Abstract
Monitoring the photosynthetic performance of plants is a major key to understanding how plants adapt to their growth conditions. Stress tolerance traits have a high genetic complexity as plants are constantly, and unavoidably, exposed to numerous stress factors, which limits their growth rates in the natural environment. Arabidopsis thaliana, with its broad genetic diversity and wide climatic range, has been shown to successfully adapt to stressful conditions to ensure the completion of its life cycle. As a result, A. thaliana has become a robust and renowned plant model system for studying natural variation and conducting gene discovery studies. Genome wide association studies (GWAS) in restructured populations combining natural and recombinant lines is a particularly effective way to identify the genetic basis of complex traits. As most abiotic stresses affect photosynthetic activity, chlorophyll fluorescence measurements are a potential phenotyping technique for monitoring plant performance under stress conditions. This review focuses on the use of chlorophyll fluorescence as a tool to study genetic variation underlying the stress tolerance responses to abiotic stress in A. thaliana.
INTRODUCTION
Environmental factors, such as heat, drought, salt and fluctuating light stress, threaten agriculture across the globe by reducing the growth and yield of many plant species. For example, according to a recent United Nations study, it was estimated that the global annual cost of lost crop yield and land degradation to salinity, in particular, is around US$ 27.3 billion (Qadir et al., 2014). The prevalence of many stress events, such as drought and heat stress events, are expected to increase with climate change (Heffernan, 2013; Teixeira et al., 2013).
The use of chlorophyll fluorescence to monitor photosynthetic performance has been utilised since the Kautsky effect was first described in 1931 (reviewed in Govindjee, 1995). The Kautsky effect describes the pattern of chlorophyll fluorescence in dark adapted leaves when exposed to continuous light. There is an initial increase in fluorescence as the PSII become saturated followed by a slow decrease as photochemical quenching occurs. Since the 1930s, the development of instruments capable of imaging chlorophyll fluorescence has provided a powerful tool in many areas of plant research including revealing spatial heterogeneity of leaf photosynthetic performance (Maxwell and Johnson, 2000); screening for metabolic perturbation (Barbagallo et al., 2003); estimating green leaf area and consequently estimating growth and leaf senescence (e.g. Barbagallo et al., 2003) and identifying components of photoprotective mechanism (Niyogi et al., 1998). In addition, chlorophyll fluorescence has also been used to study abiotic stress responses such as salt stress, drought and high light stress (Tsugane et al., 1999; Stepien and Johnson, 2009; Woo et al, 2008). However, the majority of these studies have been limited to a small number of lines using manual measurements or seedling studies. The recent development of high-throughput phenotyping platforms has allowed an expansion of the use of kinetic measures of chlorophyll fluorescence in a large number of mature plants and in a range of plant research areas, including population genetics.
A. thaliana has been used for decades in many disciplines of plant research for its great advantages: (i) its entire genome is fully sequenced and well annotated, (ii) it is a selfing species with a short life cycle, (iii) it can produce a large number of seeds and (iv) it has a broad geographic distribution, which reflects its natural genetic variation that is commonly utilised in gene mapping studies (Shindo et al., 2007). Using A. thaliana as a model plant and chlorophyll fluorescence as a photosynthetic monitoring tool are a promising combination for investigating the genetic factors involved in different stress tolerance-related traits such as germination, growth, flowering time and seed yield under various growth conditions (Koornneef et al., 2004; Lefebvre et al., 2009).
In this review, we focus on the use of chlorophyll fluorescence phenotyping, and the subsequent characterisation of quantitative trait loci (QTLs), as tools to investigate the genetic variation in the tolerance response to abiotic stress in the model plant, A. thaliana. To illustrate our chosen methodology, we present two experimental examples: 1) the investigation of the natural variation in non-photochemical quenching (NPQ) using a genome wide association (GWAS) diversity set of A. thaliana accessions under high light stress and 2) the investigation of the natural genetic variability in salinity tolerance levels using measures of photoinhibition and photosynthetic efficiency in a bi-parental A. thaliana mapping population.
Photosynthesis and the use of chlorophyll fluorescence as an indicator
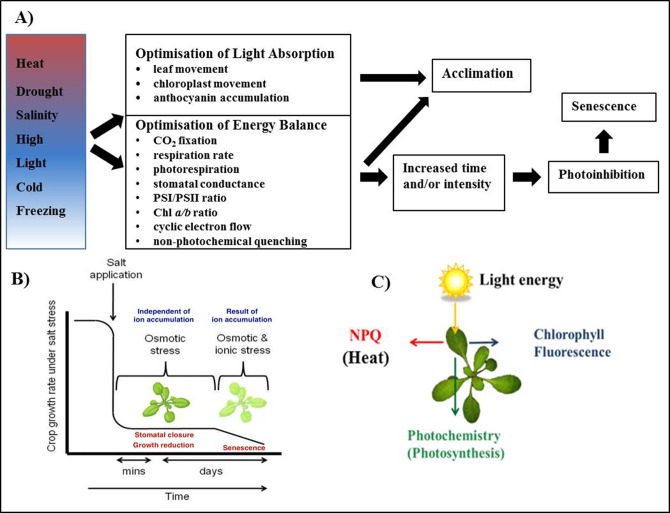
The process of photosynthesis is essential to life on Earth; the conversion of light energy from the sun to chemical energy provides the base level of most food chains. However, the frequent mild to severe abiotic stresses which plants are exposed to in the natural environment impact photosynthesis on a daily basis. Daily fluctuations in temperature, humidity and light intensity, as well as more sustained stresses such as water deficit and salinity, impact multiple cellular processes that impact photosynthetic capacity and efficiency (Figure 1A). These stresses often occur simultaneously, resulting in exacerbation of the stress. Some stresses, such as salinity, have multiple phases of salt ion accumulation that results in different negative effects on photosynthesis (Figure 1B). Plants must optimise the absorption and use of light energy to maintain growth while limiting the photooxidative damage that occurs when absorbed energy is excess to that used in CO2 fixation. Excess light absorption by the light harvesting antennae of the photosystems converts water and oxygen into reactive oxygen species (ROS), which then damages other cell components such as the photosystems I and II (PSI and PSII, respectively). Scavenging these ROS and preventing excess light absorption is energy intensive and can lead to reduced growth rate, reproduction and even death (Kasahara et al., 2002). To maintain maximum photosynthetic rate and growth, but avoid cellular damage, plants have evolved a number of mechanisms to allow short-term and long-term acclimation to changes in their environment. These involve minimising the amount of light absorbed and diverting energy from photosynthesis. Chlorophyll fluorescence can be used to quantify the effectiveness of these mechanisms. For example, photoinhibition can be monitored by measuring the change in the Fv/Fm parameter; while the change in NPQ reflects the amount of thermal dissipation, one of the photoprotective mechanisms.
Figure 1.
Abiotic stress results in a range of changes attempting to optimise light absorption and energy balance of photosynthesis.
(A) Common to all abiotic stresses, is the need to optimise light absorption and energy balance to avoid photoinhibition and allow acclimation. Sustained or high intensity stresses cause irreversible damage the photosystems and other cellular components leading to cell death. (B) Salinity, an abiotic stress factor, acts in two phases; the osmotic and the ionic. The osmotic phase is a rapid response, which results in stomatal closure and growth rate reduction. It is referred to as a shoot-ion independent effect, as the ions have not had time to accumulate to toxic levels in the shoot. It is followed by the ionic phase, which causes early leaf senescence (Munns and Tester, 2008). Reproduced with permission of Annual Review of Plant Biology, Volume 59 © by Annual Reviews, http://www.annualreviews.org. (C) Energy absorbed by the photosystems can be used in one of three ways: for photosynthesis, released as chlorophyll fluorescence or released as heat through non-photochemical quenching.
Under non-stressful conditions photosynthetic pigments, such as chlorophyll located in association with photosystems PSI and PSII, capture light energy and convert it to chemical energy via the photosynthetic pathway. When excess light is absorbed, the excess energy can be emitted as heat or chlorophyll fluorescence (Figure 1C) (Maxwell and Johnson, 2000; Baker, 2008). This model suggests firstly, that the light energy absorbed by chlorophyll activates electron transport to the primary quinone acceptor of PSII (QA), which causes the quenching of the fluorescence; a process known as photochemical quenching (Baker, 2008). Secondly, if there is additional energy that cannot be used in photochemical quenching, this excess energy could be dissipated in the form of heat, known as NPQ. The NPQ mechanism involves multiple components including a proton gradient across the thylakoid membranes, the activity of xanthophyll cycle, and the function of PsbS protein (Horton et al., 1996; Li et al., 2002; Jung and Niyogi, 2009; García-Plazaola et al., 2012). The mechanisms of how these three components affect the NPQ process will be explained below. Lastly, the excess energy could also be re-emitted as chlorophyll fluorescence. The processes of photochemical quenching, NPQ and chlorophyll fluorescence occur in competition; if one rate increases, it will result in the decrease of the other two rates. Although only 1 to 2% of the total amount of light absorbed is emitted as chlorophyll fluorescence, it can be measured with pulse-amplitude modulated (PAM) fluorometers (Maxwell and Johnson, 2000). Experimental evidence has shown that chlorophyll fluorescence could be used to determine a plant's performance in stress conditions (Kautsky, 1960; Butler, 1978; Roháček, 2002; Baker, 2008).
In addition, the photosynthetic efficiency could be calculated from the chlorophyll fluorescence measurements, which indicates how the absorbed energy is being utilized in photochemistry. Photosynthetic efficiency can be described as the fraction of light energy converted into chemical energy during photosynthesis, where the rate of photosynthesis is susceptible to changing light intensity. However, the photosynthetic efficiency can only rise to the saturation point of the photosynthetic rate, limited by maximum Rubisco activity, beyond which further increases in light intensity do not increase photosynthetic rate (Osmond, 1994). The maximum photosynthetic efficiency can be calculated as an indicator of the amount of photoinhibition occurring; as well as NPQ, the heat-dissipating process of excess light energy that is involved in photoprotective mechanisms (Maxwell and Johnson, 2000). The calculation of these parameters is described in Box 1.
Box 1. Calculations of Chlorophyll Fluorescence Parameters.
The principle of chlorophyll fluorescence measurement is straightforward and many excellent reviews exist (see Maxwell and Johnson, 2000; Murchie and Lawson, 2013). Basically, the wavelength of chlorophyll fluorescence is longer than the wavelength of absorbed light (Figure 2A). Hence, chlorophyll fluorescence yield can be observed by illuminating a plant in a known wavelength of light and measuring the amount that is re-emitted at the longer wavelength. Multiple parameters measured by PAM chlorophyll fluorescence can be correlated to abiotic stress tolerance mechanisms. In PAM chlorophyll fluorescence, plants are exposed to a program of dark and light periods while subjected to a number of regular saturating light flashes, with quantification of the resulting fluorescence (Figure 2B). Plants are initially kept in the dark for a period of time, at least 30min is recommended for Arabidopsis. This is called the dark-adapted state, which results in opening (oxidisation) of the reaction centre of PSII. Then a minimum fluorescence, Fo, is measured using a weak measuring light. It is very important to note that this measuring light intensity must be low enough to measure, but does not cause the activation of the photochemistry in PSII. Then, this is followed by a short burst of saturating actinic light (less than 1 second at several thousand µmol photons.m-2·s-1) resulting in the closure of the PSII reaction centre, because it is fully saturated. PSII later releases the maximum amount of chlorophyll fluorescence, Fm, with the thermal dissipation and photochemistry at the minimum (Maxwell and Johnson, 2000). This allows the amount of photoinhibition, or maximum quantum efficiency of PSII, to be calculated using the formula:
Healthy non-stressed Arabidopsis will have a Fv/Fm value of around 0.8 (Barbagallo et al, 2003; Hogewoning et al, 2012). Under stress conditions that can cause severe damage to PSII through photoinhibition, this damage can be observed by measuring the decrease in the Fv/Fm ratio (Figure 2C).
Subsequently, leaves are illuminated continuously with moderately excessive light energy (>700 µmol photons.m-2·s-1), commonly referred to as actinic light, which activate both photochemical and photoprotective mechanisms. Another saturating pulse of light allows the maximum fluorescence in the light, Fm′ to be measured. The operating efficiency of the primary photochemistry in PSII (ΦPSII) can then be estimated from:
where Fv′ is the difference between Fm′ and Fo· Measures of Fq′/Fm′ in light-adapted plants indicate photosynthetic efficiency, or what proportion of the absorbed light is being utilised for photochemistry. This measure, along with the light intensity, can be used to calculate the electron transport rate (ETR) using:
where PAR is the photosynthetically active radiation, the factor 0.5 accounts for partitioning of energy between PSII and PS I and the factor 0.84 accounts for the reduced light intensity that is absorbed by the leaf (Stemke and Santiago, 2011).
Continuing regular saturating flashes at one minute intervals at high light intensities allow changes in the maximal fluorescence level in the light, Fm, to be measured and the contribution of NPQ components to be calculated. After switching back to the dark, the reaction centre becomes relaxed, resulting in the relaxation of NPQ that is observed from the increasing amount of fluorescence in response to saturating flashes. NPQ can be calculated from the ratio of change in Fm and Fm′ during the illumination as shown in the equation:
Normally, the values are expected to be within the range of 0.5 – 3.5. However, it can vary greatly depending on the plant species and growth conditions (Maxwell and Johnson, 2000). When wild type plants are grown under low light, a small amount of NPQ value can be observed. However, when they are exposed to excess light for a period of time, NPQ increases until it reaches a steady state (Figure 2D).
Another commonly used chlorophyll fluorescence parameter is the level of photochemical quenching of PSII, qP. This parameter provides an indication of the proportion of open PSII reaction centres. This is calculated as:
An alternative expression used to measure the proportion of closed reactive centres, and is sometimes referred to as the “excitation pressure” on PSII, is 1-qP. The relationship of qP to the redox status is not considered linear so qL is thought to be a more accurate indicator of the redox status of PSII (Murchie & Lawson, 2013).
Photoinhibition is the decrease in photochemical capacity or increase in damage to PSII as a result of abiotic stress. It can be observed by measuring the decrease in the chlorophyll fluorescence parameter maximum quantum efficiency (Maxwell and Johnson, 2000). This parameter is frequently used as a sensitivity indicator of a plant's stress levels, either from a biotic or abiotic cause (Roháček, 2002; Baker, 2008). For instance, when wild type plants are exposed to high light stresses, maximum quantum efficiency is markedly decreased in comparison to non-stressed plants (Roháček, 2002; Baker, 2008). This occurs due to the excess energy harvested causing photo-oxidative damage to the proteins in PSII, in particular the D1 protein.
Non-photochemical quenching (NPQ) is an important mechanism by which Arabidopsis plants dissipate excess absorbed energy in the form of heat (Figure 1C). NPQ is considered to be a fast, safe and effective mechanism of photoprotection, which is able to eliminate over 75% of excessively absorbed light energy (Niyogi, 1999). There are three major components involved in NPQ, namely qE, qT and qI (Jung and Niyogi, 2009; Ihnken et al., 2011; Turan, 2012). The qE, energy-dependent quenching, is rapidly induced and relaxed within seconds to minutes. Development of qE is associated with the accumulation of a proton gradient (pH) across membranes caused by excess light (Li et al., 2002; Jung and Niyogi, 2009; Ikeuchi et al., 2016). This acidification activates violaxanthin de-epoxidase in the xanthophyll cycle resulting in accumulation of zeaxanthin, as seen in the lack of qE in the Zeaxanthin-deficient (npq1) Arabidopsis mutant (Baker, 2008; Jung and Niyogi, 2009; Ware et al., 2015). Lastly, the protonation of the PsbS protein, which is associated with the PSII antenna, induces conformational change for quenching of excitation energy, an Arabidopsis PsbS deletion mutant, npq4-1, shows absent of qE (Li et al., 2002; Baker, 2008). The presence of PsbS is more important in the induction phase, rather than the steady phase, of NPQ (Ikeuchi et al., 2016). The qT, state-transition quenching, is a mechanism of balancing excitation energy of PSII and PSI, i.e. balancing excited ions of PSII and PSI by reversible phosphorylation involved in the migration of light harvesting complex II (LHCII) pool between the two photosystems. However, the qT component is more important in algae than in plants (Müller et al., 2001). The last component, photoinhibitory quenching (qI) is quenching associated with closed reaction centres of PSII caused by photoinhibition (Ware et al., 2015). Among these three components, qE is the major component contributing to NPQ in higher plants such as Arabidopsis (Jung and Niyogi, 2009).
Photosynthetic responses to abiotic stress
Photosynthesis is affected by a range of abiotic stresses in the natural environment (for detailed review see Ashraf & Harris, 2013; Chaves et al, 2009). The nature of the effects will vary depending on the intensity of the stress and the length of stress imposition. In natural environments, an abiotic stress factor is often accompanied by one or more other factors, e.g. drought and heat stress. Hence, it is beneficial to consider the combinational effects of abiotic stresses, not just individually, on photosynthesis. Stomatal closure, for example, occurs due to many stress factors and would affect photosynthetic rate as well as the induction of photoprotective mechanisms. Furthermore, we must consider the limitations of both the light and dark cycles of photosynthesis, in addition to the sources and sinks of absorbed energy. Here, we discuss the effects of three abiotic stresses on photosynthesis: high light, salinity and heat stress.
In the middle of a summer day in temperate environments, light intensity could exceed 2000 µmol photons.m-2·s-1; an intensity that can cause photodamage to plants, such as Arabidopsis. High light stress occurs as the intensity of photosynthetically active radiation (PAR) in the natural environment varies over a day, with the change in seasons and even due to changes in cloud or canopy cover. Rapid acclimation of photosynthesis is thus required to allow maximum utilisation of the incident PAR while avoiding photodamage. To deal with these transient stresses, plants have evolved several short term stress avoidance mechanisms to optimise light absorption, including changes to leaf angle (Mullen et al., 2006) and chloroplasts location and orientation (Kagawa and Wada, 2002; Kadota et al., 2009). For more sustained stresses, a number of structural and biochemical acclimation processes occur. Damaging radiation can be screened by the accumulation of phenolic compounds in the leaf epidermis, preventing photodamage to PSII (Takahashi and Badger, 2011). Plants exposed to higher light intensities also tend to have thicker leaves due to expanded palisade cells, as well as higher stomatal density, in comparison to shaded leaves (Björkman, 1981). There are also lower amount of thylakoids per chloroplast section in higher light when compared to low light environments. Finally, low light plants contain more chlorophyll per chloroplast than sun exposed plants, with a higher chlorophyll a/b ratio in sun-exposed leaves than shade leaves (Anderson, 1986). Variation between genotypes in their acclimation mechanisms in response to a standardised stress could be studied using chlorophyll fluorescence measurements. Moderately increased irradiation increases electron transport rate, but, high irradiation will decrease PSII operating efficiency (ΦPSII; Rooijen et al., 2015) and the redox state of the primary acceptor QA (qL), which indicates the fraction of open PSII reaction centers (Cazzaniga et al., 2013). The induction of photoprotection against high light can be observed by an increase in NPQ (Niyogi, 1999; Maxwell and Johnson, 2000). Photodamage caused by very high light is indicated by a decrease in the maximum quantum efficiency (Ashraf and Harris, 2013).
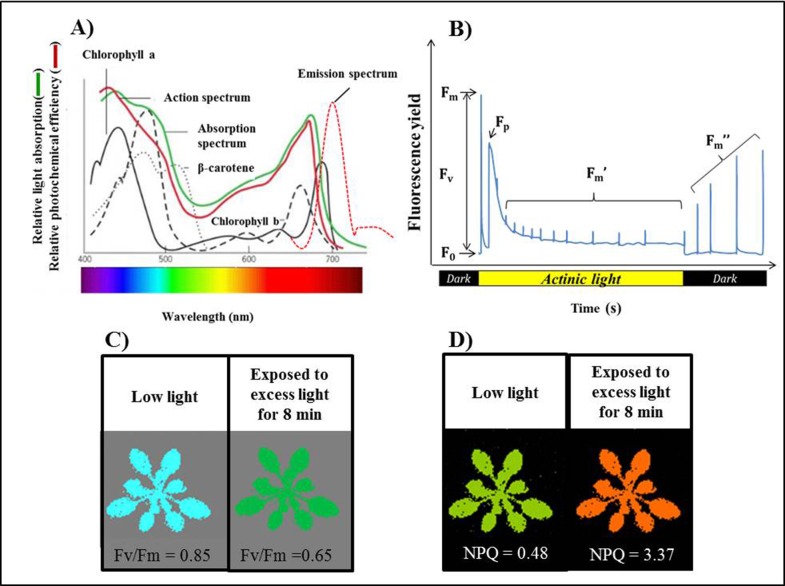
Figure 2.
The principles of Chlorophyll fluorescence.
(A) The model of absorption spectrum by different photosynthetic pigments (adapted from Karp, 2009). Reproduced with permission from John Wiley & Sons, Inc. © John Wiley & Sons, http://www.wiley.com. (B) Sequence of a typical Chlorophyll fluorescence trace measured in PlantScreen. F0 is minimum fluorescence and Fm the maximum fluorescence in the dark adapted. Fm’ represents maximum fluorescence in light. Fm” is the maximum fluorescence during dark relaxation. (C) Image of Fv/Fm for Col-0 grown under low light. The Fv/Fm values decreased from 0.85±0.03 to 0.65±0.08 when plants were exposed to excess light (750 µmol quanta m-2 s-1) for 8 min. (D) Image of NPQ measurement for Col-0 grown under low light. The NPQ values increased from 0.48±0.07 to 3.37±0.11 when plants were exposed to excess light for 8 min. Each data represent the mean (n = 16).
Furthermore, salt stress has been shown to strongly suppress photosynthetic capacity (Iyengar and Reddy, 1996; James et al., 2002; James et al., 2006). The decline in photosynthetic activity could be in the form of a direct effect; in terms of limiting gas diffusion through the stomata and mesophyll, as salinity causes stomatal closure. It could also be in the form of a secondary effect via the release of ROS and other stress signalling molecules that induce adaptive changes to the plant's metabolism (Chaves et al., 2009). In addition, Muranaka et al. (2002) have shown that, in salt treated wheat leaves, photochemical inactivation was achieved by the combinational effect of excessive absorbed energy and accumulation of excess salt ions, which reduced the stability of the PSII function. This was later demonstrated by Stepien and Johnson (2009), who confirmed this observation in Arabidopsis and Thellungiella. Other causes of photosynthetic depression in salt stress could also be due to non-stomatal related processes, such as the forced inactivation of the electron transport chain or inhibition of photochemical enzymes (Muranaka et al., 2002). Furthermore, as salt stress causes plant growth reduction, this could cause an accumulation of unutilized products of carbon assimilation in the growing tissues, which may send feedback signals to down-regulate the rate of photosynthesis (Munns and Tester, 2008).
High temperatures also affect the photosynthetic function, with different intensities of stress resulting in different outcomes (for detailed review see Sharkey and Zhang, 2010). Moderate heat stresses can result in a decrease in CO2 assimilation rates past an optimum growth temperature, due to an increase in photorespiration relative to carbon fixation (Peterhansel et al., 2010); while the electron transport rate could increase, as indicated by an increase in PSII operating efficiency (Zhang and Sharkey, 2009). For Arabidopsis, the optimal temperature for maximum carbon assimilation was around 30°C for plants grown between 15 and 27°C (Bunce, 2008). Excess energy could be dissipated by increasing NPQ and activating cyclic electron flow (Sharkey and Zhang, 2010), while protective chaperone proteins are induced to protect key proteins (Heckthorn et al., 1998). Sometimes, the electron transport rate is decreased in response to moderate heat stress as Rubisco is deactivated to reduce photorespiration (Feller et al., 1998). While there is no damage to PSII under moderate heat stress, as indicated by no change in maximum quantum efficiency, there can be an increase in qL, indicating an oxidation of PSII (Zhang and Sharkey, 2009). More extreme high temperatures may result in irreversible damage to the photosynthetic proteins, especially key PSII protein D1, and the destabilisation of thylakoid membranes; leading to a rapid decline in maximum quantum efficiency. The effects of high temperature are exacerbated when transpiration cooling is limited due to high humidity, which reduces the vapour pressure difference, or when low soil-water content reduces stomatal aperture. In these situations, the heat absorbed by the leaf from sunlight is greater than what it can dissipate through transpiration.
Measuring the temporal response to abiotic stress using high-throughput phenotyping
Understanding the temporal response to abiotic stress is important, as different mutants and natural accessions may vary in their rate of acclimation. One-off measurements may miss this temporal variation. Populations also vary in their rate of development, which may also affect their response to stress. Further to understanding how the response to stress varies over time, we also need to know how the response varies in different parts of the plant. Measuring whole rosettes allows us to focus on either the whole plant or specific leaves. This tissue and developmental specificity may be critical in understanding the abiotic stress response, as differently-aged leaves may show markedly different sensitivities to stresses as in the case of salt stress (Chaves et al., 2009). The current development of high-throughput phenotyping has allowed frequent, non-destructive measurements of stress responses in large numbers of whole plant rosettes. While this provides significant challenges in data management, image analysis automation and statistical analysis; it provides a great opportunity for the discovery and characterisation of stress responsive genes through genome mapping techniques.
In the field, plants are continually exposed to multiple stresses. However, growth chambers use standardardized or fixed conditions, which causes the plants to not be exposed to the natural combinations of light, temperature and moisture conditions that they would normally experience outdoors. In that case, their stress tolerance mechanisms and potential adaptive capability may not be challenged in ways that could be relevant to growth in the field. Dynamic growth chambers, such as the Spectral-Pheno-Climatron (SPC) (Brown et al., 2014), can mimic natural diurnal and global seasonal changes in light intensity and spectra, temperature and moisture (Figure 3A and B). The in-chamber cameras can help in monitoring and quantifying plant growth and development (Figure 3C and 3D). Size, shape and colour parameters can be extracted from the image data in the rosette area or in individual leaves and regarded as response-related phenotypes (Figure 3E). Such chambers allow reproducible controlled stresses to be applied and that the temporal stress responses of plants to be closely and continuously monitored. However, there are still some limitations on using the dynamic growth chambers system as an imitation of the conditions that plants would experience in the field. As the simulated climate is derived from the average climate data, the variability in conditions due to the weather or more extreme events is not seen. In addition, light intensities in many systems are not as high as full sunlight conditions; soil conditions are limited by pot size and soil type; and rainfall is generally not simulated.
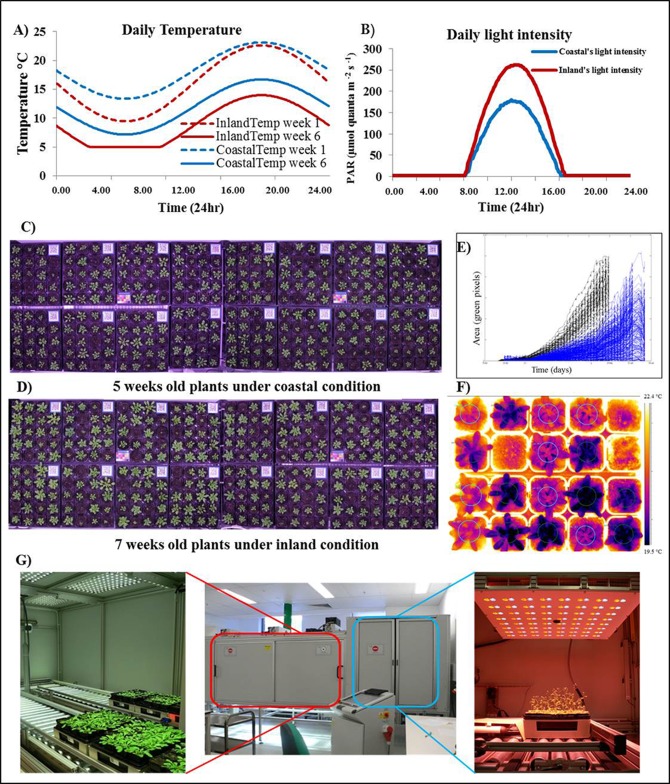
Figure 3.
High-throughput phenotyping allows temporal resolution
Diurnal light intensities and temperature modelled by the SolarCalc software to mimicked environments, coastal (blue) and inland (red). Daily temperature, dash lines represent the daily temperature at week 1 and solid lines represent the daily temperature at week 6 (A). Example of daily light intensity (B). Image capture is automated to measure plants growth and development of coastal condition plants at 5 weeks old (C) and inland condition at 7 weeks old (D). Rosette area is then calculated; black plot represent coastal plants and blue plot represent inland plants (E). Thermal images used to measure transpiration (F). The PlantScreen system for high-throughput phenotyping of cphotosynthetic function and growth (G).
Most modern phenotypic screens require a large number of plants to be phenotyped, whether the screen is an EMS mutant population or a natural population for GWAS. Hence, high-throughput methods of chlorophyll fluorescence phenotyping are necessary. A number of high-throughput phenotyping systems have been developed and are largely aimed at crop species. Some of these systems just use chlorophyll fluorescence as a measure of leaf senescence and chlorophyll content, as they are not Pulse-Amplitude Modulation (PAM) systems (e.g. Chen et al., 2014; Hairmansis et al., 2014). The PlantScreen system (Photon Systems Instruments, PSI) is an example of a system that is appropriate for a small plant, such as Arabidopsis, and can measure the full range of chlorophyll fluorescence parameters using a PAM FluorCam. This system can automatically measure up to 300 Arabidopsis plants in a single run allowing for high-throughput screening to measure the stress effects on photosynthesis, such as photoinhibition and photosynthetic efficiency, and the kinetics of stress tolerance mechanisms, such as NPQ. In addition to chlorophyll fluorescence, PlantScreen can also measure changes in transpiration and stomatal conductance using a thermal camera, plant growth using stereo RGB cameras as well as water use with individual pot weighing and watering (Figure 3F and 3G). In addition to the PlantScreen system, there are similar systems that have been either commercially developed or developed in-house at various research institutes worldwide. These include the dynamic environmental photosynthesis imager (DEPI) at Michigan State University, USA (Cruz et al., 2016) and the Scanalyzer systems from LemnaTec.
Individual timepoints could be treated as traits or multiple timepoints could be analysed collectively to find trends as well as maximum and minimum values. Data from multiple instruments could also be combined to examine the correlation between traits, e.g. transpiration and soil-water content, or growth and electron transport rate. Automation of image analysis also allows a higher-throughput and a greater range of parameters to be extracted from each experiment. Statistical analysis could be performed to determine the heritability of these phenotypes, their genetic basis and the change in heritability and genetic effects through development and in response to treatments.
Combining phenomics with genetic resources for abiotic stress response gene discovery
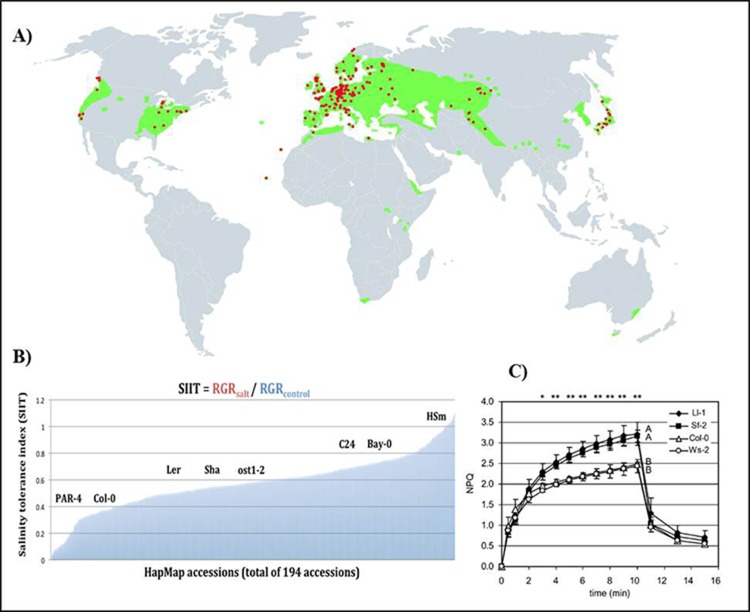
Arabidopsis is native to Europe and central Asia (Koornneef et al., 2004; Cao et al., 2011). It can be found from low to high altitude, and has now been naturalised across the world (Figure 4A). The genetic and geographic diversity of this model plant represents a capacity to adapt to a wide range of ecological habitats, providing potential to discover novel genes and alleles that could be involved in adaptive responses to the environment (Bouchabke et al., 2008). A wide natural variation in abiotic stress tolerance in natural populations of Arabidopsis has also been observed for salinity tolerance (Figure 4B; Busoms et al., 2015) and for nonphotochemical quenching (Figure 4C).
Figure 4.
Natural variation in A. thaliana in abiotic stress responses
(A) Geographic distribution of A. thaliana. The red dots represent original habitat. The green color represent areas that A. thaliana has been naturally spread (Koornneef, 2004). Reproduced with permission of Annual Review of Plant Biology, Volume 55 © by Annual Reviews, http://www.annualreviews.org. (B) Natural variation in salinity tolerance is observed across 194 accessions of the Arabidopsis HapMap population in terms of their relative growth rate (RGR) and shoot-ion independent tolerance (SIIT) index. A selection of well-known Arabidopsis accessions are high-lighted on the SIIT scale, including Col-0, C24 and the parental lines of the bi-parental mapping population Bay and Sha (unpublished). (C) four representative high and low NPQ accessions from a total of 62 A. thaliana accessions screened (Jung and Niyogi, 2009).
There are two strategies to study natural variation (Lefebvre et al., 2009). Firstly, it can be identified using a diversity panel or haplotype mapping population (HapMap) to look at common response signatures in specific stress conditions. Secondly, natural variation can be studied through searching for differences among segregating populations such as F2, recombinant inbred lines (RILs), multiparent advanced generation intercrosses (MAGIC) and advanced intercrossed lines (AIL); which have been created by crossing two or more different accessions through intercrossing to reach the F8 generation, for instance. The combination of environmental and genetic effect in these segregating populations results in a continuous variation of phenotypes.
The naturally occurring genetic variation in Arabidopsis is often represented as a continuous distribution of trait values, and therefore, it is referred to as a quantitative trait. A genomic region that contributes to quantitative variation is a so-called quantitative trait locus (QTL). QTL mapping is the most popular tool to identify the underlying genetic basis associated with particular phenotypic traits. QTL mapping can be used to detect the interaction between genes and environments as well as the internal interaction between genes (epistasis) (Keurentjes and Sulpice, 2009; Borevitz et al., 2002; Weigel, 2012). The segregation of phenotypic variation in RILs reflects the segregation of the genetic markers, which are supplied by the two parental inbred lines (Weigel, 2012; Keurentjes and Sulpice, 2009; Trontin et al., 2011). Generally, any segregating population can be used for QTL mapping. However, there are advantages in using a particular population type over another. Recombinant inbred lines (RILs) represent unique combinations of the parental genotypes where the recombinant chromosomes have been fixed through inbreeding. Hence they can be phenotyped repeatedly under different environments for numerous traits (Weigel, 2012), and allow multiple biological replicates to reduce the environmental noise and increase the power of QTL detection.
Box 2. Case study: Using natural variation in chlorophyll fluorescence parameters to screen for abiotic stress responses.
Example 1. The investigation of the natural variation in non-photochemical quenching (NPQ) using a genome wide association (GWAS) diversity set of A. thaliana accessions under high light stress
To understand the variation of the NPQ photoprotective process, 287 natural accessions of A. thaliana were used for high-throughput screening. Two NPQ mutants, npq1 (Niyogi, 1999) and npq4 (Li et al., 2000) were also included as controls. These two mutants lack most of the qE, due to the absence of Zeaxanthin and PsbS protein respectively. All plants were germinated and grown in dynamic growth chambers, with conditions mimicking two temperate environments (coastal and inland) with an early autumn germination (Figure 3A and 3B). The main differences between the two environments were that the inland condition had more diurnal variation in temperature and higher light intensity than the coastal environment. The acclimation of plants to the stress was monitored over the treatment with NPQ measured in PlantScreen (PSI) twice a week in the early afternoon. The NPQ protocol included 1) 30-min dark adaptation; 2) steady state measurements of fluorescence; 3) 8-min actinic light interval at 750 µmol photons.m-2·s-1 to measure NPQ induction and steady state, followed by 3 minute dark interval to measure NPQ relaxation.
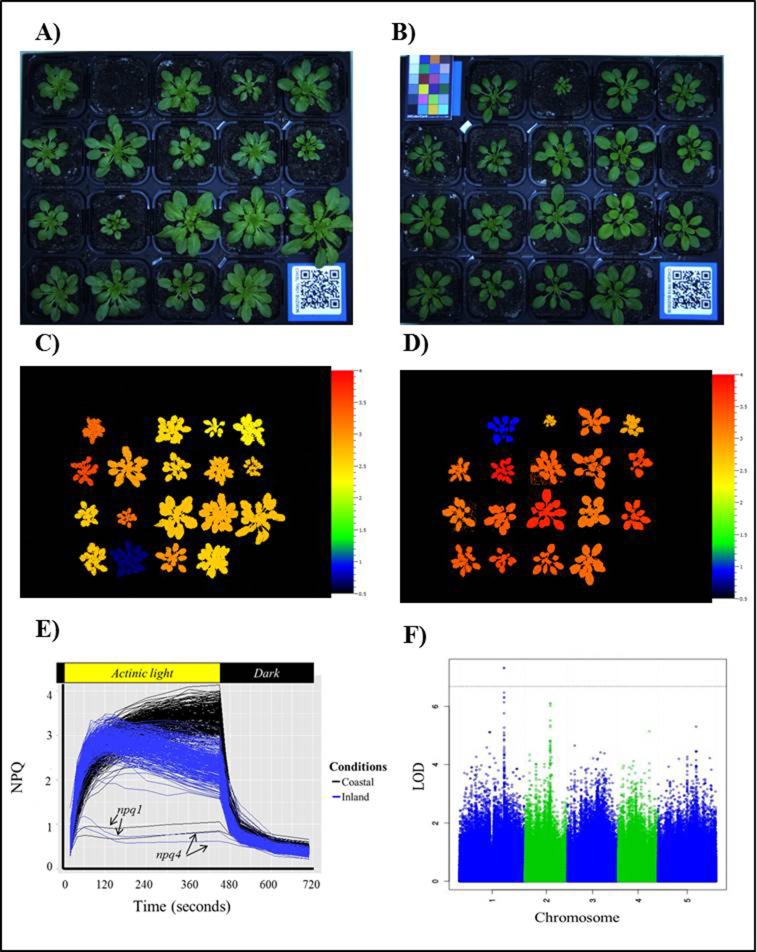
There was large variation in leaf development rates and the NPQ capacities between different accessions within each environment as well as difference in the dynamics of NPQ between the two environments (Figure 5A and 5B; Figure 5C – 5E). There was a two-fold variation in NPQ capacity within the same growth condition, while an increase of up to four-fold difference was found when comparing between non-stress and stress plants (Figure 5E). The differences in NPQ pattern and maximum capacity presented between two conditions may be due to the ability of inland plants to acclimate to the high light condition as observed by the fast induction of NPQ, rapidly dissipating excess energy. Furthermore, the decline in NPQ over time most likely indicates the activation of CO2 fixation and the subsequent de-acidification of the thylakoid lumen by the ATPase, resulting in de-activation of thermal dissipation (Munekage et al., 2002). The slower growth rate of the inland plants is likely due to the lower temperatures, as well as the high light causing photo-oxidative stress. These photosynthetic phenotypes, along with growth and developmental phenotypes measured in the Spectral-Pheno-Climatron, were then used for genome wide association studies (Atwell et al., 2010; Li et al., 2014) that account for genotype by environment interaction and time-dependent effects (e.g. Figure 5F).
Example 2: The investigation of the natural genetic variability in salinity tolerance levels using measures of photoinhibition and photosynthetic efficiency in a biparental A. thaliana mapping population
A screen of photosynthetic efficiency and photoinhibiton as an early response to salinity was undertaken in the Arabidopsis bi-parental mapping population Bay x Sha (Loudet et al., 2002). This population was chosen due to its previously documented variation in two traits related to salinity tolerance; shoot ion exclusion (Roy et al., 2013) and sensitivity of germination to salinity (Vallejo et al., 2010). Furthermore, the gene affecting Na+ exclusion, CIPK16, was positionally cloned using this population (Roy et al., 2013). The Bay x Sha population consisted of 411 lines at the F8 generation. In addition to two control lines were included; Landsberg erecta (Ler) and ost1-2, where ost is a mutant in Ler background and is impaired in stomatal aperture regulation (Mustilli et al., 2002).
The plants were grown in growth chambers with a short-day light regime to avoid early flowering. At the 10-leaf growth stage, which is approximately 21–23 days old, the plants were subjected to salt stress. As the phenotypic screen was targeting the early responses to salinity, before there has been large ion accumulation in the shoot (Figure 1B), phenotyping was performed within one hour of salt imposition. The plants were placed in a 150 mM NaCl solution tank, and left to soak to full soil saturation for 10 minutes, drain for another 10 minutes, then put through PlantScreen for RGB, fluorescence and thermal imaging. The phenotypic screen provided quantitative evidence of change in relative growth rate (RGR), photosynthetic efficiency and electron transport rate as well as changes in leaf temperature. Some phenotypes were observed to have changed rapidly as a result of the salt imposition, such as leaf temperature, which can be explained by ABA and ethylene signalling that affect stomatal aperture. This lead to a decrease in NPQ values, but the minimum and maximum fluorescence (F0 and Fm) did not display any change during the imaging period of seven consecutive days. These fluorescence parameters may be more susceptible to the late-occurring ion accumulation toxicity of salt stress.
In order to identify loci involved in the early shoot-ion independent tolerance (SIIT), the genetic variation was analysed across the population by testing for genotype by environment (G x E) interactions in a QTL mapping approach (Borevitz et al., 2002).
Mapping populations in traditional QTL analysis exhibit strong linkage and a few hundred markers are usually sufficient (Lynch and Walsh, 1998). However, the genomic resolution of QTL mapping is more limited, since a causative locus could contain dozens or hundreds of genes. Consequently, it is still a very difficult task to track down the underlying gene even if a QTL is a true positive. Furthermore, allelic diversity is limited to the two parental accessions. The advent of modern genome sequencing technology brings the availability of millions of SNP markers that cover the entire genome. At the same time, researchers strive to generate mapping populations, such as advanced intercross lines (AIL), with an increased historical recombination or select subset of unrelated individuals rather than families, as done in HapMap sets, where the linkage is expected to be very weak. With these resources, genome-wide association studies (GWAS) have been a powerful tool for detecting candidate genes of numerous traits and in different plant species such as Arabidopsis, rice and maize (e.g. Atwell et al., 2010; Huang et al., 2010; Tien et al., 2011; Huang et al., 2012).
Figure 5.
High throughput chlorophyll fluorescence imaging used in a GWAS approach to determine the genetic architecture of NPQ.
Plants are grown in climate chambers that mimic control (coastal) and stressful (inland) conditions (example of single tray in A & B respectively). Chlorophyll fluorescence at similar growth stages (16 leaves) is measured using a PSI PlantScreen (inland (C) and coastal (D)). Significant variation in NPQ induction, steady state and relaxation of NPQ was seen among the natural accessions grown under the coastal (black) and inland (blue) conditions (E). The lower lines are NPQ mutants (npq1 and npq4) which are unable to produce NPQ in both conditions. GWAS analysis is undertaken to find QTLs associated with the traits of interest (F).
Determining the genetic basis of stress response traits has been one of the major challenges in the process of crop improvement. Currently, GWAS is becoming increasingly more popular for identifying genetic variation in complex traits in natural population in a range of plant species including model species such as Arabidopsis (1001 genomes consortium, 2016) and a range of crop species (reviewed in Huang and Han, 2014). Many complex traits of biological and economical importance such as flowering time, growth rate, yield and stress tolerance have been under investigation for decades in an attempt to understand the genetic basis and mechanisms associated with those particular traits (e.g. Korte and Farlow, 2013; Li et al., 2014; Lefebvre et al., 2009). The application of high-throughput phenomics techniques to GWAS and QTL mapping allows elucidation of the genetic architecture of complex physiological traits. In addition, the temporal resolution and the wide range of related parameters that can be measured allow more statistical power when using methods such as MultiQTL analysis (Cheng et al., 2013). Moreover, the combination of high-throughput phenotyping and population genomics lends itself to phenotypic prediction, as well as systems biology approaches to understanding the genetic basis of molecular and physiological processes. The power in applying these new techniques and facilities to elucidating photosynthetic traits is that it allows greater insight into the complexity of photosynthetic responses to stress. This complexity is both due to the wide range of naturally occurring genetic diversity underlying stress responses but also how the genetic architecture of stress responses change over time in response to seasonal and diurnal variation in conditions.
CONCLUSION
Chlorophyll fluorescence parameters can now be easily measured and used as a tool to monitor photosynthetic performance in controlled and field conditions. The use of automated chlorophyll fluorescence imaging in conjunction with a selection of appropriate fluorescence parameters would increase the rate of successful photosynthetic function screening. This review provided a summary of useful chlorophyll fluorescence parameters and the examples of growth conditions that can be used to evaluate changes in photosystem II in response to environmental stress.
Stress tolerance mechanisms in plants are quite variable and very complex. The underlying genetic basis and environmental cues could play important roles in influencing a plant's survival and its ability to adapt in such stressful conditions. In this chapter, we've put forward the methods and preliminary results of high-throughput phenotyping for discovery of novel candidate genes under stress conditions. By utilizing accurate chlorophyll measurements and natural variation, we can identify QTLs for stress tolerance using bi-parental RILs or using the GWAS mapping approach. These methods have helped in clarifying our understanding of the genetic basis of photoprotection and salt tolerance.
ACKNOWLEDGEMENTS
This review was supported by grants from the ARC centre of excellence in Plant Energy Biology and the Australian National University for TR, PW, JO, RF, as well as funding from King Abdullah University of Science and Technology (KAUST) for MA and MT.
REFERENCES
- Anderson J.M. ( . 1986). Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol. 37: 93– 136. [Google Scholar]
- Ashraf M., Harris P.J.C. ( . 2013). Review: Photosynthesis under stressful environments: An overview. Photosynthetica. 51( 2): 163– 190. [Google Scholar]
- Atwell S., Huang Y. S., Vilhjálmsson B.J., Willems G., Horton M., Li Y., Meng D., Platt A., Tarone A. M., Hu T. T., Jiang R., Muliyati N.W., Zhang X., Amer M.A., Baxter I., Brachi B., Chory J., Dean C., Debieu M., Meaux J. de., Ecker J.R., Faure N., Kniskern J. M., Jones J.D.G., Michael T., Nemri A., Roux F., Sait D.E., Tang C., Todesco M., Traw M.B., Weigel D., Marjoram P., Borevitz J.O., Bergelson J., and Nordborg M. ( . 2011). Genome-wide association study of 107 phenotypes in a common set of Arabidopsis thaliana inbred lines. Nature. 465( 7298): 627– 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N.R. ( . 2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual review of Plant Biology. 59: 89– 113. [DOI] [PubMed] [Google Scholar]
- Barbagallo R.P., Oxborough K., Pallett K.E., and Baker N.R. ( . 2003). Rapid noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant physiol. 132: 485– 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman O. ( . 1981). Responses to different quantum flux densities. Encyclopedia of Plant Physiology, New Series, Lange O. L., Nobel P. S., Osmond C. B., and Zeigler H., eds., . Springer, Berlin, Vol. 12A: 57– 107. [Google Scholar]
- Borevitz J.O., Maloof J.N., Lutes J., Dabi T., Redfern J.L., Trainer G.T., Werner J.D., Asami T., Berry C.C., Weigel D., and Chory J. ( . 2002). Quantitative trait loci controlling light and hormone response in two accessions of Arabidopsis thaliana. Genetics. 160( 2): 683– 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchabke O., Chang F., Simon M., Voisin R., Pelletier G., and Durand-Tardif M. ( . 2008). Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. PLoS one. 3( 2): e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.B., Cheng R., Sirault X.R., Rungrat T., Murray K.D., Trtilek M., Furbank R.T., Badger M., Pogson B.J., and Borevitz J.O. ( . 2014). TraitCapture: genomic and environment modelling of plant phenomic data. Curr Opin Plant Biol. 18: 73– 79. [DOI] [PubMed] [Google Scholar]
- Bunce J.A. ( . 2008). Acclimation of photosynthesis to temperature in Arabidopsis thaliana and Brassica oleracea. Photosynthetica. 46 ( 4): 517– 524. [Google Scholar]
- Busoms S., Teres J., Huang X-Y, Bomblies K., Danku J., Douglas A., Weigel D., Poschenrieder C., and Salt D.E. ( . 2015). Salinity is an agent of divergent selection driving local adaptation of Arabidopsis to coastal habitats. Plant Physiology. 168( 3): 915– 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W.L. ( . 1978). Energy distribution in the photochemical apparatus of photosynthesis and photo protection in mangroves under field conditions. Plant Cell Environ. 20: 579– 588. [Google Scholar]
- Cao J., Schneeberger K., Ossowski S., Günther T., Bender S., Fitz J., Koenig D., Lanz C., Stegle O., Lippert C, Wang X., Ott F., Müller J., Alonso-Blanco C., Borgwardt K., Schmid K.J., and Weigel D. ( . 2011). Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nature Genetic. 43( 10): 956– 965. [DOI] [PubMed] [Google Scholar]
- Cazzaniga S., Osto L.D., Kong S.G., Wada M., and Bassi R. ( . 2013). Interaction between avoidance of photon absorption, excess energy dissipation and zeaxanthin synthesis against photooxidative stress in Arabidopsis. The plant journal, doi:10.1111/tpj.12314. [DOI] [PubMed] [Google Scholar]
- Chaves M. M., Flexas J. and Pinheiro C. ( . 2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 103( 4): 551– 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R., Borevitz J., and Doerge R.W. ( . 2013). Selecting informative traits for multivariate quantitative trait locus mapping helps to gain optimal power. Genetics 195( 3): 683– 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Neumann K., Friedel S., Kilian B., Chen M., Altmann T., and Klukas C. ( . 2014). Dissecting the phenotypic components of crop plant growth and drought responses based on high-throughput image analysis. Plant Cell. 26: 4636– 4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J., Savage L.J., Zegarac R., Hall C.C., Satoh-Cruz M., Davis G.A., Kovac W.K., Chen J., and Kramer D.M. ( . 2016). Dynamic environment photosynthetic imaging reveals emergent phenotypes. Cell Systems. 2: 365– 377. [DOI] [PubMed] [Google Scholar]
- Feller U., Craft-Brandner S.J., Salvucci M.E. ( . 1998). Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiology. 116: 539– 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Plazaola J.I., Esteban R., Fernández-Marín B., Kranner I., Porcar-Castell A. ( . 2012). Thermal energy dissipation and xanthophyll cycles beyond the Arabidopsis model. Photosynth Res. 113: 89– 103. [DOI] [PubMed] [Google Scholar]
- Govindjee. ( 1995). Sixty-three years since Kautsky: chlorophyll a fluorescence. Australian Journal of Plant Physiology. 22: 131– 160. [Google Scholar]
- Hairmansis A., Berger B., Tester M., and Roy S.J. ( . 2014). Image-based phenotyping for non-destructive screening of different salinity tolerance traits in rice. The Rice Journal. 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn S.A., Downs C.A., Sharkey T.D., and Coleman J.S. ( . 1998). The small methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiology. 116: 439– 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan O. ( . 2013). The dry facts. Nature. 501: S2– S3. [DOI] [PubMed] [Google Scholar]
- Hogewoning S.W., Wientjes E., Douwstra P., Trouwborst G., leperen W.V., Croce R., and Harbinson J. ( . 2012). Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves. The plant cell. 24( 5): 1921– 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Ruban A.V., and Walters R.G. ( . 1996). Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 655– 684. [DOI] [PubMed] [Google Scholar]
- Huang X. and Han B. ( . 2014). Natural variation and Genome-Wide Association Studies in crop plants. Annu. Rev. Plant Biol. 65: 531 – 551. [DOI] [PubMed] [Google Scholar]
- Huang X., Wei X., Sang T., Zhao Q., Feng Q., Zhao Y., Li C., Zhu C., Lu T., Zhang Z., Li M., Fan D., Guo Y., Wang A., Wang L., Deng L., Li W., Lu Y., Weng Q., Liu K., Huang T., Zhou T., Jing Y., W Li., Lin Z., Buckler E.S., Qian Q., Zhang Q.F., J Li., and Han B. ( . 2010). Genome-wide association studies of 14 agronomic traits in rice landraces. Nature Genetics. 42( 11): 961– 967. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhao Y., Wei X., Li C., Wang A., Zhao Q., Li W., Guo Y., Deng L., Zhu C., Fan D., Lu Y., Weng Q., Liu K., Zhou T., Jing Y., Si L., Dong G., Huang T., Lu T., Feng Q., Qian Q., Li J., and Han B. ( . 2012). Genome-wide association studies of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nature Genetics. 44( 1): 32– 39. [DOI] [PubMed] [Google Scholar]
- Ihnken S., Kromkamp J.C., and Beardall J. ( . 2011). Photoacclimation in Dunaliella tertiolecta reveals a unique NPQ pattern upon exposure to irradiance. Photosynthesis research. 110 ( 2): 123– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M., Uebayashi N., Sato F., and Endo T. ( . 2016). Physiological Functions of PsbS-dependent and PsbS-independent NPQ under Naturally Fluctuating Light Conditions. Plant Cell Physiol. 55( 7): 1286– 1295. [DOI] [PubMed] [Google Scholar]
- Iyengar E. R. R. and Reddy M. P. ( . 1996). Photosynthesis in highly salt-tolerant plants. Handbook of photosynthesis. M. Pessaraki; New York, Marcel Dekker: 897– 909. [Google Scholar]
- James R. A., Rivelli A. R., Munns R. and von Caemmerer S. ( . 2002). Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Functional Plant Biology. 29( 12): 1393– 1403. [DOI] [PubMed] [Google Scholar]
- James R. A., Munns R., Von Caemmerer S., Trejo C., Miller C. and Condon T. ( . 2006). Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl- in salt-affected barley and durum wheat. Plant Cell and Environment. 29( 12): 2185– 2197. [DOI] [PubMed] [Google Scholar]
- Jung H.-s. and Niyogi K.K. ( . 2009). Quantitative Genetic Analysis of Thermal Dissipation in Arabidopsis. Plant physiology. 150: 977– 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota A., Yamada N., Suetsugu N., Hirose M., Satio C., Shoda K., Ichikawa S., Kagawa T., Nakano A., and Wada M. ( . 2009). Short actin-base mechanism for light-directed chloroplast movement in Arabidopsis. PNAS. 106( 31): 13106– 13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., and Wada M. ( . 2002). Blue light-induced chloroplast relocation. Plant Cell Physio. 43( 4): 367– 371. [DOI] [PubMed] [Google Scholar]
- Karp G. ( . 2009). Cell and Molecular Biology: Concepts and Experiments. 6th ed JohnWiley and Sons Limited. [Google Scholar]
- Kasahara M., Kagawa T., Oikawa K., Suetsugu N., Miyao M., and Wada M. ( . 2002). Chloroplast avoidance movement reduces photo-damage in plants. Nature. 420: 829– 832. [DOI] [PubMed] [Google Scholar]
- Kautsky H., Apel W., and Amann H. ( . 1960). Chlorophyll fluoreszenz und Kohlensäureassimilation. XIII. Die Floureszenkurve und die Photo-chemie der Pflanze. Biochem. Zeit. 322: 277– 292. [Google Scholar]
- Keurentjes J.J.B., and Sulpice R. ( . 2009). The role of natural variation in dissecting genetic regulation of primary metabolism. Plant Signaling & Behavior. 4( 3): 244– 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Alonso-Blanco C., and Vreugdenhil D. ( . 2004). Naturally occurring genetic variation in Arabidopsis thaliana. Annu. Rev. Plant Biol. 55: 141– 172. [DOI] [PubMed] [Google Scholar]
- Korte A. and Farlow A. ( . 2013). The advantages and limitations of trait analysis with GWAS: a review. Plant Methods. 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., Kiani S.P., and Durand-Tardif M. ( . 2009). A focus on natural variation for abiotic constraints response in the model species Arabidopsis thaliana. Int. J. Mol. Sci. 10: 3547– 3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet O., Chaillou S., Camilleri C., Bouchez D. and Daniel-Vedele F. ( . 2002) Bay-0 x Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis. Theor Appl Genet. 104( 6–7): 1173– 1184. [DOI] [PubMed] [Google Scholar]
- Li X-p., Björkman O., Shih C., Grossman A.R., Rosenquist M., Jansson S., and Niyogi K.K. ( . 2000). A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 403( 6778): 391– 395. [DOI] [PubMed] [Google Scholar]
- Li X-p., Müller-M P., Gilmore A.M., and Niyogi K.K. ( . 2002). PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhobition. PNAS. 99( 23): 15222– 15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Cheng R., Spokas K.A., Palmer A.A., and Borevitz J.O. ( . 2014). Genetic variation for life history sensitivity to seasonal warming in Arabidopsis thaliana. Genetics. 196( 2): 569– 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., and Walsh B. ( . 1998). Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- Maxwell K., and Johnson G. N. ( . 2000). Chlorophyll fluorescence: A practical guide. Journal of experimental botany. 51( 345): 659– 68. [DOI] [PubMed] [Google Scholar]
- Mullen J.L., Weinig C. and Hangarter R.P. ( . 2006). Shade avoidance and the regulation of leaf inclination in Arabidopsis. Plant Cell and Environment. 29: 1099– 1106. [DOI] [PubMed] [Google Scholar]
- Müller P., Li X.P., and Niyogi K.K. ( . 2001). Non-photochemical quenching. A response to excess light energy. Plant Physiology. 125: 1558– 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R., and Tester M. ( . 2008). Mechanisms of salinity tolerance. Annu Rev Plant Biol. 59: 651– 681. [DOI] [PubMed] [Google Scholar]
- Munekage Y., Hojo M., Meurer J., Endo T., Tasaka M., and Shikanai T. ( . 2002). PGR5 is involved in cyclic electron flow around photosystem I and Is essential for photoprotection in Arabidopsis. Cell. 110: 361– 371. [DOI] [PubMed] [Google Scholar]
- Muranaka S., Shimizu K., and Kato M. ( . 2002). A salt-tolerant cultivar of wheat maintains photosynthetic activity by suppressing sodium uptake. Photosynthetica. 40: 509– 515. [Google Scholar]
- Murchie E.H., and Lawson T. ( . 2013). Review Paper: Chlorophyll fluorescence analysis:a guide to good practice and understanding some new applications. Journal of Experimental Botany. 64( 13): 3983– 3998. [DOI] [PubMed] [Google Scholar]
- Mustilli A.-C., Merlot S., Vavasseur A., Fenzi F., and Giraudat J. ( . 2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. The Plant Cell. 14( 12): 3089– 3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi K.K., Grossman A.R., and Björkman O. ( . 1998). Arabidopsis Mutants Define a Central Role for the Xanthophyll Cycle in the Regulation of Photosynthetic Energy Conversion. The plant cell. 10: 1121– 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi K.K. ( . 1999). Photoprotection revisited: Genetic and Molecular Approaches. Annual review of plant physiology and plant molecular biology. 50: 333– 359. [DOI] [PubMed] [Google Scholar]
- Osmond CB. ( . 1994). What is photoinhibition? Some insights from comparisons of sun and shade plants. : Baker NR, Bowyer JR, eds. . Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford: Bios Scientific Publishers; 1– 24. [Google Scholar]
- Peterhansel C., Horst I., Niessen M., Blume C., Kebeish R., Kurkcuoglu S., and Kreuzaler F. ( . 2010). Photorespiration. The Arabidopsis Book. 8: e0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir M., Quillérou E., Nangia V., Murtaza G., Singh M., Thomas R. J., Drechsel P. and Noble A. D. ( . 2014). Economics of salt-induced land degradation and restoration. Natural Resources Forum. 38: 282– 295. [Google Scholar]
- Roháček K. ( . 2002). Fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica. 40( 1): 13– 29. [Google Scholar]
- Rooijen R.V., Aarts M.G.M., and Herbinson J. ( . 2015). Natural genetic variation for acclimation of photosynthetic light use efficiency to growth irradiance in Arabidopsis. Plant Physiology. 167( 4): 1412– 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. J., Huang W., . Wang X.J., Evrard A., Schmockel S.M., Zafar Z.U., and Tester M. ( . 2013). A novel protein kinase involved in Na(+) exclusion revealed from positional cloning. Plant Cell Environ. 36( 3): 553– 568. [DOI] [PubMed] [Google Scholar]
- Sharkey T.D. and Zhang R. ( . 2010). High temperature effects on electron and photon circuits of photosynthesis. Journal of Integrative Plant Biology. 52( 8): 712– 722. [DOI] [PubMed] [Google Scholar]
- Shindo C., Bernasconi G., and Hardtke C.S. ( . 2007). Natural genetic variation in Arabidopsis: Tools, Traits and Prospects for Evolutionary Ecology. Annals of Botany. 99: 1043– 1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemke J.A., and Santiago L.S. ( . 2011) Consequences of light absorptance in calculating electron transport rate of desert and succulent plants. Photosynthetica. 49: 195– 200. [Google Scholar]
- Stepien P., and Johnson G.N. ( . 2009). Contrasting Responses of Photosynthesis to Salt Stress in the Glycophyte Arabidopsis and the Halophyte; The llungiella: Role of the Plastid Terminal Oxidase as an Alternative Electron Sink1. Plant physiology. 149: 1154– 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., and Badger M.R. ( . 2011). Photo protection in plants: a new light on photosystem II damage. Trends in plant science. 16( 1): 53– 60. [DOI] [PubMed] [Google Scholar]
- Teixeira E.I., Fischer G., van Velthuizen H., Walter C., and Ewert F. ( . 2013). Global hot-spots of heat stress on agricultural crops due to climate change. Agricultural and Forest Meteorology. 170: 206– 215. [Google Scholar]
- Trontin C., Tisne S., Bach L., and Loudet O. ( . 2011). What does Arabidopsis natural variation teach us (and does not teach us) about adaptation in plants? Current opinion in plant biology. 14( 3): 225– 31. [DOI] [PubMed] [Google Scholar]
- Tsugane K., Kobayashi K., Niwa Y., Ohba Y., Wada K., and Kobayashi H. ( . 1999). A Recessive Arabidopsis Mutant That Grows Photoautotrophically under Salt Stress Shows Enhanced Active Oxygen Detoxification. Plant cell. 11: 1195– 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan S. ( . 2012). Light Acclimation in Plants: Photoinhibition and Photoprotection. Advances in bioresearch. 3 ( 1): 90– 94. [Google Scholar]
- Vallejo A.J., Yanovsky M.J. and Botto J.F. ( . 2010). Germination variation in Arabidopsis thaliana accessions under moderate osmotic and salt stresses. Ann Bot. 106( 5): 833– 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware M.A., Belgio E., and Ruban A.V. ( . 2015). Comparison of the protective effectiveness of NPQ in Arabidopsis plants deficient in PsbS protein and zeaxanthin. Journal of Experimental Botany. 66( 5): 1259– 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D. ( . 2012). Founders Review: Natural variation in Arabidopsis thaliana: from molecular genetics to ecological genomic. Plant physiology. 158: 2– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo N. S., Badger M.R., and Pogson B.J. ( . 2008). A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods. 4: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Irfan M., Ahmad A., and Hayat S. ( . 2011). Causes of salinity and plant manifestations to salt stress. J Environ Biol. 32: 667– 685. [PubMed] [Google Scholar]
- Zhang R. and Sharkey T.D. ( . 2009). Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth. Res. 100: 29– 43. [DOI] [PubMed] [Google Scholar]
- 1001 Genome Consortium. ( 2016). 1,135 Genome Reveal the Global Pattern of Polymorphism in Arabidopsis thaliana. Cell. 166: 1– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]