Abstract
Objective
Neutrophil lymphocyte ratio (NLR) has emerged as a valuable and reliable method for follow-up of systemic inflammatory disease. We herein aimed to evaluate the role of NLR in the clinical follow-up of inflammation and also to compare its relationship with other measures, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).
Material and Methods
A total of 35 active ankylosing spondylitis (AS) and 38 healthy volunteers were included in the study. The patient group was enrolled for treatment with one anti-tumor necrosis factor (TNF) drug. Total blood count, ESR, CRP, and BASDAI score were obtained before and 3 months following the treatment. NLR was found with a mathematical calculation of the ratio of neutrophils with lymphocytes.
Results
The mean NLR value of the control group and patients was 1.90±0.89 and 2.67±1.17, respectively (p<0.05). After a 3-month course of treatment, the patient group had a mean NLR value of 1.8±0.7, which was significantly lower than pretreatment values (p<0.001). The post-treatment mean ESR, CRP, and BASDAI scores were significantly lower than mean baseline scores (p<0.001, p=0.007, p<0.001, respectively). Also, NLR was found to be correlated with BASDAI, ESR, and CRP (r=0.388, p<0.001; r=0.455, p<0.0001; and r=0.3389, p<0.005, respectively).
Conclusion
Neutrophil lymphocyte ratio could be a reliable and easily accessible method for follow-up of patients with AS.
Keywords: Ankylosing spondylitis, neutrophil lymphocyte ratio, disease activity
Introduction
Ankylosing spondylitis (AS) is a chronic, systemic, and progressive disease, characterized by axial skeleton involvement and inflammation of tendons and ligaments where they anchor the bones. AS is a prototype of a group of diseases called spondyloarthropathies (SpAs). It is common in men, with a 2–3:1 male:female ratio. The common features of AS are: chronic inflammatory back pain, sacroiliitis, spondylitis, and restrictions in spine movements; early symptoms of AS are recognized in adolescents or in young adults. The prevalence of AS in the USA is 0.52%–0.55% and 0.49% in Turkey (1–3).
Ankylosing spondylitis is progressive inflammatory disease that results in substantial functional limitations and affects patients’ daily activities. It is necessary to have the information of patients’ pain status, movement restrictions, and disease activity for an accurate follow-up. AS is diagnosed based on patients’ symptoms, blood tests, and radiological imaging. There is no standard laboratory test as a diagnostic and follow-up tool unique for SpA. Today, acute phase reactants, such as as C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) are commonly used for indicating the inflammation. These tests are useful in determining the degree of the inflammation, changes in the disease activity over time, and prognosis (4). The serum levels of C-reactive protein (CRP) and ESR are increased in peripheral joint inflammation. However, they have low sensitivity and specificity (5, 6).
Despite the predictive function of MRI in terms of disease progression, radiological imaging has a limited role in the follow-up. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is essential to measure the clinical activity of the disease and is most commonly used in daily practice (7–9).
In a recent study, neutrophil lymphocyte ratio (NLR) was found to be a potential marker for inflammation. NLR is increased in patients with cardiovascular disease; malignancies, such as colorectal, breast, and lung cancers; and inflammatory disorders, like acute pancreatitis and ulcerative colitis. The relationship between AS and NLR has not been investigated yet. In this study, we aimed to assess whether NLR has a role in predicting inflammation in patients with AS and its potential relationship with BASDAI, ESR, and CRP.
Material and Methods
A total of 35 patients, fulfilling the 1984 Modified New York Criteria for the diagnosis of AS between April 2012 and April 2013, were included in the study following local ethical committee approval (10). Having a full dose of non-steroid anti-inflammatory drugs (NSAIDs) at least 3 months and a BASDAI score above 4 or active sacroiliitis were considered for inclusion criteria. A total of 38 hospital workers of similar ages and gender without any systemic disease or history of medications were included as healthy controls. Those who had malignancy, active infection, diabetes mellitus, hypertension, renal failure, and liver failure were excluded from the study. The patient charts were investigated retrospectively. The age, sex, clinical features, HLA-B27 values, and treatment process of the patients were recorded. The patient group was enrolled for treatment with one anti-TNF drug. Total blood count, ESR, CRP, and BASDAI score were obtained before and 3 months following the treatment.
Bath ankylosing spondylitis disease activity index was used to evaluate waist and hip pain, level and duration of peripheral arthritis and enthesitis, and morning stiffness (7, 8). Total blood count evaluation was performed with impedance, optic laser scatter, and radio frequency method. ESR (mm/hour) was measured with photometric capillary flow kinetic analysis, and CRP (mg/dL) was measured with immune-turbidimetric assay. NLR was found with a mathematical calculation of the ratio of neutrophils with lymphocytes.
Statistical analysis
Statistical analyses were performed with SPSS statistical package, version 18.0 (Inc, Chicago, IL, USA). The data are given as mean and standard deviation. Kolmogorov-Smirnov test was used for defining the distribution of the variables. While student’s t-test was used to compare the variables between patients and control group, paired t-test was used to compare before and after treatment. The correlation between the variables was tested with Pearson correlation. A p value of < 0.05 was considered statistically significant.
Results
The patient group consisted of 35 patient (26 men (74.3%) and 9 women (25.7%)), and the control group consisted of 38 healthy volunteers (26 men (68.4%) and 12 women (31.6%)). Mean age of the patients and healthy controls was 38.9±11.9 and 37.3±6.9, respectively. A total of 27 patients had HLA-B27 analysis; 15 (68.2%) of them were found to be positive. Four (12.9%) patients had a history of uveitis. Thirteen (46.4%) patients had enthesitis, and 14 (43.8%) patients had peripheral arthritis.
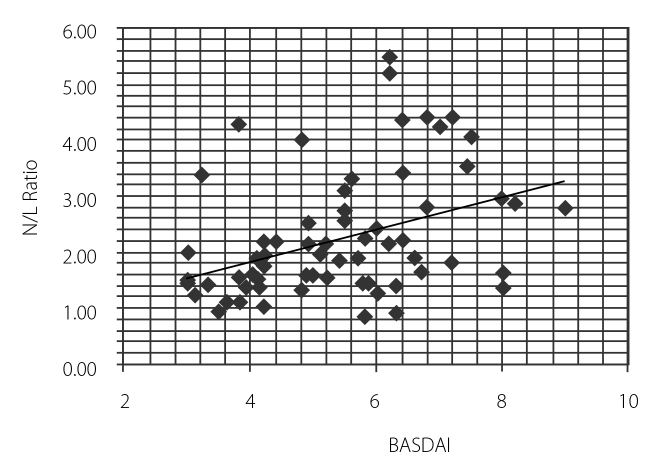
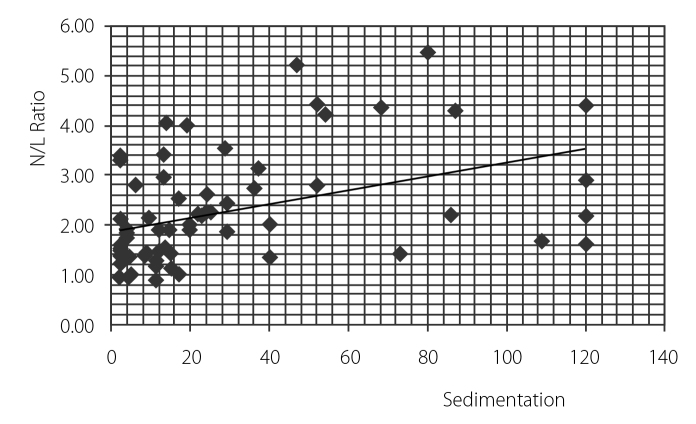
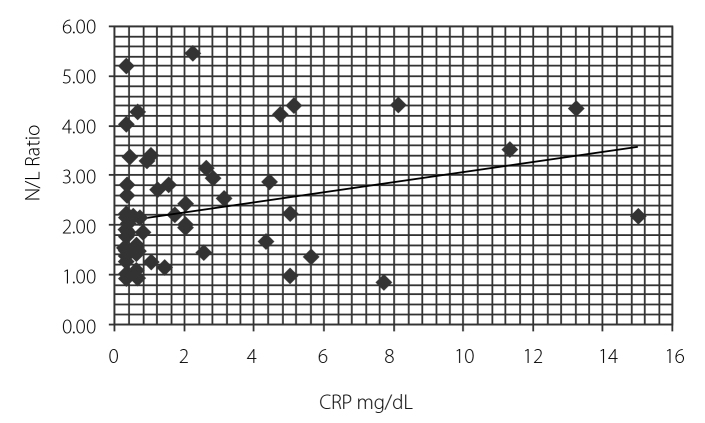
Fourteen patients (40%) took infliximab, 15 (42.9%) patients took etanercept, and 6 (17.1%) patients took adalimumab. The mean NLR value of the control group and patients was 1.90±0.89 and 2.67±1.17, respectively (p<0.05). Table 1 represents the patient and control groups’ demographics and clinical and laboratory results. After TNF-alpha treatment, the patient group had a mean NLR value of 1.8±0.7. The decrement was found to be statistically significant (p<0.001) (Table 2). Also, NLR was found to be correlated with BASDAI, sedimentation rate, and CRP (r=0.388, p<0.001; r=0.455, p<0.0001; and r=0.3389, p<0.005, respectively) (Figure 1–3).
Table 1.
Demographic and laboratory data of patients and controls.
| Patients with AS (n=35) | Healthy controls (n=38) | p value | |
|---|---|---|---|
| Age, mean±SD years | 38.91±11.90 | 37.36±6.90 | |
| Sex, male (%) | 26 (74.3) | 26 (68.4) | |
| Disease duration (years) | 9.25±8.37 | ||
| HLA-B27-positive (n=22) (%) | 15 (68.2) | ||
| A history of uveitis (n=31) (%) | 4 (12.9) | ||
| The presence of enthesitis (n=28) (%) | 13 (37.1) | ||
| Peripheral arthritis (n=32) (%) | 14 (43.8) | ||
| Patients receiving infliximab (%) | 14 (40.0) | ||
| Patients receiving etanercept (%) | 15 (42.9) | ||
| Patients receiving adalimumab (%) | 6 (17.1) | ||
| Leukocyte (×103/mm3) | 10.330±2.66 | 7.075±1.42 | <0.001 |
| Neutrophil (×103/mm3) | 6.596±2.11 | 3.975±1.04 | <0.001 |
| Lymphocyte (×103/mm3) | 2.771±1.15 | 2.235±0.54 | 0.012 |
| Hemoglobin | 12.82±1.62 | 14.31±1.20 | <0.001 |
| Platelet (×103/mm3) | 360.37±113.77 | 235.00±36.47 | <0.001 |
| NLR | 2.67±1.17 | 1.90±0.89 | <0.001 |
| MCV | 81.80±7.31 | 87.05±3.73 | <0.001 |
| RDW | 16.08±2.38 | 14.69±1.22 | <0.001 |
| MPV | 8.80±1.69 | 8.36±0.98 | 0.004 |
AS: ankylosing spondylitis; NLR: neutrophil lymphocyte ratio; MCV: mean corpuscular volume; MPV: mean platelet volume; RDW: red cell distribution width
Table 2.
Pre- and post-treatment laboratory values of patients
| Pre-treatment | Treatment 3 month ahead | p value | |
|---|---|---|---|
| Leukocyte (×103/mm3) | 10.330±2.66 | 8.019±2.004 | |
| Neutrophil (×103/mm3) | 6.596±2.11 | 4.693±1.399 | |
| Lymphocyte (×103/mm3) | 2.771±1.15 | 2.783±7.26 | |
| Hemoglobin | 12.82±1.62 | 13.73±1.55 | |
| Platelet (×103/mm3) | 360.37±113.77 | 279.42±87.44 | |
| NLR | 2.67±1.17 | 1.80±0.79 | <0.001 |
| MCV | 81.80±7.31 | 82.86±7.91 | <0.001 |
| RDW | 16.08±2.38 | 15.78±2.17 | <0.001 |
| MPV | 8.80±1.69 | 8.84±1.50 | 0.004 |
| 4+9 | 39.97±36.05 | 14.02±24.08 | <0.001 |
| CRP (mg/dL) | 2.57±3.09 | 1.28±2.08 | 0.007 |
| BASDAI | 6.57±0.93 | 4.22±0.83 | <0.001 |
NLR: neutrophil lymphocyte ratio; MCV: mean corpuscular volume; MPV: mean platelet volume; RDW: red cell distribution width; ESR: erythrocyte sedimentation rate; CRP: c-reactive protein; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index
Figure 1.
Correlation between neutrophil lymphocyte ratio and BASDAI score
Figure 2.
Correlation between neutrophil lymphocyte ratio and sedimentation levels
Figure 3.
Correlation between neutrophil lymphocyte ratio and CRP levels
Discussion
Bath ankylosing spondylitis disease activity index scores, ESR, and CRP are the common measures for determining the disease activity and patients’ response to the treatment. In this study, we found that NLR correlated with these parameters. To best of our knowledge, this is the first study showing the relation between NLR and disease activity in patients with AS.
TNF-alpha plays a major role in the pathogenesis of AS. The net effect of TNF-alpha on neutrophils is not clear; however, it has effects on interleukin (IL)-1, IL-6, IL-8, and granulocyte macrophage colony-stimulating factor. Also, it is an essential element in hematopoietic progenitor cell differentiation and maturation (11).
The relation between higher levels of NLR and systemic inflammation is not clear yet. Systemic inflammation has deleterious effects on vascular endothelial cells through decreased production of nitric oxide and prostacyclin, which causes decreased vasodilation and anti-thrombosis. Also, stimulated leukocytes have increased adherence to the vascular endothelium (12). Cytokines, like IL-1ra, IL-6, IL-7, IL-8, IL-12, and PDGF, play a considerable role in inflammation and may have an effect on increased NLR (13).
Bath ankylosing spondylitis disease activity index score was found to be reliable for measuring the disease activity; however, it has a disadvantage of creating different scores in the same patients at different attempts (14). Also, factors, like joint destruction and psychological stress, can affect the results of BASDAI. For these reasons, it should not be used as the sole tool to determine disease activity (15). Laboratory tests, like ESR and CRP, can provide additional benefits in screening patients, although their use is controversial. Clinical studies report an increase in ESR and CRP in 50%–70% of the patients (5–6). However, they can be used in the evaluation of the response to TNF-alpha treatment (16–18).
Neutrophil lymphocyte ratio is found to be related to many inflammatory diseases. Kaya et al. (19) reported NLR to be a sign of severe atherosclerosis, and they suggested NLR as a useful marker for cardiac risk stratification. NLR has been shown to be related with coronary atherosclerosis, and it is reported as an independent factor with CRP in predicting undesirable events in the hospital setting after myocardial infarction and unsuccessful attempt at primary percutaneous intervention (20, 21). Duffy et al. found a relationship between NLR and long-term mortality in patients undergoing percutaneous coronary procedures. They have explained this relationship with the effects of systemic disease (22). Furthermore, NLR has been found be an indicator for subclinical inflammation, and its association with prognosis is reported in coronary artery disease and cardiac failure (23, 24). The independent determining factor of NLR on long-term mortality was shown in myocardial infarction with or without ST-segment elevation (25, 26).
In studies of patients with ulcerative colitis, high levels of NLR were associated with disease activity, and it was suggested as a marker for intestinal inflammation (27, 28). NLR was found to be an independent predictor of mortality from Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease scores for liver cirrhosis. NLR has a predictive role in overall and cancer-specific surveillance of stomach, lung, breast, and renal cancers (29–31). Other inflammatory diseases, like acute pancreatitis and acute appendicitis, and systemic diseases, like hypertension, diabetes mellitus, and chronic renal failure, have higher levels of NLR (12, 32–35). Recently, Ahsen et al. (34) reported higher NLR levels in patients with familial Mediterranean fever than the control group (36). In another study, bone loss and inflammation were related with higher levels of NLR in elderly patients with osteoporosis (37).
Besides NLR, platelets are found to be associated with disease activity in patients with inflammatory disease. They play a significant role in inflammation and immunity, besides being major elements of hemostasis (38, 39). Mean platelet volume (MPV) is a cost-effective indicator of platelet activity (40). Recently, Soydinc et al. (39) found higher levels of MPV in patients with scleroderma than in healthy controls. Also, they found a negative correlation between MPV and disease activity.
Kisacik et al. (40) found significantly lower levels of MPV in patients with AS and rheumatoid arthritis in a study in which they investigated the role of MPV as an inflammatory marker. However, they did not find a correlation between pre-treatment MPV values and BASDAI scores. Two major limitations of the present study were the limited number of patients and limited follow-up duration.
Neutrophil lymphocyte ratio can be a valuable and reliable tool of disease activity in patients who have started anti-tumor necrosis factor (TNF) drugs for AS. Further studies with larger patient groups, longer follow-up duration, and radiographic end-points are required to verify our findings concerning the role of NLR in the inflammatory process of rheumatologic diseases.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - Y.P., E.D.; Design - Y.P., E.D., B.N.C., M.F.O.; Supervision - Y.P.; Resource - B.N.C., A.N.T., S.E.; Materials - B.N.C., A.D.; Data Collection&/or Processing - B.N.C., M.F.O., A.D., N.O.; Analysis&/or Interpretation - B.N.C., M.F.O., Y.P.; Literature Search - B.N.C., M.F.O.; Writing - B.N.C., Y.P., M.F.O.; Critical Reviews - Y.P.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Gensler L. Clinical Features of Ankylosing Spondylitis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 5th edition. Philadelphia, PA: USA: 2011. pp. 1129–34. http://dx.doi.org/10.1016/B978-0-323-06551-1.00112-3. [Google Scholar]
- 2.Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci. 2013;345:431–6. doi: 10.1097/maj.0b013e318294457f. http://dx.doi.org/10.1097/MAJ.0b013e318294457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onen F, Akar S, Birlik M, Sari I, Khan MA, Gurler O, et al. Prevalence of ankylosing spondylitis and related spondyloarthritides in an urban area of Izmir, Turkey. J Rheumatol. 2008;35:305–9. [PubMed] [Google Scholar]
- 4.Saxena A, Cronstein BN. Acute Phase Reactants and the Concept of Inflammation. In: Firestein GS, et al., editors. Kelley’s Textbook of Rheumatology. 9th edition. Philadelphia, PA: USA: 2013. pp. 818–29. http://dx.doi.org/10.1016/B978-1-4377-1738-9.00057-8. [Google Scholar]
- 5.Dougados M, Gueguen A, Nakache JP, Velicitat P, Zeidler H, Veys E, et al. Clinical relevance of C-reactive protein in axial involvement of ankylosing spondylitis. J Rheumatol. 1999;26:971–4. [PubMed] [Google Scholar]
- 6.Spoorenberg A, van der Heijde D, de Klerk E, Dougados M, de Vlam K, Mielants H, et al. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol. 1999;26:980–4. [PubMed] [Google Scholar]
- 7.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: The Bath ankylosing spondylitis disease activitiy index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 8.Akkoc Y, Karatepe AG, Akar S, Kirazlı Y, Akkoc N. A Turkish version of the Bath Ankylosing Spondylitis Disease Activity Index: reliability and validity. Rheumatol Int. 2005;25:280–4. doi: 10.1007/s00296-003-0432-y. http://dx.doi.org/10.1007/s00296-003-0432-y. [DOI] [PubMed] [Google Scholar]
- 9.Braun J, Heijde van der D, Dougados M, Emery P, Khan MA, Sieper J, et al. Staging of patients with ankylosing spondylitis: a preliminary proposal. Ann Rheum Dis. 2002;61:19–23. doi: 10.1136/ard.61.suppl_3.iii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goie The HS, Steven MM, van der Linden SM, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a comparison of the Rome, New York and modified New York criteria in patients with a positive clinical history screening test for ankylosing spondylitis. Br J Rheumatol. 1985;24:242–9. doi: 10.1093/rheumatology/24.3.242. http://dx.doi.org/10.1093/rheumatology/24.3.242. [DOI] [PubMed] [Google Scholar]
- 11.Beutler BA. The role of tumor necrosis factor in health and disease. J Rheumatol Suppl. 1999;57:16–21. [PubMed] [Google Scholar]
- 12.Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. http://dx.doi.org/10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–30. doi: 10.1016/j.critrevonc.2013.03.010. http://dx.doi.org/10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Berthelot JM, Tortellier L, Lavy-Bregeon D, Le Goff B, Maugars Y. High intraindividual week-to-week variability in BASDAI and BASFI values: are several evaluations needed before starting or stopping TNF alpha antagonist therapy for spondyloarthropathies? Joint Bone Spine. 2008;75:167–71. doi: 10.1016/j.jbspin.2007.05.018. http://dx.doi.org/10.1016/j.jbspin.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 15.de Vries MK, van Eijk IC, van der Horst-Bruinsma IE, Peters MJ, Nurmohamed MT, Dijkmans BA, et al. Erythrocyte sedimentation rate, C-reactive protein level, and serum amyloid a protein for patient selection and monitoring of anti-tumor necrosis factor treatment in ankylosing spondylitis. Arthritis Rheum. 2009;61:1484–90. doi: 10.1002/art.24838. http://dx.doi.org/10.1002/art.24838. [DOI] [PubMed] [Google Scholar]
- 16.Jung SY, Park MC, Park YB, Lee SK. Serum amyloid A as a useful indicator of disease activity in patients with ankylosing spondylitis. Yonsei Med J. 2007;48:218–24. doi: 10.3349/ymj.2007.48.2.218. http://dx.doi.org/10.3349/ymj.2007.48.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozgocmen S, Godekmerdan A, Ozkurt-Zengin F. Acute-phase response, clinical measures and disease activity in ankylosing spondylitis. Joint Bone Spine. 2007;74:249–53. doi: 10.1016/j.jbspin.2006.07.005. http://dx.doi.org/10.1016/j.jbspin.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Yildirim K, Erdal A, Karatay S, Melikoglu MA, Ugur M, Senel K. Relationship between some acute phase reactants and the Bath Ankylosing Spondylitis Disease Activity Index in patients with ankylosing spondylitis. South Med J. 2004;97:350–3. doi: 10.1097/01.SMJ.0000066946.56322.3C. http://dx.doi.org/10.1097/01.SMJ.0000066946.56322.3C. [DOI] [PubMed] [Google Scholar]
- 19.Kaya H, Ertas F, Islamoglu Y, Kaya Z, Atilgan ZA, Cil H, et al. Association between Neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost. 2014;20:50–4. doi: 10.1177/1076029612452116. http://dx.doi.org/10.1177/1076029612452116. [DOI] [PubMed] [Google Scholar]
- 20.Kalay N, Dogdu O, Koc F, Yarlioglues M, Ardic I, Akpek M, et al. Hematologic parameters and angiographic progression of coronary atherosclerosis. Angiology. 2012;63:213–7. doi: 10.1177/0003319711412763. http://dx.doi.org/10.1177/0003319711412763. [DOI] [PubMed] [Google Scholar]
- 21.Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, Celik T, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110:621–7. doi: 10.1016/j.amjcard.2012.04.041. http://dx.doi.org/10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 22.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–7. doi: 10.1016/j.amjcard.2008.05.006. http://dx.doi.org/10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–8. doi: 10.1016/j.amjcard.2010.09.039. http://dx.doi.org/10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–6. doi: 10.1016/j.amjcard.2010.03.062. http://dx.doi.org/10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 25.Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36:491–7. doi: 10.1016/j.clinre.2012.06.004. http://dx.doi.org/10.1016/j.clinre.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kücük H, Gürsoy S, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013;27:72–6. doi: 10.1002/jcla.21564. http://dx.doi.org/10.1002/jcla.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biyik M, Ucar R, Solak Y, Gungor G, Polat I, Gaipov A, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2013;25:435–41. doi: 10.1097/MEG.0b013e32835c2af3. [DOI] [PubMed] [Google Scholar]
- 28.Forget P, Machiels JP, Coulie PG, Berliere M, Poncelet AJ, Tombal B, et al. Neutrophil: lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20:650–60. doi: 10.1245/s10434-013-3136-x. http://dx.doi.org/10.1245/s10434-013-3136-x. [DOI] [PubMed] [Google Scholar]
- 29.Lee DY, Hong SW, Chang YG, Lee WY, Lee B. Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer. 2013;13:111–6. doi: 10.5230/jgc.2013.13.2.111. http://dx.doi.org/10.5230/jgc.2013.13.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, et al. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445–52. doi: 10.1159/000331494. http://dx.doi.org/10.1159/000331494. [DOI] [PubMed] [Google Scholar]
- 31.Markar SR, Karthikesalingam A, Falzon A, Kan Y. The diagnostic value of neutrophil: lymphocyte ratio in adults with suspected acute appendicitis. Acta Chir Belg. 2010;110:543–7. [PubMed] [Google Scholar]
- 32.Solak Y, Yilmaz MI, Sonmez A, Saglam M, Cakir E, Unal HU, et al. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:532–40. doi: 10.1007/s10157-012-0728-x. http://dx.doi.org/10.1007/s10157-012-0728-x. [DOI] [PubMed] [Google Scholar]
- 33.Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail. 2012;34:155–9. doi: 10.3109/0886022X.2011.641514. http://dx.doi.org/10.3109/0886022X.2012.72358. [DOI] [PubMed] [Google Scholar]
- 34.Ahsen A, Ulu MS, Yuksel S, Demir K, Uysal M, Erdogan M, et al. As a New Inflammatory Marker for familial Mediterranean fever: neutrophil-to-lymphocyte Ratio. Inflammation. 2013;36:1357–62. doi: 10.1007/s10753-013-9675-2. http://dx.doi.org/10.1007/s10753-013-9675-2. [DOI] [PubMed] [Google Scholar]
- 35.Öztürk ZA, Yesil Y, Kuyumcu ME, Bilici M, Öztürk N, Yeşil NK, et al. Inverse relationship between neutrophil lymphocyte ratio (NLR) and bone mineral density (BMD) in elderly people. Arch Gerontol Geriatr. 2013;57:81–5. doi: 10.1016/j.archger.2013.02.005. http://dx.doi.org/10.1016/j.archger.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: Linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–50. doi: 10.1038/sj.mn.7800198. http://dx.doi.org/10.1080/mic.10.3-4.335.350. [DOI] [PubMed] [Google Scholar]
- 37.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. Journal of Thrombosis and Haemostasis. 2003;1:1897–905. doi: 10.1046/j.1538-7836.2003.00304.x. http://dx.doi.org/10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin JF, Shaw T, Heggie J, Penington DG. Measurement of the density of human platelets and its relationship to volume. Br J Haematol. 1983;54:337–52. doi: 10.1111/j.1365-2141.1983.tb02109.x. http://dx.doi.org/10.1111/j.1365-2141.1983.00337.x. [DOI] [PubMed] [Google Scholar]
- 39.Soydinc S, Turkbeyler IH, Pehlivan Y, Soylu G, Goktepe MF, Bilici M, et al. Mean platelet volume seems to be a valuable marker in patients with Systemic Sclerosis. Inflammation. 2014;37:100–6. doi: 10.1007/s10753-013-9716-x. http://dx.doi.org/10.1007/s10753-013-9716-x. [DOI] [PubMed] [Google Scholar]
- 40.Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75:291–4. doi: 10.1016/j.jbspin.2007.06.016. http://dx.doi.org/10.1016/j.jbspin.2007.06.016. [DOI] [PubMed] [Google Scholar]