Abstract
Objective
Ankylosing spondylitis (AS) is a chronic inflammatory disease of the spine and sacroiliac joints of unknown etiology. Recent studies have reported increased oxidative stress, which is implicated in the pathogenesis of a number of diseases, in AS. The purpose of this study was to investigate oxidative stress and related factors in AS.
Material and Methods
Eighty-five patients with AS [36 (16–64) years; 65 male/20 female] and 56 healthy subjects [36 (21–63) years; 39 male/17 female] with no known cardiovascular risk factors were enrolled. Serum total oxidant status (TOS) and total anti-oxidant status (TAS) were studied. The Bath ankylosing spondylitis functional index (BASFI), Bath ankylosing spondylitis disease activity index (BASDAI), and Bath ankylosing spondylitis metrology index (BASMI) were calculated. A logistic regression model was used to identify the independent risk factors for TOS.
Results
No differences were observed in terms of demographic characteristics, laboratory findings, or TAS concentrations between the patient and control groups. However, the serum TOS levels were significantly higher in the AS group than in the controls (p=0.003). The comparison of cases of active (BASDAI ≥4) and inactive AS revealed significantly higher TOS levels in the active disease group. The TOS and TAS concentrations did not differ between patients treated with biological agents and those treated with conventional agents. Correlation analysis yielded significant correlations between TOS and TAS, BASMI, BASFI, BASDAI, erythrocyte sedimentation rate (ESR), and high-sensitive C-reactive protein (hs-CRP) (p<0.05; r values ranged from 0.291 to 0.452) and a positive correlation between TAS and BASMI (p<0.05; r=0.344). Based on regression analysis, BASDAI, BASMI, and hs-CRP independently predicted the TOS levels [p<0.05, R2: 0.262, and standard error of the estimate (SEE): 10.96]
Conclusion
Oxidative stress levels were higher in patients with AS than in healthy subjects. Patients with active disease status had significantly higher oxidative stress than patients with inactive disease status and healthy controls. Treatment status has no effect on TOS, and BASMI, BASDAI, and hs-CRP are independent variables associated with TOS. The TAS levels were found to be associated with only BASMI.
Keywords: Oxidative stress, spondylitis, anklylosing, outcome assessment
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease of the spine and sacroiliac joints of unknown etiology (1). Increased oxidative stress is implicated in the pathogenesis of some diseases such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). Recent studies have also reported increased oxidative stress in AS (2–5).
Under normal conditions, there is a balance between oxidative damage and antioxidant defense. Insufficient antioxidant defense or increased production of reactive oxygen species (ROS) generates a condition known as oxidative stress (6).
Oxidative stress can be measured by total antioxidant status (TAS) and total oxidant status (TOS), systematic reflections of it. Because the separate measurement of different oxidant and antioxidant molecules is not practical and their oxidant and antioxidant effects are cumulative, the total oxidant and antioxidant capacity of a sample is measured instead, providing TOS and TAS values, respectively (7).
This may help to explain the pathogenesis. Insufficient data are available concerning the relations between oxidative stress, antioxidative capacity, and disease activity or biological treatment in AS.
Further studies on the subject are needed. The primary aim of this study was to investigate oxidative stress and related factors in AS.
Material and Methods
Patients and controls
Eighty-five patients with AS meeting the Modified New York criteria (6) and with no known acute or chronic diseases were included. The exclusion criteria were hypertension (HT), diabetes mellitus (DM), hyperlipidemia, and cigarette use. Fifty-six healthy controls subject to the same exclusion criteria as the patients were recruited from among the relatives of health professionals and blood donors. Patients receiving corticosteroid therapy within 8 weeks of the beginning of the study were also excluded. The disease duration and a detailed history of biological treatment were recorded.
Patients were also evaluated using the Bath ankylosing spondylitis metrology index (BASMI) (range: 0–10)(8), Bath ankylosing spondylitis functional index (BASFI) (range: 0–10) (9, 10), and Bath ankylosing spondylitis disease activity index (BASDAI) (range: 0–10) (11, 12). Written informed consent was obtained from each subject, and research protocols were approved by the Local Ethical Committee.
Definition of variables
Hypertension
Average systolic BP ≥140 mmHg or average diastolic BP ≥90 mmHg or receiving treatment for HT (13).
Dyslipidemia
Total cholesterol level ≥260 mg/dL or low density lipoprotein (LDL) cholesterol ≥160 mg/dL or the use of lipid lowering medication (14).
Diabetes mellitus (DM)
Current use of medications prescribed to treat DM or fasting serum glucose levels ≥126 mg/dL (15).
Smoking
Any tobacco consumption in the previous 30 days.
Active AS disease
Patients with BASDAI ≥4 at the time of the study were defined as having active disease.
Laboratory tests
Blood samples were collected for tests after overnight fasting. The erythrocyte sedimentation rate (ESR), high-sensitivity C-reactive protein (hs-CRP), fasting blood glucose, and serum lipids [total cholesterol, high density lipoprotein (HDL) cholesterol, LDL cholesterol, and triglycerides] were measured using standard methods. Serum measurement of hs-CRP was performed using enzyme-linked immunosorbent assay (ELISA). The serum total oxidant status (TOS, μmol H2O2Eq/L) and total antioxidant status (TAS, μmolTroloxEq/L) were measured using the method described by Erel (16, 17).
Statistical analysis
The results are given as medians with minimum and maximum values. The comparison of continuous variables between groups was performed using the Mann–Whitney U test. Differences between categorical variables were analyzed using Fisher’s exact test. The Kruskal–Wallis test was used to compare multiple groups, and the Mann–Whitney test was used for pairwise comparisons. Relationships between variables were analyzed using Spearman’s rank correlation coefficient. A linear regression model was used to determine the independent risk factors for TOS. Enter methods was used for linear regression analysis.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) (version 16.0 SPSS Inc.; Chicago, Illinois, USA) software. A two-tailed p value of <0.05 was considered statistically significant.
Results
The study group consisted of 85 patients with AS [age 36 (16–64) years; 65 male (M)/20 female (F)] and 56 healthy controls [age 36 (21–63) years; 39 M/17 F]. No differences were determined between the groups in terms of age, sex, body mass index (BMI), waist circumferences, glucose, blood pressure, or lipid levels (p>0.05; Table 1). The mean duration of disease was 10 (1–32) years. BASDAI, BASFI, and BASMI were 2.8 (0.4–8.1), 1.85 (0–7.11), and 1 (0–8), respectively. The TOS concentrations were significantly higher in the patients than in the controls [20.9 (4.2–84.9) vs. 16.2 (3.6–74.9) μmol H2O2Eq/L; p=0.003]. The TAS levels did not differ between the groups [0.76 (0.37–1.07) vs. 0.78 (0.39–1.17) μmolTroloxEq/L; p=0.96]
Table 1.
Comparison of ankylosing spondylitis (AS) patients and healthy controls in terms of clinical and laboratory parameters
| Patients with ankylosing spondylitis (n=85) | Healthy controls (n=56) | p | |
|---|---|---|---|
| Age (years) | 39 (16–64) | 36 (21–63) | 0.25 |
| Sex (M/F) | 65/20 | 39/17 | 0.78 |
| BMI (kg/m2) | 25.1 (16.3–32.9) | 24.9 (16.9–34.3) | 0.72 |
| Waist circumference (cm) | 88 (56–104) | 83 (59–105) | 0.39 |
| ESR (mm/h) | 11 (2–70) | 3 (1–23) | <0.001 |
| Hs-CRP (mg/L) | 10.9 (0.5–17.3) | 2.7 (0.2–5.5) | <0.001 |
| Glucose mg/dL | 89 (71–114) | 89 (78–122) | 0.7 |
| Total cholesterol mg/dL | 178 (90–234) | 185 (99–244) | 0.2 |
| HDL cholesterol mg/dL | 43 (29–71) | 47 (34–78) | 0.16 |
| LDL cholesterol mg/dL | 109 (42–156) | 117 (41–154) | 0.4 |
| Triglyceride mg/dL | 82 (37–216) | 73 (44–244) | 0.53 |
| TOS (μmol H2O2Eq/L) | 20.9 (4.2–84.9) | 16.2 (3.7–74.9) | 0.003 |
| TAS (μmolTroloxEq/L) | 0.76 (0.37–1.07) | 0.78 (0.39–1.17) | 0.96 |
| Systolic blood pressure (mmHg) | 120 (90–135) | 110 (90–130) | 0.06 |
| Diastolic blood pressure (mmHg) | 75 (55–90) | 70 (60–80) | 0.08 |
ESR: erythrocyte sedimentation rate; Hs-CRP: high-sensitive C-reactive protein; BMI: body mass index; HDL: high density lipoprotein; LDL: low density lipoprotein; TOS: total oxidant status; TAS: total antioxidant status; M: male; F: female
A- Comparison of subjects with AS based on treatment
Of the 85 patients, 27 [21 M/6 F; 39 (19–55) years] were being treated with tumor necrosis factor- α (TNF-α) blocking agents (17 etanercept, 5 adalimumab, and 5 infliximab). The mean duration of treatment with biological drugs was 31.1±26 months. Fifty-eight patients [44 M/14 F; 36 (16–52) years] were receiving conventional treatments (non-steroidal anti-inflammatory drugs and/or sulfasalazine), and none had been previously treated with anti-TNF-α agents. Age, sex distribution, BMI, waist circumference, acute phase reactants, disease activity indices, and TAS and TOS concentrations were similar in patients receiving anti-TNF-α blocking agents and those on conventional drug s (p>0.05; Table 2).
Table 2.
Comparison of subjects receiving conventional treatment or anti-tumor necrosis factor-α (anti-TNF-α) drugs
| AS with conventional treatment (n=58) | AS with anti-TNF treatment (n=27) | p | |
|---|---|---|---|
| Age (years) | 36 (16–64) | 39 (19–55) | 0.23 |
| Sex (M/F) | 44/14 | 21/6 | 0.8 |
| BASFI | 2.42 (0–7.11) | 1.65 (0–6.1) | 0.34 |
| BASDAI | 4 (0.35–8.1) | 2.36 (0.64–6.46) | 0.11 |
| BASMI | 0 (0–8) | 1 (0–6) | 0.64 |
| BMI (kg/m2) | 25.2 (16.3–32.5) | 24.4 (19–32.9) | 0.94 |
| Waist circumference (cm) | 87 (56–100) | 88.5 (74–104) | 0.3 |
| ESR (mm/h) | 11 (2–70) | 9 (2–40) | 0.10 |
| Hs-CRP (mg/L) | 11.6 (0.5–17.3) | 6.9 (1–16.4) | 0.06 |
| TOS (μmol H2O2 Eq/L) | 22.9 (4.2–84.9) | 18.3 (6.3–74.7) | 0.2 |
| TAS (μmol Trolox Eq/L) | 0.73 (0.37–1.01) | 0.77 (0.45–1.07) | 0.4 |
| Total cholesterol mg/dL | 175 (90–234) | 185 (110–253) | 0.5 |
| HDL cholesterol mg/dL | 44 (29–83) | 48 (29–87) | 0.4 |
| LDL cholesterol mg/dL | 108 (42–157) | 114 (49–153) | 0.6 |
| Triglyceride mg/dL | 89 (37–236) | 86 (45–216) | 0.7 |
| Systolic blood pressure (mmHg) | 120 (90–135) | 120 (97–135) | 0.2 |
| Diastolic blood pressure (mmHg) | 70 (55–90) | 80 (57–90) | 0.02 |
ESR: erythrocyte sedimentation rate; Hs-CRP: high-sensitive C-reactive protein; BMI: body mass index; BASFI: Bath ankylosing spondylitis functional index; BASDAI: Bath ankylosing spondylitis disease activity index; BASMI: Bath ankylosing spondylitis metrology index; TOS: total oxidant status; TAS: total antioxidant status; HDL: high density lipoprotein; LDL: low density lipoprotein; AS: ankylosing spondylitis; M: male; F: female; anti-TNF: anti-tumor necrosis factor
Results are presented as median (min-max). A two-tailed p value <0.05 was regarded as statistically significant.
B- Comparison of subjects based on disease activity
Forty-four patients with AS had active disease (BASDAI >4).The TOS concentrations were significantly higher in patients with active disease than in inactive patients and healthy controls (Table 3). However, the TOS levels were not significantly different between the patients with inactive disease and the healthy controls. The TAS concentrations were similar between the groups.
Table 3.
Comparison of subjects in terms of disease activity
| Patients with active AS (n=44) | Patients with inactive AS (n=38) | Healthy controls (n=56) | |
|---|---|---|---|
| Age (years) | 38.7±10.0 | 35.2±10.1 | 36 (21–63) |
| Sex (M/F) | 34/10 | 29/9 | 39/17 |
| Waist circumference (cm) | 89 (63–108) | 87 (56–107) | 83 (59–105) |
| BMI (kg/m2) | 26 (16–34) | 24 (18–34) | 25 (16–34) |
| ESR (mm/h) | 26 (3–83)* | 12 (2–49)** | 3 (1–23) |
| Hs-CRP (mg/L) | 9.3(1.3–17.3)* | 8.0 (0.5–15.9)** | 2.7 (0.2–5.5) |
| TOS (μmol H2O2 Eq/L) | 28.6 (6.4–84.9)£ | 15.2 (4.2–76.4) | 16.2 (3.6–74.9) |
| TAS (μmolTroloxEq/L) | 0.76 (0.4–1.1) | 0.88 (0.3–1.63) | 0.78 (0.39–1.17) |
| Total cholesterol mg/dL | 184 (90–253) | 171 (110–234) | 190 (99–244 |
| HDL cholesterol mg/dL | 43 (29–87) | 47 (29–83) | 185 (99–244) |
| LDL cholesterol mg/dL | 113 (42–153) | 103 (49–157) | 47 (34–78) |
| Triglyceride mg/dL | 98 (43–236) | 74 (37–216) | 117 (41–154) |
| Systolic blood pressure (mmHg) | 120 (95–135) | 110 (97–135) | 73 (44–244) |
| Diastolic blood pressure (mmHg) | 80 (55–90) | 72 (57–90) | 16.2 (3.7–74.9) |
BMI: body mass index; ESR: erythrocyte sedimentation rate; Hs-CRP: high-sensitive C-reactive protein; TOS: total oxidant status; TAS: total antioxidant status; HDL: high density lipoprotein; LDL: low density lipoprotein; AS: ankylosing spondylitis; M: male; F: female
Results are presented as median (min-max). P values <0.05 were regarded as statistically significant.
Indicates a significant difference between active AS patients vs. healthy controls,
inactive AS patients vs. healthy subjects,
active AS patients vs healthy controls and inactive AS patients.
C- Correlation and regression analysis
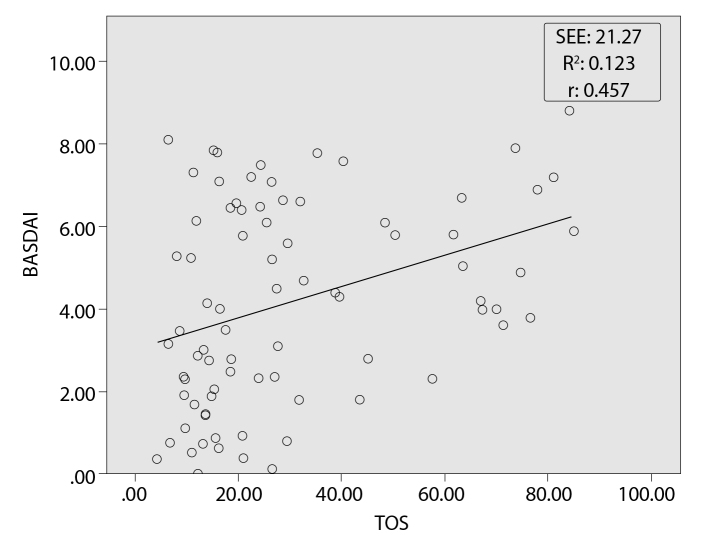
Correlation analysis yielded significant correlations between TOS and TAS, BASMI, BASFI, BASDAI, ESR, and hs-CRP (p<0.05; r=0.291, 0.451, 0.271, 0.363, 0.456, and 0.437, respectively). The highest correlation values were between TOS and BASDAI (Figure 1). In addition, a positive correlation was observed between TAS and BASMI (p<0.05; r=0.344). At regression analysis, BASMI, BASDAI, and CRP independently predicted TOS levels (Table 4). R2 was found to be 0.262, and the standard error of the estimate (SEE) was found to be 10.96 for this model.
Figure 1.
A correlation plot showing the relation between total oxidant status and bath ankylosing spondylitis disease activity index
Table 4.
Regression analysis of related factors for the total oxidant status (TOS) levels
| Variables | p | B | 95% CI |
|---|---|---|---|
| Age | >0.05 | 1.2 | 0.8–2.04 |
| BASMI | <0.05 | 2.6 | 1.6–4.7 |
| BASDAI | <0.05 | 5.3 | 1.9–8.7 |
| hs-CRP | <0.05 | 2.8 | 1.2–5.2 |
BASDAI: bath ankylosing spondylitis disease activity index; BASMI: bath ankylosing spondylitis metrology index; hs-CRP: high-sensitive C-reactive protein; CI: confidence interval
Discussion
In this study, patients with active AS patients had significantly higher TOS levels than the inactive group. The TAS levels were the same in all groups. The TOS and TAS concentrations were similar between patients treated with biologics and those receiving conventional agents.
The TOS levels were correlated with TAS, BASDAI, BASMI, and hs-CRP, but this correlation was low or moderate. The TOS levels are thought to be associated with activity and inflammation.
Markers of increased oxidative stress have previously been reported in AS (3–5). Some studies have also reported that oxidative stress is more pronounced in the active disease status (3, 4). In agreement with previous studies, we observed an increase in TOS in AS, and subgroup analysis revealed higher TOS levels in AS patients with active disease than in healthy controls. Additionally, BASDAI independently predicted the TOS levels.
Inconsistent data are available regarding the antioxidant status in AS. Yazici et al. (5) reported decreased thiol levels, Ozgocmen et al. (4) observed unchanged superoxide dismutase but increased catalase activity, and Karakoc et al. (3) reported decreased TAS levels in AS. In this study, the TAS levels did not differ between the groups. Oxidative stress originates from an imbalance in the oxidative-antioxidative status. It is therefore important to consider the complex interactions that occur between individual antioxidants in vivo. We attributed our findings for TAS to uncompensated increased protective antioxidative total response to oxidative stress. Similar to our results, a significant increase in TOS and a significant decrease in TAS have also been observed in patients with coronary artery disease. This indicates an imbalance between oxidant and antioxidant molecules (18). This situation may also contribute to cardiovascular risk in AS.
Pro-inflammatory cytokines (particularly TNF-α) have in recent years been shown to increase oxidative stress (19). A number of studies have reported a decreased in the oxidation response with a TNF-α targeting agent (20, 21). To the best of our knowledge, only one study has investigated the effect of anti-TNF-α on oxidative stress. In that study, Feijoo et al. (2) reported that infliximab therapy caused a decrease in oxidative stress biomarkers. In the current study, however, the TOS concentrations did not differ between patients treated with conventional or biological agents and healthy controls.
The exact mechanisms involved in the increased oxidative stress in AS are unknown, but the correlation of TOS with ESR and hs-CRP and increased TOS levels in active AS suggests that disease-related factors are involved.
One limitation of this study is its cross-sectional nature. The pre-treatment TOS/TAS levels were unavailable, making it difficult to discuss treatment efficacy.
In conclusion, oxidative stress increases in patients with AS, most probably mediated by inflammation. The TAS levels are insufficient to cope with the increased oxidative stress. The treatment status seems to have no effect on TOS. BASMI, BASDAI, and CRP emerge as independent related factors associated with TOS.
Footnotes
Ethics Committee Approval: Ethics Committee approval was received for this study from Dokuz Eylül University Ethical Committee.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - D.S., D.K, I.S., S.A.; Design - D.S., F.O., N.A., A.T., I.S., S.A.; Supervision - F.O., S.A., N.A.; Materials - D.S., D.K., A.T., I.S.; Data Collection and/or Processing - D.S., I.S, A.T., S.A.; Analysis and/or Interpretation - D.S., I.S., N.A., S.A.; Literature Review - D.S., D.K., A.T., S.A.; Writer - D.S., I.S., N.A., F.O., S.A., N.A.; Critical Review - D.S., D.K., S.A., I.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The author declared that this study has received no financial support..
References
- 1.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–90. doi: 10.1016/S0140-6736(07)60635-7. http://dx.doi.org/10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 2.Feijoo M, Tunez I, Tasset I, Montilla P, Ruiz A, Collantes E. Infliximab reduces oxidative stress in ankylosing spondylitis. Clin Exp Rheumatol. 2009;27:167–8. author reply 8. [PubMed] [Google Scholar]
- 3.Karakoc M, Altindag O, Keles H, Soran N, Selek S. Serum oxidative-antioxidative status in patients with ankylosing spondilitis. Rheumatol Int. 2007;27:1131–4. doi: 10.1007/s00296-007-0352-3. http://dx.doi.org/10.1007/s00296-007-0352-3. [DOI] [PubMed] [Google Scholar]
- 4.Ozgocmen S, Sogut S, Ardicoglu O, Fadillioglu E, Pekkutucu I, Akyol O. Serum nitric oxide, catalase, superoxide dismutase, and malondialdehyde status in patients with ankylosing spondylitis. Rheumatol Int. 2004;24:80–3. doi: 10.1007/s00296-003-0335-y. http://dx.doi.org/10.1007/s00296-003-0335-y. [DOI] [PubMed] [Google Scholar]
- 5.Yazici C, Kose K, Calis M, Kuzuguden S, Kirnap M. Protein oxidation status in patients with ankylosing spondylitis. Rheumatology (Oxford) 2004;43:1235–9. doi: 10.1093/rheumatology/keh317. http://dx.doi.org/10.1093/rheumatology/keh317. [DOI] [PubMed] [Google Scholar]
- 6.Motor S, Ozturk S, Ozcan O, Gurpinar AB, Can Y, Yuksel R, et al. Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. Int J Clin Exp Med. 2014;7:1089–93. [PMC free article] [PubMed] [Google Scholar]
- 7.Young I, Woodside J. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–86. doi: 10.1136/jcp.54.3.176. http://dx.doi.org/10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol. 1994;21:1694–8. [PubMed] [Google Scholar]
- 9.Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281–5. [PubMed] [Google Scholar]
- 10.Karatepe AG, Akkoc Y, Akar S, Kirazli Y, Akkoc N. The Turkish versions of the Bath Ankylosing Spondylitis and Dougados Functional Indices: reliability and validity. Rheumatol Int. 2005;25:612–8. doi: 10.1007/s00296-004-0481-x. http://dx.doi.org/10.1007/s00296-004-0481-x. [DOI] [PubMed] [Google Scholar]
- 11.Akkoc Y, Karatepe AG, Akar S, Kirazli Y, Akkoc N. A Turkish version of the Bath Ankylosing Spondylitis Disease Activity Index: reliability and validity. Rheumatol Int. 2005;25:280–4. doi: 10.1007/s00296-003-0432-y. http://dx.doi.org/10.1007/s00296-003-0432-y. [DOI] [PubMed] [Google Scholar]
- 12.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 13.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 311:507–20. doi: 10.1001/jama.2013.284427. 5. [DOI] [PubMed] [Google Scholar]
- 14.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 15.Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8–17. doi: 10.1136/ard.2007.084772. http://dx.doi.org/10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 16.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015. http://dx.doi.org/10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. http://dx.doi.org/10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Bhat MA, Mahajan N, Gandhi G. Oxidative Stress Status in Coronary Artery Disease Patients. International Journal of Sciences Biotechnology and Pharma Research. 2012;1 [Google Scholar]
- 19.Adamson GM, Billings RE. Tumor necrosis factor induced oxidative stress in isolated mouse hepatocytes. Arch Biochem Biophys. 1992;294:223–9. doi: 10.1016/0003-9861(92)90161-o. http://dx.doi.org/10.1016/0003-9861(92)90161-O. [DOI] [PubMed] [Google Scholar]
- 20.Kageyama Y, Takahashi M, Ichikawa T, Torikai E, Nagano A. Reduction of oxidative stress marker levels by anti-TNF-alpha antibody, infliximab, in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:73–80. [PubMed] [Google Scholar]
- 21.Tunez I, Feijoo M, Huerta G, Montilla P, Munoz E, Ruiz A, et al. The effect of infliximab on oxidative stress in chronic inflammatory joint disease. Curr Med Res Opin. 2007;23:1259–67. doi: 10.1185/030079907X187955. http://dx.doi.org/10.1185/030079907X187955. [DOI] [PubMed] [Google Scholar]