Abstract
The expression of CD30 receptors is one of the defining characteristics of the malignant Reed-Sternberg cells of Hodgkin lymphoma. CD30 is rarely expressed by normal cells, and is rapidly internalized, making it an ideal therapeutic target for monoclonal antibodies and for antibody-drug conjugates (ADCs). Brentuximab vedotin is the first ADC to be approved by regulatory agencies for the treatment of patients with relapsed HL, with a single agent response rate of 75%. In this review article we will discuss the current and ongoing development of brentuximab vedotin in patients with relapsed and newly diagnosed Hodgkin lymphoma.
Introduction
Hodgkin Lymphoma (HL) accounts for10% of all lymphomas and 0.6% of all cancers in the developed world. In the United States, it is estimated that 9,000 new cases will be diagnosed in 2015, and approximately 1,150 will die of their disease. HL is histologically defined by rare mononuclear Hodgkin cells and multinucleated Reed-Stemberg (HRS) cells residing in a sea of inflammatory cells. Morphologically it is known by its contiguous spread through the lymph nodes. HRS cells are derived from germinal center B cells, but phenotypically lose the expression of typical B cell markers.
Despite an excellent cure rate with modern frontline HL therapy, approximately 15% to 20% of patients are not cured with first line or second line therapy, and will require additional treatments. For those who are cured, treatment-related late toxicities continue to impact patients’ quality of life and survival. Therefore, there is need to develop new therapeutic agents to further improve the current cure rate and to reduce treatment toxicity. HRS cells are characterized by the expression of CD30 receptors. CD30 is a transmembrane glycoprotein that belongs to the tumor necrosis factor (TNF) receptor superfamily (1). CD30 expression is very restricted making it an ideal therapeutic target for monoclonal antibodies.
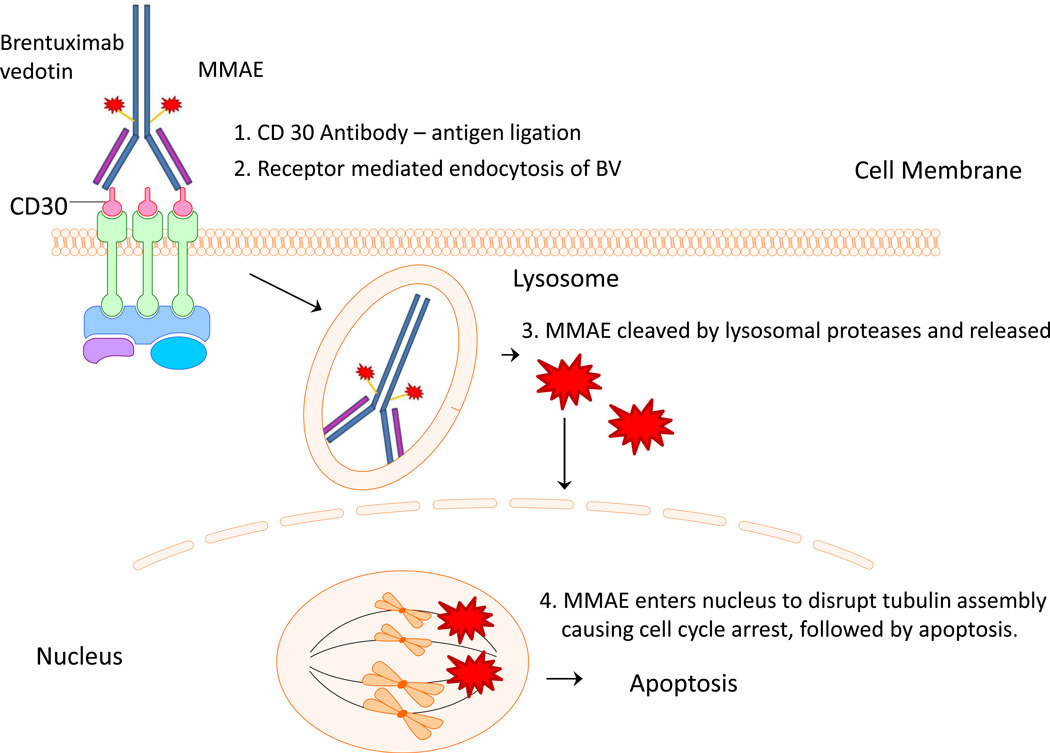
Brentuximab vedotin is an antibody-drug conjugate (ADC) that was developed by conjugating the tubulin toxin monomethyl auristatin E (MMAE) to the chimeric monoclonal anti-CD30 antibody cAC10. On average, four molecules of MMAE are conjugated to one cAC10. After binding to CD30, brentuximab vedotin in internalized and processed into lysosomal vesicles leading to the release of MMAE from the antibody by reduction or acid hydrolysis within the lysosomes. Subsequently, MMAE is released into cytoplasm and inhibits microtubule polymerization leading to cell cycle arrest followed by cell death (Figure 1). As HRS cells die, a small amount of MMAE is released into the tumor microenvironment which can kill neighboring cells by a CD30-independent manner.
Figure 1.
Brentuximab vedotin mechanism of action. As an antibody for CD30, BV then enters cell via endocytosis. In the lysosome, the cytotoxin monomethyl auristatin E gets released. When MMAE enters the nucleus, it disrupts mitosis at the microtubulin level, causing apoptosis.
The first-in-man phase I study of brentuximab vedotin was conducted in 45 patients with relapsed or refractory CD30 positive lymphomas. Brentuximab vedotin was administered by short intravenous infusion every 3 weeks. The trial demonstrated the safety of the drug and provided encouraging early efficacy data, as the treatment resulted in tumor reductions in the majority of patients (2). Subsequently, a pivotal phase 2 trial was conducted in 102 refractory or relapsed patients after autologous stem cell transplant. Remarkably, 75% of patients had a response with 34% having a complete response. With a long-term follow up, about 25% of the patients remained in remission after 4 years (3).
Treatment with brentuximab vedotin is generally well tolerated. The most common treatment-related side effects of any grade were peripheral neuropathy (42%), nausea (35%), and fatigue (34%). In 8% of patients, grade 3 or 4 neuropathy was observed, requiring dose interruption and/or reduction (3)(4). In rare cases, pancreatitis and progressive multifocal leukoencephalopathy were also reported.
Development of brentuximab vedotin in frontline therapy
Given the high response rate, and good safety profile, brentuximab vedotin is now being incorporated with several frontline regimens for the treatment of patients with classical HL. The goal of these treatment programs is to improve the cure rate, reduce the number of treatment cycles, or eliminate the need for consolidation with radiation therapy.
Initially, brentuximab vedotin was combined with ABVD for the treatment of patients with advances stage HL. In this phase-I study, brentuximab vedotin was administered every 2 weeks with each cycle of ABVD. The phase 2 the recommended dose was 1.2 mg/kg. However, when brentuximab vedotin was combined with full doses of ABVD, 40% of the patients developed interstitial pneumonitis. Subsequently, bleomycin was eliminated from the regimen and patients were treated with AVD plus brentuximab vedotin, with no further lung toxicity being observed. This novel regimen resulted in a 3-year overall survival of a 100%, and a 3-year failure free survival of 96% (5, 6). Based on these promising results, a randomized phase-3 study was initiated comparing standard ABVD with AVD plus brentuximab vedotin in a different strategy, the German Hodgkin Lymphoma Study Group incorporated brentuximab vedotin with a modified version of their BEACOPP regimen to produce the novel regimen of BrECAPP; however, little data is currently available on the safety and efficacy of this approach (7).
The outcome of standard ABVD therapy in elderly patients with HL remains disappointing, and these patients need more effective but tolerable new treatment strategies. Ongoing clinical trials with BV as a single agent are being explored specifically in the for the population of age 60 and greater with preliminary data showing an overall response rate of over 90% and complete remission seen in 70%, with a median progression free survival of 8.7 months (8). Also tested in this age group was the combination of BV with decarbazine. Although the power of the study is small, it showed overall response rate of 100% with complete response being 33% and partial response being 66% (9). Table 1.
TABLE 1. Brentuximab vedotin in Frontline Therapy.
Brentuximab vedotin trials used in frontline therapy. Listed are the preliminary results of these trials.
| Treatment | Indication | Preliminary Data | N (to date) |
|---|---|---|---|
| BV then (BV + AVD) (15) | Stage I–II, non-bulky | 14 month follow up: PFS = 90%, OS= 97% |
34 |
| ABVD then BV, instead of RT (16) |
Stage I, II Non-bulky |
100% PET negative, PFS thus far 7.6 months |
15 |
| BV +AVD +RT (17) | Stage I, II unfavorable risk | 93% PET negative | 19 |
| BV + AVD (18, 19) | Advanced stage (II bulky, IIB, III, or IV) |
3 year OS – 100%; 3 year FFS 96% | 26 |
| BV then ABVD +/− RT (20) | Stage I–IIIA | ORR =100%, CR = 91% *1 Patient with stage III had PR |
12 |
| BV monotherapy (22) | Adults > age 60 | ORR= 93%; CR = 70% ; PFS= 8.7 months |
27 |
| BV +Dacarbazine (23) | Adults > age 60 | ORR = 100% CR 33%; PR = 66% | 6 |
BV brentuximab vedotin
ABVD adriamycin, bleomycin, vinblastine, dacarbazine
AVD adriamycin, bleomycin, dacarbazine
RT radiation therapy
OS overall survival
PFS progression free survival
FFS failure free survival
ORR overall response rate
CR complete response
PR partial response
Brentuximab vedotin is also incorporated in the management of patients with early stage HL, with encouraging results. In one study, patients were initially treated with single agent brentuximab vedotin for 2 cycles, followed by 4–6 cycles of AVD plus brentuximab vedotin. With a relatively short term follow up, the 14 month progression free survival was 90% and the overall survival was 97% (10). In a different strategy, patients with unfavorable early stage HL were treated with Brentuximab vedotin + AVD for 4 cycles followed by involved field radiation therapy. Thus far, of 19 early stage patients with unfavorable prognosis, including 10 with bulky disease achieved over 90% PET negativity after 2 or 4 cycles. No patients suffered pulmonary toxicity, making this regimen safe and effective. (11).
Pre transplant salvage therapy
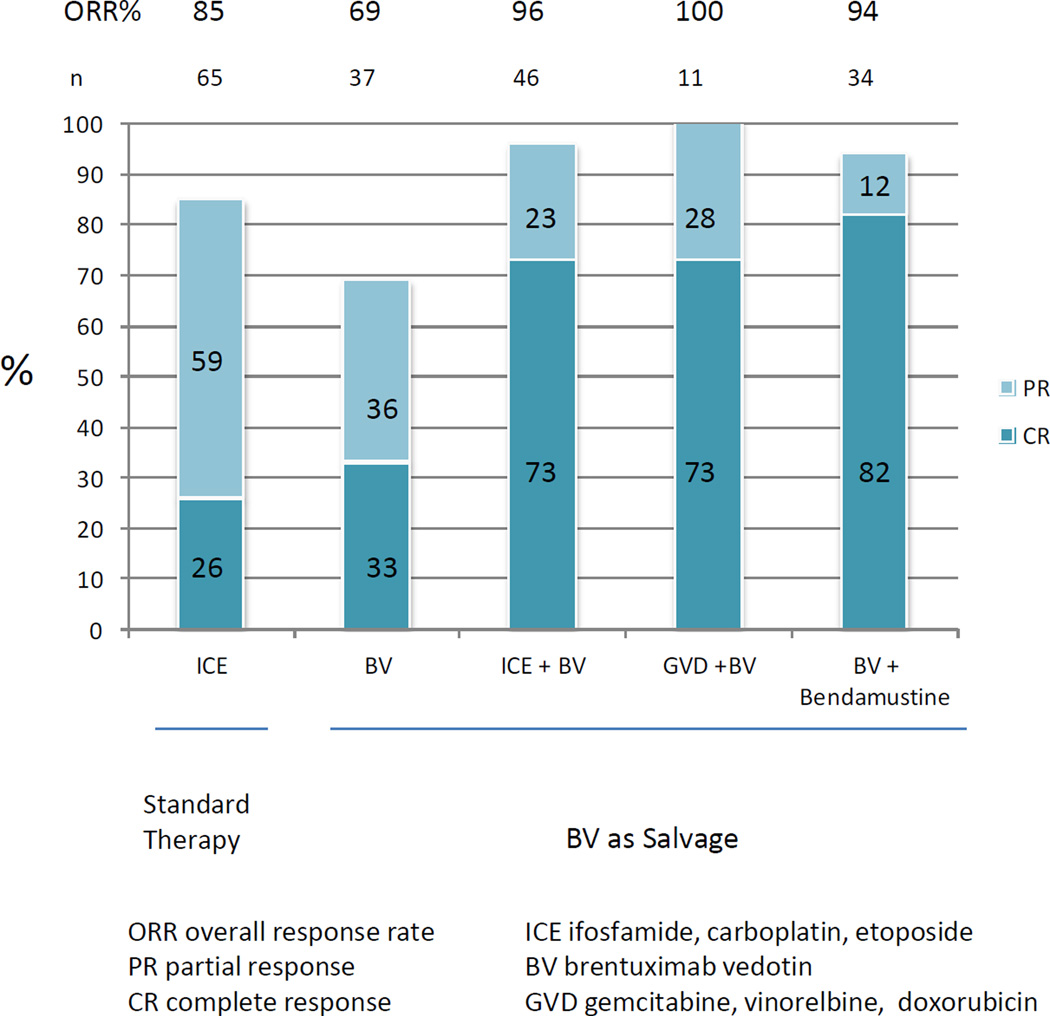
About 20–30% of HL patients have refractory or relapsed disease after frontline therapy. Patients typically undergo salvage chemotherapy followed by autologous stem cell transplant (ASCT). While many platinum-based and gemcitabine-based salvage regimens produce high response rates, exceeding 60%–70%, the complete remission rates achieved with these regimens remains rather low. To improve the CR rate of salvage regimens, brentuximab vedotin was combined with ifosfamide, carboplatin, and etoposide (ICE) (12). In this phase 2 trial brentuximab vedotin was given as a single agent for 2 cycles, with each cycle being 3 weekly doses of brentuximab vedotin followed by one week of rest. Patients who achieved PET negative response were allowed to proceed directly to stem cell collection followed by ASCT. Otherwise, patients were treated with augmented doses of ICE followed by ASCT. Using this strategy, 26% (12/46) of patients achieved PET negative disease after 2 cycles of brentuximab vedotin. The 2 year overall survival and event-free survival were 95% and 80%, respectively. In a separate phase 1/2 study brentuximab vedotin was combined with bendamustine in 45 patients with first relapse. Patients received up to 6 cycles of the combination, followed by ASCT, then maintenance with brentuximab vedotin for up to 16 doses. 82% of patients achieved CR before ASCT, with 94% ORR. (13). Brentuximab vendotin was also combined with gemcitabine, vinorelbine, and doxorubicin (GVD) on day 1 followed by brentuximab vedotin on day 8 every 3 weeks as a bridge to transplant. The ORR was 100%, with 8 of 11 patients achieving CR.(14). (Figure 2)
Figure 2.
Response rate of brentuximab vedotin used as monotherapy and combination therapy in patients that are refractory or relapsed after frontline therapy, before autosomal stem cell transplant.
Brentuximab vedotin consolidation after ASCT
Patients with relapsed and refractory HL are typically treated with second-line salvage therapy followed by ASCT. At best, this approach can cure approximately 60% of transplant eligible patients. To improve on the outcome of ASCT, 2 strategies have been explored. In the first one, as discussed earlier, brentuximab vedotin is combined with pre-transplant salvage regimens to improve the CR rate. In the second, brentuximab vedotin is administered after ASCT as a consolidation, to prevent relapsed disease. To test the second approach, a phase 3, randomized, double-blind, placebo-controlled trial comparing brentuximab vedotin consolidation with placebo in patients with high risk disease (AETHERA trial) (15). Patients were treated with either brentuximab vedotin for 16 doses or placebo, starting 30–45 days after ASCT. The 2 year PFS for patients receiving brentuximab vedotin or placebo were 65% and 45%, respectively. However, there was no difference in the overall survival, as 85% of patients in the placebo group received brentuximab vedotin after progression (15). Based on these data, brentuximab vedotin was approved by the FDA for consolidation therapy after ASCT.
Future Directions
As discussed earlier, brentuximab vedotin produces 75% response rate in patients with relapsed HL after receiving ASCT. However, despite this high response rate, the majority of responses are partial, and short lived. To improve the CR rate and response duration, it will be important to combine brentuximab vedotin with other active agents. Ongoing combinations include the histone deacetylase inhibitor mocetinostat (NCT02429375), and the mTOR inhibitor everolimus (NCT02254239, NCT01022996). With the recent reports of significant clinical activity of immune checkpoint inhibitors targeting PD1, future combination of brentuximab vedotin plus PD1 or PDL1 blocking antibodies are warranted. Should these combinations prove to be safe and highly active, this doublet can be incorporated with other active agents and tested in newly diagnosed patients with high risk features.
TABLE 2. Standard therapy versus experimental brentuximab vedotin therapy for Hodgkins lymphoma.
Standard therapy versus experimental therapy, using brentuximab vedotin at different stages of disease.
| Stage | Standard | BV Experimental |
|---|---|---|
| Front line | ABVD × 2 cycles + 20Gy IFRT | BV monotherapy |
| Early Stage | BV + Dacarbazine | |
| BV then (BV +AVD) | ||
| ABVD then BV | ||
| BV +AVD +RT | ||
| Advanced stage | ABVD × 4 cycles +30Gy IFRT | BV +AVD |
| BV then ABVD +/− RT | ||
| Pretransplant/ Salvage |
ICE × 3 cycles + BEAMS | BV monotherapy |
| ICE +BV | ||
| GVD +BV | ||
| BV + Bendamustine | ||
| Post-transplant/ Consolidation |
- | BV |
| Relapsed / Post ASCT |
BV | BV + histone-deacetylase (HDAC) inhibitor |
| BV + PD-1 inhibitor | ||
| BV + mTOR inhibitor |
ABVD adriamycin, bleomycin, vinblastine, dacarbazine
IFRT Involved-field radiation therapy
ICE ifosfamide, carboplatin, etoposide
BV brentuximab vedotin
AVD adriamycin, vinblastine, decarbazine
GVD gemcitabine, vinorelbine, doxorubicin
References
- 1.Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85(1):1–14. [PubMed] [Google Scholar]
- 2.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. The New England journal of medicine. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 3.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopal AK, Ramchandren R, O'Connor OA, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120(3):560–568. doi: 10.1182/blood-2011-12-397893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. The Lancet Oncology. 2013;14(13):1348–1356. doi: 10.1016/S1470-2045(13)70501-1. [DOI] [PubMed] [Google Scholar]
- 6.Connors JM, Ansell S, Park SI, et al. Brentuximab Vedotin Combined with ABVD or AVD for Patients with Newly Diagnosed Advanced Stage Hodgkin Lymphoma: Long Term Outcomes. Blood. 2014;124(21):292. [Google Scholar]
- 7.Eichenauer DA, Plütschow A, Kreissl S, et al. Targeted Beacopp Variants In Patients With Newly Diagnosed Advanced Stage Classical Hodgkin Lymphoma: Interim Results Of a Randomized Phase II Study. Blood. 2013;122(21):4344. [Google Scholar]
- 8.Chen RW, Palmer J, Martin P, et al. Results of a Phase II Trial of Brentuximab Vedotin As First Line Salvage Therapy in Relapsed/Refractory HL Prior to AHCT. Blood. 2014;124(21):501. [Google Scholar]
- 9.Forero-Torres A, Holkova B, Sharman JP, et al. Brentuximab Vedotin Monotherapy and in Combination with Dacarbazine in Frontline Treatment of Hodgkin Lymphoma in Patients Aged 60 Years and Above: Interim Results of a Phase 2 Study. Blood. 2014;124(21):294. [Google Scholar]
- 10.Abramson JS, Arnason JE, LaCasce AS, et al. Brentuximab vedotin plus AVD for non-bulky limited stage Hodgkin lymphoma: A phase II trial. Journal of Clinical Oncology. 2015;33(15) [Google Scholar]
- 11.Kumar A, Yahalom J, Schoder H, et al. Safety and Early Efficacy in an Ongoing Pilot Study of Brentuximab Vedotin and AVD Chemotherapy Followed By 30 Gray Involved-Site Radiotherapy for Newly Diagnosed, Early Stage, Unfavorable Risk Hodgkin Lymphoma. Blood. 2014;124(21):3085. [Google Scholar]
- 12.Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. The Lancet Oncology. 2015;16(3):284–292. doi: 10.1016/S1470-2045(15)70013-6. [DOI] [PubMed] [Google Scholar]
- 13.LaCasce A, Bociek RG, Matous J, et al. Brentuximab Vedotin in Combination with Bendamustine for Patients with Hodgkin Lymphoma who are Relapsed or Refractory after Frontline Therapy. Blood. 2014;124(21):293. [Google Scholar]
- 14.Michallet AS, Guillermin Y, Deau B, et al. Sequential combination of gemcitabine, vinorelbine, pegylated liposomal doxorubicin and brentuximab as a bridge regimen to transplant in relapsed or refractory Hodgkin lymphoma. Haematologica. 2015;100(7):e269–e271. doi: 10.3324/haematol.2015.124784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 2015;385(9980):1853–1862. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]