Abstract
Objective
Plasma interleukin-18 (IL-18) has been reported to be associated with homeostasis model assessment of insulin resistance (HOMA-IR). It also has been described as one of the factors that, in addition to insulin resistance, may also contribute to atherosclerosis. Parameters of systemic inflammation are also significantly associated with circulating IL-18. Our objective was to investigate whether IL-18 is associated with insulin resistance and atherosclerosis in patients with rheumatoid arthritis (RA) in which accelerated atherogenesis develops.
Material and Methods
Fifty-one female RA patients and 30 female controls were enrolled in the study; 31 of them were without disease-modifying antirheumatic drug (DMARD) treatment and had a relatively short disease duration. Disease activity was assessed by Disease Activity Score (DAS) 28 index. HOMA-IR method was used to detect insulin resistance. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), fasting plasma glucose (FPG), insulin, tumor necrosis factor alpha (TNF-α), and IL-18 levels were evaluated. Also, carotid intima-media thickness (cIMT) was measured.
Results
There were no differences between patients and the control group according to age, sex, and body mass index. ESR, CRP, insulin, FPG, HOMA-IR, TNF-α, IL-18 levels, and cIMT measurements were significantly high in the patient group. HOMA-IR and cIMT measurements were similar and high in both the DMARD and non-DMARD patient groups. HOMA-IR correlated with TNF-α (r=0.308, p=0.028), but no correlation was found between IL-18 and HOMA-IR. However, IL-18 was correlated positively with cIMT (r= 0.318, p=0.028) and negatively with BMI (r=−0.360, p=0.01).
Conclusion
IL-18 is associated with atherosclerosis in RA patients. However, no significant relation was found with insulin resistance. IL-18 may be a marker for early evaluation of atherosclerosis in RA patients.
Keywords: Atherosclerosis, insulin resistance, interleukin-18, rheumatoid arthritis, tumor necrosis factor alpha
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disorder of unknown etiology primarily affecting the joints and is associated with increased mortality rates (1). It is shown that this increased mortality in RA is largely attributable to cardiovascular disease, primarily atherosclerosis-related mortality and coronary heart disease (1, 2). Available data do not suggest that these associations are due to aggregation of traditional risk factors in patients with RA, or they can be explained by adverse effects from antirheumatic treatment (3). Therefore, non-traditional risk factors may also significantly contribute to the pathogenesis of atherosclerosis.
Atherosclerosis is also a chronic inflammatory disease of the arterial wall characterized by progressive accumulation of lipids, several cells, and extracellular matrix. Inflammation plays a major role throughout the development and pathogenesis of the atherosclerotic lesion (4). It was reported that inflammatory cells and mediators are present in human atherosclerotic plaque (5). The levels of inflammation markers, such as C-reactive protein (CRP), several cytokines, and other inflammatory markers known to be elevated in RA, are also elevated in ischemic injuries and predict coronary heart disease risk (6–8). Therefore, atherosclerosis could be attributed to the result of chronic inflammation, as occurs with RA. In recent years, the relation between RA and cardiovascular disease has become a particular focus of attention because of the increased recognition of the inflammatory underpinnings of atherosclerosis (4). Recently, the carotid intima-media thickness (cIMT) has been used as a non-invasive method for the assessment of atherosclerosis, and the prevalence of subclinical atherosclerosis, detected as an increase in the cIMT, is higher in patients with RA than in controls (9).
Rheumatoid arthritis is considered a typical Th1-mediated disease in which a broad range of proinflammatory mediators are released-classically, tumor necrosis factor-alpha (TNF-α) and interleukin–1 (IL–1) or interferon-gamma (IFN-γ) (2). Many additional factors have been added to this cytokine network in recent years; for instance, interleukin–18 (IL-18), a novel cytokine of the IL-1 family, identified in several autoimmune diseases, was shown to induce proliferation, cytotoxicity, and cytokine production by Th1 and NK cells (10). Overproduction of IL-18 is known to induce severe inflammatory disorders (11). In addition, IL-18 expression has been demonstrated in the synovium of patients with RA (12). IL-18 exhibits pleiotropic activities in RA, with a wide variety of effects that are influenced by the overall cytokine milieu. Several lines of evidence also indicated recently that IL-18 is also involved in atherosclerosis (13). IL-18 stimulates IFN-γ and TNF-α production and might be involved in atherosclerosis (14). IL-18 is also highly expressed in atherosclerotic plaques and seems to be a major factor in plaque destabilization (13).
Insulin resistance is known to be a major risk factor in the etiology of type 2 diabetes, hypertension, and dyslipidemia and may be a risk factor for coronary heart disease (15). Elevated concentrations of various inflammatory markers in the circulation have been reported in humans with insulin resistance-e.g., interleukin-6, TNF-α, soluble TNF receptors (sTNFRs), and CRP (16). In addition, there is some evidence that IL-18 concentrations may be linked insulin resistance (17).
The present study was therefore undertaken to compare plasma concentrations of IL-18 in patients with RA with those in age-matched control subjects and to investigate whether plasma IL-18 is associated with insulin resistance and cIMT, an early marker of atherosclerosis, in patients with RA.
Material and Methods
Patients
A total of 51 female RA patients were recruited from a hospital-based sample and included in the study. The patients met the 1987 American College of Rheumatology revised classification criteria for RA (18). The healthy control group consisted of 30 age- and body mass index (BMI)-matched female. Detailed information was obtained through a structured interview, physical examination, laboratory tests, and review of medical records of patients. We recorded the medication taken at the time of the study. Their BMI (kg/m2) was calculated. We excluded patients with diabetes mellitus, dyslipidemia, impaired fasting glucose, BMI >30/kg/m2, recent or ongoing infection, chronic inflammatory disease, or systemic disease except RA and patients taking corticosteroids and lipid-lowering or glucose-lowering agents. All patients were classified into two subgroups based on whether they received disease-modifying antirheumatic drugs (DMARDs) or not. Twenty (39.2%) patients were on DMARDs, while 31 patients (60.8%) were only on nonsteroidal anti-inflammatory drugs (NSAIDs) of non-DMARDs. Non-DMARD patients were presented as active RA being newly diagnosed.
Methods
In patients, disease activity was measured using the Disease Activity Score based on the evaluation of 28 joints (DAS28). The DAS28 score is a validated composite index containing a 28-joint count for tenderness, a 28-joint count for swelling, erythrocyte sedimentation rate (ESR), and the patient’s overall assessment of well-being. DAS 28 >5.1 is high disease activity, 5.1≥DAS 28 >3.2 is mild disease activity, and DAS 28 ≤3.2 is low disease activity, and the patient’s assessment of disease activity [measured on a 0–100-mm visual analog scale (VAS)] was taken (19). Blood was collected after an overnight fast for the measurement of complete blood count, fasting plasma glucose (FPG), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides. Total cholesterol, HDL cholesterol and triglycerides (Olympus; County Clare, Ireland), of LDL cholesterol (Randox; Crumlin, UK), and of plasma glucose. Laboratory testing was carried out using autoanalyzers and enzymatic methods (for lipids). Ethylenediaminetetraacetic acid (EDTA) plasma was obtained after low-speed centrifugation at 4°C and frozen at -80°C prior to use. Quantitative measurement of patients’ fasting insulin concentrations was conducted using an insulin kit based on a microparticle enzyme immunoassay (BioSource; INS-EASIA, Brussels, Belgium). Serum CRP and rheumatoid factor (RF) were determined using a nephelometric immunoassay (Dade Behring Inc; Newark, DE, USA), and values of more than 20 IU/mL in the RF titer were considered positive. ESR was determined by Westergren method. Serum levels of IL-18 and TNF-α were measured by enzyme-linked immunosorbent assay using ELISA kits (BioSource; International Immunoassay Kit, CA, USA). Insulin resistance was evaluated by the homeostasis model assessment of insulin resistance (HOMA-IR) (20), which was calculated as follows: HOMA-IR = fasting plasma insulin level (μU/mL) X FPG (mmol/l)/22.5. cIMT of the common carotid artery was determined using duplex ultrasonography with a high-resolution 7.5-MHz transducer (HDI 1500 C2-5, USA). cIMT was defined as the distance from the leading edge of the first echogenic line to the leading edge of the second echogenic line in the sonographic image (21). We obtained approval for the study from the local ethical committees.
Statistical analysis
The statistical analysis in this study was performed using Statistical Package of Social Sciences (SPSS) 17.0 for Windows (SPSS, Inc., Chicago, IL, USA). Results are given as mean±standard deviation (SD). The statistical significance of the differences between the groups was determined by the Mann-Whitney U-test and student t-test. Correlation was tested using Pearson and Spearman’s rank test. P-values less than 0.05 were considered significant.
Results
The demographic characteristics and results of other parameters and the comparison of these data in patients with RA (DMARD and non-DMARD) and controls are shown in Table 1. Patients with RA and control subjects were of similar age, sex, and BMI. RF was positive in 31 (60.8%) RA patients, whereas it was not positive in control subjects. Serum concentrations of total, HDL, and LDL cholesterol and triglycerides were similar among patients and controls. ESR, CRP, insulin, FPG, TNF-α, IL-18 levels, HOMA-IR, and cIMT measurements were significantly higher in patients than in the control group (Table 1). HOMA-IR and cIMT measurements were similar and high in these two patient groups (p<0.05). ESR, CRP, and TNF-α levels and DAS 28 scores were also significantly higher in patients without DMARD treatment than in the other patients (p<0.001). Of the RA patients, 11.7% (6 patients) had a plaque in the arteria carotis communis, whereas there was no plaque in the control group.
Table 1.
Clinical characteristics and comparison of parameters in patients with rheumatoid arthritis and control subjects*
| Characteristic | RA (N=51) | Non-DMARD (N=31) | DMARD (N=20) | Controls (N=30) |
|---|---|---|---|---|
| Age (yrs) | 47.29±12.69 | 47.38±13.91 | 47.15 ± 10.88 | 45.13±11.26 |
| Sex (female) | 51 | 31 | 20 | 30 |
| BMI | 24.74±2.76 | 24.29±2.81 | 25.45±2.58 | 23.96±3.18 |
| Duration of disease (months) | 49±63 | 23.45±53.86 | 89.80±56.79c | ---- |
| RF positivity | 31 | 19 | 12 | ---- |
| RF levels (IU/mL) | 145.59±162.81 | 146.46±147.04 | 144.21±192.12 | ---- |
| ESR (mm/h) | 32.60±25.86a | 35.74±28.44a | 27.75±21.03b | 12.23±5.73 |
| CRP (mg/L) | 61.99±49.57a | 73.03±49.60a | 44.87±45.56a, d | 3.05±1.23 |
| DAS | 28 4.72±1.60 | 5.10±1.48 | 4.13±1.64d | ---- |
| TNF-α (ng/mL) | 21.29±9.84a | 24.23±9.54a | 16.74±8.68a, d | 3.25±0.75 |
| IL–18 (pg/mL) | 500.6±502.3a | 567.8±621.8a | 396.5±186.3b | 322.8±202.8 |
| FPG (mg/dL) | 92.58±11.05b | 90.19±11.7 | 96.3±8.83b,d | 87.76±8.83 |
| Insulin (uIU/mL) | 10.39±1.56a | 10.54±1.46a | 10.15±1.77b | 9.17±1.20 |
| HOMA-IR | 2.39±0.54a | 2.37±0.57b | 2.42±0.49b | 1.99±0.37 |
| cIMT (mm) | 0.76±0.11a | 0.75±0.10a | 0.77±0.12b | 0.67±0.04 |
| Plaque | 6 | 2 | 4 | ---- |
Values are expressed as mean±standard deviation.
P < 0.001 vs control group,
p< 0.05 vs control group,
p < 0.001,
p< 0.05 for comparison between non-DMARD and DMARD groups.
RA: rheumatoid arthritis; Non-DMARD: patients who did not receive disease-modifying antirheumatic drugs; DMARD: patients on disease-modifying antirheumatic drugs; BMI: body mass index; RF: rheumatoid factor; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; DAS: disease activity score; FPG: fasting plasma glucose; HOMA-IR: homeostasis model assessment of insulin resistance; cIMT: carotid intima-media wall thickness
In patients with RA, there were positive correlations with DAS 28 scores and ESR (r=0.650, p=0.000), CRP (r=0.928, p=0.000), and TNF-α (r=0.883, p=0.000). HOMA-IR correlated with TNF-α (r=0.308, p=0.028), but no correlation was found between IL-18 and HOMA-IR (Figure 1). However, IL-18 correlated positively with cIMT (r=0.318, p= 0.028) and negatively with BMI (r=−0.360, p=0.01) (Figure 2). The levels of insulin also correlated with PFG, ESR, CRP, TNF-α, and DAS 28.
Figure 1.
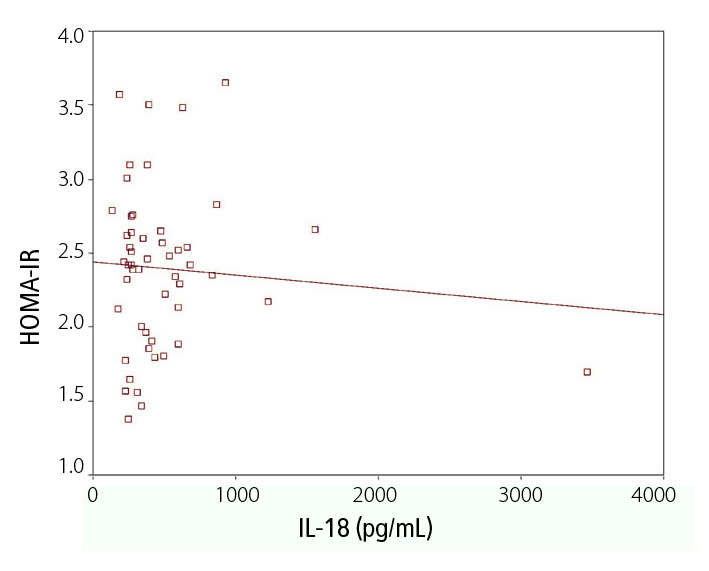
Correlation between HOMA-IR and serum IL-18 levels in patients with RA
Figure 2.
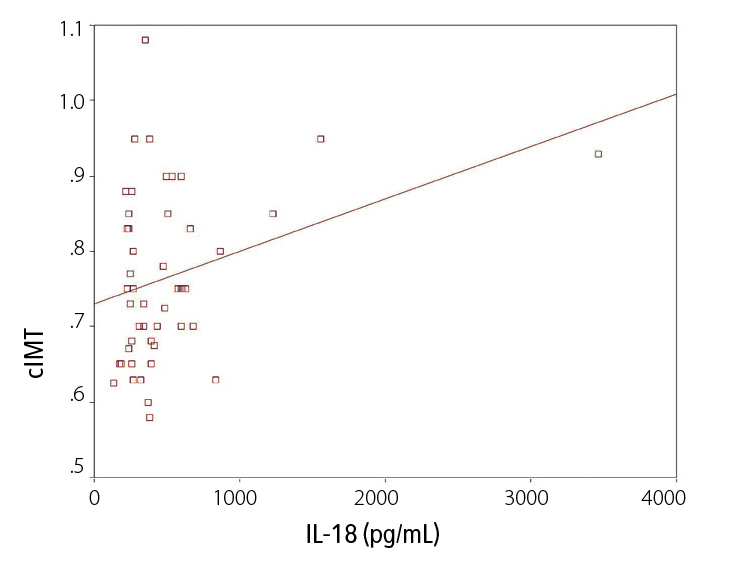
Correlation between cIMT and serum IL-18 levels in patients with RA
Discussion
In the present study, we evaluated the plasma levels of IL-18 and a possible relationship between plasma IL-18 levels and insulin resistance and atherosclerosis in patients with RA. The major findings were that there was significant elevation of plasma IL-18, TNF-α, HOMA-IR, and cIMT measurements in RA patients compared with healthy controls. Elevated IL-18 levels were significantly associated with cIMT measurements but were not correlated with HOMA-IR.
The cause of increased atherosclerosis in patients with RA is not known; however, inflammation and activated innate immunity are thought to play an important role in the development of atherosclerosis (22). Both traditional and nontraditional risk factors contribute to atherogenesis in RA. It may involve interactions between chronic vascular inflammation, corticosteroid use, augmented traditional risk factors, and hypertension (23). These multifactorial influences contribute to alterations in the vasculature and the development of atherosclerosis. As a result, accelerated atherosclerosis in RA may increase cardiovascular mortality. Using carotid ultrasonography, Del Rincon et al. confirmed a significant association between atherosclerosis and systemic inflammation in both RA and healthy individuals (24). These authors found a significant association between laboratory markers of systemic inflammation and both the cIMT and the presence of carotid plaques. Indeed, cIMT, a marker of early atherosclerosis, not only shows a strong relationship with cardiovascular disease risk factors but also predicts cardiovascular events (25).
It was reported that proinflammatory cytokines, such as TNF-α, IL-1, and IL-6, generated in the synovial tissue, can be released into systemic circulation in RA patients (26). Unlike other cytokines, elevated plasma IL-18 may be associated with atherosclerotic plaque instability, which may cause acute coronary syndrome (14). It was shown that IL-18 levels were positively associated with metabolic risk factors and that plasma IL-18 was an independent predictor of death from cardiovascular causes in patients with coronary artery disease (27). In the literature, it was reported that plasma IL-18 levels were increased in obese women, diabetes mellitus individuals, polycystic ovary syndrome, a number of autoimmune diseases, inflammatory bowel disease, psoriasis, and adult-onset Still’s disease (17, 28–30). In addition, IL-18 was detected in RA synovial membrane in macrophages, together with the lining layer of fibroblasts, and the level of IL-18 was increased in the joint and in the serum of RA patients (12). The present study confirmed that plasma concentrations of IL-18 were elevated in RA patients compared with healthy controls and also demonstrated that RA patients with high IL-18 had a greater cIMT than healthy controls. The demonstration of raised serum levels of IL-18 highlighted in this study is consistent with the hypothesis that IL-18 has a pathophysiological role in RA (12). In addition, our results suggest that elevated plasma IL-18 concentrations may be associated at least with early carotid atherosclerosis. We speculate that inflammation or plaque instability mediated by elevated plasma IL-18 concentrations could accelerate the development of atherosclerosis in RA patients. However, we found no significant difference in serum IL-18 levels and cIMT measurements between the patient groups (DMARD and non-DMARD). In addition, in this study, no correlation between disease activity and IL-18 in the serum of RA patients was found, as in the report of Bresnihan et al. (31). In contrast to our study, it was reported that RA synovial IL-18 expression correlates with ESR and disease activity following DMARD therapy (29). The reason for the distinct results between these findings and the present study may relate to differences in patient selection or the assay conditions. The failure to find a positive correlation between IL-18 levels and measures of disease activity in this study suggests that IL-18 may have limited ability to induce acute phase proteins and mediators of inflammation in RA.
Increased insulin resistance is an important risk factor for atherosclerosis (32). In the general population, insulin resistance is an established risk factor for cardiovascular disease and type 2 diabetes mellitus, and its main determinant is abdominal obesity (33). In addition to the production by immunocompetent cells, adipose tissue contributes significantly to the levels of IL-6 and TNF-α in the circulation. It is recognized that the pathogenesis of insulin resistance includes low-grade inflammation (16). It was reported that serum IL-18 levels might be a sensitive marker of the chronic inflammatory process underlying insulin resistance, especially in patients with type 2 diabetes (16, 34). In addition, the authors hypothesized that plasma levels of IL-18 would be a superior marker of insulin resistance when compared to IL-6, TNF-α, sTNFR2, and CRP. Patients with autoimmune connective tissue diseases also have increased insulin resistance (32), and it was reported to be associated with inflammation and glucocorticoid therapy in RA (34). The associations between acute-phase responses and insulin resistance are well documented in RA (35). However, the relationship between plasma IL-18 and insulin resistance in patients with RA has not been investigated previously. In our study, we then examined the relationship between plasma IL-18 and insulin resistance, a measure of HOMA-IR. HOMA-IR one of the indirect indices for the assessment of insulin resistance and correlates well with euglycemic clamp measures in men and women, younger and older adults, and obese and non-obese individuals (36). In our patients, we did not find a correlation between levels of IL-18 and HOMA-IR, similar to Aso et al. (37), indicating that other conditions are implicated in the development of the insulin resistance observed in RA. Another potential explanation for the lack of correlation between insulin resistance and serum IL-18 concentration is that patients had chronic disease and were receiving DMARDs and thus had lower disease activity. However, Dessein et al. (35) reported that abdominal obesity and patient’s assessment of disease activity were predictors of insulin resistance. In their study, patients with high-grade inflammation had increased insulin resistance as compared with patients with low-grade inflammation. These findings may explain our results. Our results suggest that elevation of plasma IL-18 levels may not enhance insulin resistance in patients with RA.
Obesity is a risk factor for coronary heart disease and mortality in the general population and may potentially contribute to the increased incidence of the CV complications observed in RA (26). However, obesity is the main determinant of insulin resistance in the general population, and previous studies of insulin resistance in RA did not report on the relative role of excess weight (35). In the present study, we found a negative correlation of plasma IL-18 with BMI, in contrast to other studies (17, 27). However, RA patients with active disease often have loss of body mass, known as rheumatoid cachexia, which predominates in skeletal muscle but also occurs in the viscera and immune system (26) and is related to cytokine production (35). Several explanations could account for the lack of correlation between BMI and serum IL-18 concentration in patients with RA. All patients were female and had a lower mean BMI, whereas all of these factors may affect the results. Another explanation for this finding may relate to the multiple sources of circulating IL-18. However, IL-18 secretion from adipose tissue has never been reported. The increase in serum IL-18 levels might also result from chronic inflammation of joints (36).
Another potential explanation for the failure to find any relationship between insulin resistance and serum IL-18 in our RA patients is that various other mechanisms may contribute to the insulin resistance, such as production of other cytokines (32). Inflammatory cytokines, such as IL-6 and TNF-α, have been shown to be released by adipose tissue and affect insulin resistance (38). Recently, many studies suggested that TNF-α might be an important mediator of insulin resistance (26, 32). TNF-α decreases the tyrosine kinase activity of the insulin receptor (26). TNF-α is also a proinflammatory cytokine that has a major role in the pathogenesis of autoimmune diseases and inflammatory disorders. Recently, it has been reported that a significant decrease in insulin resistance and disease activation was seen after infliximab treatment in most insulin-resistant patients (32). Our results suggest that plasma levels of TNF-α would be a superior marker of insulin resistance when compared to IL-18 in patients with RA.
Limitations of this study should be noted. First, all patients were female. Thus, our conclusion regarding the association between IL-18 and insulin resistance may be limited to the female gender. In addition, the menopausal status of the patients was not taken into consideration; the benefit of estrogen in atherosclerosis is well known. Second, the study was cross-sectional. The causal relationship can not be confirmed by a cross-sectional study. A prospective study is required to confirm causality among plasma IL-18 and HOMA-IR or cIMT in patients with RA. The mechanism of the association between IL-18 and insulin resistance in RA was not clarified by this study.
RA results in increased serum IL-18 and TNF-α concentrations. Our results indicate that carotid artery atherosclerosis is more prevalent in patients with RA compared to control subjects. RA should be added to the list of conditions associated with increased atherosclerosis; thus, inflammation should be controlled better. Our study implies that health care practitioners treating RA should be aware of the risk of atherosclerosis in those patients with high plasma IL-18. Our results also demonstrate an increase in circulating TNF-α concentration associated with insulin resistance in RA patients.
Footnotes
Ethics Committee Approval: The study protocol was approved by the ethics committee of our institution.
Informed Consent: All study subjects gave signed informed consent for participation in the study.
Peer-review: Externally peer reviewed.
Author contributions: Concept - M.Ş., Y.U.; Design - M.Ş., Y.U., Ş.E.T.; Supervision M.Ş., Ş.A, B.K.; Resource - M.Ş., Ş.E.T.; Materials - M.Ş., Y.U., Ş.A., B.K.; Data Collection&/or Processing - M.Ş., Y.U., Ş.A., A.K., A.Y., R.S., A.K.; Analysis&/or Interpretation - M.Ş., Ş.E.T., Y.U., B.K., A.Y.; Literature Search - M.Ş., Ş.A., A.K., R.S.; Writing - M.Ş., Ş.E.T., B.K.; Critical Reviews - B.K., A.Y., R.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. http://dx.doi.org/10.1161/01.CIR.0000054612.26458.B2. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. http://dx.doi.org/10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 3.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort no explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. http://dx.doi.org/10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. http://dx.doi.org/10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–900. doi: 10.1161/01.cir.0000042674.89762.20. http://dx.doi.org/10.1161/01.CIR.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. http://dx.doi.org/10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. http://dx.doi.org/10.1161/01.CIR.98.8.731. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. http://dx.doi.org/10.1161/01.CIR.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 9.Park YB, Choi HK, Lee SH, In BH, Lee HC, Nam CM, et al. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum. 2003;46:1714–9. doi: 10.1002/art.10359. http://dx.doi.org/10.1002/art.10359. [DOI] [PubMed] [Google Scholar]
- 10.McInnes IB, Gracie JA, Liew FY. Interleukin-18: a novel cytokine in inflammatory rheumatic disease. Arthritis Rheum. 2001;44:1481–3. doi: 10.1002/1529-0131(200107)44:7<1481::AID-ART268>3.0.CO;2-1. http://dx.doi.org/10.1002/1529-0131(200107)44:7<1481::AID-ART268>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Liew FY. The role of innate cytokines in inflammatory response. Immunology Letters. 2003;85:131–4. doi: 10.1016/s0165-2478(02)00238-9. http://dx.doi.org/10.1016/S0165-2478(02)00238-9. [DOI] [PubMed] [Google Scholar]
- 12.Gracie AJ, Forsey JR, Ling Chan W, Gilmour A, Leung PB, Greer RM, et al. A proinflammatory role for IL–18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–401. doi: 10.1172/JCI7317. http://dx.doi.org/10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–603. doi: 10.1161/hc3901.096721. http://dx.doi.org/10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 14.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–23. doi: 10.1038/386619a0. http://dx.doi.org/10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–43. doi: 10.1016/s0735-1097(02)02051-x. http://dx.doi.org/10.1016/S0735-1097(02)02051-X. [DOI] [PubMed] [Google Scholar]
- 16.Fischer CP, Perstrup LB, Eskildsen P, Pedersen BK. Elevated plasma interleukin-18 is a marker of insulin-resistance in Type 2 diabetic and non-diabetic humans. Clin Immunol. 2005;117:152–60. doi: 10.1016/j.clim.2005.07.008. http://dx.doi.org/10.1016/j.clim.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millan JL. Serum interleukin–18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. J Clin Endocrinol Metab. 2004;89:806–11. doi: 10.1210/jc.2003-031365. http://dx.doi.org/10.1210/jc.2003-031365. [DOI] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. http://dx.doi.org/10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. http://dx.doi.org/10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. http://dx.doi.org/10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–406. doi: 10.1161/01.cir.74.6.1399. http://dx.doi.org/10.1161/01.CIR.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 22.Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol. 2003;41:1071–7. doi: 10.1016/s0735-1097(03)00088-3. http://dx.doi.org/10.1016/S0735-1097(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 23.Del Rincon I, Freeman GL, Haas RW, O’Leary DH, Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413–23. doi: 10.1002/art.21397. http://dx.doi.org/10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 24.Del Rincon I, Williams K, Stern MP, Freeman GL, O’Leary DH, Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–40. doi: 10.1002/art.11078. http://dx.doi.org/10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 25.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. http://dx.doi.org/10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated with accelerated atherogenesis. Semin Arthritis Rheum. 2005;35:8–17. doi: 10.1016/j.semarthrit.2005.03.004. http://dx.doi.org/10.1016/j.semarthrit.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Hung J, McQuillan BM, Chapman CML, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268–73. doi: 10.1161/01.ATV.0000163843.70369.12. http://dx.doi.org/10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 28.Esposito K, Pontillo A, Ciotola M, di Palo C, Grella E, Nicoletti G, et al. Weight loss reduces IL–18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–6. doi: 10.1210/jcem.87.8.8781. http://dx.doi.org/10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 29.Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH, et al. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP–1-dependent pathways. Immunol Lett. 2006;103:159–66. doi: 10.1016/j.imlet.2005.10.020. http://dx.doi.org/10.1016/j.imlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Tso TK, Huang WN, Huang HY, Chang CK. Relationship of plasma interleukin–18 concentrations to traditional and non-traditional cardiovascular risk factors in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006;45:1148–53. doi: 10.1093/rheumatology/kel082. http://dx.doi.org/10.1093/rheumatology/kel082. [DOI] [PubMed] [Google Scholar]
- 31.Bresnihan B, Roux-Lombard P, Murphy E, Kane D, FitzGerald O, Dayer MJ. Serum interleukin 18 and interleukin 18 binding protein in rheumatoid arthritis. Ann Rheum Dis. 2002;61:726–9. doi: 10.1136/ard.61.8.726. http://dx.doi.org/10.1136/ard.61.8.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–6. doi: 10.1136/ard.2004.026534. http://dx.doi.org/10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grundy SM. What is the contribution of obesity to the metabolic syndrome? Endocrinol Metab Clin North Am. 2004;33:267–82. doi: 10.1016/j.ecl.2004.03.001. http://dx.doi.org/10.1016/j.ecl.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Dessein PH, Stanwix AE, Joffe BI. Cardiovascular risk in rheumatoid arthritis versus osteoarthritis: acute phase response related decreased insulin sensitivity and high-density lipoprotein cholesterolas well as clustering of metabolic syndrome features in rheumatoid arthritis. Arthritis Res. 2002;4:R5. doi: 10.1186/ar428. http://dx.doi.org/10.1186/ar428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum. 2006;54:2765–75. doi: 10.1002/art.22053. http://dx.doi.org/10.1002/art.22053. [DOI] [PubMed] [Google Scholar]
- 36.Bosch M, Lopez-Bermejo A, Vendrell J, Musri M, Ricart W, Fernandez-Real JM. Circulating IL–18 concentration is associated with insulin sensitivity and glucose tolerance through increased fat-free mass. Diabetologia. 2005;48:1841–3. doi: 10.1007/s00125-005-1859-3. http://dx.doi.org/10.1007/s00125-005-1859-3. [DOI] [PubMed] [Google Scholar]
- 37.Aso Y, Okumura K, Takebayashi K, Wakabayashi S, Inukai T. Relationships of Plasma Interleukin–18 Concentrations to Hyperhomocysteinemia and Carotid Intimal-Media Wall Thickness in Patients With Type 2 Diabetes. Diabetes Care. 2003;26:2622–7. doi: 10.2337/diacare.26.9.2622. http://dx.doi.org/10.2337/diacare.26.9.2622. [DOI] [PubMed] [Google Scholar]
- 38.Katsuki A, Sumida Y, Gabazza EC, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–5. doi: 10.2337/diacare.24.2.362. http://dx.doi.org/10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]


