Abstract
Objective
The aim of this study was to determine the tolerability and efficacy of oxaliplatin in patientswith recurrent gynecologic malignancies after carboplatin hypersensitivity reactions in comparison with conventionally used cisplatin.
Methods
Forty-six patients were treated with platinum-based chemotherapy from 2006 to 2011 and developed hypersensitivity reactions to carboplatin. Oxaliplatin was administered to 27 patients; 19 patients received cisplatin. Clinicopathologic variables, toxicity, and time-to-failure were analyzed retrospectively using descriptive statistics, Fisher exact, and independent sample permutation t tests.
Results
The median number of carboplatin cycles and cumulative dose before reaction were similar in the oxaliplatin and cisplatin groups, respectively (6 vs 7.5 cycles, P = 0.93; 980 [662] mg vs 686 [579.6] mg, P = 0.49). Non–life-threatening hypersensitivity reaction to oxaliplatin developed in 2 of 27 patients. No reactions to cisplatin occurred. The median number of oxaliplatin/cisplatin cycles was 6 in both groups. Complete response to therapy was 34.6% (oxaliplatin) and 31.6% (cisplatin); stable disease was seen in 50.0% and 36.8% of oxaliplatin- and cisplatin-treated patients, respectively (P = 0.46). Exposure to oxaliplatin resulted in less neurotoxicity than cisplatin (25.9% vs 68.4%, P = 0.01). The median number of prior chemotherapy lines in both groups was 2. The median time-to-failure was 10.8 months in oxaliplatin group and 9.8 months in cisplatin group (P = 0.86).
Conclusions
Salvage therapy with oxaliplatin after hypersensitivity reaction to carboplatin is associated with excellent tolerability and time-to-failure comparable to cisplatin. When further administration of carboplatin is precluded, oxaliplatin represents a safe and effective treatment strategy in the platinum-sensitive relapse setting. The significantly lower neurotoxicity profile makes it an attractive alternative to cisplatin.
Keywords: Carboplatin hypersensitivity, Oxaliplatin, Cisplatin
Platinum-based cytotoxic agents remain the cornerstone treatment for gynecologic cancers. Historically, combination regimens involving cisplatin were utilized as first-line therapy for ovarian cancer until superseded by carboplatin more than a decade ago. A more favorable toxicity profile, including less nephrotoxicity, neurotoxicity, and emetogenic potential, along with comparable therapeutic efficacy and convenience of outpatient administration, resulted in its wide acceptance by oncologic community. Carboplatin/taxane combinations have become the standard regimens in the adjuvant and neoadjuvant settings for patients with ovarian cancer. More recent phase 3 trials have demonstrated the utility of carboplatin-based combination regimens in endometrial and cervical cancer as well.1,2 Although their use is associated with favorable clinical outcomes, continuation of treatment is frequently hampered by development of late hypersensitivity reactions to carboplatin in patients with repeated drug exposure.3–5
The incidence of hypersensitivity to carboplatin typically peaks from 6 to 21 cycles; symptoms frequently range from mild pruritus and erythroderma to anaphylaxis.3–5 Severe reactions have been observed after a cumulative dose of 8000 mg.6 Markman et al3–5 reported 27% of patients developing allergic reactions after the seventh cycle. Such high rates of hypersensitivity limit further administration of carboplatin to patients with platinum-sensitive disease.
Unlike paclitaxel reactions that are attributed to the Cremophor EL base (polyoxyethylated castor oil), the exact mechanism of carboplatin hypersensitivity is not fully understood.7 Reactions to paclitaxel typically occur after the first or second administration.7 Usually, these can be prevented by desensitization with steroids.5,7–10 Alternatively, type 1 hypersensitivity mediated by immunoglobulin E (IgE), as well as degranulation of mast cells, is thought to be involved in carboplatin allergy.11–13 To date, desensitization with steroids and substitution with cisplatin were described in the literature with mixed success.14,15 Although many patients may not develop hypersensitivity reaction upon exposure to cisplatin, its cytotoxicity frequently precludes further use in heavily pretreated patients.
The concept of oxaliplatin substitution is based on the documented activity and tolerability of this platinum agent in advanced ovarian cancer.16,17 When used as a single agent at 100 to 130 mg/m2 every 3 weeks, the objective response rates range from 16% to 26% in heavily pretreated patients.18,19 Data from a small randomized trial demonstrate that patients treated with a combination of cyclophosphamide and oxaliplatin versus cisplatin as first-line therapy had comparable clinical outcomes—progression-free survival (13 vs 13.3 months) and overall survival (36 vs 25.1 months, not statistically significant).16,20 The main advantage of oxaliplatin-based therapy was statistically significant decrease in grade 3/4 myelosuppression, nausea/vomiting, and neurotoxicity.16,20 In addition, phase 2 study of oxaliplatin and taxotere administered as a second-line regimen to patients with platinum-sensitive disease also demonstrated favorable rates of neurotoxicity.16,17 Recent phase 2 trial evaluated combination of the triple regimen involving oxaliplatin, docetaxel, and bevacizumab showed low incidence of peripheral neuropathy and progression-free survival (16.3 months) that is similar to the results of GOG 218 and ICON 7 trials utilizing carboplatin.16,21,22 Granted its activity in recurrent platinum-sensitive disease and platinum-resistant recurrence, we wanted to evaluate whether administration of oxaliplatin after documented carboplatin hypersensitivity reaction is associated with favorable clinical outcomes that are not inferior to its predecessors—cisplatin and carboplatin. This would provide a viable therapeutic alternative to patients with platinum-sensitive disease who were unable to continue further treatment due to life-threatening allergic reactions.
PATIENTS AND METHODS
After obtaining institutional review board approval at the Roswell Park Cancer Institute, we conducted a single-institution retrospective cohort study including a total of 46 patients with previous hypersensitivity reaction to carboplatin. Medical records of all patients from June 2006 to June 2011 were reviewed. The review focused on the documentation of systemic manifestations of hypersensitivity reactions to carboplatin necessitating discontinuation of this drug and switching to an alternative platinum agent (cisplatin or oxaliplatin).
During this period, we identified 46 patients who were rechallenged with either cisplatin or oxaliplatin regimens based on insurance approval. That eliminated personal bias in selecting 1 treatment regimen over the other. Oxaliplatin was dosed at 70 mg/m2 in combination regimens and 100 mg/m2 as a single agent. Cisplatin was given at 50 mg/m2 in combination regimens. All patients were treated in the outpatient setting and received appropriate premedication with antiemetics, corticosteroids, and H1/H2 (histamine)-blockers, as per protocol. The treatment was continued until disease progression, unacceptable toxicity, or patient refusal. Clinical evaluation included physical examination, chemistry panel, and complete blood count with differential before each cycle. Toxicity was assessed according to the Eastern Cooperative Oncology Group Common Toxicity Criteria and patient self-reporting. The initial cisplatin and oxaliplatin doses could be reduced in subsequent cycles, or the cycles could be delayed by 1 week depending on the toxicity and attending physician judgment. Desensitization protocols using steroids and escalating drug concentrations (serial dilutions) were not utilized.
Radiographic imaging was used to characterize response to therapy according to the Response Evaluation Criteria in Solid Tumors criteria. In addition, the measurement of serum CA-125, where appropriate, was used to judge response to therapy. Time-to-treatment failure was defined as the time from initiation of treatment to documented disease progression, death, change of treatment due to adverse event, toxicity, or any other reason.
Patients were classified into 2 treatment groups (oxaliplatin vs cisplatin). Treatment group associations with clinicopathologic variables, toxicity, and time-to-treatment failure were analyzed retrospectively using the Fisher exact and the independent sample permutation t tests. Categorical variables were summarized using contingency tables. Continuous data were summarized by means, median, range, and SD. Adjusted odds ratios (ORs) and 95% confidence intervals were used to describe the effect of treatment (cisplatin vs oxaliplatin [reference]) on myeloid toxicity (yes vs no [reference]), neurotoxicity (yes vs no [reference]), and response (CR vs PD or persistent [reference]) outcomes. These estimates were adjusted for the results of cytoreductive surgery (suboptimal vs optimal [reference]). The 8 patients missing cytoreduction information were retained in the models using multiple imputation methods (reference, Little RJA, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2002). The complete case estimates were comparable and so were not reported. For the other toxicity outcomes, the limiting sample size was too small to support multivariable analyses. P values less than 0.05 were considered statistically significant with no adjustment for multiplicity. Time-to-treatment failure was described using Kaplan-Meier methods. All analyses were obtained using SAS/STAT software, version 9.4, copyright 2012 SAS Inc (Cary, NC).
RESULTS
We identified 46 patients who experienced hypersensitivity reaction to carboplatin and were subsequently retreated with either cisplatin or oxaliplatin based on attending physician preference and insurance approval; both groups did not differ in terms of their clinicopathologic characteristics (Table 1).
TABLE 1.
Patient characteristics
| Oxaliplatin (n = 27) | Cisplatin (n = 19) | P | ||
|---|---|---|---|---|
| Age, y | Mean | 62 (12.1) | 61.2 (6.4) | 1.0 |
| Median | 64 (25–82) | 61 (52–71) | ||
| Race | White | 26 | 18 | 0.42 |
| Hispanic | 0 | 1 | ||
| Unknown | 1 | 0 | ||
| Body mass index | Mean | 29.8 (6.2) | 27.5 (6) | 0.83 |
| Median | 30.8 (19.3–41.3) | 26.5 (19.3–38) | ||
| Cancer type | Ovary fallopian tube, primary peritoneal | 23 | 16 | 0.23 |
| Endometrium | 4 | 1 | ||
| Cervix | 0 | 2 | ||
| Histology type | Carcinosarcoma | 1 | 0 | 0.52 |
| Nonserous | 4 | 1 | ||
| Serous | 20 | 15 | ||
| Squamous cell | 0 | 1 | ||
| Unknown | 2 | 2 | ||
| Cytoreduction | Optimal | 22 | 10 | 0.17 |
| Suboptimal | 2 | 4 | ||
| Unknown | 3 | 5 |
Most patients had ovarian, fallopian, or primary peritoneal cancers (n = 39), 5 patients had uterine cancer, and 2 patients had cervical cancer. Most patients were white (n = 44). The mean body mass index was similar in both groups: 29.8 (oxaliplatin group) versus 27.5 (cisplatin group). Optimal debulking was achieved in 81.5% of patients who received oxaliplatin and 58.8% of patients treated with cisplatin, respectively. As expected, serous histology was the most prevalent type of tumor among the ovarian cancer patients: 87% patients (oxaliplatin group) versus 93.8% patients (cisplatin group).
Before switching chemotherapy regimens, 13 of 46 patients were rechallenged with carboplatin after desensitization with steroids but still manifested recurrent allergic reaction. Ultimately, carboplatin was substituted with cisplatin in 19 patients (median age, 61 years; range, 52–71 years) and 27 patients (median age, 62 years; range, 25–82 years) received oxaliplatin.
Cisplatin was substituted for carboplatin as part of combination regimens including cisplatin/paclitaxel, cisplatin/gemcitabine, and cisplatin/topotecan. Oxaliplatin was given as a single agent to 3 patients; the others were treated with one of the following combinations: oxaliplatin/gemcitabine, oxaliplatin/paclitaxel, oxaliplatin/liposomal doxorubicin, or oxaliplatin/docetaxel.
The total cumulative doses of carboplatin administered before switching platinum agents in both groups were similar: 980 (662) mg (oxaliplatin-treated patients) versus 686 (579.6) mg (cisplatin-treated patients) (P = 0.49; Table 2). This corresponds to the similar median number of carboplatin cycles: 6 (mean, 7.2) in the oxaliplatin and 7.5 (mean, 9.5) in the cisplatin cohort, respectively (P = 0.93). Non–life-threatening hypersensitivity reactions including hives, flushing, transient bronchospasm, and hypotension were observed in 2 patients treated with oxaliplatin; there were no allergic reactions in those exposed to cisplatin. Both groups received a similar number of cisplatin/oxaliplatin infusions (median, 6).
TABLE 2.
Treatment characteristics
| Oxaliplatin (n = 27) |
Cisp latin (n = 19) |
|||
|---|---|---|---|---|
| No. Patients, n (%) | No. Patients, n (%) | P | ||
| Retreated with carboplatin | No | 18 (66.7) | 15 (79.0) | 0.51 |
| Yes | 9 (33.3) | 4 (21.1) | ||
| Response to therapy | Complete response | 9 (34.6) | 6 (31.6) | 0.46 |
| Progressive disease | 4 (15.4) | 6 (31.4) | ||
| Persistent disease | 13 (50.0) | 7 (36.8) | ||
| Unknown | 1 | 0 | ||
| Hypersensitivity reaction | Yes | 2 (7.4) | 0 (0) | 0.5 |
| No | 25 (92.6) | 19 (100) | ||
| No. carboplatin cycles before HSR | Mean | 7.2 | 9.1 | 0.93 |
| Median | 6 | 7.5 | ||
| Range | 1–21 | 1–27 | ||
| SD | 4.3 | 8 | ||
| Carboplatin cumulative dose, mg | Mean | 1037.6 | 707.8 | 0.49 |
| Median | 980 | 686 | ||
| Range | 158–2940 | 73–2152 | ||
| SD | 662 | 579.6 | ||
| No. platinum cycles | Mean | 7.6 | 7.3 | 1 |
| Median | 6 | 6 | ||
| Range | 1–21 | 1–22 | ||
| SD | 4.1 | 5.8 |
Response to therapy was assessed based on radiographic imaging and tumor marker measurements (where indicated). Continued complete response to treatment was observed in 34.6% of oxaliplatin-treated patients and 31.6% of cisplatin-treated patients, whereas stable disease on imaging was reported in 50.0% and 36.8% of oxaliplatin- and cisplatin-treated patients, respectively (P = 0.46).
With regard to tolerability and toxicity of either platinum agent, we report that exposure to oxaliplatin resulted in less neurotoxicity than cisplatin (25.9% vs 68.4%; OR, 8.51 (1.96–36.90); P < 0.01; Table 3). Overall, both regimens were well tolerated by patients. Except for 2 cases of hypersensitivity to oxaliplatin, the most common reason for treatment discontinuation was disease progression or remission. There were no significant differences in myeloid toxicity in oxaliplatin group compared with cisplatin (22.2% vs 36.8%; OR, 2.16 (0.57–8.19); P = 0.26). Similar trends are seen in cases of dermatologic toxicity (1 patient in oxaliplatin group), gastrointestinal toxicity (7.4% vs 5.3%), nephrotoxicity (2 patients in cisplatin group), or emetogenic potential between oxaliplatin and cisplatin groups, respectively. The median number of chemotherapy regimens preceding oxaliplatin- and cisplatin-treated groups was 2. The median time-to-treatment failure was 10.8 months in oxaliplatin group and 9.8 months in cisplatin group (P = 0.86; Fig. 1).
TABLE 3.
Toxicity data
| Oxaliplatin Group |
Cisplatin Group |
OR for Toxicity in Cisplatin (Reference: Oxaliplatin) |
||
|---|---|---|---|---|
| No. Patients, n (%) | No. Patients, n (%) | P | Estimate (95% Confidence Interval); P | |
| Myeloid | ||||
| Yes | 6 (22.2) | 7 (36.8) | 0.33 | 2.16 (0.57–8.19); 0.26 |
| No | 21 (77.8) | 12 (63.2) | Reference | |
| Nephrotoxicity | ||||
| Yes | 0 (0) | 2 (10.5) | 0.22 | N/A |
| No | 27 (100) | 17 (89.5) | ||
| Neurotoxicity | ||||
| Yes | 7 (25.9) | 13 (68.4) | 0.01 | 8.51 (1.96–36.90); <0.01 |
| No | 20 (74.1) | 6 (31.6) | Reference | |
| GI (oral thrush) | ||||
| Yes | 2 (7.4) | 1 (5.3) | 1 | N/A |
| No | 25 (92.6) | 18 (94.8) | ||
| GI (emesis) | ||||
| Yes | 0 | 0 | N/A | |
| No | 27 (100) | 19 (100) | ||
| Dermatologic | ||||
| Yes | 1 (3.7) | 0 (0) | 1 | N/A |
| No | 26 (96.3) | 19 (100) |
N/A, not available.
FIGURE 1.
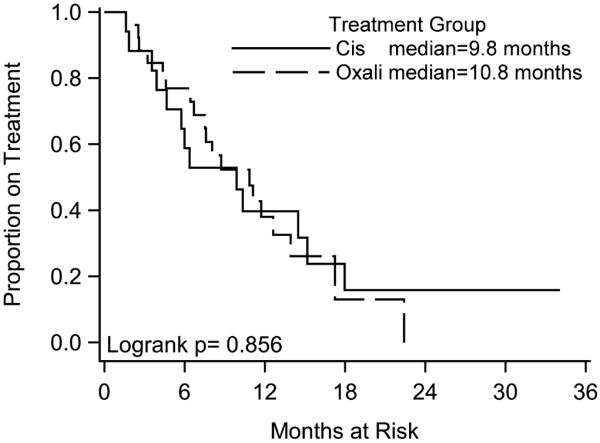
Kaplan-Meier survival curves of time-to-treatment failure.
DISCUSSION
Platinum-based chemotherapy remains the preferred therapeutic strategy for ovarian cancer patients with platinum-sensitive relapse. Repeated exposure to carboplatin frequently results in life-threatening hypersensitivity reactions that can manifest minutes to days after drug infusion and preclude its further use in salvage therapy.23 Although this is a rare occurrence during primary therapy (about 1% of cases), nearly 8% to 44% of patients will experience an allergic reaction during their second- or third-line treatment.4,24,25 Recent evidence demonstrates lower rates of carboplatin hypersensitivity with H1 and H2 blocker premedication—2.6% (all cancer patients) and 7.9% (ovarian cancer patients); however, the presence of other drug allergies was found to be one of the predisposing factors for allergic reaction to carboplatin.26 Rechallenge with carboplatin has been attempted with mixed success; however, it is generally not recommended, as 50% of patients will go on to develop anaphylaxis.3,4,14 Deaths from anaphylaxis upon reintroduction of carboplatin have been reported by Markman even after extensive desensitization with corticosteroids.27
Switching to an alternative platinum drug relies upon variations in antigenicity between these different agents. Nonetheless, the possibility of cross-reactivity still remains, and cases of subsequent cisplatin hypersensitivity have been described.14 Caiado et al28 propose utilization of skin testing for platin-specific IgE that recognizes different epitopes on carboplatin and oxaliplatin or cisplatin to select patients who are likely to tolerate retreatment with oxaliplatin after prior carboplatin sensitization. The authors suggest that carboplatin is less immunogenic than oxaliplatin, thus explaining why some patients tolerate oxaliplatin without recurrent hypersensitivity reaction.28 Authors propose oxaliplatin-specific IgE specificity and sensitivity of 75%; these results merit further evaluation in a larger sample size, as the current analysis was based on assessment of 22 patients.28 Prior studies demonstrated that administration of cisplatin without desensitization after carboplatin allergy was tolerable and allowed continuation of treatment.13 Historically, cisplatin proved to be equally efficacious to carboplatin as the first-line treatment for patients with advanced ovarian cancer; however, its toxicity profile caused it to fall out of favor, making carboplatin the ultimate drug of choice.29,30
Our goal was to investigate whether replacement of carboplatin with oxaliplatin or cisplatin would lead to continued or further objective responses and tolerability that are not inferior to carboplatin. Hypersensitivity reactions to cisplatin are not unusual in this setting either; therefore, an alternative therapeutic regimen would be invaluable, especially for those who reacted to both carboplatin and cisplatin.
The results of this study confirm that substitution of oxaliplatin or cisplatin for carboplatin is a feasible solution to treating patients with carboplatin allergy and platinum-sensitive relapse. To our knowledge, this is the first study reported in the literature comparing clinical outcomes of patients treated with oxaliplatin and cisplatin after carboplatin hypersensitivity.
Our data demonstrate that hypersensitivity reactions typically occurred after the sixth to ninth cycles of carboplatin. This is consistent with previous studies reporting that almost 50% of patients developed allergic reactions after the seventh to eighth cycle of carboplatin.4,7,14
A phase 2 trial of single-agent oxaliplatin versus paclitaxel in platinum-pretreated advanced ovarian cancer demonstrated median duration of response of 31 weeks, 16% overall response rate, and the median time-to-treatment failure of 12 weeks in the oxaliplatin arm.18 It is noteworthy that our data reveal higher response rates (34.6%) and time-to-treatment failure (10.8 months). One possible explanation of this phenomenon is administration of combination chemotherapy regimens using other agents with known activity in gynecologic cancers (eg, paclitaxel, docetaxel, gemcitabine, liposomal doxorubicin).
Our experience highlights alternative platinum rechallenge with oxaliplatin or cisplatin in patients presensitized to carboplatin therapy. Although, clinically not inferior to carboplatin, their objective clinical response rates still do not supersede those of carboplatin. Thus, future research efforts need to focus on more effective treatment strategies associated with higher secondary response rates and durable remission intervals. It is possible that a larger sample size would demonstrate statistically significant differences in clinical responses that would favor 1 platinum drug over the other.
We report 2 (7.4%) non–life-threatening reactions to oxaliplatin that confirm some degree of cross-reactivity between oxaliplatin and carboplatin. None of the 19 patients treated with cisplatin manifested recurrent hypersensitivity reaction, although cisplatin allergy has been described. Callahan et al13 reported 15% rate of cisplatin hypersensitivity in patients who have not received prior desensitization with steroids. When treatment options are limited to substituting 1 platinum analog for another versus retreating with the same drug after formal desensitization, one has to weigh the risks of cross-reactivity among platinum agents and poor glycemic control from repeated desensitization with high-dose corticosteroids. Prior attempts at simplifying Markman's desensitization protocol to shorten its administration did not decrease the rates of repeat allergic reactions.11
The retrospective nature of this review and the small sample size are the limitations of this study. Both groups treated with cisplatin and oxaliplatin have similar demographic and clinicopathologic characteristics. Differences in cytoreduction status, as well as lack of patients with cervical cancer in the oxaliplatin arm, did not significantly affect representative groups making them comparable. Our statistical analysis accounts for these differences among 2 cohorts.
Thus the observed response rates and time-to-treatment failure seen in oxaliplatin arm were not exaggerated secondary to the higher number of patients with optimal debulking and lack of patients with cervical cancer. We believe that these results are generally reflective of the trends reported in the literature and should be applicable to general patient population with platinum-sensitive relapse. In the future, it would be helpful to investigate whether therapy with cisplatin or oxaliplatin results in statistically significant differences in survival and toxicity in patients with recurrent cervical cancer.
Based on our findings, we presume that the efficacy of oxaliplatin is not inferior to cisplatin or carboplatin. This is in accordance with historical data pointing to activity of both agents in platinum-pretreated disease.18,30–32 In addition, decreased incidence of peripheral sensory neuropathy in patients exposed to oxaliplatin goes along with previously published literature.16,20 Further characterization of the cellular and molecular mechanisms of platinum hypersensitivity and subsequent validation in a prospective study are needed to optimize future treatment strategies.
In summary, our experience with salvage therapy in patients with recurrent gynecologic malignancies leads us to conclude that oxaliplatin provides clinical response rates and time-to-treatment failure that were comparable to cisplatin traditionally used in these scenarios. We confirmed that oxaliplatin-based combination regimens, as well as single-agent therapy, were associated with comparable tolerability and efficacy. Although standard desensitization protocols can still be used for patients after initial hypersensitivity reaction, we believe that the availability of other platinum agents would broaden the arsenal of therapeutic options. One of the main advantages of oxaliplatin over cisplatin is a significantly lower neurotoxicity profile without compromising objective response to therapy. Neurotoxicity may be further attenuated by dose reduction of a platinum agent, although such approach would be less desirable as it could impact clinical outcome. Thus its administration is favored in cases where continuation of platinum therapy is otherwise precluded by life-threatening allergic reactions and worsening quality of life secondary to progressive neuropathy.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Miller D, Filiaci V, Fleming G, et al. Randomized phase III non-inferiority trial of first-line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771. [Google Scholar]
- 2.Saito I, Kitagawa R, Fukuda H, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505) Jpn J Clin Oncol. 2010;40:90–93. doi: 10.1093/jjco/hyp117. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Kennedy A, Webster K, et al. Continued chemosensitivity to cisplatin/carboplatin in ovarian carcinoma despite treatment with multiple prior platinum-based regimens. Gynecol Oncol. 1997;65:434–436. doi: 10.1006/gyno.1997.4708. [DOI] [PubMed] [Google Scholar]
- 4.Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141–1145. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 5.Markman M, Hsieh F, Zanotti K, et al. Initial experience with a novel desensitization strategy for carboplatin-associated hypersensitivity reactions: carboplatin-hypersensitivity reactions. J Cancer Res Clin Oncol. 2004;130:25–28. doi: 10.1007/s00432-003-0501-3. [DOI] [PubMed] [Google Scholar]
- 6.Koshiba H, Hosokawa K, Kubo A, et al. Incidence of carboplatin-related hypersensitivity reactions in Japanese patients with gynecologic malignancies. Int J Gynecol Cancer. 2009;19:460–465. doi: 10.1111/IGC.0b013e3181a1bf2e. [DOI] [PubMed] [Google Scholar]
- 7.Robinson JB, Singh D, Bodurka-Bevers D, et al. Hypersensitivity reactions and the utility of oral and intravenous desensitization in patients with gynecologic malignancies. Gynecol Oncol. 2001;82:550–558. doi: 10.1006/gyno.2001.6331. [DOI] [PubMed] [Google Scholar]
- 8.Micha JP, Rettenmaier MA, Dillman R, et al. Single-dose dexamethasone paclitaxel premedication. Gynecol Oncol. 1998;69:122–124. doi: 10.1006/gyno.1998.4993. [DOI] [PubMed] [Google Scholar]
- 9.Onetto N, Canetta R, Winograd B, et al. Overview of taxol safety. J Natl Cancer Inst Monogr. 1991;15:131–139. [PubMed] [Google Scholar]
- 10.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 11.Jones R, Ryan M, Friedlander M. Carboplatin hypersensitivity reactions: re-treatment with cisplatin desensitization. Gynecol Oncol. 2003;89:112–115. doi: 10.1016/s0090-8258(03)00066-0. [DOI] [PubMed] [Google Scholar]
- 12.Sliesoratis S, Chikhale PJ. Carboplatin Hypersensitivity. Int J Gynecol Cancer. 2005;15:13–18. doi: 10.1111/j.1048-891x.2005.14401.x. [DOI] [PubMed] [Google Scholar]
- 13.Callahan MB, Lachance JA, Stone RL, et al. Use of cisplatin without desensitization after carboplatin hypersensitivity reaction in epithelial ovarian and primary peritoneal cancer. Am J Obstet Gynecol. 2007;197:199e1–199e5. doi: 10.1016/j.ajog.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 14.Abe A, Ikawa H, Ikawa S. Desensitization treatment with cisplatin after carboplatin hypersensitivity reaction in gynecologic cancer. J Med Invest. 2010;57:163–167. doi: 10.2152/jmi.57.163. [DOI] [PubMed] [Google Scholar]
- 15.Ottaiano A, Tambaro R, Greggi S, et al. Safety of cisplatin after severe hypersensitivity reactions to carboplatin in patients with recurrent ovarian carcinoma. Anticancer Res. 2003;23:3465–3468. [PubMed] [Google Scholar]
- 16.Herzog TJ, Monk BJ, Rose PG, et al. A phase II trial of oxaliplatin, docetaxel and bevacizumab as first-line therapy of advanced cancer of the ovary, peritoneum, and fallopian tube. Gynecol Oncol. 2014;132:517–525. doi: 10.1016/j.ygyno.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrandina G, Ludovisi M, De Vincenzo R, et al. Docetaxel and oxaliplatin in the second-line treatment of platinum-sensitive recurrence of ovarian cancer: a phase II study. Ann Oncol. 2007;18:1348–1353. doi: 10.1093/annonc/mdm136. [DOI] [PubMed] [Google Scholar]
- 18.Piccart MJ, Green JA, Lacave AJ, et al. Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: a randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology Group. J Clin Oncol. 2000;18:1193–1202. doi: 10.1200/JCO.2000.18.6.1193. [DOI] [PubMed] [Google Scholar]
- 19.Chollet P, Bensmaine A, Brienza S, et al. Single agent activity of oxaliplatin in heavily pretreated advanced epithelial ovarian cancer. Ann Oncol. 1996;7:1065–1070. doi: 10.1093/oxfordjournals.annonc.a010500. [DOI] [PubMed] [Google Scholar]
- 20.Misset JL, Vennin P, Chollet PH, et al. Multicenter phase II–III study of oxaliplatin plus cyclophosphamide vs. cisplatin plus cyclophosphamide in chemo naive advanced ovarian cancer patients. Ann Oncol. 2001;12:1411–1415. doi: 10.1023/a:1012556627852. [DOI] [PubMed] [Google Scholar]
- 21.Burger RA, Brady MF, Bookman MA, et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 22.Perren TJ, Swart AM, Pfisterer J, et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 23.Dizon DS, Sabbatini PJ, Aghajanian C, et al. Analysis of patients with epithelial ovarian cancer or fallopian tube carcinoma retreated with cisplatin after the development of a carboplatin allergy. Gynecol Oncol. 2002;84:378–382. doi: 10.1006/gyno.2001.6519. [DOI] [PubMed] [Google Scholar]
- 24.Kandel MJ, Loehr A, Harter P, et al. Cisplatinum rechallenge in relapsed ovarian cancer patients with platinum reinduction therapy and carboplatin hypersensitivity. Int J Gynecol Cancer. 2005;15:780–784. doi: 10.1111/j.1525-1438.2005.00136.x. [DOI] [PubMed] [Google Scholar]
- 25.Hendrick AM, Simmons D, Cantwell BM. Allergic reactions to carboplatin. Ann Oncol. 1992;3:239–240. doi: 10.1093/oxfordjournals.annonc.a058160. [DOI] [PubMed] [Google Scholar]
- 26.Navo M, Kunthur A, Badell ML, et al. Evaluation of the incidence of carboplatin hypersensitivity reactions in cancer patients. Gynecol Oncol. 2006;103:608–613. doi: 10.1016/j.ygyno.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Markman M. Hypersensitivity reactions to carboplatin. Gynecol Oncol. 2002;84:353–354. doi: 10.1006/gyno.2001.6513. [DOI] [PubMed] [Google Scholar]
- 28.Caiado J, Venemalm L, Pereira-Santos MC, et al. Carboplatin-, oxaliplatin-, and cisplatin-specific IgE: cross-reactivity and value in the diagnosis of carboplatin and oxaliplatin allergy. J Allergy Clin Immunol Pract. 2013;1:494–500. doi: 10.1016/j.jaip.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Ozols RF. Paclitaxel and carboplatin in the treatment of ovarian cancer. Semin Oncol. 1999;26(1 suppl 2):84–89. [PubMed] [Google Scholar]
- 30.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic oncology group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 31.Nejit JP, ten Bokkel Huinink WW, van der Burg ME, et al. Long-term survival in ovarian cancer. Eur J Cancer. 1991;27:1367–1372. doi: 10.1016/0277-5379(91)90011-2. [DOI] [PubMed] [Google Scholar]
- 32.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]


