Abstract
Outer-membrane c-type cytochrome (OM c-Cyt) complexes in several genera of iron-reducing bacteria, such as Shewanella and Geobacter, are capable of transporting electrons from the cell interior to extracellular solids as a terminal step of anaerobic respiration. The kinetics of this electron transport has implications for controlling the rate of microbial electron transport during bioenergy or biochemical production, iron corrosion, and natural mineral cycling. Herein, we review the findings from in-vivo and in-vitro studies examining electron transport kinetics through single OM c-Cyt complexes in Shewanella oneidensis MR-1. In-vitro electron flux via a purified OM c-Cyt complex, comprised of MtrA, B, and C proteins from S. oneidensis MR-1, embedded in a proteoliposome system is reported to be 10- to 100-fold faster compared with in-vivo estimates based on measurements of electron flux per cell and OM c-Cyts density. As the proteoliposome system is estimated to have 10-fold higher cation flux via potassium channels than electrons, we speculate that the slower rate of electron-coupled cation transport across the OM is responsible for the significantly lower electron transport rate that is observed in-vivo. As most studies to date have primarily focused on the energetics or kinetics of interheme electron hopping in OM c-Cyts in this microbial electron transport mechanism, the proposed model involving cation transport provides new insight into the rate detemining step of EET, as well as the role of self-secreted flavin molecules bound to OM c-Cyt and proton management for energy conservation and production in S. oneidensis MR-1.
Keywords: Shewanella oneidensis MR-1, electrochemistry, proton motive force, iron-reducing bacteira, flavin
In the anaerobic metabolism of iron-reducing bacteria, including species of Shewanella and Geobacter, insoluble extracellular materials, such as ferric oxide and carbon electrodes, serve as the final electron acceptor in the electron transport chain [1]. During this energy acquisition process, electrons are moved directly from respiratory chain to components located in the periplasm and outer membranes to extracellularly located solids through a mechanism termed extracellular electron transport (EET). In contrast to aerobic respiration, EET is potentially not limited by the diffusion kinetics of metabolites into cells [2–4]; therefore, EET-associated respiration is an important process for material and energy circulation in nature [1], iron corrosion [5], and environmental technologies, such as bioremediation and microbial fuel cells [6].
In the EET-capable bacterium Shewanella oneidensis MR-1, respiratory electrons are transported via outer membrane c-type cytochromes (OM c-Cyts), which contain heme iron centers that act as biological conduits for the movement of electrons from the periplasm to the cell exterior [7,8]. The OM c-Cyt complex is composed of three deca-heme cytochromes, MtrC, OmcA, and MtrA, and is associated with a transmembrane porin protein, MtrB (Fig. 1a). Recent biochemical and structural studies of MtrC, OmcA and MtrF, which is a homolog of MtrC, have greatly advanced the molecular-level understanding of the EET mechanism mediated in S. oneidensis MR-1. However, the kinetic parameters of the electron transport process remain unclear due to the large differences in measurements between in-vitro and in-vivo studies. Herein, we review and unify recent progresses on the EET kinetics of a single OM c-Cyt complex in S. oneidensis MR-1 and purified MtrCAB complex embedded in a lipid bilayer, which leads to a proposal of cation-limited kinetic model for EET via OM c-Cyts.
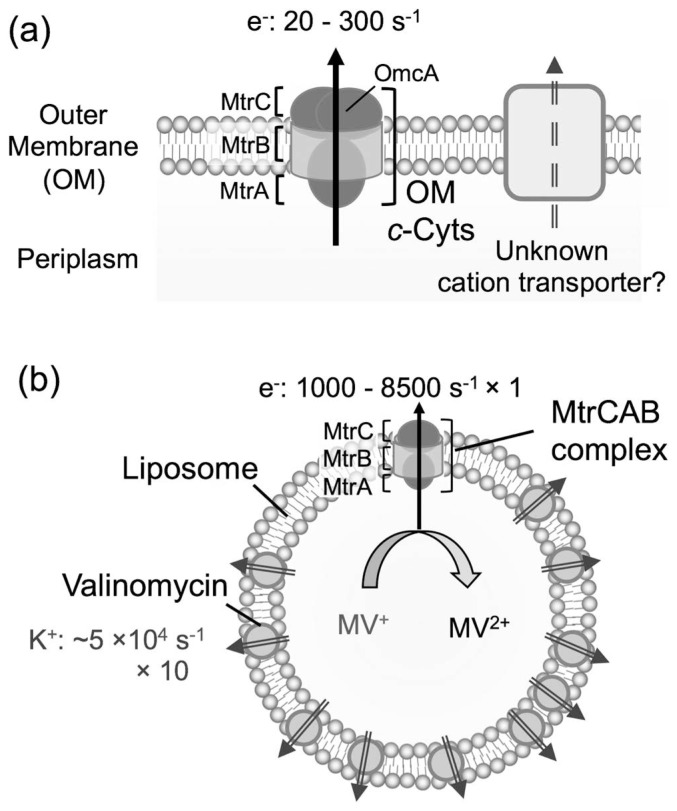
Figure 1.
Schematic illustration of electron and proton flow across (a) the outer membrane (OM) of S. oneidensis MR-1 and (b) the lipid membrane in a proteoliposome system. (a) The c-type cytochrome complex MtrCAB-OmcA, which is embedded in the OM, transports electrons from the periplasm to the cell exterior via the OM. This electron transport should associate with cationic transport. (b) Electron transport via a purified MtrCAB complex embedded in a proteoliposome coupled with potassium transport through valinomycin.
1. Discrepancy between in-vivo and in-vitro measurements of electron transport rate via single OM c-Cyt complexes
Electron flux through single OM c-Cyt complexes to electrode surfaces can be estimated from in-vivo data of microbial current production and the density of electrochemically active protein complexes. Single-cell current production measurements in electrochemical cells equipped with indium tin-doped oxide (ITO) electrodes poised at +0.4 V (vs. SHE) showed that 1.2×106 electrons per second are transported via OM c-Cyts to the electrode [2], a value that is in accordance with observations for MR-1 cells cultured in a continuous-flow microbial fuel cell system [9]. In OM c-Cyt proteins, such as MtrC and OmcA, the density of heme groups, which exchange electrons with electrodes, was directly measured by cyclic voltammetry (CV) analysis of the redox current in monolayer biofilms of MR-1 on an ITO electrode at a higher scan rate than the metabolic rate [10]. Using this approach, the heme density was determined to be 0.8 pmol cm−2 (approximately 5000 deca-heme OM c-Cyts per cell; Table 1) [10]. Notably, the number of OM c-Cyts detected by CV was consistent with the estimated amounts of MtrC and OmcA by atomic forced microscopy (AFM) [11] and Western blotting analysis of anaerobic iron-reducing cultures, suggesting that most OM c-Cyt present on the cell surface are involved in the transport of electrons to the electrode surface. From these findings, it is estimated that single OM c-Cyts are capable of transporting a maximum of 300 electrons per second. As studies of monolayer biofilms of MR-1 have reported lower rates of electron transport (Table 1) [10,12], under in-vivo conditions, electron flux per single OM c-Cyts appears to range from 20 to 300 electrons per second.
Table 1.
Summary of in-vivo studies estimating electron flux per single deca-heme in OM c-Cyt complex
| Cell conditions | Electron flux per cell | Deca-heme c-Cyt content per cell a | Rate constant (s−1) per deca-heme c-Cyt | Electron Acceptor | Potential vs SHE | Method | Ref. |
|---|---|---|---|---|---|---|---|
| Single cell (PV-4) | 1.2×106 | ~300 b | ITO electrode | 0.4 | In vivo electrochemistry | [2] | |
| Single cell (MR-1) | 1.3×106 | ~330 b | Carbon electrode | n.a. | In vivo electrochemistry | [9] | |
| Chemostat culture (MR-1) | 2.6×106 | oxygen | +0.81 | O2 sensor | [31] | ||
| Anaerobic culture (MR-1) | 90000~150000 | ferric citrate d | Western blot | [29] | |||
| 0.25±0.04 c | α-FeOOH | −0.157 | Ferrozine assay | [29] | |||
| Anaerobic culture (MR-1) | 4000 | Fe3+, d | UV-vis Absorption | [32] | |||
| Single cell (MR-1) | 4000~7000 e | Fe2O3 d | Antibody AFM | [11] | |||
| Biofilm on electrode (MR-1) | 1.8×105 | 4900 e | ~37 | ITO electrode | +0.4 | In vivo electrochemistry | [10] |
| Biofilm on electrode (PV-4) | 1.2×105 | 6000 e | ~20 | ITO electrode | +0.4 | In vivo electrochemistry | [12] |
Assumed the size of a bacteiral cell, a rod-shaped bacterium that is 0.5 by 2.0 μm.
Assumed deca-heme c-Cyt content is 4000.
Average of MtrC and OmcA estimated based on the assumption of Michaelis-Menten constant Km=0.2 M.
Electron acceptors used for the growth of cells before quantifying OmcA or MtrC.
The number of deca-heme c-Cyts at bacteira/electrode interface.
These estimated rates of in-vivo electron flux via single OM c-Cyts are one to two orders of magnitude smaller than those determined from in-vitro measurements by White et al. [13]. They constructed a proteoliposome system, in which purified MtrCAB was embedded into a lipid bilayer and used internalizaed methyl viologen as a redox indicator and electron reservoir (Fig. 1). The electron flux of MtrCAB to iron oxide reached 8700 s−1 (Table 2), which is nearly equal to the theoretical value estimated from the inter-heme distance in the crystal structure of MtrF [14] and based on an inter-heme electron hopping model [15]. Notably, although the redox potentials of electron acceptors used for in-vivo current measurements are thermodynamically more favorable for EET kinetics than those used for in-vitro studies, the in-vivo electron transfer rate is markedly slower than that of in-vitro systems. In the proteoliposomal system, the electron acceptor, γ-FeOOH, has a redox potential of −0.157 V (vs SHE), which is over 500 mV more negative than the ITO electrode used for single-cell analysis.
Table 2.
Summary of studies measuring in-vitro electron flux from purified single deca-heme cytochromes or the MtrCAB complex
| In-vitro system | Rate constant (s−1) | Electron Acceptor | E vs SHE | Ref. |
|---|---|---|---|---|
| MtrCAB complex in proteoliposome | 8,500±916 | γ-FeOOH | −0.103 | [13] |
| 1,317±33 | α-Fe2O3 | −0.121 | [13] | |
| 1,133±266 | α-FeOOH | −0.157 | [13] | |
| MtrC | (1.98±0.14)×10−3 a | α-FeOOH | n.a. | [29] |
| OmcA | (3.8±0.6)×10−3 a | α-FeOOH | n.a. | [29] |
| MtrC in total membrane | 2.94±0.54 a | α-FeOOH | n.a. | [29] |
| OmcA in total membrane | 4.84±1.1a | α-FeOOH | n.a. | [29] |
Assumed Michaelis-Menten constant Km=0.2 M
Electron flux through MtrCAB complexes in the proteoliposome is sufficiently large to account for the rate constant of in-vivo electron transport that is observed between OM c-Cyts and Fe(III) minerals and electrodes (Table 1). However, because in-vivo current production is limited by the rate of EET mediated by OM c-Cyts [3], the observed electron flux through MtrCAB in the reconstructed proteoliposome system cannot be attributed to the in-vivo data. It is known that redox mediators, such as quinones and flavins, specifically enhance the rate of EET in the presence of sufficient concentrations of suitable electron donors for microbial metabolism. If the rate of electron supply from the upstream metabolic reactions in the respiratory chain is slower than the OM c-Cyt-mediated EET rate, redox mediator-induced enhancement of EET does not occur. Therefore, the much higher electron flux that occurs in the proteoliposomal system from methyl viologen (MV) to iron oxide suggests that electron transfer via in-vivo and in-vitro OM c-Cyts has significantly different kinetics.
2. Cation-limited Kinetic Model for EET via OM c-Cyt Complexes
The MtrCAB complex in the proteoliposome system may differ from c-Cyt complexes in the OM with respect to several key factors that may influence electron transfer kinetics. Among these factors, which include cationic transport associated with EET, inter-protein interactions embedded in the OM, and the presence of OmcA in the OM c-Cyt complex, the slower transport of cationic ions across the OM most likely influences electron transport kinetics in-vivo, because charge neutrality is required to sustain continuous electron flow across the lipid bilayer membrane, which is highly impermeable for ions. In proteoliposome system, the MtrCAB complex was present at an approximately ten-fold lower concentration than that of valinomycin, which has potenital for transporting potassium at a rate of approximately 5×104 ions s−1 [16], strongly suggesting that cation transport is sufficiently fast not to limit the rate of EET. Such high cation transport capability of the proteoliposome system rationalizes the accordance of the electron transport rate constant with theoretical calculations [14,15]. In contrast, 10% to 30% of the OM in S. oneidensis MR-1 is estimated to be covered with MtrC and OmcA proteins [11], and cation export through the OM may therefore be slower than the rate of electron transport mediated by the c-Cyt complex, though the OM of gram-negative bacteria is thought to be permeable to small molecules and ions due to the presence of abundant porins and ion channels [17].
Although no studies have examined whether the in-vivo EET rate via OM c-Cyts is limited by cation transport through the OM, evidences have been presented that suggests OM proteins capable of cation transport influence EET. For example, a mutant strain of MR-1 lacking OmpW, which was recently reported to function as a cationic channel in Caulobacter crescentus [18], produced 40% less current in a microbial fuel cell than the wild-type strain [19]. In addition, transcriptional analysis of S. oneidensis MR-1 showed that the expression of OM proteins predicted to function as transmembrane porins, including OmpW, are upregulated at similar levels in OM c-Cyts under electrode respiration compared to oxygen or soluble Fe(III) conditions [20]. Therefore, cationic transport across the OM may strongly influence EET kinetics, and may possibly limit the rate of electron transport via OM c-Cyts.
3. Cation-limited EET model provides new insight into the role of flavins
Biosynthesized and secreted riboflavin and flavin mononucleotide highly enhance the rate of EET in S. oneidensis MR-1 at a few μM concentration [21,22], most likely by functioning as non-covalent binding cofactors in OM c-Cyts to facilitate diffusion-less electron transfer [3]. Under the assumption that EET-associated cation transport across the OM limits the rate of EET, bound flavin cofactors are predicted to enhance the rate of cation transport or both electron and cation transport across the OM.
The predicted functions of flavins in the EET process are consistent with the apparent conflict in the electron free energy diagram for EET with a bound flavin molecule. When heme centers in OM c-Cyts transport an electron to a bound flavin molecule, the redox potenital of flavin is approximately 200 mV more negative than that of the averaged value of ten hemes, indicating that the electron transfer reaction is unfavorable when it is terminated by the bound flavin. With respect to redox kinetics (standard rate constant k0), heme redox centers are capable of exchanging electrons at about two orders of magnitude higher rates than flavin molecules absorded on electrode [10,21,23]. Therefore, based on only the free energy of electrons, the EET rate would be reduced when flavin molecules are introduced into the respiratory chain. Notably, if flavin facilitates EET-associated proton transport, flavin-bound OM c-Cyt complexes would transport both electrons and protons, because flavin may specifically bind to β-barrel domains in MtrC or OmcA protein [24]. Although the molecular structures of the MtrC, OmcA, and MtrF proteins in MR-1 have been determined [24–26], no potential proton pathways within the proteins have been identified and the crystal structure of the proteins bound to flavin have not been determined. For these reasons, further study is required to determine how EET-associated cation transport proceeds via flavin-bound OM c-Cyts.
4. Proton management in EET-linked respiration
As respiratory electrons move across the OM and into the extracellular space, the generated proton motive force (PMF) can be stored by disrupting the charge balance across the membrane. Thus, the discharging of the OM during the simultaneous transfer of protons and electrons occurs during the metabolically driven EET. Using a newly developed method based on the membrane permeability of biosynthesized flavin, we demonstrated that the PMF is only weakly generated during EET-linked respiration [27]. As reduced flavin in the periplasm has a pKa value of 6.7, increasing the periplasmic pH will generate higher concentrations of deprotonated flavin, which can only slowly penetrate the OM, resulting in the periplasmic accumulation of flavin. When we increased membrane permeability to enhance the efflux of flavin through the OM during EET, it increased the flavin concentration in the bulk medium, indicating that the periplasmic pH values of S. oneidensis MR-1 cells under in-vivo conditions were higher than the pKa value of reduced flavin. Therefore, ATP synthesis for S. oneidensis MR-1 during EET may not be driven by PMF, but ATP may be produced by substrate-level phosphorylation as in other anaerobic respiration in MR-1 [28]. The proton-export model proposed here provokes a number of microbial physiology questions, as the possibility that the primary energy source of PMF is not utilized in iron-reducing bacteria has not been previously considered in microbial physiological models. Therefore, determining how the PMF is stored or utilized by MR-1 during EET is expected to provide more insight into the physiology of EET-capable microbes.
5. High density of OM c-Cyt complexes on the OM of S. oneidensis MR-1
In addition to cation-limited model, the observed differences in electron transfer kinetics between in-vivo and in-vitro conditions may be partially attributable to the density of OM c-Cyts on the lipid membrane surface. If the surface area of proteoliposomes are roughly approximated to be 3.0 m2 ml−1, based on the size of a pair of phosphatidylcholine lipids, the density of the MtrCAB complex is approximately 3 fmol cm−2, a value that is two orders of magnitude less than the density of OM c-Cyt complexes estimated in-vivo (Table 1). The high density of OM c-Cyts in the OM suggests that inter-complex interactions may occur with other OM proteins. Although the OM c-Cyt complex is predicted to operate as a single unit for EET, several OM c-Cyt units might form a larger complex on the OM surface. Direct observation of OM c-Cyts on the surface of whole cells by high-speed AFM is required to resolve the distribution pattern of these complexes. It is also possible that structural alterations in MtrC and OmcA resulting from the inter-complex interactions may result in slower electron transport rates in-vivo compared to those observed in the in-vitro system. Because individual MtrC and OmcA proteins have much lower electron transfer rates than the MtrCAB complex in proteoliposomes (Table 2) [29], small structural changes might strongly alter the rate of electron transport.
6. Conclusion
Although the mechanism and kinetics of electron flow during EET via OM c-Cyts have been studied for the past three decades, the influence of counter cation (e.g., protons) flow from inside to outside of OM on the kinetics of EET has not been widely considered to date [27,30]. Our model for the impact of proton transport on EET kinetics are expected to contribute to the understanding and development of methods for controlling microbial reactions, not only in microbial fuel cells, but also for microbial electrode synthesis for the production of valuable chemicals and control of iron corrosion reactions.
Acknowledgments
This work was financially supported by a Grant-in-Aid for Young Scientist B from the Japan Society for the Promotion of Science (JSPS) (KAKENHI Grant Number 26810085).
Footnotes
Conflict of Interest
The authors declare no coflict of interst.
Author Contributions
A. O., Y. T., and J. S. wrote the paper.
References
- 1.Nealson KH, Saffarini D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- 2.Liu HA, Newton GJ, Nakamura R, Hashimoto K, Nakanishi S. Electrochemical Characterization of a Single Electricity-Producing Bacterial Cell of Shewanella by Using Optical Tweezers. Angew. Chem Int Ed Engl. 2010;49:6596–6599. doi: 10.1002/anie.201000315. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto A, Hashimoto K, Nealson KH, Nakamura R. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Proc Natl Acad Sci USA. 2013;110:7856–7861. doi: 10.1073/pnas.1220823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto A, Saito K, Inoue K, Nealson KH, Hashimoto K, Nakamura R. Uptake of Self-secreted Flavins as Bound Cofactors for Extracellular Electron Transfer in Geobacter Species. Energy Environ Sci. 2014;7:1357–1361. [Google Scholar]
- 5.Dinh HT, Kuever J, Mussmann M, Hassel AW, Stratmann M, Widdel F. Iron corrosion by novel anaerobic microorganisms. Nature. 2004;427:829–832. doi: 10.1038/nature02321. [DOI] [PubMed] [Google Scholar]
- 6.Lovley DR. Bug juice: harvesting electricity with micro-organisms. Nat Rev Microbiol. 2006;4:497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- 7.Myers CR, Myers JM. Localization of Cytochromes to the Outer-Membrane of Anaerobically Grown Shewanella-Putrefaciens Mr-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidelberg JF, Paulsen IT, Nelson KE, Gaidos EJ, Nelson WC, Read TD, et al. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotechnol. 2002;20:1118–1123. doi: 10.1038/nbt749. [DOI] [PubMed] [Google Scholar]
- 9.McLean JS, Wanger G, Gorby YA, Wainstein M, McQuaid J, Ishii SI, et al. Quantification of Electron Transfer Rates to a Solid Phase Electron Acceptor through the Stages of Biofilm Formation from Single Cells to Multicellular Communities. Environ Sci Technol. 2010;44:2721–2727. doi: 10.1021/es903043p. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto A, Nakamura R, Hashimoto K. In-vivo identification of direct electron transfer from Shewanella oneidensis MR-1 to electrodes via outer-membrane OmcA-MtrCAB protein complexes. Electrochim Acta. 2011;56:5526–5531. [Google Scholar]
- 11.Lower BH, Shi L, Yongsunthon R, Droubay TC, McCready DE, Lower SK. Specific bonds between an iron oxide surface and outer membrane cytochromes MtrC and OmcA from Shewanella oneidensis MR-1. J Bacteriol. 2007;189:4944–4952. doi: 10.1128/JB.01518-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto A, Nakamura R, Ishii K, Hashimoto K. In vivo Electrochemistry of C-Type Cytochrome-Mediated Electron-Transfer with Chemical Marking. Chembiochem. 2009;10:2329–2332. doi: 10.1002/cbic.200900422. [DOI] [PubMed] [Google Scholar]
- 13.White GF, Shi Z, Shi L, Wang ZM, Dohnalkova AC, Marshall MJ, et al. Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(III) minerals. Proc Natl Acad Sci USA. 2013;110:6346–6351. doi: 10.1073/pnas.1220074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breuer M, Rosso KM, Blumberger J. Electron flow in multiheme bacterial cytochromes is a balancing act between heme electronic interaction and redox potentials. Proc Natl Acad Sci USA. 2014;111:611–616. doi: 10.1073/pnas.1316156111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polizzi NF, Skourtis SS, Beratan DN. Physical constraints on charge transport through bacterial nanowires. Faraday Discuss. 2012;155:43–62. doi: 10.1039/c1fd00098e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stark G, Ketterer B, Benz R, Lauger P. The rate constants of valinomycin-mediated ion transport through thin lipid membranes. Biophys J. 1971;11:981–994. doi: 10.1016/S0006-3495(71)86272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benz R, Jones MD, Younas F, Maier E, Modi N, Mentele R, et al. OmpW of Caulobacter crescentus Functions as an Outer Membrane Channel for Cations. PloS ONE. 2015;10:e0143557. doi: 10.1371/journal.pone.0143557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bretschger O, Obraztsova A, Sturm CA, Chang IS, Gorby YA, Reed SB, et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl Environ Microbiol. 2007;73:7003–7012. doi: 10.1128/AEM.01087-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum MA, Bar HY, Beg QK, Segre D, Booth J, Cotta MA, et al. Transcriptional Analysis of Shewanella oneidensis MR-1 with an Electrode Compared to Fe(III) Citrate or Oxygen as Terminal Electron Acceptor. PloS ONE. 2012;7:e30827. doi: 10.1371/journal.pone.0030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA. 2008;105:3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Canstein H, Ogawa J, Shimizu S, Lloyd JR. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol. 2008;74:615–623. doi: 10.1128/AEM.01387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto A, Nakamura R, Ishii K, Hashimoto K. In vivo electrochemistry of C-type cytochrome-mediated electron-transfer with chemical marking. Chembiochem. 2009;10:2329–2332. doi: 10.1002/cbic.200900422. [DOI] [PubMed] [Google Scholar]
- 24.Edwards MJ, White GF, Norman M, Tome-Fernandez A, Ainsworth E, Shi L, et al. Redox Linked Flavin Sites in Extracellular Decaheme Proteins Involved in Microbe-Mineral Electron Transfer. Sci Rep. 2015;5:11677. doi: 10.1038/srep11677. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke TA, Edwards MJ, Gates AJ, Hall A, White GF, Bradley J, et al. Structure of a bacterial cell surface decaheme electron conduit. Proc Natl Acad Sci USA. 2011;108:9384–9389. doi: 10.1073/pnas.1017200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards MJ, Baiden NA, Johs A, Tomanicek SJ, Liang LY, Shi L, et al. The X-ray crystal structure of Shewanella oneidensis OmcA reveals new insight at the microbe-mineral interface. FEBS Lett. 2014;588:1886–1890. doi: 10.1016/j.febslet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Tokunou Y, Hashimoto K, Okamoto A. Extracellular Electron Transport Scarcely Accumulates Proton Motive Force in Shewanella oneidensis MR-1. Bull Chem Soc Jpn. 2015;88:690–692. [Google Scholar]
- 28.Hunt KA, Flynn JM, Naranjo B, Shikhare ID, Gralnick JA. Substrate-Level Phosphorylation Is the Primary Source of Energy Conservation during Anaerobic Respiration of Shewanella oneidensis Strain MR-1. J Bacteriol. 2010;192:3345–3351. doi: 10.1128/JB.00090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross DE, Brantley SL, Tien M. Kinetic Characterization of OmcA and MtrC, Terminal Reductases Involved in Respiratory Electron Transfer for Dissimilatory Iron Reduction in Shewanella oneidensis MR-1. Appl Environ Microbiol. 2009;75:5218–5226. doi: 10.1128/AEM.00544-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers CR, Nealson KH. Respiration-Linked Proton Translocation Coupled to Anaerobic Reduction of Manganese(IV) and Iron(III) in Shewanella-Putrefaciens Mr-1. J Bacteriol. 1990;172:6232–6238. doi: 10.1128/jb.172.11.6232-6238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci USA. 2010;107:18127–18131. doi: 10.1073/pnas.1004880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borloo J, Vergauwen B, De Smet L, Brige A, Motte B, Devreese B, et al. A kinetic approach to the dependence of dissimilatory metal reduction by Shewanella oneidensis MR-1 on the outer membrane cytochromes c OmcA and OmcB. FEBS J. 2007;274:3728–3738. doi: 10.1111/j.1742-4658.2007.05907.x. [DOI] [PubMed] [Google Scholar]