Abstract
Objective
To define the best cut-off value for identifying Achilles tendon thickening using ultrasound (US) in patients with spondyloarthropathies (SpA) and to assess its diagnostic utility in comparison with different cut-off values used in the literature.
Material and Methods
One-hundred and one subjects (55 SpA patients and 46 age and body mass index ((BMI)-matched healthy controls (HC)) were investigated. US was performed using a MyLab70 US system (Esaote Biomedica, Genoa, Italy) with a linear probe (6–18 MHz). Three images per Achilles enthesis were stored and the antero-posterior thickness of the enthesis was measured at the level of the Achilles tendon deeper margin insertion into the calcaneal bone on the longitudinal median scan. The best cut-off value for each gender was determined by ROC curve analysis and compared to the other cut-off values in the literature: 1) 5.29 mm for both genders, and 2) 5.5 mm for females and 6.2 mm for males. The number of measurements exceeding the cut-off values as well as sensitivity (SE), specificity (SP), positive (PPV) and negative (NPV) predictive values were calculated.
Results
A significant difference was observed for Achilles enthesis thickness between genders (mean±SD: 4.6±0.7 mm in males vs. 4.0±0.8 mm in females, p<0.00) and between SpA patients and HC (mean±SD: 4.4±0.8 mm in SpA patients vs. 4.0±0.8 mm in HC, p<0.001). The ROC curve analysis revealed the best cut-off value to be 3.7 mm for females and 4.8 mm for males (SE: 43–70%, SP: 59–85%, PPV: 66–79%, NPV: 54–63%). Previously reported cut-off values were found to have high SP (91–98%) but very low SE (2–11%).
Conclusion
Achilles tendon thickness differs between genders; thus, it is crucial to refer to normal values that are specific for gender. High cut-off values, as previously suggested, showed very low SE in the current study. When Achilles enthesis thickening is used for the purpose of screening enthesitis in SpA patients, a lower cut-off value has a higher SE with slightly worse SP, PPV and NPVs.
Keywords: Achilles tendon thickness, ultrasound, enthesitis, seronegative spondyloarthropathies
Introduction
Enthesis is considered to be the target tissue of inflammation in SpAs and Achilles tendon (AT) insertion into the calcaneal bone is one of the most frequently involved enthesis (1, 2). Ultrasound (US) is thought to be the gold standard imaging technique for assessing tendons (3) and US findings for the term “enthesopathy” were clarified in Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) 7 by the US special interest group (4). Tendon thickening is one of those findings, but its presence is quite non-specific, being detectable in various pathologic conditions (5).
To date, there is a noticeable lack of a standardised method for measuring AT thickness using US. In some studies, the insertion site of the tendon was used for measuring the thickness, while in others the measurement was taken 2 or 3 cm proximal to the insertion site (6–10). Despite Schmidt et al. (8) reporting a difference in AT thickness between males and females, a single value for both genders was used in studies scoring enthesitis (6, 7, 10–12).
The aims of the present study were, therefore, to define where to measure the thickness of Achilles tendon, to determine a cut-off value and to assess its diagnostic utility in comparison with different cut-off values used in the literature.
Material and Methods
Patients
Fifty-five SpA patients, diagnosed according to the European Spondyloarthropathy Study Group criteria (13) and 46 age-, gender-, and body mass index (BMI)-matched healthy controls (HC) were included in the study. Within the group of SpA patients, 31 had ankylosing spondylitis and 24 had psoriatic arthritis. At the time of evaluation, only 21.7% of SpA patients had clinical signs of Achilles enthesitis and/or retrocalcaneal bursitis including tenderness and swelling, while 48.9 % had Achillodynia in their history.
The study was performed in the rheumatology outpatient unit of the Marmara University Hospital, Istanbul, Turkey and was approved by the Local Research Ethics Committee. Informed consent was obtained from all patients and controls.
Ultrasound
Ultrasound (US) examinations were carried out with patients in the prone position with their feet hanging off the examination table in a neutral position (90 degrees of flexion) using a MyLab70 US system (Esaote Biomedica, Genoa, Italy) equipped with a broadband 6–18 MHz linear probe. In all patients, both ATs were scanned.
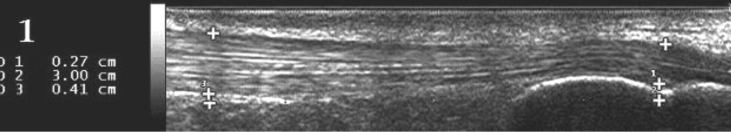
Antero-posterior AT thickness was measured on the same longitudinal median scan at two different levels: at the insertion of the AT deeper margin into the calcaneal bone and 3 cm more proximal (Figure 1).
Figure 1.
Antero-posterior Achilles tendon (AT) thickness measured on the same longitudinal median scan at two different levels: 1st measurement: At the insertion of the AT deeper margin into the calcaneal bone, 2nd measurement: 3 cm proximal to the first measurement
A set of three US images was stored for each AT using the longitudinal median scan and measurements were taken at the end of the study by the sonographer (SZA) on anonymous US images. The mean values of three measurements were used for statistical analysis.
Statistical analysis
The MedCalc software package (V.4.2.0 for Windows) was used for statistical analyses. Data are expressed as mean and standard deviation (SD). Preliminary analysis of the data showed a significant difference between the genders; thus, the statistical analysis was performed separately for each gender. Continuous variables between the two groups were compared using Mann-Whitney U or Student’s t tests where appropriate, according to the pattern of distribution. A receiver operating characteristics (ROC) curve was generated by plotting sensitivity (y axis) against 1-specificity (x axis) to find the best cut-off value for entheseal thickness. A multivariate logistic regression analysis was performed to examine relationships among age, BMI and tendon measurements.
The sensitivities, specificities, and positive and negative predictive values with the calculation of accuracies were obtained by using the following cut-off values: 1) 5.29 mm for both genders as suggested by Balint et al. (6), 2) 5.5 mm for females and 6.2 mm for males, according to Schmidt et al. (8), and 3) The cut-off values obtained in the current study.
The intra-observer reliability between different measurements of the same patient was determined using intraclass-coefficient correlation (ICC) and 95% Confidence Interval (95%CI). Three ICCs were obtained separately for both enthesis and tendon by comparing two measurements at a time and the results are given as a range of ICCs (95%CI).
Results
The age [mean (SD) years: SpA: 40.2 (10.7) vs. HC: 36.4 (10), p=0.07], BMI [mean (SD): SpA-26.9 (4.8) vs. HC-25.7 (3.6), p=0.18] and gender (female/male SpA-33/22 vs. HC-29/17, p=0.84) were not significantly different between SpA patients and HC.
Entheseal and tendon thickness measurements
Entheseal thickness measurements were observed to be significantly higher in SpA patients compared to HC, whereas no significant difference was found for AT thickness (Table 1). Both AT and enthesis were significantly thicker in males than in females (for the enthesis: 4.6±0.7 vs. 4.0±0.8 mm, p<0.001; for the tendon: 4.5±0.6 vs. 4.2±0.6 mm, p<0.001) in the whole study population. Thus, further data analysis was performed separately for each gender.
Table 1.
The thickness of Achilles tendon (mean±SD) (mm) measured with US in patients with spondyloarthropathies (SpA) and healthy controls (HC)
| SpA | HC | p | |
|---|---|---|---|
| Entheseal thickness | 4.4±0.8 | 4.0±0.8 | <0.001 |
| Females | 4.2±0.8 | 3.7±0.6 | 0.001 |
| Males | 4.7±0.6 | 4.4±0.8 | 0.07 |
| AT thickness | 4.3±0.6 | 4.3±0.5 | 0.3 |
| Females | 4.2±0.6 | 4.1±0.5 | 0.5 |
| Males | 4.6±0.6 | 4.5±0.5 | 0.6 |
US: ultrasound; AT: Achilles tendon; SpA: spondyloarthropathies; HC: healthy controls
In females, entheseal thickness was observed to be significantly higher in SpA patients compared to HC; however, no significant difference was present at the AT level. In males, both entheseal and tendon thicknesses were not significantly different in SpA patients and HC, despite a tendency towards an increased thickening of the enthesis in SpA (p=0.07).
ROC curve analysis
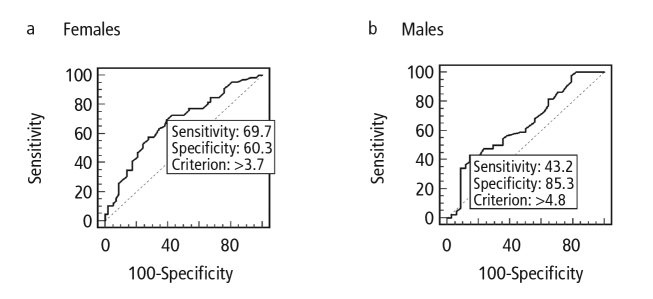
ROC curve analysis was performed in order to define the best cut-off values for obtaining the highest values of sensitivity and specificity for both genders (Figure 2). Using the cut-off value of 3.7 mm for entheseal thickness in females, the sensitivity was 70% and the specificity was 59% (24/58 of HC vs. 46/66 of SpA had high levels of entheseal thickness for this cut-off). The best cut-off value was determined as 4.8 mm for males, with a sensitivity of 43% and a specificity of 85% (The ratio of AT in males exceeding the cut-off value was 5/34 (20.6%) for HC and 19/44 (43.2 %) for SpA).
Figure 2.
a, b. ROC curve, obtained by plotting sensitivity for detecting SpA (y axis) against specificity (x axis). Healthy controls were used as the control group for determining specificity. ROC curve for entheseal thickness in females. Area under curve = 0.676 (a). ROC curve for entheseal thickness in males. Area under curve = 0.638 (b).
The average ICCs for both entheseal and tendon thickness measurements demonstrated high concordance [ICCs (95%CI) for enthesis: 0.88–0.92 (0.85–0.93), for tendon: 0.79–0.85 (0.75–0.88)].
Multivariate analysis
In multiple regression analysis, a significant correlation was found between BMI and entheseal thickness, but not age, in males (r2=0.202, p<0.001). In females, in addition to BMI, age was also weakly correlated with entheseal thickness (r2=0.060, p=0.02).
Comparison with different cut-off values
Cut-off values for Balint et al. (6) and Schmidt et al. (8) were found to have high specificities (91–98%), however with very low sensitivities (2–11%) (Table 2). The SE and SP using lower cut-off values detected in our study resulted in a higher SE (43–70%) with a slightly lower SP (59–85%).
Table 2.
Diagnostic value of different cut-off levels of entheseal thickness
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Balint et al. (6) | 10 | 96 | 73 | 47 | 54 |
| Females | 11 | 98 | 88 | 49 | 57 |
| Males | 11 | 91 | 63 | 44 | 49 |
| Schmidt et al. (8) | 4 | 99 | 80 | 46 | 48 |
| Females | 5 | 98 | 75 | 48 | 50 |
| Males | 2 | 97 | 50 | 43 | 44 |
| Current study | 53 | 74 | 70 | 57 | 62 |
| Females | 58 | 71 | 69 | 59 | 63 |
| Males | 46 | 79 | 74 | 53 | 62 |
PPV: positive predictive values; NPV: negative predictive values
Discussion
Achilles Tendon enthesopathy is a common musculoskeletal disorder. AT pathology was previously classified according to its localisation by Baxter and Clain as insertional or non-insertional tendinitis with the former occurring within 2 cm of the calcaneal insertion (14). This classification was suggested to be useful for therapeutic approaches; however, it might also be relevant to the identification of the aetiological factors of tendon pathology.
Measurement of the thickness at the proximal site of a tendon is unlikely to demonstrate the involvement in SpA, as tendon involvement as a sole pathology is rare and expected to exist only if inflammation spreads proximally or secondary to mechanical factors. A comparison between thickness of the insertion site and 3 cm above of AT in SpA was made by Olivieri et al. (9), with results emphasising that the inflammation usually extends proximally from the enthesis. In accordance with these observations, we observed that AT was thicker at the level of insertion than HC in SpA patients; therefore, we suggest measuring the thickness at the level of tendon insertion when performing the US of a SpA patient.
Schmidt et al. (8) have previously reported a difference of half a millimetre in the mean AT thickness of male and female healthy subjects. In the present study, we also found a difference between genders of the AT tendon both at the insertional level and 3 cm more proximally. This highlights the importance of defining normal values for two genders separately, to decrease false negativity in females and false positivity in males.
Many factors affect the thickness of AT, including physiological variations. In our study, the thickness of the AT enthesis was highly affected by BMI in males and by both age and BMI in females. Therefore, it is essential to include the data from BMI-, age- and gender-matched healthy controls in comparative studies when defining normal values.
The lack of a diseased control group is among the limitations of our study. Other diseases that might be considered in the differential diagnosis of SpAs, like mechanical low back pain or anatomical disorders of the foot, should also be investigated. The sonographer (SZA) was not completely blinded to the clinical data as some of the patients with SpA had characteristic phenotypes due to vertebral deformities and/or psoriatic lesions around the heel. Measurements were performed anonymously at the end of the study to partially overcome this bias. Another limitation is the low number of patients when data were separated according to gender. The lack of a significant difference in entheseal thickness in SpA vs. HC may be due to a type II error with an insufficient number of male participants.
In conclusion, AT thickness at the entheseal level seems to be a better discriminator for SpA. As the entheseal thickness differs between genders, it is crucial to refer to normal values specific for gender. High cut-off values, previously suggested, showed very low SE in the current study. When Achilles enthesis thickening is used for the purpose of screening enthesitis in SpA patients, a lower cut-off value has a higher SE with slightly worse SP, positive predictive values (PPV) and negative predictive values (NPV).
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Local Research Ethics Committee.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - S.Z.A., E.F., H.D.; Design - S.Z.A., E.F., H.D.; Supervision - P.A., W.G., S.Y., H.D.; Resource - S.Z.A., E.F., H.D.; .; Materials - S.Z.A., E.F., H.D.; P.A. Data Collection&/or Processing - S.Z.A., E.F., H.D.; P.A. Analysis&/or Interpretation -S.Z.A., E.F., P.A., W.G., S.Y., H.D. Literature Search - S.Z.A.; Writing - S.Z.A., E.F., P.A., W.G., S.Y., H.D.; Critical Reviews - E.F., P.A., W.G., S.Y., H.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Koehler L, Kuipers JG, Zeidler HK. Enthesopathy. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. New York: Mosby; 2003. pp. 1275–81. [Google Scholar]
- 2.Ball J. Enthesopthy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213–23. doi: 10.1136/ard.30.3.213. http://dx.doi.org/10.1136/ard.30.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grassi W, Filippucci E, Farina A, Cervini C. Sonographic imaging of tendons. Arthritis Rheum. 2000;43:969–76. doi: 10.1002/1529-0131(200005)43:5<969::AID-ANR2>3.0.CO;2-4. http://dx.doi.org/10.1002/1529-0131%28200005%2943:5%3C969::AID-ANR2%3E3.0.CO%3B2-4. [DOI] [PubMed] [Google Scholar]
- 4.Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–7. [PubMed] [Google Scholar]
- 5.Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006;45:508–21. doi: 10.1093/rheumatology/kel046. http://dx.doi.org/10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- 6.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–10. doi: 10.1136/ard.61.10.905. http://dx.doi.org/10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondyloarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–33. doi: 10.1002/art.10812. http://dx.doi.org/10.1002/art.10812. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt WA, Schmidt H, Schicke B, Gromnica-Ihle E. Standard reference values for musculo skeletal ultrasonography. Ann Rheum Dis. 2004;63:988–94. doi: 10.1136/ard.2003.015081. http://dx.doi.org/10.1136/ard.2003.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivieri I, Barozzi L, Padula A, De Matteis M, Pierro A, Cantini F, et al. Retrocalcaneal bursitis in spondyloarthropathy: assessment by ultrasonography and magnetic resonance imaging. J Rheumatol. 1998;25:1352–7. [PubMed] [Google Scholar]
- 10.Filippucci E, Aydin SZ, Karadag O, Salaffi F, Gutierrez M, Direskeneli H, et al. Reliability of high-resolution ultrasonography in the assessment of Achilles tendon enthesopathy in sero negative spondyloarthropathies. Ann Rheum Dis. 2009;68:1850–5. doi: 10.1136/ard.2008.096511. http://dx.doi.org/10.1136/ard.2008.096511. [DOI] [PubMed] [Google Scholar]
- 11.Alcalde M, Acebes JC, Cruz M, González-Hombrado l, Herrero-Beaumont G, Sánchez-Pernaute O. A sonographic enthesitic index of lower limbs is a valuable tool in the assessment of ankylosing spondylitis. Ann Rheum Dis. 2007;66:1015–9. doi: 10.1136/ard.2006.062174. http://dx.doi.org/10.1136/ard.2006.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Miguel E, Cobo T, Mu-oz-Fernández S, Naredo E, Usón J, Acebes JC, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68:169–74. doi: 10.1136/ard.2007.084251. http://dx.doi.org/10.1136/ard.2007.084251. [DOI] [PubMed] [Google Scholar]
- 13.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. http://dx.doi.org/10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 14.Clain MR, Baxter DE. Achilles tendinitis. Foot Ankle. 1992;13:482–7. doi: 10.1177/107110079201300810. http://dx.doi.org/10.1177/107110079201300810. [DOI] [PubMed] [Google Scholar]