Abstract
It is well known that surfactant-suspended carbon nanotube (CNT) samples can be purified by centrifugation to decrease agglomerates and increase individually-dispersed CNTs. However, centrifugation is not always part of protocols to prepare CNT samples used in biomedical applications. Herein, using carboxylated multi-walled CNTs (cMWCNTs) suspended in water without a surfactant, we developed a Boehm titrimetric method for the analysis of centrifuged cMWCNT suspensions and used it to show that the surface acidity of oxidized carbon materials in aqueous cMWCNT suspensions was enriched by ~40% by a single low-speed centrifugation step. This significant difference in surface acidity between un-centrifuged and centrifuged cMWCNT suspensions has not been previously appreciated and is important because the degree of surface acidity is known to affect the interactions of cMWCNTs with biological systems.
Keywords: Carbon nanomaterial sample preparation, Oxidized multi-walled carbon nanotube, Boehm titration, Nanotoxicology, Nanomedicine
Graphical Abstract
1. Introduction
Carbon nanotubes (CNTs) have unique physiochemical properties that make them useful for the potential diagnosis and treatment of a number of diseases, especially cancer.[1–6] An important consideration for these and other biomedical applications of CNTs is how CNT samples are prepared.[7] In brief, almost all commercial CNT products are supplied as powdered soot that contains some degree of metallic and carbonaceous impurities in addition to CNTs. To prepare samples of pristine, non-functionalized CNTs for biomedical applications, the first step typically involves sonicating a known mass of CNT soot in an aqueous solution of a surfactant (e.g., a biocompatible polymer, protein, or serum) to yield a CNT suspension,[8] and a second optional step involves centrifugation of the CNT suspension to remove heavier metal-containing CNTs, bundles, and other agglomerated materials.[9] To prepare oxidized or carboxylated CNT (cCNT) samples for biomedical applications, two methods are commonplace that do not involve the use of surfactants. One method reported by several groups is to sonicate cCNT soot in deionized water before adding the resulting aqueous cCNT suspension to cell culture medium,[10–14] while others have reported an additional centrifugation step to purify the aqueous cCNT suspension (herewith called a centrifuged cCNT suspension) before adding this material to cell culture medium or blood.[15–18] Another important consideration for biomedical applications of CNTs is therefore a thorough physiochemical characterization of the exact CNT sample that is presented to living cells or intact organisms.[19–28] At a minimum, this involves some measure of CNT structures, amounts, dimensions, porosities, impurities, and in the case of cCNTs, a measure of surface acidity, defined as the acidic groups covalently attached to cCNT surfaces and potential acidic carbonaceous substances adsorbed to cCNT surfaces (i.e., oxidative debris).[13,29–36]
The acidic surface properties of cCNTs primarily stem from the presence of carboxyl, lactonic, hydroxyl, and phenolic groups, which are generated through reaction of CNTs with acidic liquid oxidants or high-temperature oxygen.[37–39] Acidic groups on cCNT surfaces have been assessed qualitatively using thermogravimetric analysis (TGA),[40–43] semi-quantitatively using Fourier transform-infrared (FT-IR) spectroscopy,[24,44–46] and quantitatively using either fluorescence spectroscopy and dye-derivatized cCNTs,[47–49] x-ray photoelectron spectroscopy (XPS),[24,43–45,50,51] or acid-base titrimetry.[52–55] While these and other approaches have their advantages and limitations, a survey of the literature reveals that they have been used overwhelmingly to assess the surface acidity of un-centrifuged cCNT suspensions, and rarely to assess the surface acidity of cCNT suspensions purified by centrifugation. This is notable because centrifuging an aqueous cCNT suspension without a surfactant should facilitate the removal of hydrophobic, non-oxidized soot components from the supernatant, as opposed to when a surfactant is used where both oxidized and non-oxidized components would be coated by surfactant and suspended in the supernatant. In other words, the process of centrifuging an aqueous suspension of cCNT soot could selectively enrich the centrifuged sample with oxidized carbon material.
Herein, a Boehm titrimetric method for the analysis of surfactant-free suspensions of cCNTs was developed and was used to show that the surface acidities of aqueous suspensions of multi-walled cCNTs (cMWCNTs) and centrifuged cMWCNT suspensions are not equivalent. Specifically, the surface acidities of aqueous cMWCNT suspensions and centrifuged cMWCNT suspensions were 7.46 ± 0.41 and 19.09 ± 0.52 mmols/g, respectively – a result of an increase in suspended oxidized carbon material and a decrease in agglomerated materials in the centrifuged cMWCNT suspensions. This significant difference in surface acidity between un-centrifuged and centrifuged cMWCNT suspensions has not been previously appreciated and is important because the degree of surface acidity is known to affect the biodegradation rates of cCNT samples,[56,57] as well as, the in vitro and in vivo toxicity profiles of cCNT samples.[58–61]
2. Experimental
2.1. Materials and solutions
Carboxylated MWCNT (cMWCNT) soot (product SC-M10; lot 1256YJF-070510) was purchased from Nanostructured & Amorphous Materials, Inc. (Houston, TX, USA) and its properties are described in Table 1. Caution, a fine-particulates respirator and other personal protective equipment (PPE) should be worn when handling cCNT soot.[62] Sodium hydroxide and standard buffer solutions of pH 4.00 ± 0.01, 7.00 ± 0.01, and 10.00 ± 0.02 were purchased from Fisher Scientific (Houston, TX, USA). HCl concentrate (0.01 mol), potassium hydrogen phthalate (KHP), and phenolphthalein were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Table 1.
Properties of SC-M10 cMWCNT soot reported by the manufacturer.
| Synthetic Method | CVD |
| Catalytic Metals | Fe, Co, Ni |
| % Carbon Purity | >95 |
| % Metals | <3.7 |
| % Chloride | <1.0 |
| Inner Diameter (nm) | 5 – 10 |
| Outer Diameter (nm) | 10 – 20 |
| Length (μm) | 0.5 – 2 |
| Oxidizing Agents | H2SO4 & KMnO4 |
| % COOH | 1.9 – 2.1 |
2.2. Preparation and standardization of sodium hydroxide
Approximately 200 mg of NaOH was weighed and transferred to a 500.0-mL volumetric flask, dissolved, brought to volume, and stored in a 500-mL polyethylene bottle. All ~0.01 M NaOH solutions were standardized against KHP using phenolphthalein as the indicator. In brief, ~50 mg of KHP was weighed, transferred to a 125-mL Erlenmeyer flask, and dissolved in 25.0 mL of 18.2 MΩ-cm deionized water with five drops of phenolphthalein. Using a 50.00-mL buret, NaOH solutions were titrated to endpoint with KHP. This process was repeated four times to determine the mean concentration and standard deviation (SD) of the standardized NaOH. NaOH solutions were used within seven days of being standardized.
2.3. Preparation of cMWCNT suspensions and centrifuged cMWCNT suspensions
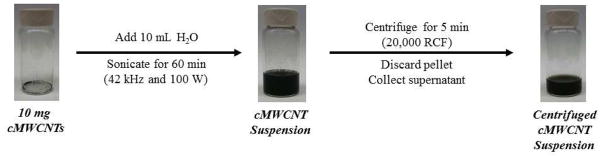
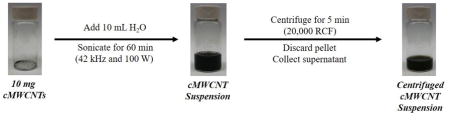
The preparation of cMWCNT suspensions and centrifuged cMWCNT suspensions started with the addition of 10.0 mL of 18.2 MΩ-cm deionized water to 10.0 mg of as-received cMWCNT soot that was weighed into a pre-cleaned, 20-mL scintillation vial (Figure 1). The mixture was sonicated for 60 min using a Branson model 2510 bath sonifier (100 W, 42 kHz) with the bath water being changed every 30 min to maintain the temperature <15 °C; bath sonication was chosen so as to avoid nanotube perturbations induced by intense probe sonication. The purification of aqueous cMWCNT suspensions involved a single low-speed centrifugation step. To accommodate the use of a benchtop centrifuge (Eppendorf, model 5424), 10.0-mL suspensions were divided by transferring 1-mL aliquots into ten 1.5-mL centrifuge tubes, which were each centrifuged for 5 min at 20,000 RCF. The top ~900 μL from each supernatant was collected carefully using a micropipette so as to not disturb the pellet and combined in a pre-cleaned scintillation vial to afford a ~9-mL sample of a centrifuged cMWCNT suspension.
Fig. 1.
Overview of the procedure to prepare cMWCNT suspensions and centrifuged cMWCNT suspensions.
2.4. Raman spectroscopy
Raman spectra were acquired using a Jobin Yvon Horiba HR800 high-resolution LabRam Raman microscope system equipped with a 250-μm entrance slit and an 1100-μm pinhole. The 633-nm laser excitation was provided by a Spectra-Physics model 127 helium-neon laser operating at 20 mW. A 50×/0.5 NA LM-Plan objective was used with a neutral density filter of 1.0. Spectral acquisition was performed with a 1.0-s integration time, a minimum overlap of 50, and a 3-subpixel average; each spectrum was presented as an average of 2 scans. Wavenumber calibration was performed using the 520.5 cm−1 line of a crystalline Si wafer. A 15-μL aliquot of either a cMWCNT suspension or centrifuged cMWCNT suspension was deposited on to a crystalline Si wafer and dried at room temperature; spectra were acquired from at least five different regions of dried material across the wafer.
2.5. UV-VIS-NIR spectrophotometry
All UV-VIS-NIR spectrophotometric analyses were performed using a Shimadzu UV-161PC spectrophotometer. All spectra were obtained as a single scan that was background corrected against a deionized water reference using a medium scan speed with 0.5-nm intervals and 1.0-cm quartz cuvettes. Spectra of cMWCNT suspensions and centrifuged cMWCNT suspensions were acquired after dilution with deionized water.
2.6. Dynamic light scattering (DLS)
The particle size distribution of cMWCNT suspensions and centrifuged cMWCNT suspensions diluted 1:10 with deionized water were analyzed by DLS using a 633-nm laser and a backscatter measurement angle of 173° (Zetasizer Nano-ZS 3600, Malvern Instruments, Worcestershire, UK). Ten consecutive 30-s runs were taken per measurement at 25 °C and the instrument was calibrated with polybead standards (Polysciences, Warrington, PA, USA). The particle size, in terms of hydrodynamic diameter, was calculated using a viscosity and refractive index of 0.8872 cP and 1.330, respectively for deionized water, and an absorption and refractive index of 0.010 and 1.891, respectively, for cMWCNTs.
2.7. Acidimetric Boehm titration of cMWCNT suspensions
Boehm titrations work on the principle that carbon surface oxygen groups have different acidities that can be neutralized by bases of different strengths; for example, weak bases such as sodium bicarbonate can be used to neutralize carboxylic acids and strong bases such as sodium hydroxide can be used to neutralize all Brønsted acids.[37–39] While the Boehm method has been applied to the analysis of oxidized carbon materials for over fifty years,[37–39,63–69] a standard protocol has rarely been followed for the analyses of cCNTs.[24,29,32,35,36,43,49,50,52,54,55,70–75] For example, there are different routines to determine endpoints, differences in base treatment times and agitation methods, differences in methods to address dissolved carbon dioxide, and differences in determining the mass of carbon material titrated. Herein, the first major parameter considered was the agitation method used to expose carbon materials to the chosen base – a process that can damage a carbon lattice and create new oxygen surface groups. The method of Oickle and co-workers was employed since they showed that shaking was a less destructive method of agitation relative to rigorous stirring and sonication.[72] Specifically, 4 mL of a 1.0-mg/mL cMWCNT suspension and 25.00 mL of standardized NaOH were combined in a 50-mL plastic vial and then shaken for 24 h to afford a NaOH-treated cMWCNT suspension. The second major factor to consider in a Boehm titration is how to minimize CO2 effects, which can distort neutralization points associated with the acidic groups present on a carbon material.[69,71,74] The method of Kim and co-workers was employed whereby re-acidification of the deprotonated sample was followed by a back-titration with NaOH.[69] Specifically, 10.00 mL of the NaOH-treated cMWCNT suspension and 20.00 mL of ~0.01 M HCl standard were combined in a 150-mL beaker that was covered using parafilm. Finally, to determine the surface acidity (i.e., the mols of acidic groups per mass of carbon material analyzed), a magnetic stir bar and a calibrated Accumet model 13–620–299A pH electrode were placed in the reaction mixture, which was titrated at room temperature (22 ± 2°C) by recording the pH after the delivery of each 0.10-mL aliquot of standardized NaOH from a 50.00-mL buret.
2.8. Acidimetric Boehm titration of centrifuged cMWCNT suspensions
The same titration protocol described above for cMWCNT suspensions was performed on centrifuged cMWCNT suspensions except for the method to determine the amount of carbon material titrated. This is because the mass of soot determined using an analytical balance during the preparation of a cMWCNT suspension can no longer be applied to a centrifuged cMWCNT suspension since a large portion of the original mass (i.e., the centrifugation pellet) is discarded. Instead, the amount of carbon material titrated in centrifuged cMWCNT suspensions was determined using UV-VIS-NIR spectroscopy and a calibration curve prepared from the π-plasmon absorbance of aqueous standards of non-centrifuged suspensions of cMWCNT soot containing known amounts of carbon material.[76,77] The accuracy of this method was assessed by a gravimetric analysis of dried centrifuged cMWCNT suspensions; cMWCNT concentrations determined using the spectrophotometric method were within 2% of the values determined using the gravimetric method.
3. Results and discussion
3.1. Elemental analysis of the as-received cMWCNT soot
Almost all CNT manufacturing processes use a carbon feedstock, metal catalysts, and heat to yield a heterogeneous CNT soot that contains metallic and carbonaceous impurities in addition to CNTs. The cMWCNT soot chosen for this work represents one of the most common commercial methods of CNT synthesis, a metal-catalyzed chemical vapor deposition (CVD) process, and a common combination of liquid oxidants, sulfuric acid and potassium permanganate, for carboxylation of pristine CNT surfaces. As shown in Table 1, this soot is reported to have a carbon content of >95% by weight, a metal content of <3.7%, and a chloride content of <1.0%. To assess the composition of the as-received material, sub-samples of the cMWCNT soot were subjected to our carbon nanomaterial elemental analysis protocol.[78] As shown in Table 2, the major findings were that the 94.37% carbon content was within 1% of the value reported by the manufacturer, the oxygen content was 4.12%, the hydrogen content was <1%, sulfur and nitrogen were not detected, and the sum of the C, H, and O weight percentages was 98.91%, indicating that the Fe/Co/Ni-catalyzed CVD cMWCNT soot was essentially halide- and metal-free.
Table 2.
Elemental percentages of as-received SC-M10 cMWCNT soot.
| cMWCNT soot (%)a | |
|---|---|
| C | 94.37 ± 0.32 |
| H | 0.43 ± 0.24 |
| N | 0.00 ± 0.00 |
| S | 0.00 ± 0.00 |
| O | 4.12 ± 0.93 |
CHNS/O analyses were performed as described previously,[78] with the exception that the cMWCNT soot was vacuum dried at 100 °C for 4 h prior to analysis. Each elemental percentage is from n = 2 independent analyses (± SD).
3.2. Spectroscopic analysis of cMWCNT suspensions and centrifuged cMWCNT suspensions
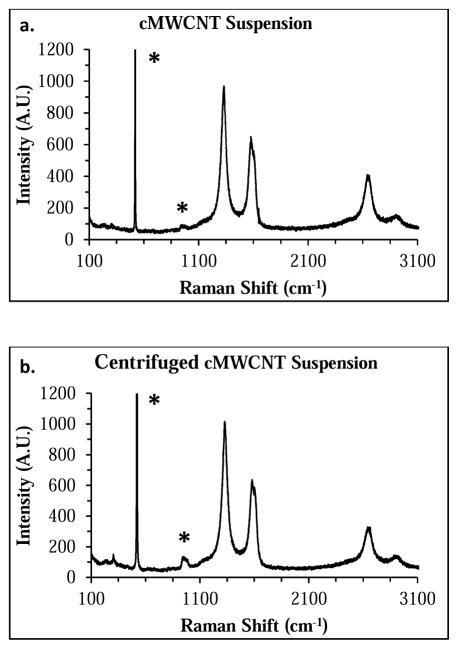
Aqueous cMWCNT suspensions and centrifuged cMWCNT suspensions (Figure 1) were characterized by Raman and UV-VIS-NIR spectroscopies. Representative 633-nm Raman spectra of a cMWCNT suspension and a centrifuged cMWCNT suspension are shown in Figure 2. Prominent Raman peaks characteristic of MWCNTs such as the disorder-induced mode (D-band) at ~1300 cm−1, the tangential stretching mode (G-band) at ~1590 cm−1, and the second-order G′-band at ~2600 cm−1 were observed in both spectra. There were no significant differences between the D/G-ratios (~1.4), the full width at half maximum (FWHM) peak widths of the D-bands (~60 cm−1), and the FWHM peak widths of the G-bands (~74 cm−1), indicating that the spectral properties of nanotubes in cMWCNT suspensions and centrifuged cMWCNT suspensions were similar.
Fig. 2.
Representative 633-nm Raman spectra of a cMWCNT suspension (a) and a centrifuged cMWCNT suspension (b). Characteristic carbon nanomaterial Raman bands (e.g., D-bands at ~1300 cm−1, G-bands at ~ 1600 cm−1, and G′-bands at ~2600 cm−1) were observed in both spectra. Peaks denoted by an asterick originate from the Si-wafer substrate.
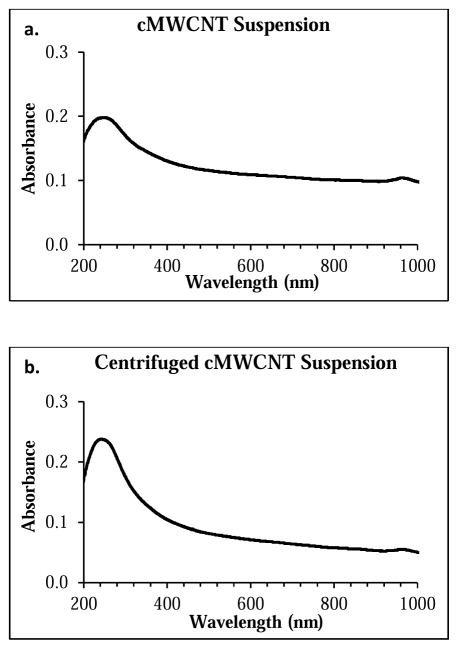
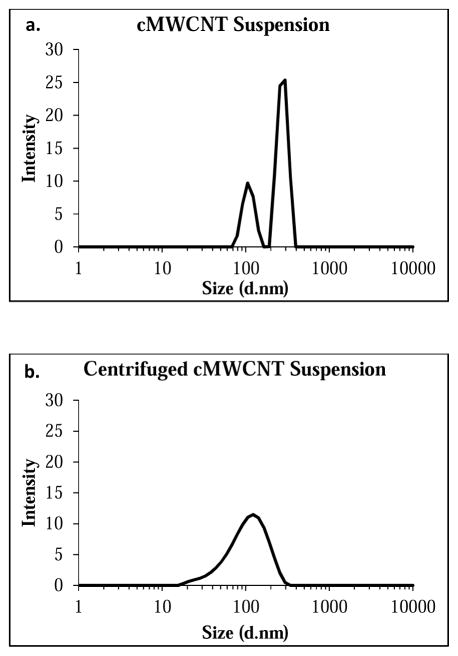
Representative UV-VIS-NIR spectra of a 20-μg/mL cMWCNT suspension and a 20-μg/mL centrifuged cMWCNT suspension are shown in Figure 3. Two main absorption features are observed in each spectrum. The first is the prominent π-plasmon absorption peak at ~240 nm resulting from the collective excitation of the π-electron systems from all sp2-hydridized carbonaceous material in the soot.[79] The absorbance of this UV peak can be used to qualitatively evaluate the degree of CNT exfoliation in aqueous CNT samples,[80,81] where an increase in the amount of individually-dispersed MWCNTs results in an increase in the peak area.[81] The second main spectral feature is a non-resonant background comprising contributions from both cMWCNTs and other materials.[82,83] Comparison of the spectra in Figure 3 reveals (i) that the area of the π-plasmon absorption peak for the cMWCNT suspension was ~54% less than that of the centrifuged cMWCNT suspension, indicative of increased agglomeration of cMWCNTs and carbonaceous material in a non-centrifuged suspension, and (ii) that the background absorbance of the cMWCNT suspension at 800 nm was ~40% greater than that of the centrifuged cMWCNT suspension, similar to the increased background absorbances observed for non-centrifuged CNT suspensions relative to centrifuged CNT suspensions.[84,85] These spectral observations were also corroborated by DLS measurements which revealed a decrease in particle size following centrifugation. As shown in Figure 4, cMWCNT suspensions displayed two distinct populations of particles, one with a hydrodynamic diameter of ~277 nm and the other with a hydrodynamic diameter of ~109 nm, while centrifuged cMWCNT suspensions displayed only one population of particles with a hydrodynamic diameter of ~115 nm, indicating that larger agglomerates were removed by low-speed centrifugation.[33]
Fig. 3.
Representative background-corrected UV-VIS-NIR absorption spectra of a 20-μg/mL cMWCNT suspension (a) and a 20-μg/mL centrifuged cMWCNT suspension (b).
Fig. 4.
Representative DLS particle size distribution of a diluted cMWCNT suspension (a) and a diluted centrifuged cMWCNT suspension (b).
The oxidation of CNTs can generate oxidative debris in the form of particulate matter such as oxidized carbon fragments (OCFs),[25] and/or molecular debris such as polyaromatic hydrocarbons (PAHs).[55] If present, both types of debris remain stably bound to cCNT surfaces at acidic and neutral pH but can be desorbed by strong base. To determine if oxidative debris was present in cMWCNT suspensions purified by centrifugation, centrifuged cMWCNT suspensions that were treated with NaOH were passed through a 0.1-μm polyvinylidene fluoride (PVDF) membrane filter followed by analysis of the filtrate using Raman and UV-VIS-NIR spectroscopies. Raman D-, G-, and G′-bands were not detected (data not shown), indicating that short cMWCNTs and OCF debris were not present in the filtrate. With the UV-VIS-NIR spectra of filtrates, no absorbance at 240 nm was observed and the background absorbance between 375 – 1100 nm was negligible (<0.01 a.u., data not shown), indicating that short cMWCNTs and OCF debris were not present in the filtrate. However, a single small peak at 289 nm with an absorbance of 0.13 a.u. was observed in suspensions of centrifuged cMWCNTs indicating a trace amount of a PAH.[55]
3.3. Titration of cMWCNT suspensions
As described in the Experimental section, cMWCNT suspensions were first exposed to a known amount of NaOH to deprotonate potential acidic groups, then a known amount of HCl was added to re-acidify the suspensions, followed by back titration with NaOH to quantify acidic surface groups. A representative titration curve and first derivative plot for a cMWCNT suspension are shown in Figure 5a. As NaOH is added, the excess HCl is neutralized to an equivalence point of pH 7. Since the amount of excess HCl is directly proportional to the amount of excess NaOH that was present in the NaOH-treated cMWCNT suspension, the determined amount of excess NaOH can be subtracted from the amount of initial NaOH added to quantitate the amount of acidic groups that were present on the cMWCNTs. Specifically, the mols of acidic groups for cMWCNT suspensions were calculated using the following equation,
Fig. 5.
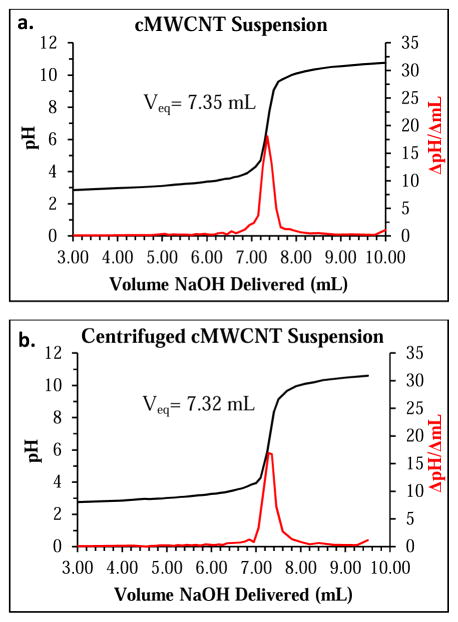
Representative titration (black) and first derivative (red) curves of a NaOH-treated cMWCNT aqueous suspension (a), and a NaOH-treated, centrifuged cMWCNT suspension (b). The equivalence point volumes (Veq) for each sample (7.39 ± 0.05 and 7.33 ± 0.02, respectively) were calculated using a second derivative (not shown).
| (1) |
where nAG denotes the mols of acidic groups for the cMWCNT suspension, [B] and VB are the concentration and volume of NaOH used to treat the cMWCNT suspension, [HCl] and VHCl are the concentration and volume of HCl added to re-acidify the reaction mixture, [NaOH] and VNaOH are the concentration and volume of titrant needed to reach the equivalence point, and Va is the volume of NaOH-treated cMWCNT suspension titrated.[71] As shown in Table 3, the mean mols of acidic groups calculated for cMWCNT suspensions was 2.96 × 10−5.
Table 3.
Acidimetric titration results for cMWCNT suspensions and centrifuged cMWCNT suspensions.
| cMWCNT Suspensions | Centrifuged cMWCNT Suspensions | |
|---|---|---|
| Acidic groups (mol) a | 2.96 × 10−5 | 2.79 × 10−5 |
| Mass of carbon material analyzed (g) b | 3.96 × 10−3 | 1.46 × 10−3 |
| Acidic groups (mmol/g) c | 7.46 ± 0.41 | 19.09 ± 0.52 |
Determined by acidimetric titrations (Figure 5) and Equation 1.
For cMWCNT suspensions, masses were determined using an analytical balance and Equation 2; for centrifuged cMWCNT suspensions, masses were determined using a UV-VIS calibration curve (Figure 6) and Equation 4.
Determined from three independent measurements (± SD) using Equation 3.
The mass of material titrated in cMWCNT suspensions was determined using the following equation,
| (2) |
where m is the weighted mass of material analyzed in grams, Vt is the volume of cMWCNT suspension treated with NaOH, and D is the initial concentration of carbon material in the suspension. As shown in Table 3, the mass of material titrated in cMWCNT suspensions was 3.96 × 10−3 g. Subsequently, the mmols of acidic groups per gram of carbon material in cMWCNT suspensions was calculated using the following equation,
| (3) |
where nAG is the mmols of acidic groups calculated using Equation 1, and m is the mass of carbon material analyzed calculated using Equation 2. As shown in Table 3, the mean surface acidity of cMWCNT suspensions, defined as the mmols of acidic groups per gram of material analyzed, was 7.46 ± 0.41 mmol/g.
3.4. Titration of centrifuged cMWCNT suspensions
To our knowledge, Boehm titrimetric methods have been performed exclusively on aqueous suspensions of as-synthesized and purified cCNT soot, and not on centrifuged cCNT suspensions. One reason for this could be the additional experimentation required to determine the mass of material titrated. In brief, when a soot product is analyzed as-received, or when a soot purification step is performed before the preparation of a cCNT suspension (e.g., a Soxhlet extraction or low-temperature heat treatment[29,31]), the mass of soot can be determined easily using an analytical balance. However, when a purification step follows the preparation of a soot suspension (as is the case with centrifuged cMWCNT suspensions), the mass of carbon material is no longer known since large portions of material can be pelleted and discarded following consecutive centrifugation steps.[86] Therefore, the same titration protocol described above for cMWCNT suspensions was performed on centrifuged cMWCNT suspensions with the exception that the amount of carbon material titrated in centrifuged cMWCNT suspensions was determined from a calibration curve prepared from the π-plasmon absorbance of aqueous standards of non-centrifuged suspensions of cMWCNT soot (Figure 6).
Fig. 6.
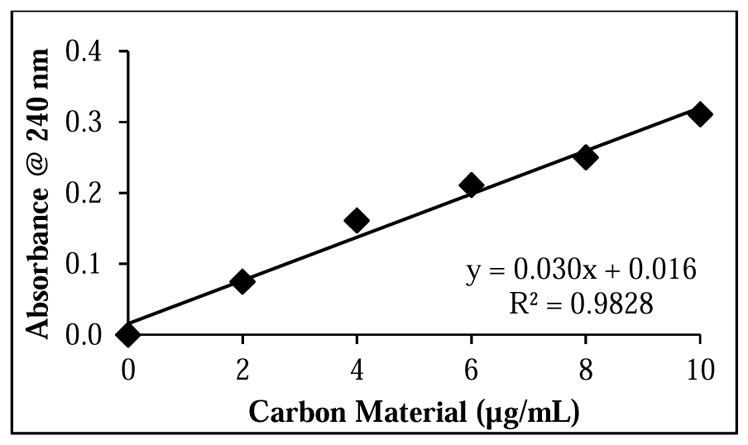
Calibration curve of carbon material in standard cMWCNT suspensions used to determine the concentration of carbon material in centrifuged cMWCNT suspensions.
A representative titration curve and first derivative plot for a centrifuged cMWCNT suspension are shown in Figure 5b, and as shown in Table 3, the mean mols of acidic groups determined for centrifuged cMWCNT suspensions using Equation 1 was 2.79 × 10−5. Next, the mass of material analyzed was determined using the following equation,
| (4) |
where y is the π-plasmon absorbance of centrifuged cMWCNT suspensions from the standard curve, and x is the concentration of carbon material in a centrifuged cMWCNT suspension in μg/mL. Next, the resulting concentration was used in Equation 2 to calculate the mass of carbon material analyzed in centrifuged cMWCNT suspensions (e.g., 1.46 × 10−3 g). Finally, the mean surface acidity of centrifuged cMWCNT suspensions was calculated using Equation 3 to be 19.09 ± 0.52 mmol/g. We know of only one other work to quantify the acidic surface groups of carbon material in centrifuged cCNT suspensions;[49] in brief, using fluorescence spectroscopy and 1-aminopyrene dye-treated samples, it was determined that MWCNTs oxidized for 24 h with nitric and sulfuric acids were 15 – 22% oxidized, which is in accordance with the 23% level of oxidation calculated for our centrifuged cMWCNT suspensions.
3.5. Enriched surface acidity of centrifuged cMWCNT suspensions
The ~40% enrichment of surface acidity for centrifuged cMWCNT suspensions relative to non-centrifuged cMWCNT suspensions can be explained as follows. Starting with a single lot of high-purity cMWCNT soot which was bath sonicated in deionized water without surfactant, a dark suspension was afforded that was observed to be free of precipitants, and that displayed carbon material concentrations on the order of 990 μg/mL. Samples of the cMWCNT suspensions were centrifuged at 20,000 RCF for 5 min to afford a small dark pellet of agglomerated material and a homogeneous dark-gray supernatant (i.e., a centrifuged cMWCNT suspension) that displayed carbon material concentrations on the order of 365 μg/mL. Raman spectroscopic analysis indicated that both suspensions and centrifuged suspensions possessed cMWCNTs of similar quality. UV-VIS-NIR spectroscopic analysis indicated an increase in suspended oxidized carbon material and a decrease in agglomerated material in centrifuged cMWCNT suspensions, evidence that centrifuged cMWCNT suspensions were enriched with oxidized carbon material. Additional evidence that cMWCNT suspensions were purified by centrifugation was provided by DLS measurements which showed that a population of larger particles were removed by centrifugation. Aqueous cMWCNT suspensions and centrifuged cMWCNT suspensions were analyzed by a Boehm titrimetric method, and interestingly, both samples displayed similar equivalence point volumes (Figure 5) and mols of surface acidic groups (Table 3). The resulting ~40% difference in the measured surface acidity (7.46 ± 0.41 vs. 19.09 ± 0.52 mmols/g) is therefore consistent with the ~37% difference in the masses of materials titrated and the hypothesis that centrifuging an aqueous cMWCNT suspension without a surfactant would remove hydrophobic, non-oxidized soot components.
4. Conclusion
The literature contains many different protocols for preparing samples of cCNTs for biomedical applications. One reason for a lack of standardized sample-preparation protocols is due to the variety of cCNT investigations. For example, if the research is concerned with a toxicity assessment of as-received cCNT soot, then the cCNT suspensions are not purified by design. Conversely, if the research requires a suspension of individually-dispersed cCNTs, an additional purification step such as centrifugation and/or filtration can be undertaken. A second reason for differing protocols is the variety in the quality of commercial cCNT soot products with additional purification steps being required for soot products containing high levels of metallic and/or carbonaceous impurities. Herein, using Boehm titrimetry and cMWCNTs suspended in water without a surfactant, the surface acidity of oxidized carbon material in aqueous cMWCNT suspensions was shown to be enriched by ~40% by a single low-speed centrifugation step. In conclusion, an important consideration for biomedical applications of cCNTs is a thorough physiochemical characterization of the exact cCNT sample that is presented to living cells or organisms. Therefore, determining if a cCNT sample-preparation protocol enriches surface acidity is critical with respect to making accurate assessments concerning the interactions of cCNTs with biological systems.
HIGHLIGHTS.
Boehm titrimetric method for oxidized CNT suspensions purified by centrifugation
Surface acidity affects CNT biodegradation rates and CNT toxicity profiles
Centrifugation of surfactant-free samples of oxidized CNTs enriches surface acidity
Acknowledgments
The authors thank the Semiconductor Research Corporation Engineering Research Center for Environmentally Benign Semiconductor Manufacturing (Grant 425-048) and the National Institute for Environmental Health Sciences (Grant R15-ES023666) for support of this work, and Steven O. Nielsen and Mihaela C. Stefan for helpful scientific discussions. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the Semiconductor Research Corporation or the National Institutes of Health.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009;2(2):85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4(10):627–633. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 3.Liang F, Chen B. A review on biomedical applications of single-walled carbon nanotubes. Curr Med Chem. 2010;17(1):10–24. doi: 10.2174/092986710789957742. [DOI] [PubMed] [Google Scholar]
- 4.Ji S-R, Liu C, Zhang B, Yang F, Xu J, Long J, et al. Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta - Reviews on Cancer. 2010;1806(1):29–35. doi: 10.1016/j.bbcan.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Vashist SK, Zheng D, Pastorin G, Al-Rubeaan K, Luong JHT, Sheu F-S. Delivery of drugs and biomolecules using carbon nanotubes. Carbon. 2011;49(13):4077–4097. [Google Scholar]
- 6.Gong H, Peng R, Liu Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv Drug Deliv Rev. 2013;65(15):1951–1963. doi: 10.1016/j.addr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Tabakman SM, Chen Z, Dai H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat Protoc. 2009;4(9):1372–1381. doi: 10.1038/nprot.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yehia HN, Draper RK, Mikoryak C, Walker EK, Bajaj P, Musselman IH, et al. Single-walled carbon nanotube interactions with HeLa cells. J Nanobiotechnol. 2007;5(8):17. doi: 10.1186/1477-3155-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297(5581):593–596. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 10.Jos A, Pichardo S, Puerto M, Sanchez E, Grilo A, Camean AM. Cytotoxicity of carboxylic acid functionalized single wall carbon nanotubes on the human intestinal cell line Caco-2. Toxicol in Vitro. 2009;23(8):1491–1496. doi: 10.1016/j.tiv.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Yan B. Cell cycle regulation by carboxylated multiwalled carbon nanotubes through p53-independent induction of p21 under the control of the BMP signaling pathway. Chem Res Toxicol. 2012;25(6):1212–1221. doi: 10.1021/tx300059m. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Dong X, Song L, Zhang H, Liu L, Zhu D, et al. Carboxylation of multiwalled carbon nanotube enhanced its biocompatibility with L02 cells through decreased activation of mitochondrial apoptotic pathway. J Biomed Mater Res A. 2014;102(3):665–673. doi: 10.1002/jbm.a.34729. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Hu S, Smith EF, Ruenraroengsak P, Thorley AJ, Menzel R, et al. Aqueous cationic, anionic and non-ionic multi-walled carbon nanotubes, functionalised with minimal framework damage, for biomedical application. Biomaterials. 2014;35(17):4729–4738. doi: 10.1016/j.biomaterials.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Liu Y, Peng D. Carboxylation of multiwalled carbon nanotube attenuated the cytotoxicity by limiting the oxidative stress initiated cell membrane integrity damage, cell cycle arrestment, and death receptor mediated apoptotic pathway. J Biomed Mater Res A. 2015;103(8):2770–2777. doi: 10.1002/jbm.a.35416. [DOI] [PubMed] [Google Scholar]
- 15.Cheng X, Zhong J, Meng J, Yang M, Jia F, Xu Z, et al. Characterization of multiwalled carbon nanotubes dispersing in water and association with biological effects. J Nanomater. 2011;2011(938491):12. [Google Scholar]
- 16.Vlasova II, Vakhrusheva TV, Sokolov AV, Kostevich VA, Gusev AA, Gusev SA, et al. PEGylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol Appl Pharm. 2012;264(1):131–142. doi: 10.1016/j.taap.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Vakhrusheva TV, Gusev AA, Gusev SA, Vlasova II. Albumin reduces thrombogenic potential of single-walled carbon nanotubes. Toxicol Lett. 2013;221(2):137–145. doi: 10.1016/j.toxlet.2013.05.642. [DOI] [PubMed] [Google Scholar]
- 18.Orecna M, De Paoli SH, Janouskova O, Tegegn TZ, Filipova M, Bonevich JE, et al. Toxicity of carboxylated carbon nanotubes in endothelial cells is attenuated by stimulation of the autophagic flux with the release of nanomaterial in autophagic vesicles. Nanomed Nanotechnol Biol Med. 2014;10(5):939–948. doi: 10.1016/j.nano.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Belin T, Epron F. Characterization methods of carbon nanotubes: A review. Mater Sci Eng B. 2005;119(2):105–118. [Google Scholar]
- 20.Itkis ME, Perea DE, Jung R, Niyogi S, Haddon RC. Comparison of analytical techniques for purity evaluation of single-walled carbon nanotubes. J Am Chem Soc. 2005;127(10):3439–3448. doi: 10.1021/ja043061w. [DOI] [PubMed] [Google Scholar]
- 21.Freiman S, Hooker S, Migler K, Arepalli S. NIST recommended practice guide, special publication 960-19: Measurement issues in single wall carbon nanotubes. NIST; Gaithersburg, MD: 2008. p. 78. [Google Scholar]
- 22.Richman EK, Hutchison JE. The nanomaterial characterization bottleneck. ACS Nano. 2009;3(9):2441–2446. doi: 10.1021/nn901112p. [DOI] [PubMed] [Google Scholar]
- 23.Boverhof DR, David RM. Nanomaterial characterization: Considerations and needs for hazard assessment and safety evaluation. Anal Bioanal Chem. 2010;396(3):953–961. doi: 10.1007/s00216-009-3103-3. [DOI] [PubMed] [Google Scholar]
- 24.Wepasnick KA, Smith BA, Bitter JL, Fairbrother DH. Chemical and structural characterization of carbon nanotube surfaces. Anal Bioanal Chem. 2010;396:1003–1014. doi: 10.1007/s00216-009-3332-5. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Mikoryak C, Li S, Bushdiecker D, Musselman IH, Pantano P, et al. Cytotoxicity screening of single-walled carbon nanotubes: Detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol Pharmaceutics. 2011;8(4):1351–1361. doi: 10.1021/mp2001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pumera M, Ambrosi A, Chng ELK. Impurities in graphenes and carbon nanotubes and their influence on the redox properties. Chem Sci. 2012;3(12):3347–3355. [Google Scholar]
- 27.Salamon AW. The current world of nanomaterial characterization: Discussion of analytical instruments for nanomaterial characterization. Environ Eng Sci. 2013;30(3):101–108. [Google Scholar]
- 28.Wang R, Hughes T, Beck S, Vakil S, Li S, Pantano P, et al. Generation of toxic degradation products by sonication of Pluronic® dispersants: Implications for nanotoxicity testing. Nanotoxicology. 2012;7(7):1272–1281. doi: 10.3109/17435390.2012.736547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdejo R, Lamoriniere S, Cottam B, Bismarck A, Shaffer M. Removal of oxidation debris from multi-walled carbon nanotubes. Chem Commun. 2007;(5):513–515. doi: 10.1039/b611930a. [DOI] [PubMed] [Google Scholar]
- 30.Salzmann CG, Llewellyn SA, Tobias G, Ward MAH, Huh Y, Green MLH. The role of carboxylated carbonaceous fragments in the functionalization and spectroscopy of a single-walled carbon-nanotube material. Adv Mater. 2007;19(6):883–887. [Google Scholar]
- 31.Fogden S, Verdejo R, Cottam B, Shaffer M. Purification of single walled carbon nanotubes: The problem with oxidation debris. Chem Phys Lett. 2008;460(1–3):162–167. [Google Scholar]
- 32.Harun MH, Whitby R, Dahlan KZ, Nik Salleh NG, Alias MS, Mohamed M, et al. Removal of oxidative fragments from chemically functionalized multi-walled carbon nanotubes. Seminar R&D Nuclear Malaysia. 2010;43(26):6. [Google Scholar]
- 33.Heister E, Lamprecht C, Neves V, Tilmaciu C, Datas L, Flahaut E, et al. Higher dispersion efficacy of functionalized carbon nanotubes in chemical and biological environments. ACS Nano. 2010;4(5):2615–2626. doi: 10.1021/nn100069k. [DOI] [PubMed] [Google Scholar]
- 34.Stefani D, Paula AJ, Vaz BG, Silva RA, Andrade NDF, Justo GZ, et al. Structural and proactive safety aspects of oxidation debris from multiwalled carbon nanotubes. J Hazard Mater. 2011;189(1–2):391–396. doi: 10.1016/j.jhazmat.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Worsley KA, Kondrat RW, Pal SK, Kalinina I, Haddon RC. Isolation and identification of low molecular weight carboxylated carbons derived from the nitric acid treatment of single-walled carbon nanotubes. Carbon. 2011;49(15):4982–4986. [Google Scholar]
- 36.Wu Z, Hamilton RF, Jr, Wang Z, Holian A, Mitra S. Oxidation debris in microwave functionalized carbon nanotubes: Chemical and biological effects. Carbon. 2014;68:678–686. [Google Scholar]
- 37.Boehm H-P, Diehl E, Heck W, Sappok R. Surface oxides of carbon. Angew Chem, Int Ed. 1964;3(10):669–677. [Google Scholar]
- 38.Boehm HP. Chemical identification of surface groups. In: Eley DD, Pines H, Weisz PB, editors. Advances in Catalysis. Vol. 16. Academic Press; 1966. pp. 179–274. [Google Scholar]
- 39.Boehm HP. Surface oxides on carbon and their analysis: A critical assessment. Carbon. 2002;40(2):145–149. [Google Scholar]
- 40.Shieh Y-T, Liu G-L, Wu H-H, Lee C-C. Effects of polarity and pH on the solubility of acid-treated carbon nanotubes in different media. Carbon. 2007;45(9):1880–1890. [Google Scholar]
- 41.Zhang M, Yudasaka M, Ajima K, Miyawaki J, Iijima S. Light-assisted oxidation of single-wall carbon nanohorns for abundant creation of oxygenated groups that enable chemical modifications with proteins to enhance biocompatibility. ACS Nano. 2007;1(4):265–272. doi: 10.1021/nn700130f. [DOI] [PubMed] [Google Scholar]
- 42.Samori C, Sainz R, Menard-Moyon C, Toma FM, Venturelli E, Singh P, et al. Potentiometric titration as a straightforward method to assess the number of functional groups on shortened carbon nanotubes. Carbon. 2010;48(9):2447–2454. [Google Scholar]
- 43.Gong H, Kim S-T, Lee JD, Yim S. Simple quantification of surface carboxylic acids on chemically oxidized multi-walled carbon nanotubes. Appl Surf Sci. 2013;66:219–224. [Google Scholar]
- 44.Zhao C, Ji L, Liu H, Hu G, Zhang S, Yang M, et al. Functionalized carbon nanotubes containing isocyanate groups. J Solid State Chem. 2004;177(12):4394–4398. [Google Scholar]
- 45.Stobinski L, Lesiak B, Koverr L, Toth J, Biniak S, Trykowski G, et al. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J Alloys Compd. 2010;501(1):77–84. [Google Scholar]
- 46.Wepasnick KA, Smith BA, Schrote KE, Wilson HK, Diegelmann SR, Fairbrother DH. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon. 2011;49(1):24–36. [Google Scholar]
- 47.Dementev N, Feng X, Borguet E. Fluorescence labeling and quantification of oxygen-containing functionalities on the surface of single-walled carbon nanotubes. Langmuir. 2009;25(15):7573–7577. doi: 10.1021/la803947q. [DOI] [PubMed] [Google Scholar]
- 48.Dementev N, Ronca R, Borguet E. Oxygen-containing functionalities on the surface of multi-walled carbon nanotubes quantitatively determined by fluorescent labeling. App Surf Sci. 2012;258:10185–10190. [Google Scholar]
- 49.Nishikiori H, Tanigaki T, Endo M, Fujii T. Quantitative characterization of acidic groups on acid-treated multi-walled carbon nanotubes using 1-aminopyrene as a fluorescent probe. Carbon. 2014;66:560–566. [Google Scholar]
- 50.Li J, Chen C, Zhang S, Wang X. Surface functional groups and defects on carbon nanotubes affect adsorption-desorption hysteresis of metal cations and oxoanions in water. Environ Sci: Nano. 2014;1(5):488–495. [Google Scholar]
- 51.Gilbertson LM, Goodwin DG, Taylor AD, Pfefferle L, Zimmerman JB. Toward tailored functional design of multi-walled carbon nanotubes (MWNTs): Electrochemical and antimicrobial activity enhancement via oxidation and selective reduction. Environ Sci Technol. 2014;48(10):5938–5945. doi: 10.1021/es500468y. [DOI] [PubMed] [Google Scholar]
- 52.Hu H, Bhowmik P, Zhao B, Hamon MA, Itkis ME, Haddon RC. Determination of the acidic sites of purified single-walled carbon nanotubes by acid-base titration. Chem Phys Lett. 2001;345(1–2):25–28. [Google Scholar]
- 53.Bustero I, Ainara G, Isabel O, Roberto M, Ines R, Amaya A. Control of the properties of carbon nanotubes synthesized by CVD for application in electrochemical biosensors. Microchim Acta. 2006;152(3/4):239–247. [Google Scholar]
- 54.Gonzalez-Guerrero AB, Mendoza E, Pellicer E, Alsina F, Fernandez-Sanchez C, Lechuga LM. Discriminating the carboxylic groups from the total acidic sites in oxidized multi-wall carbon nanotubes by means of acid-base titration. Chem Phys Lett. 2008;462(4–6):256–259. [Google Scholar]
- 55.Wang Z, Shirley MD, Meikle ST, Whitby RLD, Mikhalovsky SV. The surface acidity of acid oxidised multi-walled carbon nanotubes and the influence of in-situ generated fulvic acids on their stability in aqueous dispersions. Carbon. 2009;47(1):73–79. [Google Scholar]
- 56.Zhao Y, Allen BL, Star A. Enzymatic degradation of multiwalled carbon nanotubes. J Phys Chem A. 2011;115(34):9536–9544. doi: 10.1021/jp112324d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russier J, Menard-Moyon C, Venturelli E, Gravel E, Marcolongo G, Meneghetti M, et al. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale. 2011;3(3):893–896. doi: 10.1039/c0nr00779j. [DOI] [PubMed] [Google Scholar]
- 58.Bushdiecker DK., II . Masters thesis. The University of Texas; Dallas, Richardson, Texas, USA: 2013. Analysis and cytotoxicity of nitric acid oxidized single-walled carbon nanotubes. [Google Scholar]
- 59.Jain S, Thakare VS, Das M, Godugu C, Jain AK, Mathur R, et al. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem Res Toxicol. 2011;24(11):2028–2039. doi: 10.1021/tx2003728. [DOI] [PubMed] [Google Scholar]
- 60.Singh RP, Das M, Thakare V, Jain S. Functionalization density dependent toxicity of oxidized multiwalled carbon nanotubes in a murine macrophage cell line. Chem Res Toxicol. 2012;25(10):2127–2137. doi: 10.1021/tx300228d. [DOI] [PubMed] [Google Scholar]
- 61.Gilbertson LM, Melnikov F, Wehmas LC, Anastas PT, Tanguay RL, Zimmerman JB. Toward safer multi-walled carbon nanotube design: Establishing a statistical model that relates surface charge and embryonic zebrafish mortality. Nanotoxicology. 2015:10. doi: 10.3109/17435390.2014.996193. early online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Working safely with nanomaterials. [accessed March 2013];OSHA Fact Sheet. https://www.osha.gov/Publications/OSHA_FS-3634.pdf.
- 63.Bandosz TJ, Jagiello J, Contescu C, Schwarz JA. Characterization of the surfaces of activated carbons in terms of their acidity constant distributions. Carbon. 1993;31(7):1193–1202. [Google Scholar]
- 64.Contescu A, Contescu C, Putyera K, Schwarz JA. Surface acidity of carbons characterized by their continuous pK distribution and Boehm titration. Carbon. 1997;35(1):83–94. [Google Scholar]
- 65.Contescu A, Vass M, Contescu C, Putyera K, Schwarz JA. Acid buffering capacity of basic carbons revealed by their continuous pK distribution. Carbon. 1998;36(3):247–258. [Google Scholar]
- 66.Lopez-Ramon MV, Stoeckli F, Moreno-Castilla C, Carrasco-Marin F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon. 1999;37(8):1215–1221. [Google Scholar]
- 67.Strelko V, Jr, Malik DJ, Streat M. Characterisation of the surface of oxidised carbon adsorbents. Carbon. 2002;40(1):95–104. [Google Scholar]
- 68.Chen JP, Wu S. Acid/base-treated activated carbons: A characterization of functional groups and metal adsorptive properties. Langmuir. 2004;20(6):2233–2242. doi: 10.1021/la0348463. [DOI] [PubMed] [Google Scholar]
- 69.Kim YS, Yang SJ, Lim HJ, Kim T, Park CR. A simple method for determining the neutralization point in Boehm titration regardless of the CO2 effect. Carbon. 2012;50(9):3315–3323. [Google Scholar]
- 70.Gorgulho HF, Mesquita JP, Gonçalves F, Pereira MFR, Figueiredo JL. Characterization of the surface chemistry of carbon materials by potentiometric titrations and temperature-programmed desorption. Carbon. 2008;46(12):1544–1555. [Google Scholar]
- 71.Goertzen SL, Theriault KD, Oickle AM, Tarasuk AC, Andreas HA. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon. 2010;48(4):1252–1261. [Google Scholar]
- 72.Oickle AM, Goertzen SL, Hopper KR, Abdalla YO, Andreas HA. Standardization of the Boehm titration: Part II. Method of agitation, effect of filtering and dilute titrant. Carbon. 2010;48(12):3313–3322. [Google Scholar]
- 73.Hanelt S, Orts-Gil G, Friedrich JRF, Meyer-Plath A. Differentiation and quantification of surface acidities on MWCNTs by indirect potentiometric titration. Carbon. 2011;49(9):2978–2988. [Google Scholar]
- 74.Kim YS, Yang SJ, Lim HJ, Kim T, Lee K, Park CR. Effects of carbon dioxide and acidic carbon compounds on the analysis of Boehm titration curves. Carbon. 2012;50(4):1510–1516. [Google Scholar]
- 75.Doroodmand MM, Shafie Z. Solid-based titrimetry as a straightforward method for simultaneous detection of hydroxyl and carboxylic functional groups during evaluation of the acidity of nanocarbons. Sens Actuators A. 2014;207:32–38. [Google Scholar]
- 76.Jiang L, Gao L, Sun J. Production of aqueous colloidal dispersions of carbon nanotubes. J Colloid Interface Sci. 2003;260(1):89–94. doi: 10.1016/s0021-9797(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 77.Attal S, Thiruvengadathan R, Regev O. Determination of the concentration of single-walled carbon nanotubes in aqueous dispersions using UV-Visible absorption spectroscopy. Anal Chem. 2006;78(23):8098–8104. doi: 10.1021/ac060990s. [DOI] [PubMed] [Google Scholar]
- 78.Braun EI, Pantano P. The importance of an extensive elemental analysis of single-walled carbon nanotube soot. Carbon. 2014;77:912–919. doi: 10.1016/j.carbon.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pichler T, Knupfer M, Golden MS, Fink J, Rinzler A, Smalley RE. Localized and delocalized electronic states in single-wall carbon nanotubes. Phys Rev Lett. 1998;80(21):4729–4732. [Google Scholar]
- 80.Rausch J, Zhuang R-C, Mader E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Composites Part A. 2010;41(9):1038–1046. [Google Scholar]
- 81.Yu J, Grossiord N, Koning CE, Loos J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon. 2007;45(3):618–623. [Google Scholar]
- 82.Landi BJ, Ruf HJ, Evans CM, Cress CD, Raffaelle RP. Purity assessment of single-wall carbon nanotubes, using optical absorption spectroscopy. J Phys Chem B. 2005;109(20):9952–9965. doi: 10.1021/jp044990c. [DOI] [PubMed] [Google Scholar]
- 83.Jacobsen NS, Pantano P. Determining the percentages of semi-conducting and metallic single-walled carbon nanotubes in bulk soot. Carbon. 2011;49(6):1998–2006. [Google Scholar]
- 84.Tan Y, Resasco DE. Dispersion of single-walled carbon nanotubes of narrow diameter distribution. J Phys Chem B. 2005;109(30):14454–14460. doi: 10.1021/jp052217r. [DOI] [PubMed] [Google Scholar]
- 85.Naumov AV, Ghosh S, Tsyboulski DA, Bachilo SM, Weisman RB. Analyzing absorption backgrounds in single-walled carbon nanotube spectra. ACS Nano. 2011;5(3):1639–1648. doi: 10.1021/nn1035922. [DOI] [PubMed] [Google Scholar]
- 86.Lim HJ, Lee K, Cho YS, Kim YS, Kim T, Park CR. Experimental consideration of the Hansen solubility parameters of as-produced multi-walled carbon nanotubes by inverse gas chromatography. Phys Chem Chem Phys. 2014;16(33):17466–17472. doi: 10.1039/c4cp02319f. [DOI] [PubMed] [Google Scholar]