 mouse strains challenged with influenza A(H1N1)pdm09 virus. Other investigators have reported on varying susceptibilities in the same mouse breed to infections with different influenza virus strains. But mouse models also differ in their overall genetic make-up. BALB/c, C57BL/2 and DBA.2 are inbred mice, whereas Swiss-Webster mice are outbred. This would potentially confound results obtained with this particular strain when innate immune responses of the host, such as SAA levels, are the focus of the study. The robust response of DBA.2 mice with an approximately 1000-fold acute increase in SAA levels at 3 dpi implies that DBA.2 mice are much more susceptible to influenza virus infections than C57BL/2 or Swiss-Webster mice. This observation has also been made by others (Dengler et al., 2012; Pica et al., 2011). DBA.2 mice offer the additional advantage of being susceptible to influenza viruses that have not been mouse-adapted (Boon et al., 2010; Kim et al., 2013). Therefore, several investigators have proposed the use of DBA.2 mice as a suitable small animal model to study influenza virus pathogenicity and for the evaluation of anti-influenza drug efficacies. The fact that 40% of the control mice died in our experiments apparently not explainable by exposure to influenza virus make this particular strain less than ideal for influenza virus research in our opinion, because of their sensitivity to procedural trauma (i.e., unexpected rougher handling of the mice due to the aggressive nature of the mouse strain or the sensitivity to the oral gavage procedure). C57BL/6 mice also showed the characteristic SAA response after experimental infection with influenza A(H1N1)pdm09 virus. However, the response was less robust and was not perturbed by oseltamivir treatment. Similar to the DBA.2 strain, BALB/c mice showed a very strong SAA response but unlike DBA.2 mice, BALB/c mice normally require influenza virus strains to be mouse-adapted for full lethality. This is clearly a disadvantage as the adaptation process may lead to changes in the viral genome that, besides an increased pathogenicity, might also affect host responses in an unpredictable fashion. Despite this shortcoming, the BALB/c strain still remains one of the most utilized small animal hosts for influenza virus research. As already noted, it is likely that the difference in observed SAA levels between the different mice strains is due to the difference in genetic background of the strains. The two major acute-phase reactants SAA1 and SAA2 have been intensely studied in BALB/c mice but little is known about the expression pattern of SAA2 in C57BL/6, Swiss-Webster, and DBA.2 mice. Future studies should address this point.
Finally, although we did not systematically address the question of protein stability under storage conditions, our data indicate that long-term storage (over 12 months at -80°C) and repeated freeze-thaw cycles did not lead to a decline in the quantified levels of SAA from the same samples.
5. Conclusions
Our studies show that mouse serum levels of SAA can be used as an early diagnostic biomarker of influenza virus infection in BALB/c, C57BL/6, Swiss-Webster, and DBA.2 mice. Its prognostic value, however, is most apparent when the infecting virus induces severe weight loss and causes mortality within 5-8 days after virus exposure. In BALB/c mice lethally challenged with influenza A(H1N1)pdm09, animals with high SAA levels measured at 3 dpi (> 300 μg/ml in Experiment 3 and 700 μg/ml in Experiment 4) later all succumbed to death, whereas animals with low SAA levels survived. However, at this point it is not possible to propose a general threshold value since the SAA concentrations varied with viral inoculum. Strain differences were seen after therapeutic oseltamivir phosphate treatment, with BALB/c and DBA.2 mice showing the strongest response. The apparent stability of the SAA protein under storage conditions coupled with the relative ease of quantification from blood samples, which do not require any special assay considerations, further make this serological biomarker attractive, particularly for large scale experiments involving BALB/c mice, the most commonly used mouse strain in influenza virus research.
Supplementary Material
Fig. S1 (Supplement to Fig. 5A and 5B). SAA time course in BALB/c mice infected with influenza A(H3N2) virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected with mouse-adapted influenza A/Victoria/3/75 (H3N2) virus (1LD100) and treated with oseltamivir (+4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). Control animals (uninfected, n=10) received dilution vehicle (MEM) only. At specific time points (0-7 dpi), animals were cheek-bled for serum collection. The experiment was terminated 21 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 2.23. Oseltamivir-treated animals gained weight significantly faster during convalescence (12-16 dpi) compared to placebo-treated animals. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2 and 3 dpi in the placebo group (****P < 0.0001, compared to control). (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment significantly reduced SAA levels to uninfected control levels at 2 and 3 dpi. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S2 (Supplement to Fig. 5C and 5D). SAA time course in BALB/c mice infected with influenza B virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected with mouse-adapted influenza B/Sichuan/379/99 virus (2LD90) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 6.55. Oseltamivir-treated animals lost significantly less weight at 1 and 3-7 dpi compared to placebo-treated animals. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2 and 3 dpi in the placebo group (**P < 0.01 and ****P < 0.0001, respectively, compared to control). (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment significantly reduced SAA levels to uninfected control levels at 3 dpi. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S3 (Supplement to Fig. 6A and 6B). SAA time course in C57BL/6 mice infected with influenza A(H1N1)pdm09 virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected (1LD100) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. One animal in the oseltamivir-treated group was found dead at 1 dpi and was excluded from further analysis. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 8.03. Oseltamivir-treated animals lost significantly less weight at 4-7 dpi compared to placebo-treated animals. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated in infected animals at 3 and 4 dpi compared to uninfected control mice. (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment lowered SAA levels slightly at 3dpi, but this was not statistically significant. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 DPI. (F) Lung hemorrhage scores at 6 DPI. (G) Lung weights at 3 DPI. (H) Lung weights at 6 DPI. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S4 (Supplement to Fig. 6C and 6D). SAA time course in Swiss-Webster mice infected with influenza A(H1N1)pdm09 virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected (1LD100) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. One animal in the oseltamivir-treated group was found dead at 1 dpi and was excluded from further analysis. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 2.51. Oseltamivir treatment protected from weight loss only at 6 and 11 dpi. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2 and 3 dpi in the placebo group compared to the uninfected control group. (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment lowered SAA levels, but this was not statistically significant. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Note that all placebo mice were dead by 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S5 (Supplement to Fig. 6E and 6F). SAA time course in DBA.2 mice infected with influenza A(H1N1)pdm09 virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected (1LD100) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. One animal in the oseltamivir-treated group was found dead at 1 dpi and was excluded from further analysis. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 7.40. Oseltamivir treatment did not protect from weight loss but deferred death by 2 days. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2-4 dpi in the placebo group compared to the uninfected control group. (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment significantly reduced SAA levels to uninfected control levels at 3 dpi. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Highlights.
Acute phase reactants were assessed as potential biomarkers of influenza virus infection in BALB/c mice.
Serum amyloid A (SAA) was the most promising biomarker tested.
Influenza virus infection also elicited a transient spike in SAA levels in C57BL/2, Swiss-Webster, and DBA.2 mice.
SAA levels were significantly lowered by antiviral treatment in BALB/c and DBA.2 mice.
SAA levels indicated disease status at day 3 post infection and were associated with disease outcome in BALB/c mice.
Acknowledgments
We acknowledge the support of the Institute for Antiviral Research at Utah State University in conducting these studies. Further funding for this project came from the National Institutes of Health (NIH), contract number HSN272201000039I/HHSN27200012/A66, task order number A66. We thank Dr. Nabil N. Youssef for critical reading of the manuscript and Brett L. Hurst for performing the cytokine analysis and practical assistance in the laboratory.
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at (URL to follow).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnard DL. Animal models for the study of influenza pathogenesis and therapy. Antiviral Res. 2009;82:A110–122. doi: 10.1016/j.antiviral.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, Kumar A, Lim M, Shindo N, Penn C, Nicholson KG. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. New Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- Belser JA, Wadford DA, Pappas C, Gustin KM, Maines TR, Pearce MB, Zeng H, Swayne DE, Pantin-Jackwood M, Katz JM, Tumpey TM. Pathogenesis of pandemic influenza A (H1N1) and triple-reassortant swine influenza A (H1) viruses in mice. J Virol. 2010;84:4194–4203. doi: 10.1128/JVI.02742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon AC, deBeauchamp J, Krauss S, Rubrum A, Webb AD, Webster RG, McElhaney J, Webby RJ. Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model. J Virol. 2010;84:7662–7667. doi: 10.1128/JVI.02444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier NM, Lowen AC. Animal models for influenza virus pathogenesis and transmission. Viruses. 2010;2:1530–1563. doi: 10.3390/v20801530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler L, May M, Wilk E, Bahgat MM, Schughart K. Immunization with live virus vaccine protects highly susceptible DBA/2J mice from lethal influenza A H1N1 infection. Virol J. 2012;9:212. doi: 10.1186/1743-422X-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Powell ML, Meitin C, Small PA., Jr Virus infection as a stressor: influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol Behav. 1989;45:591–594. doi: 10.1016/0031-9384(89)90078-4. [DOI] [PubMed] [Google Scholar]
- Foyn Bruun C, Sletten K, Marhaug G. Mouse serum amyloid A (SAA) proteins isolated by two-dimensional electrophoresis: characterization of isotypes and the effect of separate and combined administrations of cytokines, dexamethasone and lipopolysaccharide (LPS) on serum levels and isotype distribution. Clin Exp Immunol. 1998;111:231–236. [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. New Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Galiano M, Johnson BF, Myers R, Ellis J, Daniels R, Zambon M. Fatal cases of influenza A(H3N2) in children: insights from whole genome sequence analysis. PloS One. 2012;7:e33166. doi: 10.1371/journal.pone.0033166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glojnaric I, Cuzic S, Erakovic-Haber V, Parnham MJ. The serum amyloid A response to sterile silver nitrate in mice and its inhibition by dexamethasone and macrolide antibiotics. Int Immunopharmacol. 2007;7:1544–1551. doi: 10.1016/j.intimp.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Prithviraj P, Dolinar T, Brittenham GM. Interleukin 1 administration in mice produces hypoferremia despite neutropenia. J Clin Invest. 1988;82:1934–1938. doi: 10.1172/JCI113812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashisaka K, Yoshioka Y, Yamashita K, Morishita Y, Fujimura M, Nabeshi H, Nagano K, Abe Y, Kamada H, Tsunoda S, Yoshikawa T, Itoh N, Tsutsumi Y. Acute phase proteins as biomarkers for predicting the exposure and toxicity of nanomaterials. Biomaterials. 2011;32:3–9. doi: 10.1016/j.biomaterials.2010.08.110. [DOI] [PubMed] [Google Scholar]
- Hulten C, Sandgren B, Skioldebrand E, Klingeborn B, Marhaug G, Forsberg M. The acute phase protein serum amyloid A (SAA) as an inflammatory marker in equine influenza virus infection. Acta Vet Scand. 1999;40:323–333. doi: 10.1186/BF03547012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebekk A, Skogen B, Husby G, Marhaug G. Transformation of amyloid precursor SAA to protein AA and incorporation in amyloid fibrils in vivo. Scand J Immunol. 1985;21:283–287. doi: 10.1111/j.1365-3083.1985.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Hamada K, Sumitomo N, Okamoto H, Sakakibara B. Serum amyloid A, cytokines, and corticosterone responses in germfree and conventional mice after lipopolysaccharide injection. Biosci Biotechnol Biochem. 1999;63:1006–1010. doi: 10.1271/bbb.63.1006. [DOI] [PubMed] [Google Scholar]
- Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, Marjuki H, Barman S, Webster RG, Webby RJ. Adaptation of pandemic H1N1 influenza viruses in mice. J Virol. 2010;84:8607–8616. doi: 10.1128/JVI.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isersky C, Page DL, Cuatrecasas P, DeLellis RA, Glenner GG. Murine amyloidosis: immunologic characterization of amyloid fibril protein. J Immunol. 1971;107:1690–1698. [PubMed] [Google Scholar]
- Julander JG, Hagloch J, Latimer S, Motter N, Dagley A, Barnard DL, Smee DF, Morrey JD. Use of plethysmography in assessing the efficacy of antivirals in a mouse model of pandemic influenza A virus. Antiviral Res. 2011;92:228–236. doi: 10.1016/j.antiviral.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Kim JI, Park S, Lee S, Lee I, Heo J, Hwang MW, Bae JY, Kim D, Jang SI, Park MS, Park MS. DBA/2 mouse as an animal model for anti-influenza drug efficacy evaluation. J Microbiol. 2013;51:866–871. doi: 10.1007/s12275-013-3428-7. [DOI] [PubMed] [Google Scholar]
- Kindy MS, de Beer FC. A mouse model for serum amyloid A amyloidosis. Methods Enzymol. 1999;309:701–716. doi: 10.1016/s0076-6879(99)09046-1. [DOI] [PubMed] [Google Scholar]
- Kushner I, Broder ML, Karp D. Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J Clin Invest. 1978;61:235–242. doi: 10.1172/JCI108932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke RP, Sipe JD, Pollock PS, Ignaczak TF, Glenner GG. Isolation of a low-molecular-weight serum component antigenically related to an amyloid fibril protein of unknown origin. Proc Natl Acad Sci USA. 1975;72:1473–1476. doi: 10.1073/pnas.72.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Lamirande EW, Subbarao K. The mouse model for influenza. Curr Protoc Microbiol. 2009:Unit 15G.13.11–15G.13.30. doi: 10.1002/9780471729259.mc15g03s13. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Sonoda S, Urano T, Yamada T, Okada M. Monitoring both serum amyloid protein A and C-reactive protein as inflammatory markers in infectious diseases. Clin Chem. 1993;39:293–297. [PubMed] [Google Scholar]
- Pica N, Iyer A, Ramos I, Bouvier NM, Fernandez-Sesma A, Garcia-Sastre A, Lowen AC, Palese P, Steel J. The DBA.2 mouse is susceptible to disease following infection with a broad, but limited, range of influenza A and B viruses. J Virol. 2011;85:12825–12829. doi: 10.1128/JVI.05930-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomorska-Mol M, Kwit K, Pejsak Z, Markowska-Daniel I. Analysis of the acute-phase protein response in pigs to clinical and subclinical infection with H3N2 swine influenza virus. Influenza Other Respi Viruses. 2014;8:228–234. doi: 10.1111/irv.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomorska-Mol M, Markowska-Daniel I, Pejsak Z. Acute phase protein response during subclinical infection of pigs with H1N1 swine influenza virus. Vet Microbiol. 2012;159:499–503. doi: 10.1016/j.vetmic.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Sarov I, Shainkin-Kestenbaum R, Zimlichman S, Winikoff Y, Chaimovitz C, Pras M. Serum amyloid A levels in patients with infections due to cytomegalovirus, varicella-zoster virus, and herpes simplex virus. J Infect Dis. 1982;146:443. doi: 10.1093/infdis/146.3.443. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R, Zimlichman S, Winikoff Y, Pras M, Chaimovitz C, Sarov I. Serum amyloid A (SAA) in viral infection: rubella, measles and subacute sclerosing panencephalitis (SSPE) Clin Exp Immunol. 1982;50:503–506. [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH, Gilbert J, Moscon B, Pedersen G, Burger R, Warren RP. Utilization of pulse oximetry for the study of the inhibitory effects of antiviral agents on influenza virus in mice. Antimicrob Agents Chemother. 1992;36:473–476. doi: 10.1128/aac.36.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF. Experimental disease models of influenza virus infections: recent developments. Drug Discov Today Dis Models. 2004;1:57–63. [Google Scholar]
- Sidwell RW, Wong MH, Bailey KW, Barnard DL, Jackson MK, Smee DF. Utilization of alpha-1-acid glycoprotein levels in the serum as a parameter for in vivo assay of influenza virus inhibitors. Antiviral Chem Chemother. 2001;12:359–365. doi: 10.1177/095632020101200606. [DOI] [PubMed] [Google Scholar]
- Sladkova T, Kostolansky F. The role of cytokines in the immune response to influenza A virus infection. Acta Virol. 2006;50:151–162. [PubMed] [Google Scholar]
- Smee DF, Barnard DL. Methods for evaluation of antiviral efficacy against influenza virus infections in animal models. Methods Mol Biol. 2013;1030:407–425. doi: 10.1007/978-1-62703-484-5_31. [DOI] [PubMed] [Google Scholar]
- Smith W, Manch MD, Andrewes CH, Lond MD, Laidlaw PP. A virus obtained from influenza patiens. Lancet. 1933;222:66–68. [Google Scholar]
- Song MS, Pascua PN, Choi YK. Virulence of pandemic (H1N1) 2009 influenza A polymerase reassortant viruses. Virulence. 2011;2:422–426. doi: 10.4161/viru.2.5.17267. [DOI] [PubMed] [Google Scholar]
- Stark GV, Long JP, Ortiz DI, Gainey M, Carper BA, Feng J, Miller SM, Bigger JE, Vela EM. Clinical profiles associated with influenza disease in the ferret model. PloS one. 2013;8:e58337. doi: 10.1371/journal.pone.0058337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tape C, Tan R, Nesheim M, Kisilevsky R. Direct evidence for circulating apoSAA as the precursor of tissue AA amyloid deposits. Scand J Immunol. 1988;28:317–324. doi: 10.1111/j.1365-3083.1988.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkalcevic VI, Hrvacic B, Pasalic I, Erakovic Haber V, Glojnaric I. Immunomodulatory effects of azithromycin on serum amyloid A production in lipopolysaccharide-induced endotoxemia in mice. J Antibiot. 2011;64:515–517. doi: 10.1038/ja.2011.14. [DOI] [PubMed] [Google Scholar]
- Torzewski M, Waqar AB, Fan J. Animal models of C-reactive protein. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/683598. 683598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H, Sato J, Tsuda H, Fujiwara Y, Yamada T, Fujimura A, Koshimizu TA. Serum amyloid A upsurge precedes standard biomarkers of hepatotoxicity in ritodrine-injected mice. Toxicology. 2013;305:79–88. doi: 10.1016/j.tox.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- van der Laan JW, Herberts C, Lambkin-Williams R, Boyers A, Mann AJ, Oxford J. Animal models in influenza vaccine testing. Expert Rev Vaccines. 2008;7:783–793. doi: 10.1586/14760584.7.6.783. [DOI] [PubMed] [Google Scholar]
- Whicher JT, Chambers RE, Higginson J, Nashef L, Higgins PG. Acute phase response of serum amyloid A protein and C reactive protein to the common cold and influenza. J Clin Pathol. 1985;38:312–316. doi: 10.1136/jcp.38.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ZX, Jones JE, Anderson GP, Gualano RC. Oseltamivir treatment of mice before or after mild influenza infection reduced cellular and cytokine inflammation in the lung. Influenza Other Respi Viruses. 2011;5:343–350. doi: 10.1111/j.1750-2659.2011.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman O, Rogowski O, Aviram G, Mizrahi M, Zeltser D, Justo D, Dahan E, Arad R, Touvia O, Tau L, Tarabeia J, Berliner S, Paran Y. C-reactive protein serum levels as an early predictor of outcome in patients with pandemic H1N1 influenza A virus infection. BMC Infect Dis. 2010;10:288. doi: 10.1186/1471-2334-10-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 (Supplement to Fig. 5A and 5B). SAA time course in BALB/c mice infected with influenza A(H3N2) virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected with mouse-adapted influenza A/Victoria/3/75 (H3N2) virus (1LD100) and treated with oseltamivir (+4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). Control animals (uninfected, n=10) received dilution vehicle (MEM) only. At specific time points (0-7 dpi), animals were cheek-bled for serum collection. The experiment was terminated 21 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 2.23. Oseltamivir-treated animals gained weight significantly faster during convalescence (12-16 dpi) compared to placebo-treated animals. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2 and 3 dpi in the placebo group (****P < 0.0001, compared to control). (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment significantly reduced SAA levels to uninfected control levels at 2 and 3 dpi. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S2 (Supplement to Fig. 5C and 5D). SAA time course in BALB/c mice infected with influenza B virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected with mouse-adapted influenza B/Sichuan/379/99 virus (2LD90) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 6.55. Oseltamivir-treated animals lost significantly less weight at 1 and 3-7 dpi compared to placebo-treated animals. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2 and 3 dpi in the placebo group (**P < 0.01 and ****P < 0.0001, respectively, compared to control). (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment significantly reduced SAA levels to uninfected control levels at 3 dpi. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S3 (Supplement to Fig. 6A and 6B). SAA time course in C57BL/6 mice infected with influenza A(H1N1)pdm09 virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected (1LD100) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. One animal in the oseltamivir-treated group was found dead at 1 dpi and was excluded from further analysis. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 8.03. Oseltamivir-treated animals lost significantly less weight at 4-7 dpi compared to placebo-treated animals. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated in infected animals at 3 and 4 dpi compared to uninfected control mice. (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment lowered SAA levels slightly at 3dpi, but this was not statistically significant. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 DPI. (F) Lung hemorrhage scores at 6 DPI. (G) Lung weights at 3 DPI. (H) Lung weights at 6 DPI. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S4 (Supplement to Fig. 6C and 6D). SAA time course in Swiss-Webster mice infected with influenza A(H1N1)pdm09 virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected (1LD100) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. One animal in the oseltamivir-treated group was found dead at 1 dpi and was excluded from further analysis. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 2.51. Oseltamivir treatment protected from weight loss only at 6 and 11 dpi. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2 and 3 dpi in the placebo group compared to the uninfected control group. (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment lowered SAA levels, but this was not statistically significant. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Note that all placebo mice were dead by 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.
Fig. S5 (Supplement to Fig. 6E and 6F). SAA time course in DBA.2 mice infected with influenza A(H1N1)pdm09 virus and effect of antiviral treatment on SAA levels. Mice (n=20) were intranasally infected (1LD100) and treated with oseltamivir (+ 4 hpi, 20 mg/kg/d, bid × 5) or saline (placebo). At specific time points (0-7 dpi), animals were cheek-bled for serum collection. One animal in the oseltamivir-treated group was found dead at 1 dpi and was excluded from further analysis. The experiment was terminated 14 days post-virus inoculation. Survival (A) and body weight (B) were monitored daily. The hazard ratio was 7.40. Oseltamivir treatment did not protect from weight loss but deferred death by 2 days. (C) Time course of SAA as determined from a single ELISA assay. SAA levels were significantly elevated at 2-4 dpi in the placebo group compared to the uninfected control group. (D) SAA amounts in serum samples from individual animals taken 3 dpi. Oseltamivir treatment significantly reduced SAA levels to uninfected control levels at 3 dpi. Symbols with a white dot in the center indicate that animals later died. (E) Lung hemorrhage scores at 3 dpi. (F) Lung hemorrhage scores at 6 dpi. (G) Lung weights at 3 dpi. (H) Lung weights at 6 dpi. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared to placebo.


