Abstract
Global analyses of cancer transcriptomes demonstrate that ADAR (adenosine deaminase, RNA-specific)-mediated RNA editing dynamically contributes to genetic alterations in cancer, and directly correlates with progression and prognosis. RNA editing is abundant and frequently elevated in cancer, and affects functionally and clinically relevant sites in both coding and non-coding regions of the transcriptome. Therefore, ADAR and differentially edited transcripts may be promising biomarkers or targets for therapy.
Cancer cells acquire the ability to avoid checkpoint controls, proliferate continuously, and become motile as a result of genomic dysfunction. Research has consequently focused on DNA-level mutations that affect the function of specific proteins (DNA repair factors, cell cycle regulators, modulators of chromatin states, enzymatic modifiers of DNA, etc.) or the ability of particular proteins (e.g., transcription factors) to bind to DNA and regulate transcription. Generally, DNA mutations can be broadly classed into those that alleviate tumor-suppressive functions and those that promote oncogenic pathways, and cancer is often the combined outcome of loss of function in both.
Three papers published recently in Cell Reports and Cancer Cell [1,2,3] reveal a new, formerly unappreciated level of genetic complexity in cancer, demonstrating that mutations at the RNA level also have implications in the progression and prognosis of clinical disease. These transcriptomic-level mutations, known as RNA editing events, are catalyzed by two sets of deaminases, APOBEC1 (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 1) and APOBEC3A (in human), which convert cytosine to uracil (C-to-U), and ADARs, a family of enzymes that deaminate adenosine to form inosine (A-to-I), which is interpreted by the cellular machinery as guanosine. A-to-I editing is very widespread in the human genome [4] and spans both coding and non-coding regions, mainly within highly repetitive Alu elements, which can form the requisite double-stranded structure for ADAR-mediated editing. RNA editing has been shown to create different protein isoforms [5], modulate alternative splicing [6], and regulate transcript stability by modifying microRNAs and microRNA binding sites [7,8]. In the context of cancer, as this recent work shows, RNA editing may serve as an additional, and much more dynamic and flexible, counterpart to genomic-level mutations.
To date, research on the effects of RNA editing in cancer have been largely restricted to specific edited sites. For instance, in hepatocellular carcinoma, ADAR-mediated editing of AZIN1 (antizyme inhibitor 1) is increased, and this helps to drive the progression of disease through increased cell proliferation [9]. Similarly, in colorectal cancer, increased editing of RHOQ (Ras homolog family member Q) promotes invasion potential through deregulation of actin remodeling [10]. However, these studies do not fully address the role of ADAR in these cancers, because editing in both cases is much more widespread, potentially affecting thousands of sites within tens of transcripts. By contrast, these recent reports [1,2,3] collectively highlight the global effects of ADAR-mediated RNA editing on human cancer. Several themes emerge from this work.
RNA Editing is Abundant in Human Cancer
Using data from The Cancer Genome Atlas (TCGA), both Han et al. and Paz-Yaacov et al. report abundant RNA editing across many types of human cancer, and note that the mutational load due to RNA editing is of the same order as that contributed by DNA mutations.
RNA Editing Is Dysregulated in Human Cancer
In most of the types of cancer studied, including breast, thyroid, and lung, editing rates were higher in tumor samples compared to the paired normal tissue [1,2,3]. Notably, Paz-Yaacov and collaborators found that a new metric they devised, the Alu editing index (AEI), was a good predictor of patient outcome. Specifically, patients with liver, head and neck, and breast cancers showed improved survival rates when exhibiting lower AEI, a measure of the averaged editing rates of adenosines in Alu elements, weighted by their relative expression levels [3]. However, not all cancers show elevated editing; indeed, chromophobe renal cell carcinoma and renal papillary cell carcinoma showed significantly reduced A-to-I editing (or ‘underediting’) in tumors compared to normal tissues [2].
RNA Editing Occurs in Functionally and Clinically Relevant Sites
A major challenge in cancer research is to distinguish passenger events from driver events. Paz-Yaacov et al. found 60 recoding editing events that were dysregulated in cancer compared to normal tissues; similarly, they found dysregulation in thousands of editing sites that disrupt potential microRNA sites [3]. Han et al. found that 12 of the 17 cancers studied contain ‘clinically relevant edited sites’, which were identified by performing differential analyses between editing levels and tumor subtypes and stages and correlation analysis with patient survival rates; these sites were distributed in non-coding RNAs, introns, and coding regions [2]. They demonstrated that the nonsynonymous editing of AZIN1, GRIA2 (glutamate receptor, ionotropic, AMPA 2) and COG3 (component of oligomeric Golgi complex 3) conferred significantly increased cell survival. They also discovered an association between editing and drug sensitivity, showing that mutations in AZIN1, for instance, resulted in resistance to the insulin-like growth factor 1 receptor (IGF-1R) inhibitor BMS536924.
The Increase in ADAR-Mediated Editing Is Due Largely to an Increase in ADAR1 Expression
While there are multiple ADARs in humans (ADAR1, ADAR2, and ADAR3), ADAR1 was found to be the primary contributor to RNA editing in the context of cancer [1,2,3], modulating the frequency and number of sites edited. Fumagalli et al. further demonstrated that, in breast cancer, ADAR1 expression was elevated as a result of aberrantly high copy number owing to chromosomal duplication. They also showed that ADAR1 expression and therefore editing are part of a broader type I interferon response, which is the result of the chronic inflammatory environment of the tumors. Conversely, a lack of ADAR1 resulted in decreased cell proliferation and increased apoptosis.
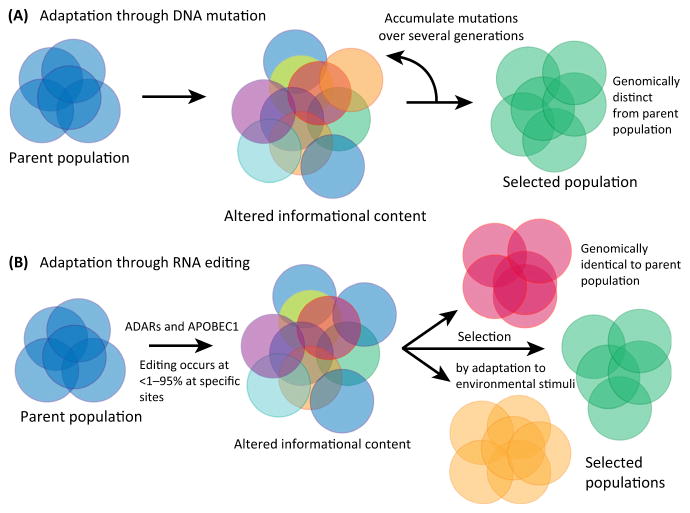
These new studies raise the exciting possibility that, exactly as was the case for DNA mutation, RNA editing may also be a highly dynamic modulator of disease progression and manifestations. RNA editing events could, in aggregate, drive cancer progression and the development of drug sensitivity by actively targeting related cellular pathways. Such targeting, while leaving no trace on the genome, could transiently generate distinct subsets of cells with specific resistance profiles, which would then be selected on the basis of features that can be propagated onward even in the absence of editing (Figure 1). Future work at the single cell level will help determine the existence of such populations (akin to tumor stem cells) and document their emergence.
Figure 1.
Adding RNA Editing to Current Paradigms of Cancer Progression.
(A) Cancer model of DNA mutation, selection, and adaptation. (B) RNA editing contribution to selection and adaptation. Abbreviations: ADAR, adenosine deaminase, RNA-specific; APOBEC1, apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 1.
The widespread involvement of ADAR in a wide variety of cancers suggests that ADAR and its targets are excellent candidate biomarkers of cancer progression. Further studies will be necessary to determine whether targeting clinically relevant sites is feasible, given the lower rates of editing, and whether small-molecule modulation of ADAR activity, to reverse editing to normal tissue levels, is a viable therapeutic avenue. Finally, these studies would predict that other emerging epitranscriptomic (RNA-level) modifications (e.g., APOBEC1-mediated C-to-U editing, N6-adenosine methylation and pseudouridylation) will also be active contributors to disease progression, with potentially key roles, especially in diseases that are known to have a genetic basis but which have not demonstrated a high degree of association with specific DNA mutations.
References
- 1.Fumagalli D. Principles governing A-to-I RNA editing in the breast cancer transcriptome. Cell Rep. 2015;13:277–289. doi: 10.1016/j.celrep.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han L, et al. The genomic landscape and clinical relevance of A-to-I RNA editing in human cancers. Cancer Cell. 2015;28:515–528. doi: 10.1016/j.ccell.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paz-Yaacov N, et al. Elevated RNA editing activity is a major contributor to transcriptomic diversity in tumors. Cell Rep. 2015;13:267–276. doi: 10.1016/j.celrep.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 4.Bazak L, et al. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res. 2014;24:365–376. doi: 10.1101/gr.164749.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald LW, et al. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacol. 1999;21:82S–90S. doi: 10.1016/S0893-133X(99)00004-4. http://www.ncbi.nlm.nih.gov/pubmed/10432493. [DOI] [PubMed] [Google Scholar]
- 6.Rueter SM, et al. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- 7.Laganà A, et al. miR-EdiTar: a database of predicted A-to-I edited miRNA target sites. Bioinformatics. 2012;28:3166–3168. doi: 10.1093/bioinformatics/bts589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoshan E, et al. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat Cell Biol. 2015;17:311–321. doi: 10.1038/ncb3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med. 2013;19:209–216. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han SW, et al. RNA editing in RHOQ promotes invasion potential in colorectal cancer. J Exp Med. 2014;211:613–621. doi: 10.1084/jem.20132209. [DOI] [PMC free article] [PubMed] [Google Scholar]