Highlights
-
•
Extended fermentation strategy for G. lucidum mycelium in shake flask and bioreactor.
-
•
Repeated-batch fermentation affected the morphology with reduced fermentation time.
-
•
Ovoid pellet favours exopolysaccharide production in a shake flask.
-
•
Starburst-like pellet favours exopolysaccharide production in a bioreactor.
Keywords: Ganoderma lucidum, Morphology, Ovoid pellet, Starburst-like pellet, Repeated-batch fermentation
Abstract
The morphology of Ganoderma lucidum BCCM 31549 mycelium in a repeated-batch fermentation (RBF) was studied for exopolysaccharide (EPS) production. RBF was optimised for time to replace and volume to replace. G. lucidum mycelium showed the ability to self-immobilise and exhibited high stability for repeated use in RBF with engulfed pellets. Furthermore, the ovoid and starburst-like pellet morphology was disposed to EPS production in the shake flask and bioreactor, respectively. Seven RBF could be carried out in 500 mL flasks, and five repeated batches were performed in a 2 L bioreactor. Under RBF conditions, autolysis of pellet core in the shake flask and shaving off of the outer hairy region in the bioreactor were observed at the later stages of RBF (R4 for the shake flask and R6 for the bioreactor). The proposed strategy showed that the morphology of G. lucidum mycelium can withstand extended fermentation cycles.
1. Introduction
Medicinal mushrooms have been shown to be the most popular choice of traditional medicine, especially those from the phylum Basidiomycota such as Ganoderma lucidum, Ganoderma atrum, Ganoderma tsugae and Ganoderma applanatum [1]. Although all have been used in traditional Chinese medicine for the prevention and treatment of human diseases [2], Ganoderma lucidum (Fr.) Karst (Polyporaceae) has been the organism of choice for creating useful bioproducts [2], [3], with a significant number of reports in the literature attesting to its benefits [4], [5], [6].
To date, the effects of inoculation concentration [7], dissolved oxygen [8], pH [9], [10], environmental conditions (e.g. shear rate and catabolite repression) [11], medium composition [12], two-stage culture process [13], pH-shift and dissolved-oxygen transfer (DOT)-shift integrated fed-batch fermentation [14], and oxygen supply [15] have been studied for G. lucidum fermentation strategy. Most existing research has focused on improving the fermentation process to increase metabolite activity [16]. However, only one study on increasing metabolite productivity by reducing the fermentation time, cost for seed culture and inoculation between each fermentation cycle has been reported [17]. Similarly, this work addresses how morphological characteristics play an important role in the extended fermentation cycle.
Repeated-batch fermentation (RBF) is an adaptation of an existing technique or alternative strategy in which the medium, or a portion of the medium, is removed and fresh medium introduced periodically or repeatedly without changing the existing culture [17], [18]. Based on this, RBF differs from fed-batch techniques as it has the advantage of reducing the fermentation time, cost for seed culture, medium usage, and inoculation requirements between fermentation cycles [19]. RBF has been shown to enhance the productivity of microbial fermentations [18], [20], as it requires less time for washing and sterilising the bioreactor, omits the seed preparation time, results in high growth rates, and shortens the initial inoculation procedure between batches [19]. These advantages have the potential to lead to significant savings in terms of both time and labour, as yield remains constant with reduced fermentation time [21]. Consequently, this process enables the reuse and storage of pellets while also maintaining their long-term cell activity [22], especially for the production of bioactive metabolites [18], [22], [23].
G. lucidum morphology is an important factor that affects the rheological properties of the fermentation broth, and its control is highly desired from an industrial perspective [24]. Generally, two growth forms, the filamentous and the pelleted form, can be observed in most fungal fermentations and the pelleted form is usually less viscous than the filamentous form [22], [25]. Pellets are characterised by mycelia developing into stable, spherical aggregates consisting of a relatively dense, branched and partially intertwined network of hyphae [26]. A number of reports have addressed the factors influencing G. lucidum morphology, rheology and production of fungal exopolysaccharide (EPS) [8], [25], [27]. Amongst many aspects of morphology, the effect of long continuous culture (RBF) has not been studied to date.
In order to investigate this effect, this work focused on morphological relationship and the optimisation of RBF to reduce fermentation time while enhancing EPS productivity. In addition, the optimum morphology for EPS production during RBF cycles was determined.
2. Materials and methods
2.1. Microorganism and medium
G. lucidum BCCM 31549 stock culture was obtained from the Belgian Coordinated Collections of Microorganisms (BCCM/MUCL), [Agro] Industrial Fungi and Yeast Collection (Leuven, Belgium). The fungus was transferred onto potato dextrose agar (PDA, Oxoid Limited, Hampshire, UK) to avoid any contamination and ensure sustainability, as shown in previous research [3], [17]. PDA plates were inoculated and incubated at 30 °C for 7 days, and stored at 4 °C. The compositions of media was, in g/L: Glucose 50, Yeast Extract (YE) 1, KH2PO4 0.5, K2HPO4 0.5, MgS04 0.5 and NH4Cl 4, unless otherwise stated.
2.2. Fermentation conditions in shake flask and bioreactor
The method for inoculum preparation of Wan et al. [17] for G. lucidum BCCM 31549 was used and involved two seed culture stages, both cultivated for 10 days at 30 °C, initial pH 4 and 100 rpm. Four mycelial agar squares (5 mm × 5 mm) from a 10-day-old plate were inoculated into a 500 mL Erlenmeyer flask containing 100 mL of medium (first seed culture). To produce additional growing hyphae tips, mycelium from the first seed culture were then homogenised using a sterile Warring blender for 20 s. This material was used as the inoculum for the second seed culture (500 mL Erlenmeyer flask containing 200 mL medium) and then transferred to the bioreactor; during inoculum production, it was inoculated into new fresh medium during the late exponential phase (from day 9 to day 11), meaning that cells were biochemically most active and in the optimum physiological state. EPS fermentation was performed in a 500 mL (20 mL working volume) shake flask and a 2.5 L stirred-tank (STR) bioreactor (New Brunswick Bioflow 3000, Edison L.N, USA) [2 L working volume]. 20% (v/v) of the seed culture was used to inoculate the fermenter, unless otherwise stated. The cultivation was carried out at 30 °C with pH maintained at 4.0, dissolved oxygen (DO) was controlled, aeration rate was at 2.0 vvm, and agitation speed was controlled at 100 rpm. The compositions of media were, in g/L: Glucose 30, KH2PO4 0.5, K2HPO4 0.5, MgSO47H2O 0.5, YE 1 and NH4Cl 4.
2.3. Repeated-batch fermentation
RBF was carried out for the maximum possible number of cycles. To determine a suitable broth replacement ratio, the existing fermentation broth was removed at a pre-determined broth replacement ratio [50% to 90% (v/v)] and replaced with fresh medium to permit continuous growth of G. lucidum mycelium (Table 1). To determine the appropriate broth replacement time point, three batch fermentation growth phases were obtained from the shake flask. These were designated as increasing EPS concentration (at the end of logarithmic growth phase), highest EPS concentration (transition phase) and stabilizing EPS concentration (stationary phase). During RBF, the fermentation cycle was started until it achieved the highest growing point (i.e. the organism became too viscous, or autolysis had occurred). At this point, the existing medium fermentation mixture was replenished and the process was repeated for the subsequent cycles that affected morphology.
Table 1.
Mode of operation (broth replacement ratio) of repeated-batch processes by G. lucidum BCCM 31549 in the shake flask.
| Experiments | Working culture (v/v) | Harvesting percentage (v/v) | Fresh broth replacement (v/v) |
|---|---|---|---|
| A | 10% | 90% | 90% |
| B | 20% | 80% | 80% |
| C | 30% | 70% | 70% |
| D | 50% | 50% | 50% |
*Total working volume: 200 mL in a 500 mL Erlenmeyer flask.
2.4. Analytical methods
2.4.1. Exopolysaccharide (EPS)
EPS was obtained from the harvested fermentation broth. From this broth, supernatant was collected through centrifugation at 8000 rpm for 15 min. Crude EPS was precipitated from the supernatant by the addition of four volumes of 95% (v/v) ethanol and left overnight at 4 °C to form one volume of cell-free filtrate. The precipitate was then separated by centrifugation at 10,000 rpm for 15 min, a process that was repeated twice. The precipitate was then filtered through a pre-dried and weighted GF/C filter paper and washed twice with 5 mL of 95% (v/v) ethanol, as described by Wan et al. [17]. Precipitate was then placed in a desiccator and dried for 24 h at room temperature to a constant weight before the weight of EPS was estimated. All assays were carried out in triplicate.
2.4.2. Image analysis
A light microscope (Nikon OPIPHOT-2, Japan) with a coupled camera (JVC, TK-C1381 Colour Video Camera) was used to assess the morphology details of the collected samples [17]. Five mL of culture samples were re-suspended in 5 mL of a fixative solution, according to the technique defined by Packer and Thomas [28], and maintained at 4 °C until assessment. Observation of mycelium in the samples required fixative solution which was prepared by mixing 13 mL of 40% (v/v) formaldehyde, 5 mL of glacial acetic acid and 200 mL of 50% (v/v) ethanol. A 0.1 mL aliquot of each fixed sample was transferred to a slide, air dried and stained with methylene blue [9]. The microscopic images for samples from increasing RBF cycles were compared.
2.5. Statistical analysis
All analyses were carried out in triplicate and the respective mean ± S.D determined using GraphPad Prism 5 (Version 5.01) software and shown as error bars as described in the earlier work [17]. Where error bars were not visible, it was assumed that they were smaller than the size of the symbol. A t-test was used for plotting fermentation graphs, and one-way ANOVA and post-hoc analysis (Bonferroni’s Multiples Comparison Test) were used to compare kinetic parameters.
3. Results and discussion
3.1. Morphological observation of the shake flask fermentation
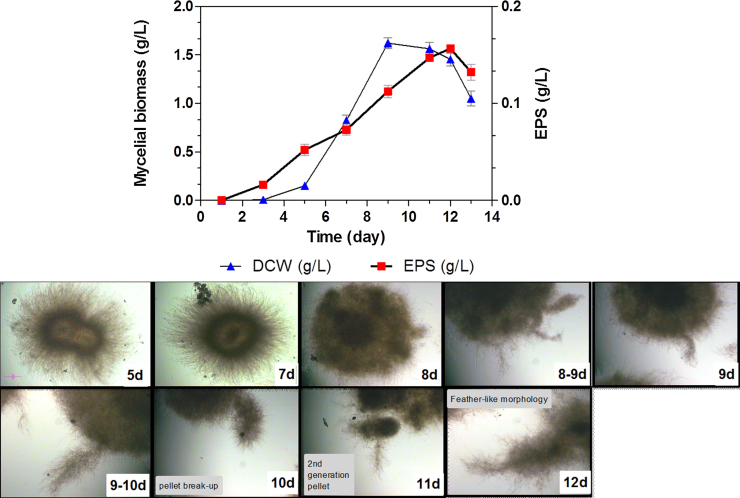
Shake flask batch fermentation was performed to illustrate the initial hyphal growing tips and the effect on EPS production prior to the implementation of RBF (Fig. 1). Based on the time profile, the fermentation process had successfully followed the normal fungal growth curve pattern whereas the morphological changes showed that G. lucidum grew with a pellet morphology that changed over time. The mycelial biomass started to increase at the day 5 (0.44 g/L) and accelerated rapidly at day 8, reaching its highest value at day 9 (1.62 g/L). Meanwhile, EPS was low during the first 5 days because the mycelium was still in the growth stage (from day 4 to day 9). Subsequently, the highest level of EPS was achieved at day 12 (0.16 g/L). This result is comparable with Wagner et al. [25], in that the EPS production rate was low during the first generation pellet (at day 5) formation and accelerated during the second stage (at day 11). Furthermore, Yang and Liau [17], [29] have also observed that the newly formed EPS can stick to the mycelia pellet, thus likely lessening the secretion of additional EPS into the media.
Fig. 1.
Time profile and morphological changes in mycelia during shake flask batch-fermentation of Ganoderma lucidum BCCM 31549. All other fermentation conditions were constant: 50 g/L glucose and 10% (v/v) inoculum. Images were taken at 4-fold magnification. Bar = 150 μm. 5–12 d indicates fermentation period in days. T-test was performed on the graph data, and both means were shown to be significantly different (P < 0.05).
The observed rapid EPS production was attributed to the liberation of second generation pellets (at day 11), demonstrated by morphological changes as illustrated in Fig. 1. According to Wagner et al. [25] and Fazenda et al. [8], second generation pellets are characterized as ovoid in shape although cultures also contained several first generation pellets with protuberances (starburst appearance). These were subsequently released (detached from the primary pellet surface) to generate a feather-like morphology which can be observed at day 12 (Fig. 1).
Morphological changes were shown to correlate with time profile. At the early stages (from day 5 to day 7), the mycelium displayed a pellet ball with significant hairiness, a behaviour potentially due to self-immobilisation. These pellets functioned as an immobiliser to provide the fungus some protection in the liquid medium, thus helping to avoid any detrimental shear effects [30], [31]. Later on, several long hyphal tips were observed to protrude from the pellet surfaces and increasing in length to become protuberances at day 8. These protuberances were defined as a newly-formed pellets protruding from the parent surfaces, a finding in support of previous work by Kim et al. [9] who stated that pellets originally produce protuberances during the liberation of the first generation to second generation pellets.
As growth progressed, the protuberances increased in length and width to give the pellet a starburst appearance by day 9. This morphology was associated with the starting point of the EPS acceleration phase on the same day. The next day (day 10), the protuberances began to detach from the first generation pellet, a process which could be categorised as early pellet break-up. These events continued until the protuberances were completely broken up by day 11 (full pellet break-up). These broken-up pellets transformed in shape into a feather-like morphology at the time of release on day 12, and seemed to be the morphological form most closely linked to EPS production. On this day, maximum EPS concentration (0.16 g/L) was observed while the biomass reduced as the cells entered the death phase. Kim et al. [9] also described this relationship between morphology and EPS production, whereby the liberation of second generation pellets was thought to be due to pellet break-up, thus initiating EPS production.
3.2. Repeated-batch fermentation
To investigate long-term stability and performance of G. lucidum, RBF was implemented in this study. This technique was accomplished by substituting a certain portion of the mature fungal culture fluid with fresh medium. This approach has been shown to improve the productivity of fungal bioproducts as it reduces the time required for growth of seed culture, inoculation, and cleaning and sterilisation of the fermenter between each fermentation cycle [17], [19]. Therefore, in the present study, we sought to develop an effective strategy for the RBF process, mainly to increase EPS productivity. Optimisation experiments were carried out in shake flasks to find a suitable broth replacement ratio and broth replacement time point for this process.
3.2.1. Effect of broth replacement ratio
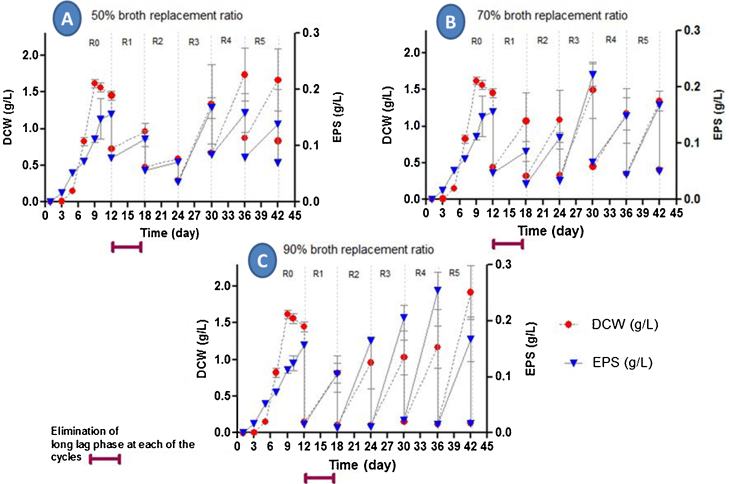
As shown in Fig. 2, experiments with different broth replacement ratios were implemented for the repeated-batch culture, and the various broth replacement ratios were set as 50% (100 mL), 70% (140 mL) and 90% (180 mL). During the broth replacement procedure, these volumes were withdrawn from three separate 500 mL (200 mL of total working volume) flasks after 288 h (12 days) of fermentation, and the same volume of fresh medium was then added into the flask. Samples were withdrawn at 144 h (6 day) intervals and analysed for mycelial biomass, EPS and mycelial morphology. The data were processed and compared to previous work by Yang et al. [23], which also used an RBF technique to cultivate the fungus Rhizopus arrhizus.
Fig. 2.
Effect of broth replacement ratio on (dry cell weight) DCW and EPS production during RBF of G. lucidum BCCM 31549 in the shake flask at (v/v): (A) 50%, (B) 70% and (C) 90%. All other fermentation conditions were the same as for Fig. 1. R1–R7 indicates fermentation repetition in cycles.
Based on the results displayed in Fig. 2, the three broth replacement ratios were capable of tolerating the continuous production of biomass and EPS for up to 5 cycles (R5) during RBF. The cells of G. lucidum were reused, resulting in the elimination of lag phases during the subsequent culture and considerable enhancement of EPS productivity (g/L day−1). During the RBF cycles (R1−R5), adaptations of the cultures to their growth conditions were observed, starting from the second (R2) to the third (R3) cycle for all broth replacement ratios. During these first two cycles, the fungus adapts to the new conditions and some sub-populations may enter the death phase due to the stress of addition of new medium. Later on, the fungus was able to stabilise at the fourth (R4) and fifth (R5) cycles, and the cultures kept producing EPS until the environment became too viscous (turbid) for any further EPS production.
3.2.1.1. Morphological characteristics at different broth replacement ratios
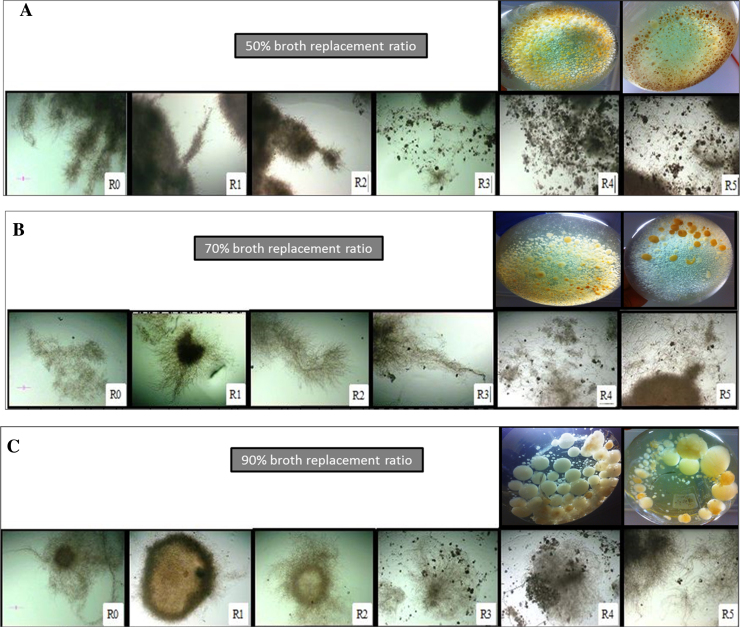
Using a light microscope and coupled camera, morphological features following different broth replacement ratios were compared. Fig. 3A, B, and C show the evaluation of morphological changes during the RBF of G. lucidum, using 50%, 70% and 90% broth replacement ratios (v/v), respectively. Among all figures, significant autolysis (indicated by pellet colouration) was noticeable only from the third cycle (R3) onwards.
Fig. 3.
Comparison of morphological changes during shake flask RBF on G. lucidum BCCM 31549 using 50% broth replacement ratio (A), 70% broth replacement ratio (B), and 90% broth replacement ratio (C), respectively. Images were taken at 4-fold magnification. Bar = 150 μm. R0–R5 indicates fermentation repetition in cycles. Macroscopic changes in pellet colour were observed at R4 and R5.
For the 50% broth replacement ratio (Fig. 3A) and following batch fermentation (R0), pellets exhibited longer protuberances during the first RBF cycle (R1) and these protuberances (branch-points) kept increasing in length until second generation protuberances were produced by the second cycle (R2). By the third cycle (R3), coloured pellets were observed and increased through the fourth cycle (R4) and before ending at the fifth cycle (R5). Pellets turned yellow at R4 and became brownish at R5, as observed in the flasks. Pellet colouration seemed to be associated with partial disintegration of pellets, possibly indicating autolysis.
As shown in Fig. 3B, the 70% broth replacement ratio started with feather-like pellets during R1 which increased in hairiness at both R2 and R3. The increasing number of filaments caused the feather-like pellets to disperse completely during R4 and terminate at R5. This strategy favoured EPS production for G. lucidum mycelium, and showed the lowest quality of coloured (yellowish-brown) pellets. This coloration was reduced at R4 and R5, compared with the 50% broth replacement ratio.
By contrast, the 90% broth replacement ratio showed a larger pellet with a dense core at R1 (Fig. 3C). Later on, this large pellet reduced in size (under macroscopic observation) but increased in hairiness at R2. By the third cycle (R3), the mycelial filament was completely dispersed in the culture with an increasing number of coloured pellets which related to the incidence of cell autolysis. These autolysing pellets were increased at R4 and completely reduced at R5. This broth replacement strategy was incapable of producing a better morphology for EPS production compared with the 70% broth replacement strategy. Macroscopically, the pellet size was the largest compared with others at the R4 and R5 stage. Overall, from the third cycle (R3) to the fifth cycle (R5), the highest amount of pellet autolysis was observed at the 50% broth replacement ratio (brown pellets) followed by the 90% (yellowish pellets) and 70% (fewer yellowish-brown pellets) ratios. The differences in pellet colouration of 90% and 70% suggested that an 80% broth replacement strategy would generate less autolysis. As described in our previous work [17], this strategy was considered optimal for RBF.
3.2.2. Effects of broth replacement time point
The purpose of these experiments was to identify the optimum time point at which to carry out broth replacement. As shown in Table 2, broth replacement time points were set at A = end of logarithmic growth phase (day 11), B = transition phase (day 12), and C = stationary phase (day 13). Based on Table 3, a sustainable EPS level was observed in the three experiments (repeated batch A–C). Repeated-batch cells have shown their ability to undergo repeated use and seven batches could be fermented consecutively using a 500 mL flask, meaning that G. lucidum cells demonstrated robustness to the RBF process.
Table 2.
Experimental design to determine the effect of broth replacement time point during G. lucidum BCCM 31549 RBF using shake flask.
| Repeated-batch | Time point (day)a | Phases |
|---|---|---|
| A | 11 | At the end of logarithmic phase |
| B | 12 | Transition phase |
| C | 13 | Stationary phase |
Time points were obtained from the normal shake flask batch fermentation based on their EPS production curve from Fig. 1.
Table 3.
Effect of different broth replacement time points (growth phase) on EPS productivity by G. lucidum BCCM 31549 repeated-batch fermentation.c
| Time pointa | Kineticsb | RBF cyclesd |
Sum (R1–R7) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | |||
| A | |||||||||
| P EPS (g/L day −1) | 0.027 | 0.028 | 0.032 | 0.030 | 0.030 | 0.031 | 0.029 | 0.207 ± 0.002 | |
| Q EPS/x [(g/g) day −1] | 0.028 | 0.023 | 0.024 | 0.019 | 0.023 | 0.022 | 0.014 | 0.153 ± 0.004 | |
| B | |||||||||
| P EPS (g/L day −1) | 0.027 | 0.030 | 0.033 | 0.029 | 0.030 | 0.029 | 0.028 | 0.206 ± 0.002 | |
| Q EPS/x [(g/g) day −1] | 0.024 | 0.024 | 0.017 | 0.021 | 0.022 | 0.031 | 0.025 | 0.164 ± 0.004 | |
| C | |||||||||
| P EPS (g/L day −1) | 0.028 | 0.021 | 0.020 | 0.037 | 0.024 | 0.036 | 0.033 | 0.199 ± 0.07 | |
| Q EPS/x [(g/g) day −1] | 0.018 | 0.020 | 0.012 | 0.030 | 0.015 | 0.022 | 0.030 | 0.147 ± 0.007 | |
A = at the end of logarithmic phase, B = transition phase and C = stationary phase.
P x (g/L day1) = biomass productivity, P EPS (g/L day1) = EPS productivity and Q EPS/x [(g/g) day1] = specific production of EPS.
Fermentations were carried out in shake flasks with conditions and medium compositions of [(g/L): Glucose 50, KH2PO4 0.5, K2HPO4 0.5, MgSO47H2O 0.5, YE 1, NH4Cl 4], 10% (v/v) inoculum, 100 rpm, initial pH 4, temperature at 30 °C, and 6 day interval between cycles.
(R1–R7) indicates repetition, in cycles.
Overall, consistent EPS productivity (P EPS) of 0.021–0.037 g/L day−1 and specific production of EPS (Q EPS/x) of 0.012–0.031 (g/g) day−1 were obtained for all time points (A–C). On average, these time points produced a balanced value for P EPS (A = 0.029, B = 0.029 and C = 0.028 g/L day−1) and Q EPS/x (A = 0.022, B = 0.023 and C = 0.021 (g/g) day −1) for the average of seven cycles.
Meanwhile, time points B and C displayed the highest EPS productivity (P EPS) of 0.033 g/L day−1 at the third cycle (R3) and 0.036 g/L day−1 at the fourth cycle (R4). No significant differences were observed (P > 0.05) on EPS productivity between time points B and C. However, time point B was chosen over A and C as the ideal broth replacement time point for RBF as it achieved the highest total value of Q EPS/x [0.164 (g/g) day −1]. The reason for these higher values was attributed to morphological differences between the time points.
3.2.2.1. Morphological changes during broth replacement time points
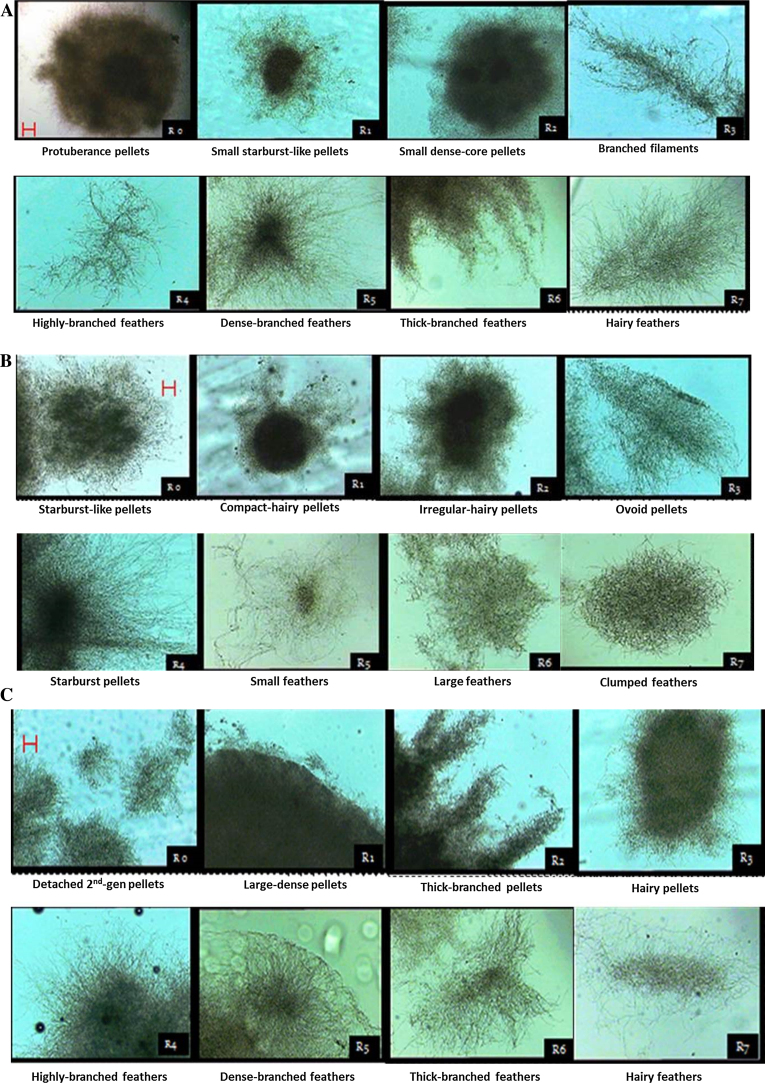
Fig. 4A–C show a comparison of morphological changes during RBF at different broth replacement time points. Each phase started at a different initial time point, which was at day 11 for A, day 12 for B and day 13 for C. These time points were associated with diverse initial morphology (R0), namely protuberance pellets for A, starburst-like pellets for B and detached second generation pellets for C. These features can be explained in that each had a different pellet morphology which was related to the previous batch fermentation shown in Fig. 1. In addition, G. lucidum produces different pellet morphologies from the first cycle (R1) to the seventh cycle (R7).
Fig. 4.
Comparison of morphological changes during shake flask RBF on G. lucidum BCCM 31549 with a specific broth replacement time point (End of logarithmic phase: A, Transition phase: B, and Stationary phase: C, respectively). Images were taken at 4-fold magnification. Bar = 150 μm. R0–R7 indicates fermentation repetition in cycles.
For time point A (Fig. 4A), at the first cycle (R1), the protuberance pellets from batch (R0) detached from the pellet surfaces and formed scattered (small) starburst-like pellets. These pellets may have coagulated with mycelial fragments at the second cycle (R2), thus forming a small dense-core structure. Reaching the third cycle (R3), this dense-core pellet structure broke into several branched filaments which were later torn into a higher-branched network termed ‘feathers’ by the fourth cycle (R4). By the fifth cycle (R5), torn filament-branches had tied with the outer mycelia, thus creating dense-branched feathers. These dense-branched feathers thickened into intertwined branched-feathers by the sixth cycle (R6) which later deteriorated into hairy feathers at the seventh cycle (R7) in Fig. 4A.
For time point B (Fig. 4B), the morphology initially showed starburst-like pellets (R0). At this time, the pellets were at the start of the pellet break-up or the onset of production of second progeny from the first pellet, thus suggesting an active growing environment for the fungus. Later, these pellets were compacted and became hairy at R1. These hairy pellets were intertwined with each other and became irregular at R2. They disintegrated during the following cycle and formed smoother ovoid pellets at the third cycle (R3) which was the critical morphology for EPS production. Later, these pellets engulfed the nearby mycelia and formed starburst pellets at the fourth cycle (R4) which later broke into small feathers at the fifth cycle (R5). These hyphal aggregates increased in size and became large at the sixth cycle (R6), then clumped together at the seventh cycle (R7).
For time point C (Fig. 4C), the second generation pellets were completely dispersed during the initial stage (R0). These dispersed new pellets were intertwined with each other and then formed larger dense structure during the first cycle (R1). Later on, protuberances extended from the pellet surfaces, forming thick-branched pellets during the second cycle (R2). The hairy feathers surrounding the thick-branched pellet detached from the branch’s tips and generated small hairy pellets at the third cycle (R3). The hair surrounds the pellets, which elongated and increased in number before becoming highly-branched feathers. The hairy structure entangled again and clumped at the fifth cycle (R5) and burst from the core at the sixth cycle (R6). Ovoid pellets were observed at the seventh cycle (R7), once the hyphal elements growing outwards (hairy feathers) had been sheared off.
These time points showed a variety of pellet morphologies across the batches, although only some resulted in EPS production. According to work by Wagner et al. [25], EPS production by G. lucidum is highest upon formation of ovoid pellets. As observed in the present study, time point B produced ovoid pellets at R3 while time point C managed to produce ovoid pellets at R7. Time point A was incapable of producing ovoid pellets throughout the cycles. The formation of ovoid pellets at R3 of time point B was chosen for future work.
With the implementation of the RBF technique, there was evidence of fungal rejuvenation and renewed growth by the culture due to the influx of fresh nutrient supplies at each repeated-batch cycle, and possibly also due to the reduction in levels of metabolic waste compounds in the culture medium following dilution with fresh medium. Our culture produced a series of hairy pellets, consisting of active tip regions which may contribute to the success of the RBF strategy.
Because the same fungal cell culture was used for RBF, it was deemed critical to maintain the EPS concentration below the restricting level by replenishing the media at an appropriate fermentation time, as described by Wenyan et al. [32]. In the present study, RBF using shake flask had to be stopped at the seventh cycle due to pellet colour changes (possibly indicating autolysis) and potential toxic metabolite build up which may have disrupted the morphology. Therefore, experiments in the bioreactor should be performed to determine the highest cycle number for RBF that can be endured.
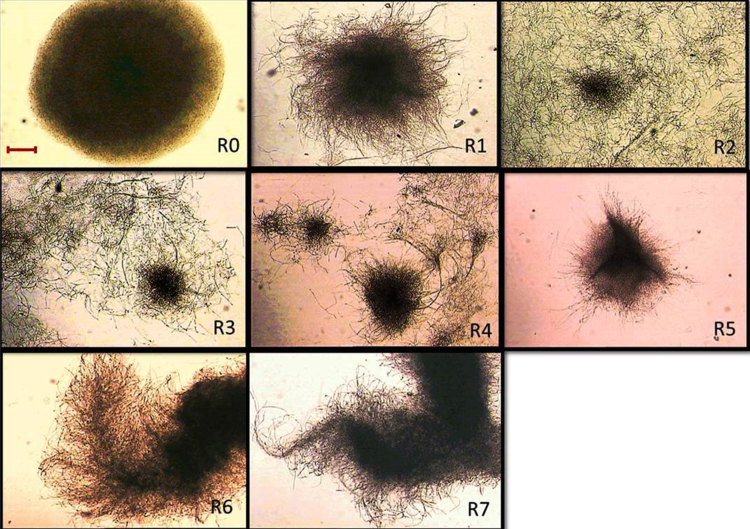
3.3. Morphological characteristics in a 2.5 L bioreactor
RBF was carried out in a bioreactor, whereby a mycelial suspension was used to inoculate a 2.5 L bioreactor containing 2L working media with a complete non-baffled reactor [17]. At the transition phase of the fermentation process, 80% of the fermented broth (1.6 L) was removed and fresh medium was pumped into the bioreactor using a peristaltic pump. Fermented samples from the bioreactor were analysed for EPS productivity, and when maximum EPS productivity was detected the fermented broth was replaced. Different values in EPS production were linked to morphology, as the physical state of the fungus generated different EPS levels. Therefore, the morphological changes observed during the RBF process in a bioreactor were compared, as shown in Fig. 5.
Fig. 5.
Morphological changes in a bioreactor repeated-batch fermentation of G. lucidum BCCM 31549. Conditions and medium compositions were as for shake flask, except 30 g/L of glucose and 20% (v/v) inoculum were used. Images were taken at 4-fold magnification. Bar = 150 μm. The baffle was removed in this strategy.
Morphologically, this fungus formed pellets throughout the batch fermentation (R0) at the tenth day. The pellets had degraded or dispersed at the first cycle (R1) to generate starburst-like pellets with mycelial strands protruding from the surface. The fungus grew exponentially in the second cycle (R2), and the culture became cloudier (under macroscopic observation) than the previous cycle. Vigorous mycelial growth was observed as the morphology showed a dense fluffy structure as the pellet shape disintegrated.
It is proposed that the fluffy-like pellets were linked to the liberation of the second generation of fungal progeny (R1 to R2), as mentioned in the shake flask work, and were associated with the rise in EPS production. This event continued to the third cycle (R3) as the intertwined mycelia started to fuse together to form denser pellets. With the growth increase, the engulfed mycelia haltingly formed pellets at the fourth cycle (R4). Later, an irregular-pellet shape was generated during the fifth cycle (R5), the morphology of which was typically a starburst-like pellet associated with EPS production during the present RBF cycles.
After R5, the fungus lost its ability to sustain a high growth rate through the sixth cycle (R6), possibly due to loss of vigour arising from successive subculturing events (RBF). At R6, the fungus showed an absence of small hyphal elements, indicating reduced growth conditions. The same morphology was observed during the last cycle (R7), as a thicker, branched and denser structure was present. To conclude, the highest cycle that G. lucidum BCCM 31459 can tolerate under bioreactor RBF was the fifth (R5). This successful RBF process was compared with that of batch fermentation.
3.4. Comparison of batch and repeated-batch fermentation
Table 4 shows the kinetic parameters of batch and RBF methods for G. lucidum fermentation, both in the shake flask and bioreactor (based on previous work by Wan et al. [17]). For the shake flask, EPS production was 1.3 fold higher for RBF than for batch. The EPS productivity of RBF was a considerable 2.5 times higher than for the batch method itself. Specific production of EPS was doubled for RBF, demonstrating the effectiveness of RBF in EPS production. Through application of RBF, the productivity of EPS was increased within only 6 days of total fermentation time, compared with 32 days for the batch method.
Table 4.
Comparison of batch and repeated-batch fermentation of G. lucidum BCCM 31549 in shake flask and bioreactor.
| Fermentation technique c | Total preparation time (days) | EPS production, (g/L) | EPS productivity, P EPS (g/L day−1) | Specific production of EPS, Q EPS/x [(g/g) day−1] | Yield EPS, (g EPS/g GLU) |
|---|---|---|---|---|---|
| Batch in flaska | 32 | 0.16 ± 0.02 | 0.013 ± 0.001 | 0.009 ± 0.001 | 0.030 ± 0.01 |
| Repeated-batch in flask | 6 (up to 7 cycles) |
0.20 ± 0.03 | 0.033 ± 0.001 | 0.018 ± 0.003 | 0.042 ± 0.01 |
| Original work from Wan et al. [17] Batch in bioreactorb |
40 | 8.08 ± 1.0 | 0.81 ± 0.02 | 0.51 ± 0.03 | 0.89 ± 0.07 |
| Repeated-batch in bioreactor | 5 (up to 5 cycles) |
4.64 ± 0.6 | 0.93 ± 0.02 | 0.49 ± 0.08 | 0.73 ± 0.02 |
*One way ANOVA was carried out for each row with a P value of <0.0001, and the pairing was considered significant with a P value of 0.0485.
Fermentations in shake flasks were carried out under conditions of [(g/L): Glucose 50, KH2PO4 0.5, K2HPO4 0.5, MgSO47H2O 0.5, YE 1, NH4Cl 4], 100 rpm, initial pH 4, 10% inoculum (v/v) and temperature 30 °C.
Fermentations in the bioreactor were carried out under conditions of [(g/L): Glucose 30, KH2PO4 0.5, K2HPO4 0.5, MgSO47H2O 0.5, YE 1, NH4Cl 4], 100 rpm, initial pH 4, 20% (v/v) inoculum (bioreactor) and temperature at 30 °C.
Total preparation time was calculated based on 10 days plating time, 10 days inoculum fermentation, and 10 days for seed culture (only for bioreactor).
G. lucidum cells displayed a reasonable tolerance to repeated use. Five batches could be fermented consecutively in a 2 L bioreactor from a total of seven batches. In contrast to batch culture in a bioreactor, the time for EPS production in a bioreactor was reduced greatly from 40 to 5 days and was slightly higher, while EPS production, specific production of EPS, and EPS yield were observed at levels comparable to the batch itself.
Overall, without the requirement for extra energy (time) and resources (media), RBF was shown to be a superior fermentation technique than the batch method. One possible reason for this conclusion might be the synergistic effect of reused mycelial cells in adaptation to RBF.
4. Conclusion
In the shake flask, repeated-batch cultures survived the autolysis event until the fifth cycle. The transition growth phase of the broth replacement time point generated the ideal morphology for EPS production at the third cycle of RBF, showing ovoid pellets in the shake flask. In the bioreactor, EPS levels were ideal, with starburst-like pellets observed at the fifth cycle of RBF. Morphologically, the robustness of G. lucidum mycelium to survive RBF cycles contributed to the generation of a sustainable EPS level. With RBF, EPS productivity increased both in the shake flask and bioreactor.
Conflict of interest
The authors declare that there are not conflicts of interest.
Acknowledgements
This work was financially supported by the Majlis Amanah Rakyat (MARA) London under Malaysian Government Sponsorship.
References
- 1.Yang H., Min W., Bi P., Zhou H., Huang F. Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum. Biochem. Eng. J. 2013 [Google Scholar]
- 2.Paterson R.R. Ganoderma—a therapeutic fungal biofactory. Phytochemistry. 2006;67(18):1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Wan-Mohtar W.A., Young L., Abbott G.M., Clements C., Harvey L.M., McNeil B. Antimicrobial properties and cytotoxicity of sulfated (1 3)-beta-d-glucan from the mycelium of the mushroom Ganoderma lucidum. J. Microbiol. Biotechnol. 2016;(6):26. doi: 10.4014/jmb.1510.10018. [DOI] [PubMed] [Google Scholar]
- 4.Ruan W., Popovich D.G. Ganoderma lucidum triterpenoid extract induces apoptosis in human colon carcinoma cells (Caco-2) Biomed. Prev. Nutr. 2012;2(3):203–209. [Google Scholar]
- 5.Liu Y.J., Shen J., Xia Y.M., Zhang J., Park H.S. The polysaccharides from Ganoderma lucidum: are they always inhibitors on human hepatocarcinoma cells? Carbohydr. Polym. 2012;90(3):1210–1215. doi: 10.1016/j.carbpol.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 6.Li Y.B., Liu R.M., Zhong J.J. A new ganoderic acid from Ganoderma lucidum mycelia and its stability. Fitoterapia. 2012 doi: 10.1016/j.fitote.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Fang Q.H., Tang Y.J., Zhong J.J. Significance of inoculation density control in production of polysaccharide and ganoderic acid by submerged culture of Ganoderma lucidum. Process Biochem. 2002;37(12):1375–1379. [Google Scholar]
- 8.Fazenda M.L., Harvey L.M., McNeil B. Effects of dissolved oxygen on fungal morphology and process rheology during fed-batch processing of Ganoderma lucidum. J. Microbiol. Biotechnol. 2010;20(4):844–851. doi: 10.4014/jmb.0911.11020. [DOI] [PubMed] [Google Scholar]
- 9.Kim H.M., Park M.K., Yun J.W. Culture pH affects exopolysaccharide production in submerged mycelial culture of Ganoderma lucidum. Appl. Biochem. Biotechnol. 2006;134(3):249–262. doi: 10.1385/abab:134:3:249. [DOI] [PubMed] [Google Scholar]
- 10.Fang Q.H., Zhong J.J. Effect of initial pH on production of ganoderic acid and polysaccharide by submerged fermentation of Ganoderma lucidum. Process Biochem. 2002;37(7):769–774. [Google Scholar]
- 11.Yang F.C., Yang M.J., Cheng S.H. A novel method to enhance the mycelia production of Ganoderma lucidum in submerged cultures by polymer additives and agitation strategies. J. Taiwan Inst. Chem. E. 2009;40(2):148–154. [Google Scholar]
- 12.Chang M.-Y., Tsai G.-J., Houng J.-Y. Optimization of the medium composition for the submerged culture of Ganoderma lucidum by taguchi array design and steepest ascent method. Enzyme Microb. Technol. 2006;38(3–4):407–414. [Google Scholar]
- 13.Fang Q.H., Zhong J.J. Two-stage culture process for improved production of ganoderic acid by liquid fermentation of higher fungus Ganoderma lucidum. Biotechnol. Prog. 2002;18(1):51–54. doi: 10.1021/bp010136g. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y.J., Zhang W., Zhong J.J. Performance analyses of a pH-shift and DOT-shift integrated fed-batch fermentation process for the production of ganoderic acid and ganoderma polysaccharides by medicinal mushroom Ganoderma lucidum. Bioresour. Technol. 2009;100(5):1852–1859. doi: 10.1016/j.biortech.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y.J., Zhong J.J. Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of ganoderma polysaccharide and ganoderic acid. Enzyme Microb. Technol. 2003;32(3):478–484. [Google Scholar]
- 16.Maftoun P., Malek R., Abdel-Sadek M., Aziz R., Enshasy H.E. Bioprocess for semi-industrial production of immunomodulator polysaccharide pleuran by Pleurotus ostreatus in submerged culture. J. Sci. Ind. Res. 2013;72:655–662. [Google Scholar]
- 17.Wan W.A.A.Q.I., Latif N.A., Harvey L.M., McNeil B. Production of exopolysaccharide by Ganoderma lucidum in a repeated-batch fermentation. Biocatal. Agric. Biotechnol. 2016;6:91–101. [Google Scholar]
- 18.Birhanli E., Yesilada O. Enhanced production of laccase in repeated-batch cultures of Funalia trogii and Trametes versicolor. Biochem. Eng. J. 2010;52:33–37. [Google Scholar]
- 19.Qu L., Ren L.J., Sun G.N., Ji X.J., Nie Z.K., Huang H. Batch, fed-batch and repeated fed-batch fermentation processes of the marine thraustochytrid Schizochytrium sp. for producing docosahexaenoic acid. Bioprocess Biosyst. Eng. 2013;36(12):1905–1912. doi: 10.1007/s00449-013-0966-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Chen X., Qi B., Luo J., Shen F., Su Y., Khan R., Wan Y. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresour. Technol. 2014 doi: 10.1016/j.biortech.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Naritomi T., Kouda T., Yano H., Yoshinaga F., Shigematsu T., Morimura S., Kida K. Influence of broth exchange ratio on bacterial cellulose production by repeated-batch culture. Process Biochem. 2002;38(1):41–47. [Google Scholar]
- 22.Birhanli E., Yesilada O. Increased production of laccase by pellets of Funalia trogii ATCC 200800 and Trametes versicolor ATCC 200801 in repeated-batch mode. Enzyme Microb. Technol. 2006;39(6):1286–1293. [Google Scholar]
- 23.Yang X., Wang B., Cui F., Tan T. Production of lipase by repeated batch fermentation with immobilized Rhizopus arrhizus. Process Biochem. 2005;40(6):2095–2103. [Google Scholar]
- 24.Park J.P., Kim Y.M., Kim S.W., Hwang H.J., Cho Y.J., Lee Y.S., Song C.H., Yun J.W. Effect of aeration rate on the mycelial morphology and exo-biopolymer production in Cordyceps militaris. Process Biochem. 2002;37(11):1257–1262. [Google Scholar]
- 25.Wagner R., Mitchell D.A., Sassaki G.L., de Almeida Amazonas M.A. Links between morphology and physiology of Ganoderma lucidum in submerged culture for the production of exopolysaccharide. J. Biotechnol. 2004;114(1–2):153–164. doi: 10.1016/j.jbiotec.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Park J.P., Kim Y.M., Kim S.W., Hwang H.J., Cho Y.J., Lee Y.S., Song C.H., Yun J.W. Effect of aeration rate on the mycelial morphology and exo-biopolymer production in Cordyceps militaris. Process Biochem. 2002;37(11):1257–1262. [Google Scholar]
- 27.Wagner R., Mitchell D.A., Sassaki G.L., Amazonas M.A.L.D., Berovic M. Current techniques for the cultivation of Ganoderma lucidum for the production of biomass, ganoderic acid and polysaccharides. Food Technol. Biotechnol. 2003;41(4):371–382. [Google Scholar]
- 28.Packer H., Thomas C. Morphological measurements on filamentous microorganisms by fully automatic image analysis. Biotechnol. Bioeng. 2004;35(9):870–881. doi: 10.1002/bit.260350904. [DOI] [PubMed] [Google Scholar]
- 29.Yang F.-C., Liau C.-B. The influence of environmental conditions on polysaccharide formation by Ganoderma lucidum in submerged cultures. Process Biochem. 1998;33(5):547–553. [Google Scholar]
- 30.Yang F., Ke Y., Kuo S. Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enzyme Microb. Technol. 2000;27(3–5):295–301. doi: 10.1016/s0141-0229(00)00213-1. [DOI] [PubMed] [Google Scholar]
- 31.Rogalski J., Szczodrak J., Janusz G. Manganese peroxidase production in submerged cultures by free and immobilized mycelia of Nematoloma frowardii. Bioresour. Technol. 2006;97(3):469–476. doi: 10.1016/j.biortech.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Wenyan J., Jingbo Z., Zhongqiang W., Shang-Tian Y. Stable high-titer n-butanol production from sucrose and sugarcane juice by Clostridium acetobutylicum JB 200 in repeated batch fermentations. Bioresour. Technol. 2014:0960–8524. doi: 10.1016/j.biortech.2014.04.047. [DOI] [PubMed] [Google Scholar]