Abstract
Objective
To evaluate the clinical utility of a novel radiotracer, 99mTc-glucosamine, in assessing disease activity of both rheumatoid arthritis (RA) and ankylosing spondylitis (AS).
Material and Methods: Twenty-five patients with RA (nine males and 16 females) and 12 patients with AS (all male) at various stages of disease were recruited for the study. A clinical history and examination was performed, followed by the measurement of hematological, biochemical, and autoimmune serological parameters to assess disease activity. 99mTc-glucosamine was intravenously administered and scans were compared with other imaging modalities, including plain X-ray, magnetic resonance imaging (MRI), and bone scans.
Results
In patients with AS, 99mTc-glucosamine scans were more capable of identifying active disease and differentiating between inflammatory and non-inflammatory causes. In patients with RA, 99mTc-glucosamine accumulated at all known sites of disease involvement. Uptake was most pronounced in patients with active untreated disease. The relative tracer activity in the involved joints increased with time compared with that in the adjoining soft tissue, liver, and cardiac blood pool. Using Spearman’s correlation coefficient, there was a positive correlation among glucosamine scan scores, C-reactive protein (p=0.048), and clinical assessment (p=0.003), which was not noted with bone scans.
Conclusion
The radiotracer was well tolerated by all patients, with no adverse reactions. 99mTc-glucosamine imaging could detect spinal inflammation in AS. With respect to RA, 99mTc-glucosamine was a viable alternative to 99mTc-labeled methylene diphosphonate nuclear bone scans for imaging inflamed joints and had the added advantage of demonstrating a significant clinical correlation between disease activity and scan findings.
Keywords: Glucosamine, rheumatoid arthritis, ankylosing spondylitis, nuclear scan
Introduction
In the assessment of arthritis, conventional radiological imaging techniques, such as plain X-ray, computed tomography (CT), power Doppler ultrasound, and magnetic resonance imaging (MRI), are often used while evaluating the extent of disease progression on the basis of morphological and structural changes. Equally useful, but less often used, is the positron emission tomography (PET) to detect early synovitis, which is based on an increased metabolic activity before any structural change occurs. Each modality has advantages and disadvantages, and the type of modality selected depends on the information sought and the ease of access. By far, the most commonly used imaging modality is plain X-ray, despite knowing that structural changes, such as erosions, may not be evident in early disease. Alternative techniques, such as ultrasound, are more accessible and cost effective than MRI or PET scanning but are limited by being highly observer dependent (1). MRI with its now easier access and greater resolution of joint structure and pathology is disadvantaged in only being able to screen single joints/regions at a time and possibly by high costs. Bone scans reflect secondary bone reaction to the surrounding tissue rather than a direct representation of what is happening to the synovium.
Imaging techniques that reveal synovial tissue metabolic activity are increasingly becoming popular and correlate well with clinical parameters of the disease. There is no doubt that PET scanning offers the best imaging modality for assessing metabolically active tissue and has been extensively used in oncology to stage tumors. 18-Fluorodeoxyglucose (18F-FDG), a glucose analog, is the most regularly used agent in PET. It accumulates at sites of inflammation and rapid cell turnover owing to the greater metabolic requirements of cells at these sites. Beckers et al. (1) studied the utility of 18F-FDG PET in assessing synovitis. They found a significant correlation between joint count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and affected joints on ultrasound, and positive joints on PET. Similarly, Goerres et al. (2) studied the utility of 18F-FDG PET in assessing synovitis before and after infliximab administration and in detecting extra-articular synovial inflammation such as bursitis. They noted a positive correlation between clinical assessment and PET and determined that 18F-FDG PET can detect extra-articular inflammation. In other rheumatic conditions, such as vasculitis, Walter et al. (3) found 18F-FDG PET to be highly worthwhile in assessing large vessel disease. Despite the utility of PET, it is limited by high costs, short half-life of the 18F-FDG radionuclide, and lack of accessibility.
Glucosamine, which is an amino saccharide that is a cartilage constituent and is often used for treating osteoarthritis, can be labeled with the radioisotope technetium99m to form 99mTc-glucosamine. As an analog of glucose, it is more quickly metabolized in inflamed than in non-inflamed tissues. Yang et al. (4, 5) reported that 99mTc-glucosamine is valuable in imaging tumors and their response to therapy. Zhang et al. (6) revealed that 99mTc-glucosamine is valuable in imaging mesothelioma, while Schechter et al. (7) examined individuals with non-small cell lung cancer. Regarding the use of 99mTc-glucosamine in inflammatory arthritic lesions, Kumar et al. (8) reported that it could serve as a biomarker for inflammatory arthritis. Thus, 99mTc-glucosamine scintigraphy, including 18F-FDG scintigraphy, enables the detection of inflammation on the basis of the metabolic activity of tissues. The difference between 99mTc-glucosamine and 18F-FDG is the cost and access. 99mTc-glucosamine can be locally produced by a nuclear scientist in a major nuclear medicine department and administered to a patient, as one would for a bone scan, and it is easy to produce, cheap to administer, and accessible. Other similar analogs of glucosamine, such as N-[2,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-3-yl] acrylamide, have been demonstrated to have anti-arthritic and immunomodulatory effects in adjuvant-induced arthritis but are yet to be tested in humans as imaging agents (9).
In this study, we extended our prior pilot investigations and evaluated 99mTc-glucosamine imaging modality for its clinical utility, limitations, and applications, particularly in patients with rheumatoid arthritis (RA) and ankylosing spondylitis (AS). 99mTc-glucosamine joint uptake was correlated with MRI, bone scans, and in three cases, the effect of anti-tumor necrosis factor (anti-TNF) treatment on scanning before and after therapy.
Material and Methods
Ethics approval
All patients provided their written informed consent. The Western Sydney Local Health District Human Research Ethics Committee granted ethics approval for this study.
Study population
Patients fulfilling the American College of Rheumatology revised criteria for RA (10) were recruited from private consulting rooms or outpatient hospital clinics. All the patients were aged >18 years, with variable disease duration, disease activity, and drug treatment. Exclusion criteria included any patient with an allergy to glucosamine or seafood and any patient with end-stage renal or hepatic disease, pregnancy, or lactation. Patients were required to discontinue any glucosamine therapy 3 days prior to injecting the radiotracer. There were no restrictions regarding anti-rheumatic medications that the patients were taking. None of the recruited patients suffered from diabetes mellitus. There was no history of trauma or infection involving any of the joints or adjoining tissues in the 6 months preceding the acquisition of scans. There was no history of malignancy.
Patients with a definite diagnosis of AS on the basis of the Assessment of SpondyloArthritis International Society criteria for axial spondyloarthritis (11) were recruited from private rooms or outpatient hospital clinics. Similarly to RA, all the patients were aged >18 years and had the same exclusion and inclusion criteria. Patient disease varied with respect to duration, activity, and treatment.
Patient evaluation
Each patient had history and physical examinations that were documented by a rheumatologist prior to glucosamine scanning. For patients with RA, the following clinical parameters were measured: age, sex, age at onset, duration of disease, early morning stiffness, treatment, and active joint count as defined by both tender and swollen joints. Investigations included ESR, CRP, rheumatoid factor (RF), anti-citrullinated cyclic peptide antibodies (anti-CCP), anti-nuclear antibodies (ANA), extractable nuclear antigen (ENA), full blood count (FBC), X-ray of joints, bone scan, and MRI. Clinical scores (grades 0–3) were given to each patient on the basis of their joint count (refer to Table 1). For patients with AS, the clinical parameters that were recorded included age, sex, age at onset, duration of disease, treatment, ESR, CRP, RF, anti-CCP, ANA, ENA, FBC, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), X-ray grading of sacroiliitis, bone scan, and MRI.
Table 1.
Grading scales used to measure clinical activity, glucosamine scan uptake, bone scan uptake, MRI and X-ray activity
| Grading Scores | Grades |
|---|---|
| Clinical | 0 Nil disease; 0 joints affected*; examination normal |
| 1 Mild disease; <10 small joints affected | |
| 2 Moderate disease; 10–20 small joints or 1–3 large joints affected | |
| 3 Severe disease; >20 small joints or ≥4 large joints affected | |
|
| |
| Glucosamine Scan | 0 Nil uptake |
| 1 Minor uptake | |
| 2 Mild uptake | |
| 3 Moderate uptake | |
| 4 Severe uptake | |
| 5 Marked uptake | |
|
| |
| Bone Scan | 0 Nil uptake |
| 1 Minor uptake | |
| 2 Mild uptake | |
| 3 Moderate uptake | |
| 4 Severe uptake | |
| 5 Marked uptake | |
|
| |
| MRI (Joint Specific) | 0 Normal |
| 1 Mild synovitis, without marrow oedema on T1 or STIR/T2FS | |
| 2 Advanced synovitis, with marrow oedema on T1 or STIR/T2FS | |
| 3 Erosions, without chondral loss | |
| 4 Erosions, with chondral loss | |
| 5 Ankylosis, synovitis and chondral loss, with or without erosions | |
|
| |
| Sacro-iliac joints (X-ray) | 0 Normal |
| 1 Some blurring of joint margins | |
| 2 Minimal sclerosis, with some erosions | |
| 3 Definite sclerosis on both sides of joints; severe erosions with widening of joint space, with or without ankylosis | |
| 4 Complete ankylosis | |
MRI: magnetic resonance imaging; STIR: short tau inversion recovery imaging; FS-T2: fat-suppressed T2-weighted images
Joints were considered as affected if they were both tender and swollen.
Radiopharmaceuticals
Radiochemists at Westmead Hospital produced 99Tc-glucosamine by labeling sterile glucosamine (AZPA International, Yantai Dongchong Biochemical Co. Ltd.; Shandong, China) using the method described by Yang et al. (4, 5) The radiochemical purity was >98%. A 400-MBq dose of 99mTc-glucosamine (containing 1–2 mg glucosamine) in a bolus of 0.5 mL of normal saline was intravenously administered to each patient via a cannula using the vein in either cubital fossa over 15 s and was flushed with 0.9% normal saline.
Imaging
Glucosamine scans
All patients underwent a glucosamine scan, which was reviewed and reported by a nuclear medicine physician. Tracer uptake in the joints was correlated with the activity in the neighboring muscle tissue and liver and in the cardiac blood pool at three scanning time points (7 min, 15 min, and 3 h). The joints were graded and scored (Table 1). The joint scores were added, and a total value was provided for each patient. The individual score for each joint was correlated with the individual score given for MRI, bone scan, and clinical grading.
Imaging Parameters
All scans were conducted using a dual-head gamma camera (Siemens eCam; Siemens Healthcare Pty. Ltd., Bayswater, VIC, Australia) with low-energy high-resolution collimators. Dynamic images of the most clinically affected joints were acquired at the time of administering the tracer, with a frame rate of 1 frame/s for 60 s. This was followed by a whole-body blood pool sweep, which was acquired over 7 min. Whole-body sweeps were then acquired at 15 min and 3 h after injection in all patients (14 cm/min). Five-minute static images of selected joints/regions of interest were also acquired following the whole-body sweep images, as determined by the clinical history and scan findings. Immediately following acquisition of the 3-h whole-body sweep and static scans, single-photon emission CT (SPECT) images were acquired of one body region (area of interest) as determined by the clinical history. A 128×128 matrix was used for all SPECT acquisitions. The rotational radius was <13 cm in all cases. A total of 64 projections were acquired at 30 s per view with the camera heads following a non-circular orbit, resulting in a total scan time of 16 min. The quality of SPECT images in retrospect suggests a larger dose or prolonged acquisition times of >30 s may be of benefit. In this study, these parameters were kept constant, and no variations were examined in any patient. The projection data were visually checked for patient motion using the cine display and sinograms. SPECT data were reconstructed by iterative reconstruction using eight subsets and six iterations. A Gaussian filter with full width at half maximum=10 mm was used post-reconstruction and was corrected for attenuation according to Chang’s method (12) (μ=0.15/cm).
Bone scan
In this study, a routine three- phase technetium (99mTc) medronic acid (99mTc-MDP) bone scan was obtained from the patient if current (0–6 months) or requisitioned to enable comparison with MRI and the glucosamine scan.
MRI
Standard T1-, T2-, and fat-suppressed weighted images were obtained using a Siemens 3T GE machine (Siemens Healthcare Pty. Ltd.; Bayswater, VIC, Australia). MRI scores were given for each joint examined (Table 1).
Statistical analysis
The statistical software package IBM SPSS version 22 was used to analyze the data. Two-tailed tests with a significance level of 5% were used. Spearman rank correlation was used to quantify the association between continuous variables of interest.
Results
Demographics of patients with AS
Table 2 summarizes the patient population with AS who were examined and is further divided into three groups depending on disease duration. The first group, which includes four patients, had a recent diagnosis of the disease (<10 years) and were symptomatic. For one of these, anti-TNF therapy was initiated during the study. Six patients had a disease duration of between 10 and 20 years, and two patients had long-standing disease of >30 years’ duration, resulting in spinal ankylosis of the sacroiliac (SI) joints and calcification of the anterior and posterior longitudinal ligaments, giving rise to a bamboo spine. Other clinical parameters measured in all patients (data not shown) were FBC, RF, anti-CCP, anti-double-stranded DNA antibodies (ds-DNA), ENA, and ANA.
Table 2.
Ankylosing spondylitis patient characteristics
| Characteristics | Mean and SD (n=12) | Mean and SD (<10 Years) | Mean and SD (10–20 Years) | Mean and SD (>20 Years) |
|---|---|---|---|---|
| Clinical Features | ||||
| Age (Years) | 41.17±14.10 | 33.00±9.90 | 39.50±11.61 | 62.50±4.95 |
| Sex (M:F) | 12:0 | 4:0 | 6:0 | 2:0 |
| Age of Onset (Years) | 26.58±9.56 | 28.00±8.17 | 22.83±10.21 | 35.00±7.07 |
| Duration of Disease (Years) | 13.83±8.61 | 3.75±1.71 | 16.67±3.98 | 25.50±0.71 |
| Pain Score (0–10) | 4.44±3.70 | 3.88±3.47 | 4.30±3.91 | 6.00±5.66 |
| EMS (mins) | 77.96±141.12 | 128.75±234.53 | 55.00±57.18 | 12.50±0 |
| Schober’s Test | 11.19±6.34 | 15.00±0 | 14.17±0.76 | 1.00±1.41 |
| BASDAI | 3.82±3.10 | 4.46±3.78 | 3.22±3.10 | 4.38±3.97 |
|
| ||||
| Serological Features | ||||
| HLA-B27 (Positive:Negative) | 11:1 | 4:0 | 6:0 | 1:1 |
| CRP (mg/L) | 9.89±11.69 | 7.13±7.37 | 14.10±15.19 | 3.50±2.12 |
| ESR (mm/hr) | 17.64±20.68 | 11.33±13.58 | 23.00±25.95 | 11.00±12.73 |
|
| ||||
| Radiological Features | ||||
| X-Ray Score (0–4) | 2.20±1.53 | 1.50±1.50 | 1.90±1.43 | 4.00±0 |
SD: standard deviation; EMS: early morning stiffness; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; HLA-B27: human leukocyte antigen B27; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate
Role of 99mTc-glucosamine in patients with AS
In each of the 12 patients with AS, tracer uptake in the lumbar–sacral region on the planar studies was within normal limits. However, on the SPECT studies, diffuse abnormally increased tracer uptake centered on the SI joints was apparent in three cases. Each of these three patients was diagnosed 2–4 years prior to the acquisition of the scans (group one) and none had ever received any anti-TNF therapy. The inflammatory markers (ESR and CRP) and white cell counts were within normal limits.
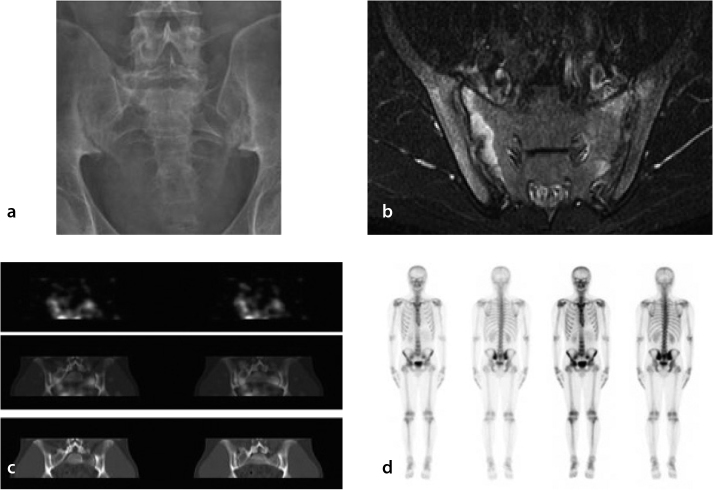
Figure 1 is a composite photograph of a 20-year-old patient with a 4-year history of back pain and a BASDAI of 5.6 imaged by four different modalities. Plain X-ray shows the presence of bilateral sacroiliitis. The bone scan shows minimal increase in the SI joints and MRI shows the presence of erosions and bilateral sacroiliitis. The planar glucosamine scan was non-specific (not shown). However, when SPECT views of the pelvis were taken they revealed a region of increased enhancement at the SI joints. Two distinct patterns of tracer uptake were identified: more intense and diffuse tracer uptake in the vicinity of the SI joints, which is consistent with an inflammatory process/arthropathy, and a more linear distribution confined to the SI joints, which was more indicative of degenerative change.
Figure 1.
a–d. Composite images from a patient with AS for comparing various imaging modalities. The subunits are plain X-ray in panel (a), MRI in panel (b), glucosamine scan in panel (c), and bone scan in panel (d). On plain X-ray (panel A), there is sclerosis in both SI joints with erosive changes present. The joint spaces did not appear to be widened. Radiographically, these findings were consistent with Grade 2 sacroiliitis. On the axial short-TI inversion recovery (T1 STIR) MRI image (panel B), there is edema on both sides of the SI joints (right greater than left), which is associated with enhancement suggestive of active inflammatory change. The SI joints were slightly widened with subtle erosive changes bilaterally. There was also minor thickening and enhancement of the synovium. On the 99mTc-glucosamine scans (panel C), diffuse increased tracer uptake was noted in the vicinity of each SI joint. On the early-phase images of the nuclear bone scan there was hyperemia in the SI regions (panel D). On the planar and SPECT images, there was intensely increased uptake in the SI joints bilaterally, which was consistent with bilateral sacroiliitis (panel D).
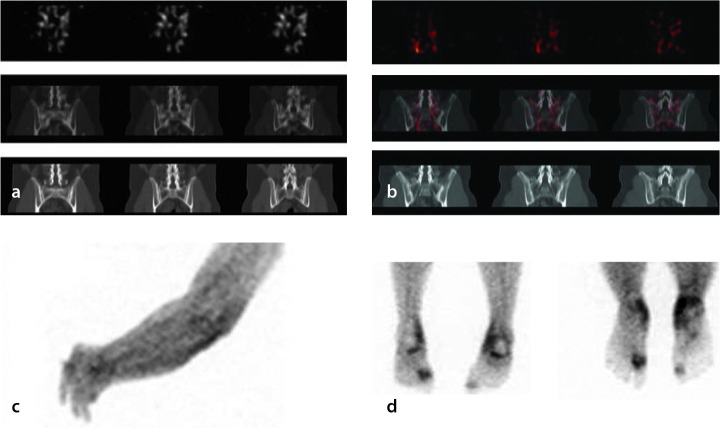
Figure 2 compares the glucosamine scans of a patient with recent-onset active disease (Figure 2a; <5 years; BASDAI 7.5) with those of a patient having long-standing inactive AS (Figure 2b; 19 years; BASDAI 1.32). Again, there were no differences between the cases on the planar scans. However, on the SPECT scans, tracer uptake in the vicinity of the SI joints in the latter patient was less avid and essentially confined to the SI joints. An additional value was the ability of glucosamine scans to identify peripheral joint involvement and the presence of tendonitis/soft-tissue edema in certain cases (Figure 2c, d). As demonstrated, bone scans and glucosamine uptake vary and are not always congruent. Glucosamine predominantly reflects soft-tissue uptake pathology, whereas MDP bone scans predominantly reflect bone uptake.
Figure 2.
a–d. Comparison of glucosamine scans between two patients with AS having different disease activity. The scan in panel A is taken from a patient with active AS (BASDAI 7.5) and the scan in panel B is from a patient with quiescent disease (BASDAI 1.3). The 99mTc-glucosamine scans in the patient with active disease (panel A) demonstrated diffusely increased tracer uptake in the vicinity of the SI joints, which is consistent with active sacroiliitis. In the patient with quiescent disease (panel B), minimal tracer uptake was localized in a vertically linear pattern to the SI joints, which is consistent with degenerative change. Panel C shows glucosamine uptake in a patient with AS and peripheral left forearm lymphedema. Panel D shows enthesitis in a patient with AS. Plain X-ray revealed large bilateral plantar calcaneal bony spurs.
RA patient demographics
Table 3 summarizes the demographics of patients with RA. As expected, there were more females than males in the RA group and the age at onset was later than that in the AS group. There were seven patients with a disease duration of >20 years. The majority of these patients had deforming arthritis consisting of metacarpophalangeal (MCP) subluxation, ulnar deviation, and multiple boutonniere and swan neck deformities. Six of these seven patients were on anti-TNF therapy and in disease remission. The seventh patient, despite the deformities of the fingers, was asymptomatic and managed on disease-modifying anti-rheumatic drugs only. Six patients had a disease duration of 10–20 years, four of whom were on anti-TNF therapy. The remaining 12 had a disease duration of <10 years with variable degrees of control. Similarly to AS, the clinical parameters measured in all patients were FBC, RF, anti-CCP, ds-DNA, ENA, and ANA.
Table 3.
Rheumatoid arthritis patient characteristics
| Characteristics | Mean and SD (n=25) |
|---|---|
| Clinical Features | |
| Age | 54.08±14.22 |
| Sex (M:F) | 8:17 |
| Age of Onset (Years) | 40.76±12.92 |
| Duration of Disease (Years) | 12.94±10.91 |
| Pain Score (0–10) | 3.39±2.50 |
| Early Morning Stiffness (Mins) | 44.35±80.12 |
| Clinical Score (0–3) | 1.28±1.21 |
| Serological Features | |
| RF (Positive:Negative) | 24:1 |
| CRP (mg/L) | 12.56±25.24 |
| ESR (mm/hr) | 30.74±25.96 |
| Radiological Features | |
| Glucosamine Scan Score (0–80) | 23.56±10.48 |
| Bone Scan Score (0–80) | 21.38±8.55 |
| MRI Score (0–5) | 3.25±1.14 |
SD: standard deviation; RF: rheumatoid factor; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; MRI: magnetic resonance imaging
Role of 99mTc-glucosamine in patients with RA
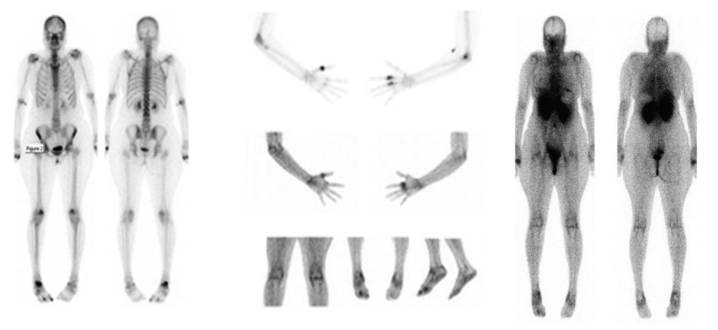
Figure 3 highlights the basic differences between a bone scan (a) and a glucosamine scan (b) in an RA patient. In the latter scans, the radionuclide was localized to the synovium rather than bone and outlined joints with increased metabolic activity. This was a pleasing finding, with the appearance being similar to the blood pool images on a bone scan. Surprisingly, very little tracer was localized to the cartilage. Whether uptake occurred in the cartilage or the adjacent bone with its better blood supply and more metabolically active nature was difficult to determine. The increased localization of the radiotracer was in the synovium and not in the synovial fluid. Aspiration of synovial fluid following glucosamine injection revealed that <1% of the injected 99mTc-glucosamine was within the synovial fluid. One advantage of glucosamine scans over MRI and plain X-ray was their ability to identify synovitis in all the involved joints. When there was bone involvement, bone scans demonstrated more avid radionuclide uptake than glucosamine scans (Figure 4).
Figure 3.
Direct comparison between bone scan and glucosamine scan in a 43-year-old female with active RA. Both glucosamine scan (panel B) and bone scan (panel A) show increased tracer uptake in areas of active disease. Intensely increased bone uptake is noted in the left first MCP joint, right second and third MCP joints, proximal right radiohumeral joint, and right talonavicular joint, and is in keeping with highly active arthropathy. Less active arthropathy was seen in several left MCP joints, the left wrist, left elbow, and the patellofemoral joints. Only minimally increased uptake was noted at the shoulders. In addition, there was increased uptake involving the posterosuperior right calcaneus, which was consistent with an enthesopathy. Less active changes of enthesopathy were noted around the right cuboid, the trochanters, the proximal left radius and left navicular medially.
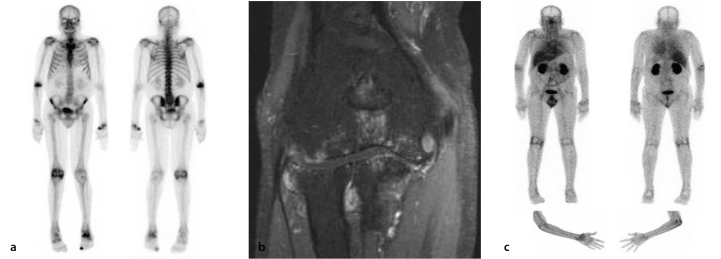
Figure 4.
Comparison between bone scan and glucosamine scan in a male RA patient with long-standing RA (15 years of disease) affecting primarily the right elbow. On the bone scan (panel A), there is increased tracer uptake (osteoblastic activity) in several joints, including the right elbow, the left wrist, and the right knee. There is a corresponding increase in 99mTc-glucosamine uptake seen in these joints on the glucosamine scans (panel C), which is consistent with active RA. Conversely, there was increased bone tracer uptake in the left ankle but minimal 99mTc-glucosamine uptake, which suggests a predominantly bone-related abnormality, rather than active RA, in the left ankle. T1 coronal fat saturation with contrast MRI (panel B) of the patient’s elbow revealed marked synovitis, effusion, and marginal erosive change with full-thickness chondral loss involving all compartments of the elbow joint, which is consistent with a long-standing erosive arthropathy with secondary osteoarthritis. Osteoarthritic changes are primarily seen in the medial compartment.
Not all joints demonstrated increased uptake following the injection of glucosamine. Increased uptake in clinically affected joints was evident. However, both glucosamine scans and bone scans still demonstrated tracer uptake in a number of joints considered inactive. Clinical correlation between physical examination and the expected outcome on scanning was best noted with glucosamine scans (Table 4).
Table 4.
Rheumatoid arthritis patient correlations
| Pain Score | Early Morning Stiffness | Clinical Score | CRP | ESR | Glucosamine Scan Score | Bone Scan Score | MRI Score Pain Score | ||
|---|---|---|---|---|---|---|---|---|---|
| Pain Score | Correlation Coefficient | 1.000 | .380 | .543** | .242 | .056 | .053 | .229 | .048 |
| Sig. (2-tailed) | . | .090 | .007 | .290 | .830 | .408 | .586 | .888 | |
| N | 23 | 21 | 23 | 21 | 17 | 23 | 8 | 11 | |
| Early Morning | Correlation Coefficient | .380 | 1.000 | .400 | .083 | −.157 | .381 | .089 | −.128 |
| Stiffness | Sig. (2-tailed) | .090 | . | .058 | .721 | .533 | .073 | .834 | .725 |
| N | 21 | 23 | 23 | 21 | 18 | 23 | 8 | 10 | |
| Clinical | Correlation Coefficient | .543** | .400 | .400 | .444* | .108 | .565** | .489 | .337 |
| Score | Sig. (2-tailed) | .007 | .058 | .058 | .034 | .659 | .003 | .219 | .284 |
| N | 23 | 23 | 23 | 23 | 19 | 25 | 8 | 12 | |
| CRP (mg/L) | Correlation Coefficient | .242 | .083 | .444* | 1.000 | .427 | .416* | −.162 | .127 |
| Sig. (2-tailed) | .290 | .721 | .034 | . | .068 | .048 | .728 | .710 | |
| N | 21 | 21 | 23 | 23 | 19 | 23 | 7 | 11 | |
| ESR (mm/hr) | Correlation Coefficient | .056 | −.157 | .108 | .427 | 1.000 | .175 | −.600 | −.305 |
| Sig. (2-tailed) | .830 | .533 | .659 | .068 | . | .475 | .208 | .425 | |
| N | 17 | 18 | 19 | 19 | 19 | 19 | 6 | 9 | |
| Glucosamine | Correlation Coefficient | .408 | .381 | .565** | .416* | .175 | 1.000 | .524 | .257 |
| Scan Score | Sig. (2-tailed) | .053 | .073 | .003 | .048 | .475 | . | .183 | .420 |
| N | 23 | 23 | 25 | 23 | 19 | 25 | 8 | 12 | |
| Bone | Correlation Coefficient | .229 | .089 | .489 | −.162 | −.600 | .524 | 1.000 | −.632 |
| Scan Score | Sig. (2-tailed) | .586 | .834 | .219 | .728 | .208 | .183 | . | .368 |
| N | 8 | 8 | 8 | 7 | 6 | 8 | 8 | 4 | |
| MRI Score | Correlation Coefficient | .048 | −.128 | .337 | .127 | −.305 | .257 | −.632 | 1.000 |
| Sig. (2-tailed) | .888 | .725 | .284 | .710 | .425 | .420 | .368 | . | |
| N | 11 | 10 | 12 | 11 | 9 | 12 | 4 | 12 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; MRI: magnetic resonance imaging
Glucosamine scans before and after anti-TNF treatment
Three patients were imaged before and after commencement of anti-TNF therapy to determine if glucosamine scanning could predict the response to therapy and identify changes in disease activity over time. The first case was imaged 3 months after commencing treatment and the second patient (Figure 5a) was imaged 8 months later. In both cases, the scans demonstrated a mild reduction in each of the affected joints (upper and lower limbs). The third patient was rescanned a year later (Figure 5b) and a mild reduction in tracer uptake was noted in most, but not all, of the affected joints. These conclusions were largely based on visual and semi-quantitative analyses. A more quantitative analysis will need to be performed for reassurance. In general, these findings correlated well with the patients’ clinical course and improvement.
Figure 5.
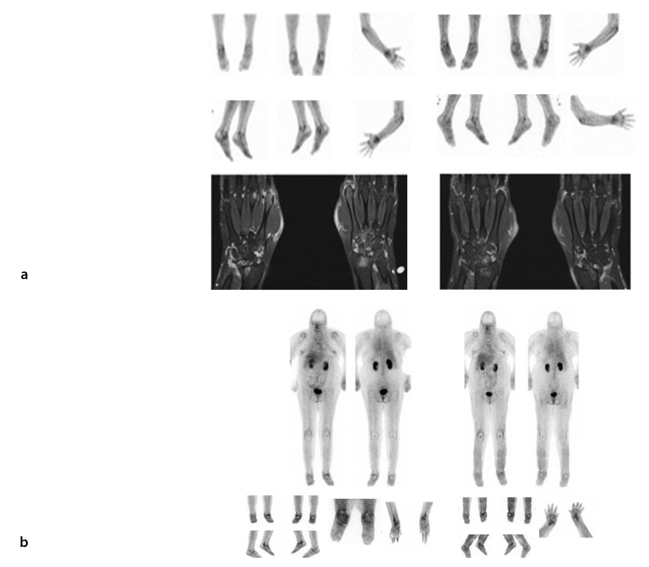
Glucosamine imaging pre- and post-anti-TNF therapy. Evaluation of anti-TNF therapy on glucosamine scans. Panel A shows the glucosamine and MRI scans of a 37-year-old female with RA. Scans were carried out before commencing treatment with biologics and 8 months after treatment. The patient had clinically improved and this was reflected in the glucosamine scan, which demonstrated reduced uptake in the affected areas. Initial T1 coronal fat saturation with contrast MRI revealed marked effusions, synovitis, bony edema, erosive changes, and degenerative cyst formation involving the radiocarpal, ulnarcarpal, intercarpal, and second through fifth carpometacarpal (CMC) joints bilaterally. There was mild involvement of the first CMC and first MCP joints. Marked synovitis and effusions involving several MCP joints were most conspicuous within the second MCP and fourth proximal interphalangeal joints. Mild changes in flexor tenosynovitis were noted. Repeat T1 coronal fat saturation with contrast MRI at 8 months later revealed active erosive arthropathy, with areas of enhancement centered primarily in the carpus at the wrist and to a lesser extent in the metacarpals. The amount of edema was reduced when compared with the previous scan. Panel B of Figure 5 shows the glucosamine images of a patient with long-standing RA that had anti-TNF therapy ceased because of an infected (L) mid-foot with draining sinus. Following a period of antibiotic therapy and observation, the arthritis was exacerbated (before), requiring the recommencement of anti-TNF therapy. One year later, after commencing treatment, the patient was significantly better with no evidence of infection and 99mTc-glucosamine was reduced, particularly in the left ankle and wrists.
Discussion
In a previous pilot study we identified the ability of 99mTc-glucosamine to differentiate between synovial and bone uptake in a number of rheumatic conditions, and determined that there was a different pattern of localization compared with bone scans (13). These differences included changes in localization patterns between active and quiescent disease in patients with RA, enhanced uptake in scleroderma lung (manuscript submitted), and enhanced uptake in patients with vasculitis (data not shown). In this study we extended these observations to patients with AS and a larger cohort of patients with RA.
In patients with AS, we noted that 99mTc-glucosamine planar scintigrams poorly correlated with the clinical appraisal of AS disease activity. However, SPECT analysis, which enables better contrast and resolution, demonstrated abnormally increased tracer uptake, which was suggestive of active AS, in three of the four patients with early-onset disease. Although we have not strictly defined the tracer uptake parameters, there were definitely two distinct patterns noted: a more diffuse tracer-avid pattern, which was consistent with active inflammation, and a less extensive, subtle pattern that appeared to reflect degenerative changes. More advanced algorithms of image reconstruction and analysis would be expected to provide clearer delineation of such changes. These promising results are exciting and require further confirmation in the evaluation of patients with more pronounced AS inflammatory back pain. 99mTc-glucosamine scintigrams may also provide opportunities to examine patients with non-radiographic axial spondyloarthritis. The greatest benefits of 99mTc-glucosamine scans may be as an adjunct to other imaging modalities in the detection of acute-on-chronic disease, where anatomical modalities tend to be inaccurate, and in the detection of subclinical disease. Potentially, 99mTc-glucosamine scintigrams may enable a more thorough and accurate assessment of disease activity and differentiation of the causation of symptoms and signs such as back pain (inflammatory or non-inflammatory), with obvious clinical implications in patient management.
In patients with RA, the absence of increased glucosamine uptake in joints correlated well with quiescent disease. In such patients, joint-specific clinical symptoms, such as pain and joint stiffness, were invariably due to mechanical factors and/or chronic osteoarthritis. Conversely, avid joint tracer uptake was indicative of clinically active disease, which necessitated modulation of therapy. There was also a third subset of patients in whom mildly increased joint uptake of 99mTc-glucosamine was apparent. This third group may harbor subclinical disease, which was not readily apparent using conventional tool for assessment. Further evaluation of such cases may be necessary to elucidate the significance of these observations.
In summary, 99mTc-glucosamine is a new clinical imaging tool that may be useful to the clinician in the decision-making process. This agent accumulates at sites of known active disease but, more importantly, it also accumulates at sites of subclinical disease and sites of quiescent disease that may prove useful in monitoring disease progression. Like bone scans, it lacks structural definition. However, unlike bone scans, the pattern of tracer distribution in the joint could be useful in distinguishing between cartilage (osteoarthritis) and synovial pathology (inflammatory synovitis) and thereby specifically identifying synovitis. The extent of its sensitivity compared with ultrasound or bone scans is still not adequately answered and further studies are needed. This imaging technique lacks the sophistication of MRI to define the joint and show pathology, but has the advantage of being able to scan the whole body. The extent of body involvement correlates well with CRP levels. The technique is cheap, readily available, and easy to perform.
Acknowledgments
We are grateful to Prof. Yang for intellectual discussions and providing the initial sample of ethylene dicysteine. Similarly, we are grateful to the many of the Rheumatology Clinic staff (Dr. J. Oliver, Dr. M. Collins, Dr. A. Jordan, Dr. W. Lau, Dr. R. Sinnathurai) involved in patient recruitment. We are grateful to Prof. V. Kumar in the Nuclear Medicine Department, who was involved in the preparation and handling of radioactive glucosamine and with the technical assistance provided by the Nuclear Medicine staff (Ms. E. Wong; Mr. D Kumar; and Mr. D. Boddeti). We are thankful to the rheumatologists of the Rheumatology Department including Doctors. G. Howe, D. Spencer, H. Bak, H. Englert for helpful discussions and providing patients for this study. We also thank to Mrs. K. Blyth (biostatistician) for the assistance with the statistics and analysis of data.
Footnotes
Author Contributions: Concept - N.M.; Design - N.M.; Supervision - N.M.; Funding - N.M.; Materials - E.A., B.C., ; Data Collection and/or Processing - M.A., E.A., B.C.; Analysis and/or Interpretation - N.M., K.P., A.M., R.D.C., S.A.; Literature Review - N.M.; Writer - N.M., M.A., K.P., R.D.C., S.A.; Critical Review - N.M., M.A., K.P., R.D.C., S.A.
Financial Disclosure: The authors declared that this investigator initiated study was funded by an unconditional research grant in aid from AbbVie Pty. Ltd. Pharmaceutical, 32-34 Lord Street, Botany NSW, Australia, 2019.
References
- 1.Beckers C, Ribbens C, Andre B, Marcelis S, Kaye O, Mathy L, et al. Assessment of disease activity in rheumatoid arthritis with (18)F-FDG PET. J Nucl Med. 2004;45:956–64. [PubMed] [Google Scholar]
- 2.Goerres GW, Forster A, Uebelhart D, Seifert B, Treyer V, Michel B, et al. F-18 FDG whole-body PET for the assessment of disease activity in patients with rheumatoid arthritis. Clin Nucl Med. 2006;31:386–90. doi: 10.1097/01.rlu.0000222678.95218.42. http://dx.doi.org/10.1097/01.rlu.0000222678.95218.42. [DOI] [PubMed] [Google Scholar]
- 3.Walter MA, Melzer RA, Schindler C, Müller-Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32:674–81. doi: 10.1007/s00259-004-1757-9. http://dx.doi.org/10.1007/s00259-004-1757-9. [DOI] [PubMed] [Google Scholar]
- 4.Yang DJ, Kim C, Schechter NR, Azhdarinia A, Yu DF, Oh CS, et al. Imaging with 99mTc-ECDG Targeted at the Multifunctional Glucose Transport System: Feasibility Study with Rodents. Radiology. 2003;226:465–73. doi: 10.1148/radiol.2262011811. http://dx.doi.org/10.1148/radiol.2262011811. [DOI] [PubMed] [Google Scholar]
- 5.Yang D, Yukihiro M, Yu D, Ito M, Oh C, Kohanim S, et al. Assessment of therapeutic tumor response using 99mTc-Ethylenedicysteine-Glucosamine. Cancer Biother Radiopharm. 2004;19:443–56. doi: 10.1089/cbr.2004.19.443. http://dx.doi.org/10.1089/1084978041979625. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Bryant J, Kong F, Yu D, Mendez R, Kim E, et al. Molecular imaging of mesothelioma with 99mTc-ECG and 68Ga-ECG. J Biomed Biotechnol. 2012;2012:232863. doi: 10.1155/2012/232863. http://dx.doi.org/10.1155/2012/232863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schechter N, Erwin W, Yang D, Kim E, Munden R, Forster K, et al. Radiation dosimetry and biodistribution of 99mTc-ethylene dicysteine-deoxyglucose in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2009;36:1583–91. doi: 10.1007/s00259-009-1135-8. http://dx.doi.org/10.1007/s00259-009-1135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Ali M, Angelides S, Manolios N. Synthesis and characterisation of 99mTc-Glucosamine and 99mTc-His-CP and evaluation of their utility in imaging inflammatory arthritis. ANZ Nucl Med. 2007;38:1–13. [Google Scholar]
- 9.Shah S, Jawed H, Awan S, Anjum S, Simjee S. The anti-arthritic and immunomodulatory effects of NHAG:A novel glucosamine analogue in adjuvant –induced arthritis. Biomed Res Int. 2013;2013:487610. doi: 10.1155/2013/487610. http://dx.doi.org/10.1155/2013/487610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. http://dx.doi.org/10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Sieper J, van der Heijde D, Landewé R, Brandt J, Burgos-Vagas R, Collantes-Estevez E, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS) Ann Rheum Dis. 2009;68:784–8. doi: 10.1136/ard.2008.101501. http://dx.doi.org/10.1136/ard.2008.101501. [DOI] [PubMed] [Google Scholar]
- 12.Chang L-T. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;25:638–43. http://dx.doi.org/10.1109/TNS.1978.4329385. [Google Scholar]
- 13.Angelides S, El-Mashaleh M, Anagnostou M, Howe G, Spencer D, Kumar V, et al. The role of 99mTc-labelled glucosamine (99mTc-ECDG) in the evaluation of rheumatic joint disease: a screening experience. Nucl Med Commun. 2014;35:655–68. doi: 10.1097/MNM.0000000000000096. http://dx.doi.org/10.1097/MNM.0000000000000096. [DOI] [PubMed] [Google Scholar]