Abstract
Cerebral mass-like lesion (MLL) is a rare form of Neuro-Behçet’s (NB) disease. There is currently no detailed knowledge on this issue in the literature. Our aim was to describe a Behçet’s disease (BD) patient with MLL, followed by a clinical analysis in light of the available literature regarding BD patients who suffered from an MLL or tumefactive lesion in the brain. We conducted a review of the English literature to analyse data on MLL in BD. The Pub-Med, Web of Science, Proquest and Ovid databases were searched for articles or abstracts using the term “Behçet’s disease” combined with one of the following terms: mass-like lesion, tumour-like lesion and tumefactive lesion. We compared clinical and laboratory features of BD patients with MLL with NB patients. We found 12 cases plus our case (6 male, 7 female; mean age: 40 years) with BD who developed MLL alongside BD. Five out of 13 BD patients (38%) had a history of BD before the onset of neurological symptoms. In 8 patients (62%), BD was diagnosed after the onset of neurological involvement. Headache, hemiparesis, dizziness, aphasia, nausea and vomiting were the presenting manifestations of NB patients with MLL. Genital ulceration, eye involvement, skin lesion and arthritis/arthralgia were less commonly reported in NB patients with MLL compared to NB patients without MLL. NB disease should be considered in the differential diagnosis of cerebral MLL even when other cardinal manifestations of BD are absent. Mucocutaneous manifestations, eye and joint involvement may be seen less often in these patients.
Keywords: Behçet’s disease, mass-like lesion, neuro-Behçet, tumefactive lesion, pseudo-tumour lesion
Introduction
Behçet’s disease (BD) is a chronic, relapsing, inflammatory syndrome, characterised by recurrent oral, genital ulceration and inflammation of the eye (1). The diagnosis is mostly based on the clinical criteria described by Turkish dermatologist Hulusi Behçet in 1937. Since then, the involvement of many other organs has been described and Behçet’s disease is now known as a multisystem disorder (2). The disease is recognised as a systemic vasculitis arising in almost all tissues (3, 4). The addition of central nervous system (CNS) involvement in patients who fulfill the criteria for BD outlined by the International Study Group for Behçet’s disease (ISG), is usually referred to as neuro-Behçet’s syndrome (NB), and is one of the most serious causes of long-term morbidity and mortality (3, 5). The frequency of CNS involvement is reported to range from 2.2 to 50% (1). The lesions are most commonly distributed in the brainstem, diencephalon, basal ganglia and internal capsula (5). The manifestations are cranial nerve palsies, cerebellar ataxia, and corticospinal tract signs (5). Mass-like lesions constitute a rare form of neuro-Behçet’s disease. We herein report an NB case with a mass-like lesion along with cases involving a mass-like lesion reported in the literature.
Material and Methods
We conducted a review of the English literature to analyse data on mass-like lesions in BD. The Pub-Med, Web of Science, Proquest and Ovid databases were searched for articles or abstracts using the term “Behçet’s disease” combined with one of the following terms: mass-like lesion, tumour-like lesion, and tumefactive lesion. We compared BD patients with mass-like lesions with NB patients reported by Akman-Demir et al. (6).
Our patient gave informed consent prior to inclusion in this study.
Case Presentation
A 27 year-old female patient with weakness in the left arm and left leg was admitted to our clinic in July 2010. She had been previously admitted to another hospital with headache and weakness in the left arm with a balance disorder 8 years ago. In that hospital, the hyperintense lesion in pons was detected by magnetic resonance imaging (MRI) examination. Nodular enhancement was seen in the corresponding areas (Figure 1). The diagnosis being considered was glioma. Chemotherapy including temozolamide (Temodal, Orion Corporation, Turku, Finland), following radiotherapy, was used to treat the patient. Her balance disorder resolved moderately; however, weakness in the left extremities continued. In 2008, the patient was readmitted to hospital with similar symptoms as well as a headache and vomiting. MRI showed the persistence of a brain-stem lesion. This lesion was considered to be possibly due to vasculitis or demyelinating diseases. Prednisolone (Deltacortril, Pfizer) (50mg/day, in tapering doses) and azathioprine (AZA) (Imuran, Heuman Pharma GmbH, Germany) (100 mg/day) were given to the patient. The patient was admitted to our hospital for a second opinion. When she presented to our clinic, she had weakness in both lower and upper extremities. It was then learnt that the patient had suffered from recurrent painful mouth ulcers (2–4 times a month) since the age of ten years. Genital ulcers had also developed on her labium majus when she was 16, which healed with scarring. She also reported papulopustular skin lesions located on her face and chest region. However, the patient was not investigated for the diagnosis of Behçet’s disease during this period and was treated with topical drugs.
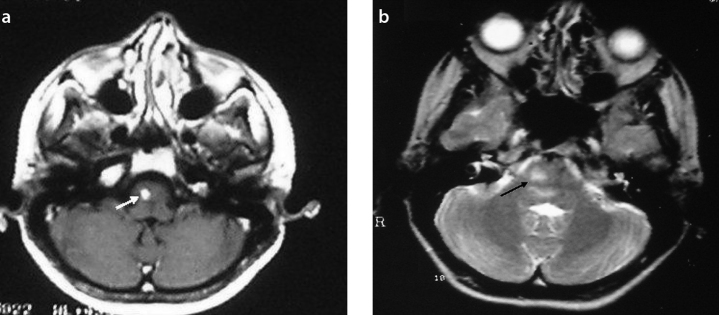
Figure 1.
a, b. T1-weighted axial MR image shows a right-sided lesion with nodular enhancement (white arrow) (a). The hyperintense lesion in the pons is seen on T2-weighted axial MR image (black arrow) (b)
On examination, her blood pressure was 100/70 mmHg and her heart rate was 72 beats/min. Body temperature was 36.2°C. Physical examination revealed weakness in the left extremities. There were no abnormal findings in complete blood counts, or biochemical and urine analysis. Chest X-ray and electrocardiography were also normal. Her erythrocyte sedimentation rate and C-reactive protein levels were 16 mm/hr (0–10 mm/hr) and 0.3 mg/dL (0–0.8 mg/dL), respectively. Pathergy test and HLA-B5 was negative. She was diagnosed as having NB and her therapy was switched to cyclophosphamide (1 gr/monthly) along with an increased dose of oral prednisolone (to 1 mg/kg or 50 mg/day). During the 6 months of therapy, minimal improvement was observed in her clinical manifestations and neurological symptoms. Cerebral MRI examination showed the almost complete regression of the brain-stem lesion compared to previous cerebral MRI studies (Figure 2).
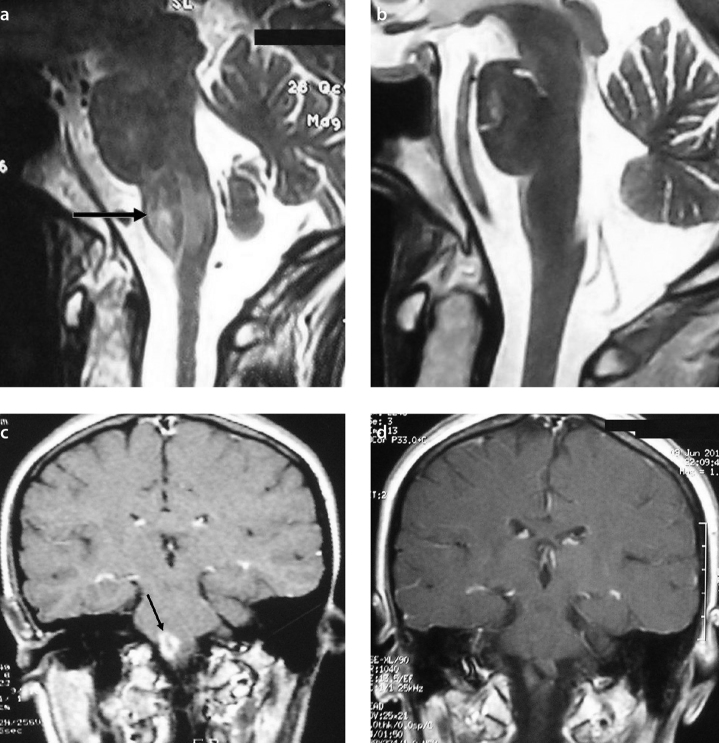
Figure 2.
a–d. Comparison between previous (a) and present (b) T2-weighted mid-sagittal MR images demonstrates the resolution of the hyperintense lesion in the brainstem (c). Complete enhancement regression was observed following assessment of the previous and present MRI examinations (d)
Results
In 6 articles and 5 abstracts, we identified 12 cases (6 males, 6 females; mean age 40 years) with mass-like lesions identified during the course of BD. Their clinical and laboratory findings are shown in Table 1 alongside those of the current patient. Six of the 13 BD patients (46%) had a history of BD before the onset of neurological symptoms (1, 2, 4, 7–10). In 7 patients (56%), BD was diagnosed after the onset of the neurological involvement. Headache (1, 4, 5, 7, 11, 12), hemiparesis (4, 10, 13), hemiplegia (14), dizziness (15), aphasia (7), and nausea and vomiting were the presenting manifestations of NB patients with mass-like lesions (Table 1).
Table 1.
Clinical and demographic features of the NB patients with MLL
| Cases | Age/Sex | BD findings | Complaints at admission | Physical examination | MRI and/or CT lesions | Biopsy | Treatment | Time of BD diagnosis (before or after neurologic manifestations) |
|---|---|---|---|---|---|---|---|---|
| Appenzeller et al. (1) | 43/female | OU GU Bilateral AU |
Headache Photophobia Asthenia |
Drowsiness, left arm and facial paresis, right eyelid ptosis | Thalamic lesion with mass effect and contrast enhancement | Gliosis with gemistocytic astrocytes | Steroid CYC | Before |
| Tuzgen et al. (4) | 59/female | OU, GU | Left hemiparesis, lethargy, sensations of bad smell, headache | Left hemiparesis, bilateral papilloedema vascular proliferation | Right frontotemporal mass causing shift | Reactive gliosis, panvasculitis, thrombosis, extensive infarction, | Right frontotemporal craniotomy and subtotal excision | After |
| Tuzgen et al. (4) | 45/female | OU, GU, monoarthritis | Headache, right hemiparesis, somnolence | Right hemiparesis | Mass in the left mesodiencephalic junction | Biopsy was not performed | Steroid | After |
| Heo et al. (5) | 47/male | OU, GU, uveitis | Headache Right side Weakness Vomiting |
Right hemiparesis, dysarthria | GdT1I showed ring enhancing lesions with internal hypointensities with subtle perilesional oedema at left side of the pons and parietal cortex | Frequent perivascular lymphocytic cuffing, focal necrotic lesion, microglial nodules, foci of necrosis, foamy hystiocytic collections, reactive gliosis | Steroid | Before |
| Darmoul et al. (7) | 38/Male | Folliculitis | Headache, aphasia, disturbed consciousness | Right hemiplegia | Pseudotumoral lesion in the left capsulo-thalamic region extending to the homolateral peduncle | Biopsy was not performed | Steroid Immunsupresive theraphy | After |
| Imoto et al. (9) | 50/male | OU, GU, Skin eruption | Dystasia dysbasia | Left hemiparesis (+) left Babinski reflex dysarthria | Low density area with significant mass effect in the right basal ganglia and thalamus with ring like enhancement following contrast injection | Large numbers of chronic inflammatory cells mainly in the Virchow robin spaces | Steroid | After |
| Schmolck et al. (10) | 39/male | OU, GU | Recurrent aseptic meningitis, diplopia, dysarthria | Right sensorimotor hemiparesis with hyperreflexia, right sided dysmetria, disdiadochokinesis, ataxic gait | Lesion in the left thalamus with patchy contrast enhancement and mass effect | Steroid CYC | Before | |
| Matsuo et al. (11) | 33/male | OU, GU, EN | Nausea, vomiting, headache | Mild right hemiparesis | Mass lesion at the left basal ganglia extending to the ventral side of the midbrain | Mild reactive gliosis | Steroid | Before |
| Bennett et al. (12) | 23/Male | OU, GU, papulopustular rash | Headache, fever, progressive right sided weakness | Right homonymous hemianopia, right spastic hemiparesis | Mass lesion involving the left temporal lobe | Perivascular inflammatory infiltrate, no neoplasia | Steroid, AZA | Before |
| Yoshimura et al. (13) | 41/Female | OU, GU, EN | Mental deterioration, right hemiparesis | Poor mental activity, dysarthria and right hemiparesis | Homogeneous hypodense lesion of the left lenticulothalamic region, mild mass effect | Ruled out a tumour but did not show any specific diagnosis | Steroid | After |
| Ben Taarit et al.14 | 26/Female | OU, GU | Left hemiplegia | Pseudotumoral lesion in the protuberance and the right cerebral pedicule | Steroid | After | ||
| Park et al.15 | 52/female | OU, EN like skin lesion and arthritis | Dizziness, nausea, vomiting | Brun’s nystagmus | T2WI showed high signal intensity in the right cerebellar hemisphere, post medulla, pons with an isosignal central mass, GdT1WI showed homogenously enhancing mass like lesion inside the T2 high signal intensity | Biopsy was not performed | Steroid AZA | After |
| Presented case | 27/Female | OU, GU, skin lesions | Headache, vomiting | Left hemiparesis | hyperintense lesion in the brainstem | Biopsy was not performed | Steroid AZA | After |
NB: neuro-Behçet; MLL: mass-like lesion; BD: Behçet’s disease; MRI: magnetic resonance imaging; CT: computerised tomography; OU: oral ulcer; GU: genital ulcer; AU: anterior uveitis; EN: erythema nodosum; CYC: cyclophosphamide; AZA: azathiopurin
The affected regions of the brain were the pons and thalamus in 11 patients. However, a mass-like lesion was located in the frontotemporal region and temporal region in 2 patients. A brain biopsy was performed in 7 patients, and histological findings showed the presence of perivascular inflammation and various degrees of gliosis. Reactive gliosis, panvasculitis, thrombosis and extensive infarction have been showed in an NB patient with a mass-like lesion by Tuzgen et al. (4). Yoshimura reported no specific pathological findings in a patient with BD (13).
Genital ulceration, eye involvement, skin lesions and arthritis/arthralgia were less commonly reported in NB patients with mass-like lesions compared to NB patients without this type of lesion (Table 2).
Table 2.
Comparison of clinical features of the patients with NB with those BD patients with mass-like lesion
| NB patients [6] Group 1 n=200 |
NB patients with mass-like lesion Group 2 n=13 |
|
|---|---|---|
| Mean age (admission) | 33 | 40 |
| M/F | 3.4 | 0.8 |
| Aphtae | 200 (100%) | 12 (92.3%) |
| GU | 188 (94%) | 11 (84.6%) |
| Eye involvement | 129 (66%) | 2 (15.3%) |
| Skin Lesions | 166 (84%) | 7 (53.8%) |
| Arthritis/arthralgia | 112 (56%) | 2 (15.4%) |
| Thrombophlebitis | 66 (33%) | ? |
| Arterial involvement | 7 (3.5%) | ? |
| Pathergy | 166 (83%) | Reported only in 1 patient as positive |
| GIS | 6 (3%) | ? |
| Pulmonary | 14 (7%) | ? |
M: Male; F: female; GU: genital ulceration; GIS: gastrointestinal system; NB: neuro-Behçet; BD: Behçet’s disease
Discussion
Our patient’s clinical manifestations, including oral and genital ulcers and papulopustular lesions, met the ISG criteria for BD (16). Because neurological symptoms developed alongside the mucocutaneous manifestations of BD, the mass-like lesion was considered secondary to BD rather than due to other diseases such as multiple sclerosis, sarcoidosis or tumour. Cerebrospinal fluid was not examined because of the risk of herniation and stimulating a pathergy reaction with the LP needle. Brain biopsy was not performed for the same reasons and also due to a lack of experienced staff. Therefore, it is difficult to say whether the mass-like lesion in this patient was secondary to inflammatory or a tumour-development process. Considering the fact that glioma is a malignant tumour and its prognosis is not good, the mass in the brain was considered unlikely to be a tumour. Complete resolution of the mass-like lesion after treatment with glucocorticoid and AZA may imply that this lesion was the result of a vasculitic process such as BD. An interesting finding in this patient was the fact that they had mucocutaneous manifestations suggestive of BD prior to the development of neurological symptoms. It can be speculated that the early diagnosis of BD in this patient might have prevented the development of neurological involvement and its poor outcome.
The reported frequency of CNS involvement in BD varies from 2.2 to 50% (1). Diagnosis can be made clinically because most cases with CNS involvement occur after the onset of manifestations of BD (11, 15). It has been reported that the appearance of neurological symptoms takes several years after the onset of symptoms that are diagnostic of BD (11). The diagnosis can be difficult to make when central nervous system involvement precedes the mucocutaneous involvement or when the history of mucocutaneous lesions is incomplete or absent (15). Moreover, the presence of a mass-like lesion in these patients will further increase the diagnostic difficulties. In this group, BD was diagnosed after the onset of neurological involvement in 8 patients with BD (61.5%; Table 1).
Neuro-Behçet can be seen in two different patterns: parenchymal and non-parenchymal involvement (2). Parenchymal involvement is the most common type, seen in 82% of cases with BD; the pathological process tends to produce focal lesions, mainly in the brainstem, basal ganglia, diencephalic structures and internal capsules (2). Pathological findings of acute parenchymal involvement includes perivascular cuffing with lymphocytes, neutrophils and macrophages, demyelination with vasculitis, areas of necrosis, glial proliferation and apoptotic neuronal loss (3, 17, 18). In the chronic progressive form of the disease, inflammatory infiltration remains, with a lower predominance of lymphocytes, axonal loss and gliosis (18). Autopsy studies and biopsy specimens of the patients with NB disease showed vasculitis with a venous predominance (19, 10), but there was no evidence of fibrinoid necrosis (3). Perivascular infiltration of sudanophilic foam-cells has been described in both forms of NB (17). However, there is limited knowledge about the histopathological findings of mass-like lesions in NB. Perivascular lymphocytic infiltration without necrosis, secondary gliosis, petechial haemorrhage, and demyelination have all been reported (15, 18). Also, plasma cell infiltration, hyaline thickening of the vascular wall and ischemic necrotic foci can be seen (15). The histological findings of a mass-like lesion in the literature are similar to the findings of acute and chronic progressive NB disease. Siva et al. (19) suggested that mass-like lesions in NB patients could be caused by acute inflammatory oedematous lesions as these kinds of lesions are promptly resolved by glucocorticoids. However, even though there are limited data, the biopsy findings of mass-like lesions do not seem to be different from the results of cerebral biopsies obtained from NB cases.
Magnetic resonance imaging is currently the most sensitive tool for diagnosing NB. However, there are still limitations, as there is overlap in the differential diagnosis with tumours, abscesses and multiple sclerosis (5) and a stereotactic biopsy may be necessary. The most common lesions seen on MRI in NB patients extend from the brainstem to the basal ganglia. None of the cases reported previously had characteristic MRI signs. In our patient, MRI revealed a mass-like lesion located close to the brainstem and she complained of headache and weakness in the left side of the body. Headache, hemiparesis, dizziness, aphasia, nausea and vomiting were the most common presenting manifestations of NB patients with mass-like lesions.
High dose corticosteroids are usually used for the treatment of NB disease, followed by immunosuppressive therapy with cyclophosphamide or azathioprine (9); this was the common therapy for the published cases. These treatment modalities result in a dramatic improvement in 83% of patients (9). In this review, 12 patients with mass-like lesion received glucocorticoids and this treatment resolved their clinical manifestations.
In conclusion, NB disease should be considered a differential diagnosis in the presence of cerebral mass-like lesions even though other manifestations of BD are not obvious.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: Written informed consent was obtained from the patient.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - N.Ş.Y.B., S.Ş., T.K., C.K.; Design - N.Ş.Y.B., S.Ş., T.K., C.K.; Supervision - N.Ş.Y.B., C.K.; Resource - N.Ş.Y.B., S.Ş., T.K., C.K.; Materials - N.Ş.Y.B., C.K.; Data Collection&/or Processing - N.Ş.Y.B., C.K.; Analysis&/or Interpretation - N.Ş.Y.B., C.K.; Literature Search - N.Ş.Y.B., C.K.; Writing - N.Ş.Y.B., S.Ş., T.K., C.K.; Critical Reviews - C.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Appenzeller S, Castro de R, de Queiroz LS, Madegan L, Soledade C, de Zanardi VA, et al. Brain tumor like lesion in Behçet disease. Rheumatol Int. 2006;26:577–80. doi: 10.1007/s00296-005-0082-3. http://dx.doi.org/10.1007/s00296-005-0082-3. [DOI] [PubMed] [Google Scholar]
- 2.Serdaroğlu P. Behçet’s disease and the nervous system. J Neurol. 1998;245:197–205. doi: 10.1007/s004150050205. http://dx.doi.org/10.1007/s004150050205. [DOI] [PubMed] [Google Scholar]
- 3.Al-Araji A, Kidd DP. Neuro-Behçet’s disease: epidemiology, clinical characteristics, and management. Lancet Neurol. 2009;8:192–204. doi: 10.1016/S1474-4422(09)70015-8. http://dx.doi.org/10.1016/S1474-4422%2809%2970015-8. [DOI] [PubMed] [Google Scholar]
- 4.Tuzgen S, Kaya AH, Erdincler D, Oguzoglu SA, Ulu O, Saip S. Two cases of Neuro-Behçet’s disease mimicking cerebral Tumor. Neurol India. 2003;51:376–8. [PubMed] [Google Scholar]
- 5.Heo JH, Lee ST, Chu K, Kim M. Neuro-Behçet’s disease mimicking multiple brain tumors: diffusion-weighted MR study and literature review. J Neurol Sci. 2008;264:177–81. doi: 10.1016/j.jns.2007.07.029. http://dx.doi.org/10.1016/j.jns.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Akman-Demir G, Serdaroglu P, Tasçi B. Clinical patterns of neurological involvement in Behçet’s disease: evaluation of 200 patients. The Neuro-Behçet Study Group. Brain. 1999;122:2171–82. doi: 10.1093/brain/122.11.2171. http://dx.doi.org/10.1093/brain/122.11.2171. [DOI] [PubMed] [Google Scholar]
- 7.Darmoul M, Habib Bouhaouala M, Smida H, Hedi Dougui M. Pseudo-tumoral neuro-Behçet’s disease. Rev Neurol. 2006;162:643–7. doi: 10.1016/s0035-3787(06)75060-3. http://dx.doi.org/10.1016/S0035-3787%2806%2975060-3. [DOI] [PubMed] [Google Scholar]
- 8.Given CA, 2nd, Stevens BS, Lee C. The MRI appearance of tumefactive demyelinating lesions. AJR Am J Roentgenol. 2004;182:195–9. doi: 10.2214/ajr.182.1.1820195. http://dx.doi.org/10.2214/ajr.182.1.1820195. [DOI] [PubMed] [Google Scholar]
- 9.Imoto H, Nishizaki T, Nogami K, Sakamoto K, Nomura S, Akimura T, et al. Neuro-Behçet’s disease manifesting as a neoplasm-like lesion. Neurol Med Chir (Tokyo) 2002;42:406–9. doi: 10.2176/nmc.42.406. http://dx.doi.org/10.2176/nmc.42.406. [DOI] [PubMed] [Google Scholar]
- 10.Schmolck H. Large thalamic mass due to neuro-Behçet disease. Neurology. 2005;65:436. doi: 10.1212/01.wnl.0000179219.97769.6a. http://dx.doi.org/10.1212/01.wnl.0000179219.97769.6a. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K, Yamada K, Nakajima K, Nakagawa M. Neuro-Behçet disease mimicking brain tumor. AJNR Am J Neuroradiol. 2005;26:650–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett DL, McCabe DJ, Stevens JM, Mifsud V, Kitchen ND, Giovannoni G. Tumefactive neuro-Behçet disease. Neurology. 2004;63:709. doi: 10.1212/01.wnl.0000130357.51278.cf. http://dx.doi.org/10.1212/01.WNL.0000130357.51278.CF. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura J, Toyama M, Sekihara Y, Tamatani S, Nagai H, Fujita S, et al. Behçet disease mimicking a thalamic tumor. No Shinkei Geka. 2001;29:527–31. [PubMed] [Google Scholar]
- 14.Ben Taarit C, Turki S, Ben Maïz H. Pseudotumoral neurobehçet: a case report. J Mal Vasc. 2002;27:93–5. [PubMed] [Google Scholar]
- 15.Park JH, Jung MK, Bang CO, Park HK, Sung KB, Ahn MY, et al. Neuro-Behçet’s disease mimicking a cerebral tumor: a case report. J Korean Med Sci. 2002;17:718–22. doi: 10.3346/jkms.2002.17.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 17.Borhani Haghighi A, Pourmand R, Nikseresht AR. Neuro-Behçet disease. A review. Neurologist. 2005;11:80–9. doi: 10.1097/01.nrl.0000156343.16797.c4. http://dx.doi.org/10.1097/01.nrl.0000156343.16797.c4. [DOI] [PubMed] [Google Scholar]
- 18.Hirohata S. Histopathology of central nervous system lesions in Behçet’s disease. J Neurol Sci. 2008;267:41–47. doi: 10.1016/j.jns.2007.09.041. http://dx.doi.org/10.1016/j.jns.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Siva A, Altintas A, Saip S. Behçet’s syndrome and the nervous system. Curr Opin Neurol. 2004;17:347–57. doi: 10.1097/00019052-200406000-00017. http://dx.doi.org/10.1097/00019052-200406000-00017. [DOI] [PubMed] [Google Scholar]