Highlights
-
•
Bioimprinting of proteins is used for production of selective binding sites for aflatoxin.
-
•
An inert protein, ovalbumin was used for the bioimpriting.
-
•
When stabilizing the imprinted molecule by internal cross-linking, it was possible to use the same imprint more than 25 times.
-
•
A limit of detection of 6 × 10−12 M was observed.
Keywords: Imprinted protein, Bioimprinting, Capacitance, Aflatoxin
Abstract
A strategy for the detection of aflatoxin B1 using a capacitive biosensor has been studied. The use of proteins for the generation of sites with high specificity against aflatoxin B1 are produced via bioimprinting. This technique has become a tool for the detection of aflatoxin B1 using a capacitive biosensor. The results demonstrate the ability to generate specific interactions with aflatoxin B1 with a linear relation between signals registered and log concentration of the target aflatoxin in the concentration range of 3.2 × 10−6 to 3.2 × 10−9 M when using ovalbumin as framework for the bioimprinting.
1. Introduction
Aflatoxins are natural food contaminants and represent a risk for human health. The four common aflatoxins present in nature are B1, B2, G1 and G2 the names of which are based on their fluorescent properties (blue or green) under UV light [3]. Among these four aflatoxins, the most abundant and dangerous aflatoxin is the B1 [34], [17] which can represent up to 80% of the total aflatoxin content in a given biomass [3], [37].
The assaying of aflatoxins using biosensors are frequently performed using antibodies [8], [16] or fractions of antibodies [24] for direct detection [1] or indirect detection [29].
The terms molecularly imprinted polymer and bioimprinting have been used to describe the method of fabrication of sites with specificity for a certain analyte on a surface of a macromolecule by using denaturation and refolding in the presence of a target molecule/analyte. These sites can be created with a synthetic polymer [11], [30], [9] or a biopolymer such as a protein (bioimprinting) [21]. Proteins are complex structures which in their native state have their own internal structural disposition given by functional groups, as covalent or non-covalent interactions [6].
Bioimprinting processes have been used to mimic specific sites for modification of biological molecules (like in enzyme reactions). When enzymes are used in anhydrous or microaqueous organic solvents, they keep a structural memory which may be lost in aqueous systems where the protein molecule can undergo conformational changes. This memory effect has been improved by the use of various different stabilization techniques [38], [14] as cross-linking using glutaraldehyde to stabilize enzymes in aminated supports [13], mimic receptors [5], or to introduce a new catalytic activity in proteins [21].
The bioimprinting procedure consists of a few basic steps: (1) unfolding the conformation of the starting protein under acidic conditions; (2) addition of imprinting molecules (or template) to allow them the interaction with the denatured protein to form new molecular configurations with some binding sites; (3) cross-linking the protein with a bifunctional reagent to stabilize the new molecular protein conformation; (4) dialysis of the protein to remove template molecules [22], [21]. The cross-linked biopolymer is formed around a molecule that acts as a template. After removal of the template, an imprint containing functional groups capable of chemical interaction remains in the imprinted biopolymer [33], [12]. The most frequently used cross-linker is glutaraldehyde, which is able to rapidly react with several residues (e.g. amines, thiols, phenols and imidazole) present in proteins generating more thermal and chemical stability [26].
In this report, a capacitive biosensor assay based on the current-pulse method was used to measure the aflatoxin concentration using an imprinted protein immobilized on a gold electrode surface. The measurements of aflatoxin B1 clearly demonstrated that bioimprinting is a useful new tool when designing new binding structures.
2. Materials and methods
2.1. Materials
Anti-Aflatoxin B1, aflatoxin B1, thioctic acid, N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), sodium cyanoborohydride, bovine serum albumin (BSA) and ovalbumin were purchased from Sigma–Aldrich (Steinheim, Germany), 1-dodecanethiol was obtained from Aldrich (Milwaukee, USA). All other chemicals used were of analytical grade. All buffers were prepared with water treated via a MilliQ system (18 ΩM cm) (Bedford MA, USA), in the rest of this paper called MilliQ water. The buffers were filtered and degassed before use. Sample from Brazilian nuts containing contaminated and non-contaminated nuts were kindly provided by Tahuamanu S.A. Company (Pando, Bolivia).
3. Methods
3.1. Gold electrode cleaning procedure
Gold electrodes prepared on silica wafers with a diameter of 3 mm and a thickness of 4000 Å were used in all experiments [35]. The electrode chips were cleaned by soaking in acetone for 5 min and ethanol for 5 min, in both cases under sonication. After each step the electrode was rinsed with MilliQ water and dried when flushing with pure nitrogen gas.
3.2. Immobilization procedure
The gold electrodes were treated with thioctic acid (250 mM in ethanol) for 12–18 h at room temperature for insulation via a self-assembled monolayer over the electrode surface. The electrodes were then rinsed with MilliQ water and dried with pure nitrogen gas [20]. The activation of the carboxylic groups was performed using 1% EDC (w/v) in dry acetonitrile for 5 h, rinsed with MilliQ water and dried with pure nitrogen gas. The bioimprinted molecule was added (30 μL) to the electrode and left in room temperature over night, then washed with potassium phosphate buffer 10 mM pH 7.4 and dried with nitrogen gas. A final immobilization step with 1-dodecane thiol (10 mM in ethanol) was used to block pin holes on the electrode (incubation time 20 min).
3.3. Bioimprinting
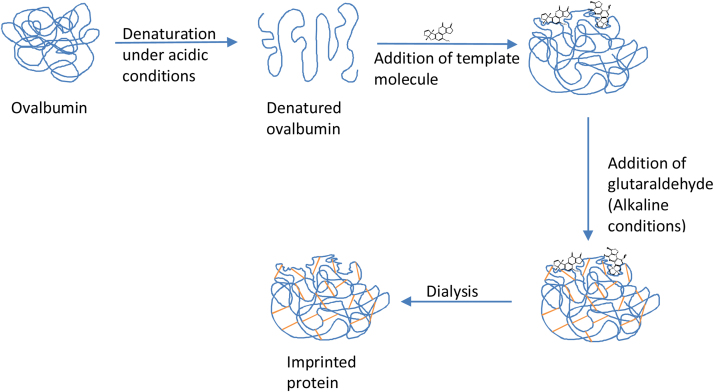
The bioimprinting was performed using the method of Liu et al. [21]; with some modifications: 1 mg of protein (ovalbumin) was dissolved in 1 mL of MilliQ water and stirred for 1 min, adjusted to pH 3.0 with 0.1 M HCl and stirred at room temperature for 10 min, then 100 μL of template molecule, aflatoxin B1 (200 μg/mL in methanol) was added and stirred for 10 additional min. After this, the pH was adjusted to 8.0 with NaOH 0.1 M, then 100 μL of glutaraldehyde 1% v/v was added and the mixture was stirred at 4 °C for 30 min. Thereafter, the imprinted protein was left overnight at 4 °C, then dialyzed for 48 h against 10 mM of potassium phosphate buffer, pH 7.4. As a control, a non-imprinted protein was prepared under the same conditions, but excluding the template molecule (aflatoxin B1). The procedure is schematically presented in Fig. 1.
Fig. 1.
Scheme of bioimprinting process.
3.4. Electrode characterization by cyclic voltammetry
The electrochemical characterization of the electrode was realized by cyclic voltammetry using a 8 PGSTAT12 potentiostat (Eco Chemie, The Netherlands). The electrochemical cell contained a working electrode (modified electrode), a reference electrode (Ag/AgCl) and an auxiliary electrode (Pt). A solution of 10 mM K3[Fe(CN)6] in 0.1 M KCl was used as a redox system with a potential range of −0.25 V to 0.7 V and a scan rate of 50 mV/s.
3.5. Capacitive measurement
An automated capacitive system device was used (CapSenze Biosystems AB, Lund, Sweden), where the capacitance measurement is assayed via the current pulse method [10]. The electrochemical cell is composed of three electrodes: a working electrode (modified gold electrode), and two platinum wire reference and auxiliary electrodes. Potassium phosphate running buffer (10 mM pH 7.4) was used as carrier of the analyte (aflatoxin B1) and cross-reactive substances with a flow rate of 100 μL/min. The regeneration of the electrode was carried out using 250 μL of glycine-HCl 25 mM (pH 2.0) to dissociate the binding between the printed protein and the analyte.
3.6. Sample preparation and cross-linking
The sample was prepared as follow: 5 g of nut sample was ground and put in a glass container, 25 mL of methanol 70% v/v was added and shaken for 30 min, then filtered through a polypropylene filter with 0.45 μm pore size. A volume of 1 mL of the filtered sample solution was diluted with 1 mL of potassium phosphate buffer (10 mM, pH 7.4) and mixed.
3.7. Reproducibility
The reproducibility test was performed using the printed protein cross-linked with glutaraldehyde (as Schiff́s base) and the structure was stabilized by reducing the Schiff’s bases using a volume of 30 μL of sodium cyanoborohydride solution (1.6 mg of sodium cyanoborohydride in 0.5 mL of 1 M NaOH) after immobilization of the imprinted protein. Reproducibility test was performed measuring repeated injections of the analyte (aflatoxin B1) utilizing the same concentration (3.2 × 10−9 M) continuously for about 40 cycles, where each cycle comprised injection-analysis-regeneration-equilibration. The change in capacitance was monitored and the relative standard deviation (RSD) was calculated in both cases.
4. Results and discussion
4.1. Cyclic voltammetry
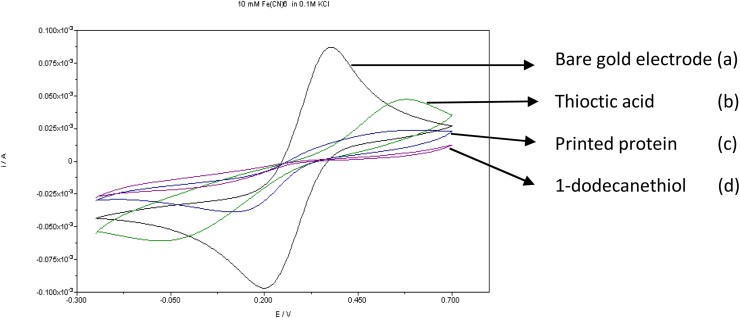
Cyclic voltammetry is a widely used technique for electrochemical characterization of electrodes. The insulation properties of electrodes were analyzed using ferro/ferricyanide [15]. The insulation of the gold electrode was recorded after each modification step using cyclic voltammetry (Fig. 2).
Fig. 2.
Cyclic voltammetry for monitoring the insulating effect of the different modification steps on the gold electrode using ferro/ferricyanide solution. (a) Bare electrode, (b) Thioctic acid (SAM), (c) Imprinted protein, (d) 1-dodecanethiol.
The purpose to use cyclic voltammetry was to evaluate insulation properties and to verify the immobilization of the imprinted protein. The protein reacts due to the presence of an amine functional group, binding covalently to thioctic acid after the activation with EDC [28], [20].
4.2. Bioimprinting design
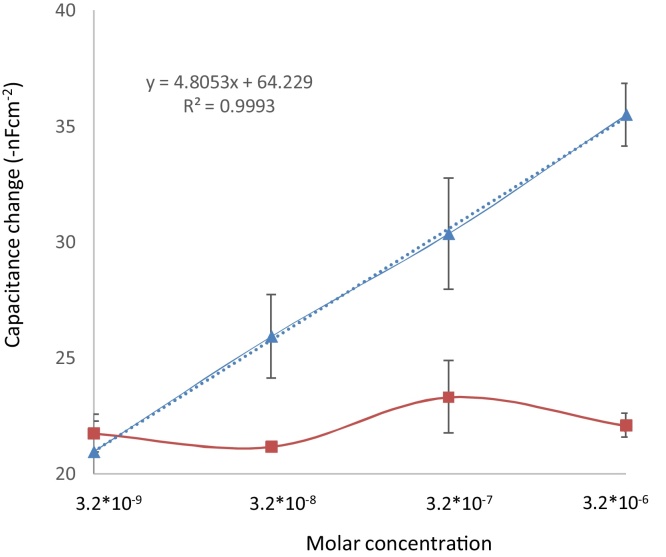
Bovine serum albumin was used as scaffold for the bioimprinting of aflatoxin B1, but due the high hydrophobicity which characterizes certain pockets on BSA [18] no change in capacitance was registered in any of the concentrations used for the detection after the injection-regeneration cycles (data not shown). However, when ovalbumin was used as platform for bioimprinting of aflatoxin B1 it was possible to quantify the change in capacitance when different concentrations of aflatoxin B1 were measured (Fig. 3) following the injection-analysis-regeneration-equilibration cycle.
Fig. 3.
Difference in response of capacitive sensing for printed protein (triangle) and non-printed protein (square).
For the bioimprinting process, the target molecules interact with the denatured ovalbumin via groups forming specific binding properties [21]. The new conformation was then fixed using glutaraldehyde as bifunctional cross-linker to induce intramolecular crosslinking. As an increased glutaraldehyde concentration used during crosslinking increases the probability of modification of functional groups from the protein is raised. However, as long as the target molecule is bound, some protection of the binding site can be expected. This would be in analogy to that active sites of enzymes are protected by substrates or inhibitors during chemical modification processes, e.g. immobilization [39], [31]. The interaction properties between aflatoxin B1 and the imprinted protein is caused by hydrogen bonds and hydrophobic interactions [19].
4.3. Capacitive sensing using bioimprinted capture molecule
Imprinted ovalbumin was used for the capacitive biosensor assay of aflatoxin B1, with potassium phosphate, 10 mM, pH 7.4 used as running buffer and glycine-HCl (0.1 M, pH 2.0) as regeneration buffer.
The analysis of different concentrations of aflatoxin B1 implied the presence of pockets with specific binding sites capable of recognizing the target molecule [5], [32], [21]. There is a marked difference in capacitive response when using aflatoxin B1 as target analyte in the imprinted protein as compared to when non-imprinted protein was used. A linear working range from 3.2 × 10−6 M to 3.2 × 10−9 M was obtained for the log of concentration of aflatoxin vs the change in capacitance registered when using the imprinted protein. These results can be compared with those from immunosensors used for aflatoxin detection (Table 1).
Table 1.
Comparison of the bioimprinting technique in capacitive biosensor with other immune sensors.
| Biosensor | LOD | LOQ | Linearity | Reference |
|---|---|---|---|---|
| Fiber optic immunosensor | – | – | 2–100 ng/mL | [25] |
| SPR using antibodies | – | 3 ng/mL | 3–98 ng/mL | [8] |
| SPR using antibodies | 0.2 ng/g | – | 1–10 ng/mL | [36] |
| SPR using single-chain antibodies fragment | 0.37 ng/mL single scFv | – | 0.37–12 ng/mL | [24] |
| 0.19 ng/mL doble scFv | – | 0.19–24 ng/mL | ||
| Enzyme immune biosensor | 0.1 ng/mL | – | 0.5–10 ng/mL | [23] |
| NRL array biosensor | 0.6–1.4 ng/mL for nut products | – | – | [29] |
| SPR using neutrophil porcine elastase | 0.97 ng/mL | 3.1 ng/mL | 1.67–17.8 ng/mL | [7] |
| Immune-capacitive biosensor | 2.74 × 10−3 ng/mL | 4.31 × 10−3 ng/mL | 1 × 10−4 – 10 ng/mL | [16] |
| Graphene field effect capacitive sensor | 1 × 10−4 pg/mL | – | 10−4 pg/mL − 1 pg/mL | [2] |
| Bioimprinting | 1.97 × 10−3 ng/mL | – | 1 ng/mL − 1000 ng/mL | This article |
The capacitance response when low molecular weight molecules (e.g. aflatoxin) bind with a thin dense layer, may result in the same change in capacitance as when a high molecular weight molecule (e.g. protein) interacts with thicker less dense layer [4].
4.4. Reproducibility
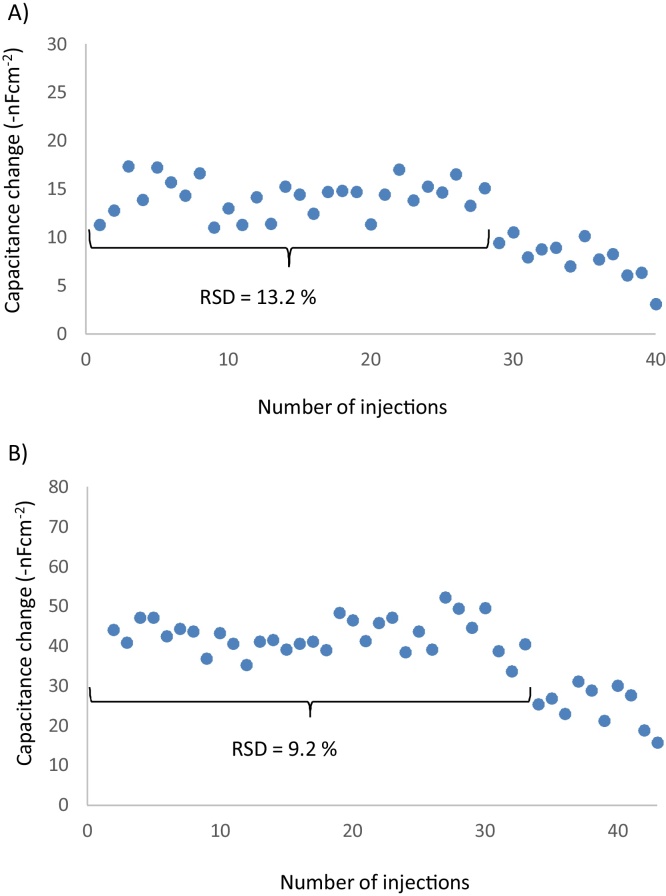
A repeatability study was performed using sequential injections of aflatoxin (3.2 × 10−9 M). the capacitance change showed little variation over 28 injections with non-reduced Schiff’s bases (Fig. 4A), and over 32 injections after the Shiff bases were reduced with a cyanoborohydride solution (Fig. 4B).
Fig. 4.
Reproducibility for protein imprinting. (A) Reaction with glutaraldehyde as Schiff’s base. (B) Stabilized Schiff’s bases with cyanoborohydride solution.
For the assay reproducibility, the cleaning procedure of the electrode surface is of crucial importance [4]. Influencing factors such as loss of activity can e.g. be the use of the low pH regeneration buffer or the utilized glutaraldehyde which can have an unstable conformation and hence react reversibly with amino groups under acidic conditions [27], [21]. This conformation has been stabilized using cyanoborohydride solution after the immobilization of the imprinted protein (Fig. 4B), reducing the RSD from 13.2% to 9.5% and increasing the number of injections from 28 to 32.
4.5. Cross reactivity
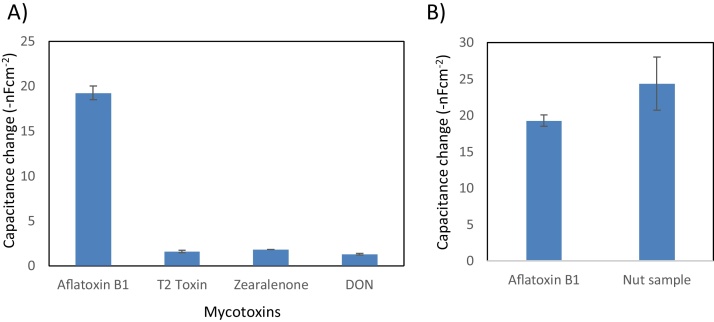
Non-specific interactions to the affinity binder is an important characteristic. In this study possible interferences with other mycotoxin molecules with similar molecular weight were studied. Three mycotoxins were utilized; deoxylivalenol (DON), zearalenone, and T2 toxin which all of them also are produced by fungal species (Fig. 5a).
Fig. 5.
A) Cross reactivity of imprinted ovalbumin for aflatoxin B1 detection. B) Sample detection: standard of aflatoxin B1 3.2 × 10−9 M (left), nut sample (right).
The results from the cross-reactivity test are shown in Fig. 5. All three mycotoxins induced non-specific capacitance changes, however significantly lower than that registered from a matching aflatoxin B1 standard solution. Thereafter, nut samples were prepared to test on the imprinted protein electrodes. The signal was evaluated using the standard curve (Fig. 3) obtaining an aflatoxin concentration of 1.5 × 10−7 M which is comparable with our previous study using the same sample but instead analyzed with an immunobiosensor [16]. The LOD was statistically calculated with an obtained value of 6.3 × 10−12 M, which again is comparable with previous work when antibodies against aflatoxin B1 was utilized as capture agent on the sensor surface.
5. Conclusions
Bioimprinting with aflatoxin B1 was shown to be a feasible method for creating highly specific binding sites for capacitive biosensing which makes it possible to detect aflatoxin at low concentrations (3.2 × 10−6 M to 3.2 × 10−9 M) using ovalbumin as platform. The aflatoxin B1 can generate binding sites on the surface of the imprinted protein. When the aflatoxin is injected into the system the interaction with the imprinted protein gives response in the capacitive sensor. The results can be compared with those from other immunosensors for aflatoxin detection [22], [8], [36], [23]. However, acidic conditions during regeneration has been observed to cause activity loss. Furthermore, improved stability by reduction of the Schiff bases on the imprinted protein using cyanoborohydride after the immobilization of the imprinted protein over the electrode could be achieved.
Conflict of interests
None.
Acknowledgement
This work was supported by the Swedish International Development Cooperation (SIDA) as a bilateral program between Sweden and Bolivia.
References
- 1.Bacher G., Pal S., Kanungo L., Bhand S. A label-free silver wire based impidimetric immunosensor for the detection of aflatoxin M1 in milk. Sens. Actuators B: Chem. 2012;168:223–230. [Google Scholar]
- 2.Basu J., Datta S., RoyChaudhuri C. A graphene field effect capacitive immunosensor for sub-femtomolar food toxin detection. Biosens. Bioelectron. 2015;68:544–549. doi: 10.1016/j.bios.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 3.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16(3):497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergveld P. A critical evaluation of direct electrical protein detection methods. Biosens. Bioelectron. 1991;6:55–72. doi: 10.1016/0956-5663(91)85009-l. [DOI] [PubMed] [Google Scholar]
- 5.Braco L., Dabulis K., Klibanov A.M. Production of abiotic receptors by molecular imprinting of proteins. Proc. Natl. Acad. Sci. U. S. A. 1990;87:274–277. doi: 10.1073/pnas.87.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carraro A.A.C. Albumin protein and functional properties of gelation and foaming. Sci. Agric. 2006;63:291–298. [Google Scholar]
- 7.Cuccioloni M., Mozzicafreddo M., Barocci S., Ciuti F., Pecorelli I., Eleuteri A.M., Spina M., Fioretti E., Angeletti M. Biosensor-based screening method for the detection of aflatoxins B1-G1. Anal. Chem. 2008;80:9250–9256. doi: 10.1021/ac801612w. [DOI] [PubMed] [Google Scholar]
- 8.Daly S.J., Keating G.J., Dillon P.P., Manning B.M., O’Kennedy L., Lee H.A., Morgan M.R. Development of Surface Plasmon Resonance-based immunoassay for aflatoxin B1. J. Agric. Food Chem. 2000;48(11):5097–5104. doi: 10.1021/jf9911693. [DOI] [PubMed] [Google Scholar]
- 9.Dias A.D., Kingsley D.M., Corr D.T. Recent advances in bioprinting and applications for biosensing. Biosensors. 2014;4:111–136. doi: 10.3390/bios4020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlandsson D., Teeparuksapun K., Mattiasson B., Hedström M. automated flow-injection immunosensor based on current pulse capacitive measurement. Sens. Actuators B. 2014;190:295–304. [Google Scholar]
- 11.Ertürk G., Berillo D., Hedström M., Mattiasson B. Microcontact-BSA imprinted capacitive biosensor for real-time, sensitive and selective detection of BSA. Biotechnol. Rep. 2014;3:65–72. doi: 10.1016/j.btre.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faber K. fourth. Springer-Verlag; Berlin: 2000. Biotransformations in Organic Chemistry; pp. 400–401. [Google Scholar]
- 13.Fernandez-Lorente G., Palomo J.M., Mateo C., Munilla R., Ortiz C., Cabrera Z., Guisan J.M., Fernandez-Lafuente R. Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules. 2006;7:2610–2615. doi: 10.1021/bm060408+. [DOI] [PubMed] [Google Scholar]
- 14.Fishman A., Cogan U. Bio-imprinting of lipases with fatty acids. J. Mol. Catal. B: Enzym. 2003;22:193–202. [Google Scholar]
- 15.Gao Z., Wang G., Li P., Zhao Z. Electrochemical and spectroscopic studies of cobalt-hexacyanoferrate film modified electrodes. Electrochim. Acta. 1991;36:147–152. [Google Scholar]
- 16.Gutierrez R.A.V., Hedström M., Mattiasson B. Screening of self-assembled monolayer for aflatoxin B1 detection using immune-capacitive sensor. Biotechnol. Rep. 2015;8:144–151. doi: 10.1016/j.btre.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid A.S., Tesfamariam I.G., Zhang Y., Zhang Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention (Review) Oncology Letters. 2013;5:1087–1092. doi: 10.3892/ol.2013.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haskard C.A., Li-Chan E.C.Y. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS-) fluorescent probes. J. Agric. Food Chem. 1998;46:2471–2677. [Google Scholar]
- 19.Li X., Li P., Zhang Q., Li Y., Zhang W., Ding X. Molecular characterization of monoclonal antibodies against aflatoxins: a possible explanation for the highest sensitivity. Anal. Chem. 2012;84:5229–5235. doi: 10.1021/ac202747u. [DOI] [PubMed] [Google Scholar]
- 20.Limbut W., Kanatharana P., Mattiasson B., Asawatreratanakul P., Thavarungkul P. A comparative study of capacitive immunosensors based on self-assembled monolayers formed from thiourea, thioctic acid, and 3-mercaptopropionic acid. Biosens. Bioelectron. 2006;22:233–240. doi: 10.1016/j.bios.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Liu L., Zhang K., Rena X., Luob G., Shen J. Bioimprinted protein exhibits glutathione peroxidase activity. Anal. Chim. Acta. 2004;504:185–189. [Google Scholar]
- 22.Liu J., Luo G., Gao S., Zhang K., Chen X., Shen J. Generation of a glutathione peroxidase-like mimic using bioimprinting and chemical mutation. Chem. Commun. 1999:199–200. [Google Scholar]
- 23.Liu Y., Qin Z., Wu X., Jiang H. Immune-biosensor for aflatoxin B1 based bio-electrocatalytic reaction on micro-comb electrode. Biochem. Eng. J. 2006;32:211–217. [Google Scholar]
- 24.Lynsey D., Daly S., Baxter A., Haughey S., O’Kennedy R. Surface Plasmon Resonance-based immunoassay for the detection of aflatoxin B1 using single-chain antibody fragments. Spectrosc. Lett. 2005;38:229–245. [Google Scholar]
- 25.Maragos C.M., Thompson V.S. Fiber-optic immunosensor for mycotoxins. Nat. Tox. 1999;7:371–376. doi: 10.1002/1522-7189(199911/12)7:6<371::aid-nt86>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Migneault I., Dartiguenave C., Bertrand M.J., Waldron K.C. Glutaraldehyde: behaviour in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques. 2004;37(5):790–802. doi: 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]
- 27.Okuda K., Urabe I., Yamada Y., Okada H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991;71:100–105. [Google Scholar]
- 28.Pei R., Cheng Z., Wang E., Yang X. Amplification of antigen-antibody interactions based on biotin labeled protein-streptavidin network complex using impedance spectroscopy. Biosens. Bioelectron. 2001;16:355–361. doi: 10.1016/s0956-5663(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 29.Sapsford K.E., Taitt C.R., Fertig S., Mooreb M.H., Lassman M.E., Maragos C.M., Shriver-Lake L.C. Indirect competitive immunoassay for detection of aflatoxin B1 in corn and nut products using the array biosensor. Biosens. Bioelectron. 2006;21:2298–2305. doi: 10.1016/j.bios.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Schirhagl R. Bioapplications for molecularly imprinted polymers. Anal. Chem. 2014;86:250–261. doi: 10.1021/ac401251j. [DOI] [PubMed] [Google Scholar]
- 31.Silva C.J.S.M., Sousa F., Gubitz G., Cavaco-Paulo A. Chemical modification of proteins using glutaraldehyde. Food Technol. Biotechnol. 2004;42:51–56. [Google Scholar]
- 32.Slade C.J. Molecular (or bio-) imprinting of bovine serum albumin. J. Mol. Catal. 2000;9:97–105. [Google Scholar]
- 33.Slade C.J., Vulfson E.N. Introduction of catalytic activity in proteins by lyophilisation in the presence of a transition state analogue. Biotechnol. Bioeng. 1998;57:211–215. doi: 10.1002/(sici)1097-0290(19980120)57:2<211::aid-bit9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Squire R.A. Ranking animal carcinogens: a proposed regulatory approach. Science. 1981;214:877–880. doi: 10.1126/science.7302565. [DOI] [PubMed] [Google Scholar]
- 35.Teeparuksapun K., Kanatharana P., Limbut W., Thammakhet C., Asawatreratanakul P., Mattiasson B., Wongkittisuksa B., Limsakul C., Thavarungkul P. Disposable electrodes for capacitive immunosensor. Electroanalysis. 2009;21(9):1066–1074. [Google Scholar]
- 36.Van deer Gaag B., Spath S., Dietrich H., Stigter E., Boonzaaijer G., Van Osenbruggen T., Koopal K. Biosensors and multiple mycotoxin analysis. Food Control. 2003;14:251–254. [Google Scholar]
- 37.Vandercammen G., (2010), New EU aflatoxin levels and sampling plan, Global agricultural information network, Gain report number: E50018.
- 38.Yilmaz E. Combining the bioimprinting technique with lipase immobilization for interesterification. World J. Microbiol. Biotechnol. 2002;18:621–625. [Google Scholar]
- 39.Zaborsky O.R. CRC Press; Cleveland, OH: 1973. Immobilized Enzymes. [Google Scholar]