Abstract
Previous work suggests domestic poultry are important contributors to the emergence and transmission of highly pathogenic avian influenza throughout Asia. In Poyang Lake, China, domestic duck production cycles are synchronized with arrival and departure of thousands of migratory wild birds in the area. During these periods, high densities of juvenile domestic ducks are in close proximity to migratory wild ducks, increasing the potential for the virus to be transmitted and subsequently disseminated via migration. In this paper, we use GPS dataloggers and dynamic Brownian bridge models to describe movements and habitat use of free-grazing domestic ducks in the Poyang Lake basin and identify specific areas that may have the highest risk of H5N1 transmission between domestic and wild birds. Specifically, we determine relative use by free-grazing domestic ducks of natural wetlands, which are the most heavily used areas by migratory wild ducks, and of rice paddies, which provide habitat for resident wild ducks and lower densities of migratory wild ducks. To our knowledge, this is the first movement study on domestic ducks, and our data show potential for free-grazing domestic ducks from farms located near natural wetlands to come in contact with wild waterfowl, thereby increasing the risk for disease transmission. This study provides an example of the importance of movement ecology studies in understanding dynamics such as disease transmission on a complicated landscape.
Keywords: domestic poultry, disease transmission, dynamic Brownian bridge movement model
1. Introduction
1.1. Role of domestic ducks in avian influenza
Highly pathogenic avian influenza (HPAI) subtype H5N1 is a zoonotic virus that has caused mortality to humans in nearly 60% of reported cases from 16 countries (World Health Organization 2015). The virus originated in China, and domestic poultry are important contributors to the emergence and transmission throughout Asia (Gilbert et al. 2006, 2008, Songserm et al. 2006, Gauthier-Clerc et al. 2007, Cappelle et al. 2014). Low-pathogenic strains of avian influenza (LPAI), which occur naturally in wild waterfowl, can become highly pathogenic when introduced to a high-density host population, such as a poultry farm (Webby and Webster 2001). High densities of free-grazing domestic ducks raised in habitats overlapping those used by wild waterfowl create the risk of disease transmission through direct or indirect contact. In some cases, domestic ducks carry H5N1 asymptomatically and can shed the virus for several weeks, thereby promoting virus persistence and evolution over time (Chen et al. 2004, Hulse-Post et al. 2005, Sturm-Ramirez et al. 2005). Many areas of southern China are characterized by high densities of domestic poultry, high human population densities and irrigated paddy fields that provide valuable habitat for wild waterfowl (Takekawa et al. 2010a). Studies show that together these conditions are associated with the emergence, persistence and transmission of H5N1 (e.g., Martin et al. 2011, Cappelle et al. 2014). In parts of Asia, spatial analyses of H5N1 outbreaks show a very strong association with domestic poultry density (Gilbert et al. 2006). In areas where free-grazing domestic ducks share habitats with migratory wild birds, potential exists for the virus to be transmitted and subsequently disseminated via migration (Takekawa et al. 2010b).
1.2. Coupling of poultry and rice production in China
Production of free-grazing domestic ducks in China is often closely coupled with rice farming (Muzaffar et al. 2010). As of 2012, China produced over one-quarter of the world’s poultry and approximately 40% of poultry in Asia, and a large portion of these poultry are free-grazing domestic ducks raised in southern China’s rice fields (FAOSTAT 2014). Poultry production cycles often coincide with timing of rice farming because rice paddy habitat provides abundant food sources for growing juvenile ducks (Gilbert et al. 2007). During wetter months, flooded rice paddies provide habitat for prey items such as arthropods, mollusks and other invertebrates (Stafford et al. 2010). After harvest, waste seeds, husks and invertebrates provide a rich and varied food source for wild waterfowl and free-grazing domestic poultry.
Several studies identify the high density of free-grazing domestic ducks in intensive rice cropping areas as a risk factor for H5N1 persistence and transmission (e.g., Olsen et al. 2006, Xiao et al. 2007). The area around Poyang Lake, Jiangxi Province, China, is characterized by intensive poultry farming in rice paddies that border natural wetlands along the edge of the lake (Takekawa et al. 2010a). These wetlands support hundreds of thousands of overwintering migratory waterbirds, including globally significant wintering populations of several species, including swan geese (Anser cygnoides) and white-naped cranes (Grus vipio) (Harris and Zhuang 2010). Farmers in the area herd flocks of domestic ducks to forage in rice paddies and natural wetlands used by wild waterfowl, providing opportunities for H5N1 transmission (Takekawa et al. 2010a). Domestic duck production cycles in Poyang Lake region are synchronized with arrival and departure of thousands of migratory wild birds in the area. During these periods, high densities of juvenile domestic ducks, which are more susceptible to H5N1 infection than mature individuals, are in close proximity to migratory wild ducks (Cappelle et al. 2014). Some wild waterfowl that have been experimentally infected with H5N1 shed the virus asymptomatically, suggesting infected wild birds may be able to spread H5N1 to other regions during migration (Brown et al. 2008, Keawcharoen et al. 2008, Gaidet et al. 2010, Nemeth et al. 2013).
There is little knowledge of movement behavior and habitat use of free-grazing domestic ducks. Studies speculating that spatial overlap of domestic and wild ducks occurs have done so without empirical data of domestic duck movements. To fully understand the risk of H5N1 transmission between domestic poultry and wild birds, we must first understand how free-grazing domestic ducks use the landscape.
1.3. Objectives
Here, we quantify habitat use and availability of free-ranging domestic ducks in the Poyang Lake Basin, China, by combining spatiotemporal analyses of domestic duck movements with availability of four habitat types on the landscape. We assume that the relative use of specific habitats by free-grazing domestic ducks directly influences the degree of spatiotemporal overlap with wild birds and the potential risk of disease transmission. We expect that disproportionately high domestic duck use of rice paddies frequented by resident wild ducks (Cappelle et al. 2014) or of natural wetlands used by migratory wild birds (Takekawa et al. 2010b) would indicate a relatively high probability of overlap between domestic and wild ducks. High densities of domestic ducks near natural wetlands provide an additional mechanism for potential virus spread throughout the migratory flyway through interaction with wild birds. Conversely, relatively high domestic duck use of upland areas, ponds, ditches and channels might suggest a lower overall probability of overlap and disease transmission risk between wild and domestic populations. This study aims to improve our understanding of the relationship between free-grazing domestic duck habitat use and the potential for disease transmission to and from wild bird populations.
2. Materials and methods
2.1. Study area
Poyang Lake, located in China’s Jiangxi Province, has historically been the largest freshwater lake in China, encompassing approximately 3585 km2 at normal water levels as of 1998, down 25% from 1958 (Shankman and Liang 2003, Shankman et al. 2006). Water levels in Poyang Lake have fluctuated seasonally for decades, yet in recent years fluctuations have been far more pronounced due to changes in Yangtze River discharge patterns caused by the newly constructed Three Gorges Dam (Zhang et al. 2014). Traditionally, the lake has been a major wintering area for waterfowl, supporting the largest concentrations of wintering waterbirds in east Asia, with an estimated population of 425,000 ± 69,000 birds from 2003 to 2008 (Qian et al. 2011). Poyang Lake lies within the East Asian–Australasian Flyway, a migratory corridor that includes Guangdong Province, China, which is recognized as the epicenter of HPAI H5N1 (Webby and Webster 2001). An estimated 14,000,000 ducks (both free-grazing and non-free-grazing) were raised annually in the Poyang Lake area during the mid-2000s (Cappelle et al. 2014), making the ratio of domestic to wild ducks in the region more than 25 to 1. The majority of poultry in the Poyang Lake area are raised in small-scale farms (average of ca. 2000 ducks per farm) with minimal to low biosecurity levels (Muzaffar et al. 2010, Cappelle et al. 2014). The two major duck production cycles in Poyang Lake occur in February–March and in October–November (Cappelle et al. 2014).
2.2. Acquiring movement data
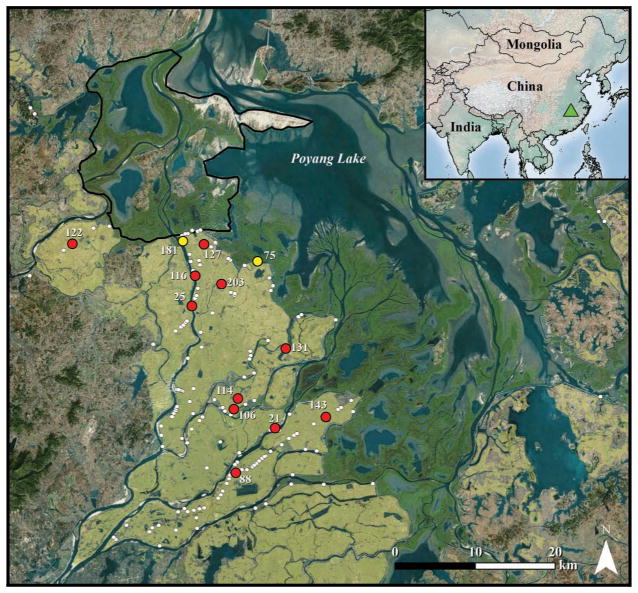
We marked 25 free-grazing domestic ducks at 13 poultry farms (1–3 marked ducks per farm) in the Poyang Lake region from late October through early December 2007 (Figure 1). Of these 25 birds, five were marked at farms located within 1.5 km of the boundary between agricultural land and natural wetlands (hereafter, border farms). All marked birds were laying ducks raised for egg production rather than for meat consumption. We attached 24 g battery-powered GPS dataloggers (Sirtrack, Hawkes Bay, New Zealand) via backpack harnesses (Miller et al. 2005) made from Teflon ribbon (Bally Ribbon Mills, Bally, PA, USA). We programmed dataloggers to record one GPS location every 10 min for the duration of battery life to capture detailed movement behavior. GPS loggers recorded locations at ~5 m accuracy. Because birds returned to shelters every evening and remained there through the night, we only included diurnal locations (those occurring between morning and evening nautical twilight periods, approximately 05:45 to 18:15 local time) in our analyses. To prevent temporal bias of locations, we excluded data from days where locations did not span the entire duration of daylight hours. For each bird, we defined the farm shelter as the mean center point of all nocturnal locations, from which we measured distances to all diurnal locations. We followed protocols approved by University of Oklahoma Institutional Animal Care and Use Committee (Animal Use Statement R12-019).
Figure 1.
Map of Poyang Lake area with free-grazing domestic duck shelters surveyed in October 2007 is shown in white. Farms with marked birds are shown in red (farms > 1.5 km from natural wetlands) and yellow (border farms) and labeled by farm number. Green triangle in inset indicates relative location of Poyang Lake within China. Dark green areas bordering southern edge of Poyang Lake indicate natural wetland. Tan shading indicates rice paddy habitat. Black outline delineates the Poyang Lake National Nature Reserve. Base imagery source: ESRI, DigitalGlobe, GeoEye, i-cubed, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, swisstopo, and the GIS User Community, 2015.
2.3. Estimating utilization distributions using dynamic Brownian bridge movement models
We ran dynamic Brownian bridge movement models (dBBMMs) using the ‘move’ package (Kranstauber and Smolla 2014) in Program R (R Foundation for Statistical Computing 2015) to estimate one utilization distribution (UD) for each full day of locations for each bird. A UD is a probability density representing an animal’s relative frequency of occurrence in space and time. In a sequence of three locations, the dBBMM assumes constant movement between the first and third location, which are connected by a Brownian bridge, while the second location is treated as an independent observation. The dBBMM estimates the Brownian motion variance (σ2m) by maximizing the likelihood of observing the second location assuming random movement between successive locations and normally distributed location errors. To allow σ2m to vary withchanges in behavior over time, the dBBMM calculates separate σ2m values for subsets (windows) of locations along the movement path. Within a sliding window with w locations, the dBBMM determines whether there is a behavioral change by comparing model fit using one or two estimates of σ2m. Specifically, the model uses Bayesian information criterion values to compare the log-likelihood of using one σ2m value for the whole window with the log-likelihood of a window split into two parts at a breakpoint located anywhere within the window. Because σ2m estimation requires at least three locations, the dBBMM requires a margin (m) with a minimum of three locations at the start and end of each window in which no breakpoints can be estimated. Larger window sizes (w) increase reliability in σ2m estimation but also increase the chance of missing short-term changes in behavior. Larger values of m enhance the power to identify behavioral changes in the sliding window but increase the chance of missing breakpoints in the margin (Kranstauber et al. 2012). We used w = 31 locations and m = 11 locations for all analyses based on Kranstauber et al. (2012) and visual inspection of example results from our own data. For each bird, we summed the pixel values of all their UDs and then rescaled the cumulative pixel values to sum to 1. The resulting UD represented the proportional amount of time occupied for each pixel across that bird’s range for the full duration it was marked.
2.4. Percent area and relative use by land cover type
We used Google Earth aerial imagery from 2010 to 2012 (version 7.1.2, Google, Mountain View, CA) to classify areas surrounding individual farms into four land cover types: pond/ditch, rice paddy, upland, and natural wetland. We intersected free-grazing domestic duck UD polygons (constructed using the 99% UD cumulative probability contour) with land cover type in ArcGIS 10.2 (Environmental Systems Research Institute, Inc., Redlands, CA) to determine percent area of each land cover type within each UD. For each bird, we used Geospatial Modelling Environment (Beyer 2014) to sum all UD pixel values within a land cover type to calculate relative use of each habitat.
2.5. Temporal habitat use patterns
We used the R package moveud (Collier 2013) to extract the σ2m estimate for each individual time step within a bird’s overall dBBMM and create a 50% UD contour for every pair of sequential locations (Byrne et al. 2014). We calculated proportional habitat use for each time step by intersected UD contours with land cover in ArcGIS 10.2. Finally, we indexed each time step by the time of the first location in each pair of locations, and for every 30 min time period (06:00–06:30, 06:30–07:00, etc.) we calculated the average proportion of each land cover type contained within all 50% UD contours. For each marked bird, we defined available habitat as the total area encompassed by all 99% UD contours from birds at that farm. We did not use a fixed-distance radius from farm centers to define available habitat because barriers such as large river channels often prevented free-grazing domestic ducks from accessing certain habitats within these surrounding areas.
3. Results
Farmers generally released free-grazing domestic ducks from shelter areas to begin foraging within 1 h of sunrise and ducks usually returned within 1 h of sunset. The average (±STDDEV) total number of diurnal locations we received per transmitter was 119.0 ± 47.4 over a span of 2.5 ± 1.5 days, or one location every 15.3 ± 7.8 min during diurnal periods.
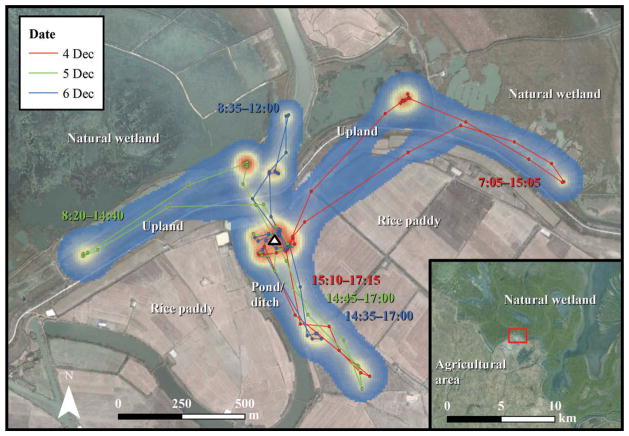
The primary habitat types used by free-grazing domestic ducks depended on the availability of nearby natural wetlands. Marked ducks in border farms foraged extensively in natural wetlands, spending an average of 51.5% ± 16.9% of daylight hours in these areas. One marked bird from a border farm located at the southern edge of the Poyang Lake National Nature Reserve occurred in natural wetlands 75% of the time (Figure 2). Relative use of natural wetlands was nearly four times higher than in rice paddies (51.5% vs. 13.2% of daylight hours). In areas without nearby natural wetland habitat, free-grazing domestic ducks primarily foraged in rice fields, where they spent 47.5% ± 26.3% of daylight hours. Presence of nearby natural wetland habitat had little effect on relative use of upland habitat; however, birds in border farms used natural wetlands far more than rice fields and man-made ponds and ditches.
Figure 2.
Diurnal locations, paths and utilization distribution estimating relative use of a free-grazing domestic duck over three days in December 2007 near Poyang Lake. Blue shading indicates areas of relatively low use, while orange and red areas indicate areas of high use. Time labels are colored by date and correspond to individual foraging trips away from the farm shelter. White triangle denotes farm shelter. Red square on inset indicates farm location in relation to boundary between agricultural area and natural wetland. Base imagery source: Google Earth (version 7.1.2, Google, Mountain View, CA, 2015).
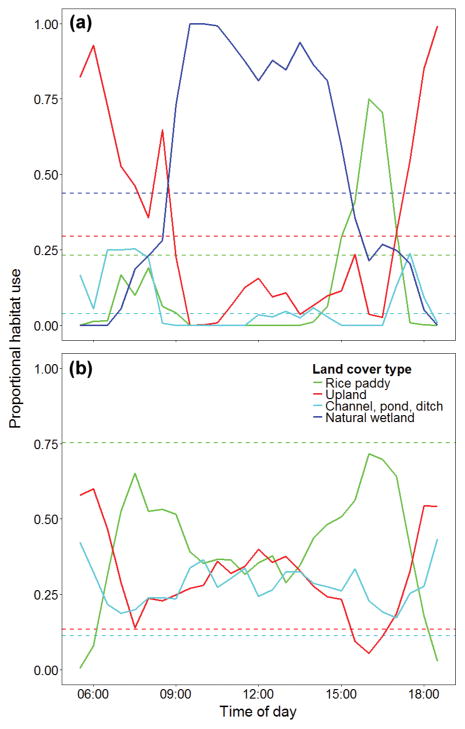
Free-grazing domestic ducks from border farms used natural wetlands disproportionately more than their availability on the landscape during diurnal hours, with peak use occurring between 08:30 and 15:30 hours (Figure 3). In both types of farms, upland habitats received disproportionately high use during early morning and late evening, when birds generally stayed near the farm center. Rice paddies received relatively low use relative to their availability in both types of farms, and peak use times often coincided with foraging bouts.
Figure 3.
Average proportional habitat use of habitat types within 50% UD contours bounding 30 min time steps for (a) 20 birds at farms > 1.5 km from natural wetlands and (b) 5 birds from farms ≤ 1.5 km from natural wetlands (border farms). Dashed horizontal lines represent the proportional availability of each habitat type within 1.5 km of farm center.
The frequency, duration, and maximum distance from the farm shelter during foraging bouts were variable within and across individuals, and across days. Marked ducks generally foraged within 1 km of the farm shelter, but occasionally traveled as far as 1.5 km. On average, free-grazing domestic ducks covered an estimated 46.7 ± 27.0 ha, based on the 99% cumulative probability contour of their UD (e.g., Figure 2). On a given day, nearly half of the marked birds (48.4% ± 14.5%) exhibited multiple foraging bouts during the day, usually once in the morning, followed by a return to the shelter for several hours midday, then a second foraging excursion in the afternoon. In six of seven farms with location data from multiple marked birds from the same day, marked individuals traveled within the same flock during foraging bouts in rice paddies or natural wetlands. However, one bird used a spatially distinct foraging area and exhibited different movement patterns than other marked birds from the same farm, indicating that it belonged to a separate flock.
4. Discussion
Our data show that in late 2007 a portion of free-grazing domestic ducks in the Poyang Lake basin regularly foraged in natural wetlands used by wild waterfowl, making contact (either directly or indirectly) between domestic and wild ducks likely. Movements and habitat use of marked domestic ducks in border farms exemplified a situation conducive to direct interaction with potential for HPAI transmission between domestic and wild birds. Our analyses of temporal habitat use indicate that free-grazing domestic ducks in these farms showed disproportionately high use of natural wetlands relative to their availability on the landscape. Although relatively few of the birds in our analyses were marked in border farms, these farms are representative of many farms in the region. In total, 28 of 166 (17%) duck shelters surveyed in October 2007 (reported in Cappelle et al. 2014) were located within 1.5 km of natural wetlands surrounding Poyang Lake (Figure 1). If free-grazing domestic ducks in these farms exhibit similar movement patterns and habitat use to those from border farms in our analyses, there is a high likelihood of spatiotemporal overlap between wild and domestic ducks throughout the entire zone where agricultural lands abut natural wetlands. Telemetry locations from a previous study of 13 resident and 15 migratory wild ducks marked with satellite transmitters in Poyang Lake in March and November 2007 (Takekawa et al. 2010b; Table 1) show a strong association with natural wetland habitats. Over 90% of the 127 diurnal locations (Argos Doppler locations; ~1–10 km accuracy) from migratory wild ducks in the Poyang Lake basin between October and April were in natural wetlands on the southern and western edges of Poyang Lake, with a center of abundance in the Poyang Lake National Nature Reserve. Approximately 20,000,000 poultry are raised annually within the counties containing large portions of this reserve (Cappelle et al. 2014). Of the 2886 diurnal locations from resident wild ducks in the region (GPS locations; ± 18.5 m accuracy), 63% were in natural wetlands, with individuals frequently alternating between rice paddies and natural wetland habitats (Hill et al. 2012). Future studies describing movements and habitat use of free-grazing domestic ducks that focus marking efforts on border farms would help clarify the extent to which free-grazing domestic ducks use natural wetlands on a larger scale, and indicate whether or not our results are representative across seasons and years.
Table 1.
Summary of diurnal satellite tracking locations in the Poyang Lake basin from wild ducks captured at Poyang Lake, China, in March and November 2007 (Takekawa et al. 2010b).
| Species | n | Data type
|
|
|---|---|---|---|
| Argos | GPS | ||
| Migratory wild ducks | |||
| Baikal teal (Anas formosa) | 2 | 4 | – |
| Common teal (Anas crecca) | 4 | 56 | – |
| Eurasian wigeon (Anas penelope) | 2 | 8 | – |
| Falcated teal (Anas falcata) | 5 | 30 | – |
| Garganey (Anas querquedula) | 1 | 25 | – |
| Northern pintail (Anas acuta) | 1 | 4 | – |
| Resident wild ducks | |||
| Spot-billed duck (Anas poecilorhyncha) | 12 | – | 2679 |
| Mallard (Anas platyrhynchos) | 1 | – | 207 |
| Total | 28 | 127 | 2886 |
Differences in feeding schedules and the extent to which farmers herded their flocks are among many potential factors that might help explain variation in movement patterns and habitat use across farms. The variation in spatiotemporal patterns we observed within some farms may influence disease persistence and transmission. Greater variation in movement patterns and habitat use within a farm would result in larger overall area covered by a group of ducks from that farm, thereby increasing the likelihood of spatiotemporal overlap with wild species. After foraging during the day, free-grazing domestic ducks return to shelters with hundreds to thousands of other ducks. Shelters with high densities of ducks may provide opportunity for LPAI contracted from wild birds to mutate into HPAI H5N1. Subsequent interactions between infected domestic ducks and wild waterfowl may also provide opportunity for reintroduction of HPAI into wild populations (Muzaffar et al. 2010).
Even without spatial overlap between migratory wild ducks and domestic poultry, resident wild species may function as conduits for H5N1 virus transmission between the two groups by alternating between using wetland habitats preferred by migratory species and rice paddies used by free-grazing domestic ducks (Hill et al. 2012). Because avian influenza virus can persist in water and feathers for up to several months at certain temperatures, temporal overlap between wild and domestic birds may not be necessary for virus transmission (Domanska-Blicharz et al. 2010, Yamamoto et al. 2010). Resident wild species such as mallards (Anas platyrhynchos), spot-billed ducks (Anas poecilor-hyncha) and swan geese (A. cygnoides) are commonly raised in captivity and sold at markets, as many Chinese consumers prefer these species over domestic fowl (Takekawa et al. 2010a). These species may be more likely to associate with flocks of phenotypically identical wild birds.
Our movement data build upon results of Cappelle et al. (2014), who found that intensive poultry farming in the Poyang Lake basin is synchronized with wild duck migration and that free-grazing domestic ducks share rice paddies with nonmigratory wild ducks. We describe movement behavior and habitat use of free-grazing domestic ducks on a smaller scale and identify border farms as areas within the Poyang Lake basin that may have the highest risk of H5N1 transmission between domestic and wild birds. Because we only marked laying ducks, we cannot use our results to make inferences on movement behavior and habitat use of meat ducks in the Poyang Lake area that may have different production systems and cycles. Given that there are two major duck production cycles in this area, it is unclear whether domestic ducks have similar patterns in movement and habitat use during the spring season in a different rice-growing stage. Further, annual fluctuation in water levels likely alters local land use practices and the availability of different habitat types for both wild and domestic birds. Nevertheless, this study provides an example of how analysis of movement ecology in relation to other biological entities or environmental factors is essential to fully understand dynamics such as disease transmission in a complicated landscape.
Acknowledgments
We thank the poultry farmers of the Poyang Lake region for collaboration on this study. The use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Funding
This work was supported by the National Institutes of Health [NIH 1R01AI101028-01A1]; United States Geological Survey; National Aeronautics and Space Administration Wild–Domestic Duck Interface and H5N1 Transmission (NASA) Public Health Program [NNX11AF66G]; National Science Foundation – National Institutes of Health – Ecology of Infectious Diseases program; United Nations Food and Agricultural Organization; National Institutes of Health – Fogarty International Center [R01-TW007869].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Beyer HK. [Accessed 29 January 2015];Geospatial modelling environment software. 2014 [online]. Available from: www.spatialecology.com.
- Bin MS, et al. Rice production systems and avian influenza: interactions between mixed-farming systems, poultry and wild birds. Waterbirds. 2010;33(sp1):219–230. doi: 10.1675/063.033.s116. [DOI] [Google Scholar]
- Brown JD, Stallknecht DE, Swaynet DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerging Infectious Diseases. 2008;14:136–142. doi: 10.3201/eid1401.070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME, et al. Using dynamic Brownian bridge movement modelling to measure temporal patterns of habitat selection. Journal of Animal Ecology. 2014;83:1234–1243. doi: 10.1111/1365-2656.12205. [DOI] [PubMed] [Google Scholar]
- Cappelle J, et al. Risks of avian influenza transmission in areas of intensive free-ranging duck production with wild waterfowl. EcoHealth. 2014;11:109–119. doi: 10.1007/s10393-014-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, et al. The evolution of H5N1 influenza viruses in ducks in southern China. Proceedings of the National Academy of Sciences. 2004;101:10452–10457. doi: 10.1073/pnas.0403212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B. moveud: dynamic Brownian bridge movement model individual time-step utilization distributions [online] [Accessed 7 July 2015];R package version 1.0.1. 2013 Available from: http://www.rnr.lsu.edu/bret/BretWebSiteDocs/ByrneDBBMM_1.0.zip.
- Domanska-Blicharz K, et al. H5N1 high pathogenicity avian influenza virus survival in different types of water. Journal of Avian Diseases. 2010;54:734–737. doi: 10.1637/8786-040109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Gaidet N, et al. Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: dispersal ranges and rates determined from large-scale satellite telemetry. Journal of Applied Ecology. 2010;47:1147–1157. doi: 10.1111/j.1365-2664.2010.01845.x. [DOI] [Google Scholar]
- Gauthier-Clerc M, Lebarbenchon C, Thomas F. Recent expansion of highly pathogenic avian influenza H5N1: a critical review. Ibis. 2007;149:202–214. doi: 10.1111/j.1474-919X.2007.00699.x. [DOI] [Google Scholar]
- Gilbert M, et al. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerging Infectious Diseases. 2006;12:227–234. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, et al. Avian influenza, domestic ducks and rice agriculture in Thailand. Agriculture, Ecosystems & Environment. 2007;119:409–415. doi: 10.1016/j.agee.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, et al. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proceedings of the National Academy of Sciences. 2008;105:4769–4774. doi: 10.1073/pnas.0710581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Zhuang H. An ecosystem approach to resolving conflicts among ecological and economic priorities for Poyang Lake wetlands. IUCN; Gland, Switzerland: 2010. Unpublished report. [Google Scholar]
- Hill NJ, et al. Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos) Molecular Ecology. 2012;21:5986–5999. doi: 10.1111/j.1365-294X.2012.05735.x. [DOI] [PubMed] [Google Scholar]
- Hulse-Post DJ, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proceedings of the National Academy of Sciences. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J, et al. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1) Emerging Infectious Diseases. 2008;14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranstauber B, et al. A dynamic Brownian bridge movement model to estimate utilization distributions for heterogeneous animal movement. Journal of Animal Ecology. 2012;81:738–746. doi: 10.1111/j.1365-2656.2012.01955.x. [DOI] [PubMed] [Google Scholar]
- Kranstauber B, Smolla M. [Accessed 29 January 2015];Move: visualizing and analyzing animal track data. R package version 1.2.475. 2014 [online]. Available from: http://cran.R-project.org/package=move.
- Martin V, et al. Spatial distribution and risk factors of highly pathogenic avian influenza (HPAI) H5N1 in China. PLoS Pathogens. 2011;7:e1001308. doi: 10.1371/journal.ppat.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, et al. Spring migration of northern pintails from California’s Central Valley wintering area tracked with satellite telemetry: routes, timing, and destinations. Canadian Journal of Zoology. 2005;83:1314–1332. doi: 10.1139/z05-125. [DOI] [Google Scholar]
- Nemeth NM, et al. Experimental infection of bar-headed geese (Anser indicus) and ruddy shelducks (Tadorna ferruginea) with a clade 2.3.2 H5N1 highly pathogenic avian influenza virus. Veterinary Pathology. 2013;50:961–970. doi: 10.1177/0300985813490758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B, et al. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Qian F, Yu C, Jiang H. Aerial and ground surveys of wintering waterbirds in Poyang Lake basin. In: Prentice C, editor. Conservation of flyway wetlands in East and West/Central Asia; Proceedings of the Project Completion Workshop of the UNEP/GEF Siberian Crane Wetland Project; 14–15 October 2009; Harbin, China. Baraboo, WI: International Crane Foundation; 2011. [Google Scholar]
- R Foundation for Statistical Computing. [Accessed 10 May 2015];R: A language and environment for statistical computing. 2015 [online]. Available from: http://www.R-project.org.
- Shankman D, Keim BD, Song J. Flood frequency in China’s Poyang Lake region: trends and teleconnections. International Journal of Climatology. 2006;26:1255–1266. doi: 10.1002/(ISSN)1097-0088. [DOI] [Google Scholar]
- Shankman D, Liang Q. Landscape changes and increasing flood frequency in China’s Poyang Lake region. The Professional Geographer. 2003;55:434–445. doi: 10.1111/0033-0124.5504003. [DOI] [Google Scholar]
- Songserm T, et al. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerging Infectious Diseases. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JD, Kaminski RM, Reinecke KJ. Avian foods, foraging, and habitat conservation in world rice fields. Waterbirds. 2010;33(Special Publication 1):133–150. doi: 10.1675/063.033.s110. [DOI] [Google Scholar]
- Sturm-Ramirez, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? Journal of Virology. 2005;79:11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa JY, et al. Victims and vectors: highly pathogenic avian influenza H5N1 and the ecology of wild birds. Avian Biology Research. 2010a;3:51–73. doi: 10.3184/175815510X12737339356701. [DOI] [Google Scholar]
- Takekawa JY, et al. Migration of waterfowl in the East Asian flyway and spatial relationship to HPAI H5N1 outbreaks. Avian Diseases. 2010b;54:466–476. doi: 10.1637/8914-043009-Reg.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Webster RG. Emergence of influenza a viruses. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2001;356:1817–1828. doi: 10.1098/rstb.2001.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. [Accessed 6 January 2015];Monthly Risk Assessment Summary: Influenza at the Human-Animal Interface. 2015 [online]. Available from: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_6January2015.pdf.
- Xiao X, et al. Remote sensing, ecological variables, and wild bird migration related outbreaks of highly pathogenic H5N1 avian influenza. Journal of Wildlife Diseases. 2007;43:S40–S46. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, et al. Persistence of avian influenza virus (H5N1) in feathers detached from bodies of infected domestic ducks. Applied Environmental Microbiology. 2010;76:5496–5499. doi: 10.1128/AEM.00563-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, et al. An investigation of enhanced recessions in Poyang Lake: comparison of Yangtze river and local catchment impacts. Journal of Hydrology. 2014;517:425–434. doi: 10.1016/j.jhydrol.2014.05.051. [DOI] [Google Scholar]