Abstract
Background
In Western countries the prevalence of Helicobacter pylori (H. pylori) infection may be declining but there is a lack of recent longitudinal population studies. We evaluated the changing epidemiology over a 23-year period in Sweden.
Materials and methods
In 1989, the validated Abdominal Symptom Questionnaire (ASQ) was mailed to a random sample of inhabitants (ages 22–80 years) in a Swedish community, and 1097 (87%) responded. H. pylori serology was analysed in a representative subsample (n = 145). Twenty-three years later, the ASQ was mailed again using similar selection criteria, and 388 out of 1036 responders had an upper endoscopy with assessment of H. pylori and corpus atrophy status.
Results
The prevalence of positive H. pylori serology decreased from 37.9% (1989) to 15.8% (2012), corresponding to a decrease in odds of 75% per decade (odds ratio (OR): 0.25; 95% confidence interval (CI): 0.11–0.59, p = 0.001) independent of age, gender, body mass index (BMI) and level of education, with a pattern consistent with a birth cohort effect. The prevalence increased with increasing age (p = 0.001). The prevalence of H. pylori on histology in 2012 was 11.4% (95% CI 8.6–15.0). The prevalence of corpus atrophy on serology and/or histology in 2012 was 3.2% (95% CI 1.8–5.5); all cases were ≥57 years old.
Conclusion
The stomach is healthier in 2012 compared with 1989. H. pylori prevalence in adults has decreased over the last two decades to a level where clinical management might be affected.
Keywords: Helicobacter pylori, corpus atrophy, epidemiology, population-based, longitudinal
Introduction
Humans have probably been living in symbiosis with Helicobacter pylori (H. pylori) from time immemorial. The earliest evidence of H. pylori infection dates back 58,000 years.1 The true infection rate at different time epochs is uncertain, although an increase in prevalence during the 19th century is assumed from an increase in H. pylori associated diseases.2 It is one of few infections that causes a chronic inflammatory response without causing disease or symptoms in a majority of those infected. The infection will cause peptic ulcer disease, gastric cancer or mucosa-associated lymphoid tissue (MALT) type lymphoma in only a minority of infected individuals.3,4 Subclinical malabsorption of nutrients may appear in older individuals with H. pylori because of associated gastric atrophy.5 The diseases related to H. pylori are proportionally uncommon and mostly arise in elderly individuals.
The proportion of infected adults decreases with increased prosperity,6–8 and most people are likely infected in childhood. The decrease in prevalence in older age in developed countries is presumed to represent a birth cohort effect9 so that when the next generation of children grow up they will likely retain their lower prevalence throughout their lives. A decrease of H. pylori infection in adults from Europe and North America has been observed, but there are only a few longitudinal population-based cohort studies published, most of them confirming this.10–13
Respected clinical guidelines recommend that younger patients with uninvestigated dyspepsia should no longer be investigated by the ‘test & treat’ strategy when the prevalence falls below 10–20%.6,14 Thus, a decrease in the prevalence of H. pylori infection would result in important implications for clinical practice.
The aim of this study is to investigate longitudinally how the natural history of H. pylori infection in an adult Swedish population has changed over 23 years, and also to evaluate the current status of chronic corpus gastritis in a Swedish population.
Methods
The study is a part of the LongGerd study, a longitudinal population based study on gastrointestinal symptoms, with surveys in 1988, 1989, 1995, as previously described,15,16 and in 2011/2012, outlined in Figure 1. We report the natural history of H. pylori infection assessed using serology from two LongGerd substudies in 1989 and in 2012, and also the prevalence of gastric corpus atrophy in 2012.
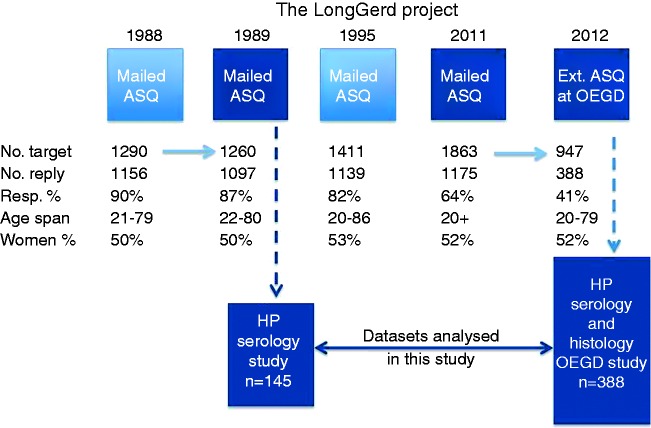
Figure 1.
The overall LongGerd study project flowchart showing the steps with the postal Abdominal Symptom Questionnaire (ASQ) surveys targeting equally sampled samples of the adults in the Östhammar community, Sweden, on four occasions, and the subsample studies on two occasions analysed in this study: the first on Helicobacter pylori (H. pylori) serology in 1989 (n = 145) and the second on H. pylori detected by both serology and histology in 2012 (n = 388). The sample approached by mail in 1989 originates from the 1988 mail study. The 1995 mail study is included to visualise the complete sampling procedure, as individuals for the 2011 study (n = 305 as shown in Figure 2) were recruited here. The two parts of the project used in this study are highlighted in dark blue. Target population size, response rate and age range and gender distribution in responders are also shown. OEGD = oesphagogastroduodenoscopy.
Settings
The 1989 study
The municipality of Östhammar had 21,338 inhabitants on 1 January 1988, living in either urban (67%) or rural (33%) areas. At the time, the distribution by age, gender, family size, income, occupation and other socioeconomic variables was largely similar to the national average. Ninety-six percent of the residents were Swedish citizens.15
The 2011/2012 study
In December 2010, the municipality had 21,373 inhabitants. The distribution of inhabitants living in either urban or rural areas, age, gender, family size, income and occupation was still similar while the level of education was slightly better than the national average. Ninety-two percent of the residents were Swedish citizens.17 In September 2011, 16,680 inhabitants aged 20 years and older lived in the community.
Procedures
Mail surveys overview
The four mail surveys are outlined in Figure 1. The previous studies from 1988, 1989 and 1995 have been described in detail.15,16 In short, a validated questionnaire on abdominal and gastrointestinal symptoms, the Abdominal Symptom Questionnaire (ASQ)15,18,19 was mailed to all adults in the Östhammar community born on day 3, 12 or 24 of each month, a sampling procedure equivalent to random sampling that allowed us to follow the same participants over time.
The questionnaire in the 1989 study was sent to the same participants as in the 1988 study. In 1995 all new inhabitants 20–27 years old with the same dates of birth were also included.16 An identical sampling procedure was followed in the 2011 survey for all inhabitants 20 years of age or older. The number of new participants in the 1995 and the 2011 studies are shown in Figure 2, sampling level A.
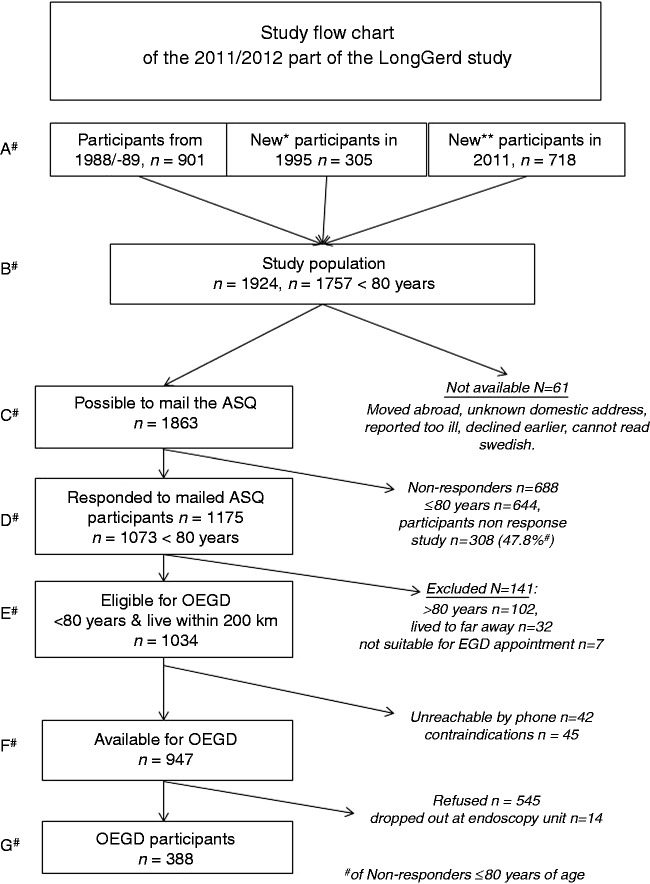
Figure 2.
The study flow chart of ‘the 2011/2012 ASQ questionnaire and oesophagogastroduodenoscopy (OEGD) part’ of the LongGerd study. *New participants ≤27 years old in 1995. **Further new participants (20+ in the community, and those who moved in) in 2012. #Different sample levels as explained in the text.
1989 Mail survey
In the 1989 survey, 1097 (87.2%) individuals responded to the ASQ. The non-responders did not differ from the responders aside from a slight overrepresentation of young men among the latter15 and the high participation rate suggests excellent generalisability.
H. pylori serology study 1989
Among the 1097 responders in the 1989 mail study, 150 stratified case-controls matched for age, gender and socioeconomic status, 50 with the irritable bowel syndrome (IBS), 50 with dyspepsia (including reflux symptoms) and 50 symptom-free,20 visited a research laboratory. Of those responders, 145 provided a blood sample for H. pylori serology (see Figure 1) and 55 (38%) were seropositive. There were no statistical significant differences in terms of being H. pylori positive between the three symptom groups (33%, 33% and 48%, respectively, p = 0.4), and there was no significant association between H. pylori seropositivity and education level or gender.20
The 145 individuals with H. pylori serology did not differ in statistically significant ways from the other responders in the 1989 mail survey, in terms of age (mean 47.7 vs 48.9 years) or proportion with higher education (30.1% vs 30.9%), although there were significantly more women (63.4% vs 50.1%, p = 0.003). This was due to the sampling strategy based on IBS, a diagnosis more common in women.
2011 Mail survey
A flowchart for the 2011 mail survey and 2012 oesophagogastroduodenoscopy (OEGD) study is presented in Figure 2. In September 2011, a new ASQ mail survey to a representative sample 20 years or older in the community was conducted using the same sampling procedure as in previous surveys (n = 1645). After exclusion of two individuals with protected identity, nine individuals who had moved out of the country and four individuals who had denied participation in the prior studies, 1630 community residents remained. In addition, we included all participants from the three prior investigations who were still alive but had moved out of the community (n = 294). This strategy gave a final study population of 1924 individuals, 1757 of them below 80 years old (sampling level B in Figure 2). Because 61 individuals were unavailable (reasons given in Figure 2), 1863 individuals (sampling level C) were mailed the ASQ in late 2011. A total of 1175 individuals (63.1%) responded. Of the responders, 1073 individuals were <80 years old (sampling level D).
Of the 688 non-responders, 644 were <80 years old and were eligible for the OEGD study. The 644 non-responders were significantly younger (mean 46.4 years) than the 1034 responders eligible for OEGD (mean 48.3, 95% CI: 45.2–51.3 years old, p < 0.001 adjusted for gender) but there was no significant difference in proportion of women between non-responders (48.0%) and responders (51.7%, p = 0.21 adjusted age). They were approached by telephone in the non-response study and 308 (47.8%) replied, 64 replied but declined further questions, 119 did not reply on three attempts, 113 had no official phone number, and 40 were not called. We asked all who replied seven key ASQ symptom questions and their level of education. There were no significant difference in proportion with higher education (73.7% vs 60.5%, p = 0.47 adjusted age and gender) prevalence of reflux symptom (heartburn and/or acid regurgitation) (22.4% vs 24.5%, p = 0.40) or epigastric pain or discomfort (10.4% vs 10.8%, p = 0.41 adjusted age and gender) between the 308 interviewed non-responders compared to the responders <80 years (Level D in Figure 2). Any non-response bias therefore appears to be minimal in the 2011 mail survey.
H. pylori serology and histology OEGD study 2012
The ASQ study was performed by mail in November–December 2011, and the OEGD study was carried out in January–April 2012. The continuing sampling procedure is shown in Figure 2. As described above, 1175 of the responders to the 2011 mail survey, 1073 individuals were <80 years of age (sampling level D in Figure 2). As 32 individuals lived more than 200 km from the research centre and seven could not make time during the OEGD study, 1034 individuals were eligible for the OEGD study (sampling level E).
Experienced research assistants, with special training in contraindications, invited the eligible participants to the research endoscopy unit by telephone, gave verbal information on informed consent and sent those who accepted OEGD written information and an appointment for the OEGD. Of the 1034 individuals eligible for OEGD, 42 could not be reached by telephone on three attempts. Forty-five had medical contraindications, leaving 947 available for the OEGD study (sampling level F in Figure 2). Of the 947 available individuals, 402 (42.5%) accepted to participate and signed a written informed consent form. Of these, 14 individuals refused the OEGD onsite or discontinued the procedure early and 388 (40.8%) completed the OEGD (sampling level G in Figure 2).
Although responders to the ASQ below <80 years of age, level D in Figure 2, were slightly older than non-responders to the ASQ (mean age difference 1.9 years), there were no significant differences regarding age, gender distribution or level of education between the OEGD participants (level G) and those eligible for endoscopy but who declined the procedure (545 + 14 individuals at level F). Hence endoscopy participants are considered representative of the study population.
OEGD
Just before the OEGD, blood samples for standard blood tests and serology were taken and stored in −70° C. At the OEGD, biopsies for histology were taken from multiple sites.
Five experienced endoscopists participated. A consensus meeting led by an external expert (Lars Lundell) reviewed multiple video recordings according to the study protocol before the study commenced. Each endoscopist was monitored on the first day by the project leader (LA).
The endoscopists were unaware of the medical history including H. pylori status or any current or previous symptoms. The participants were offered pharyngeal local spray. No sedation was used except 5–10 mg diazepam sublingual in 20 cases. The OEGD findings were recorded and locked before a complete medical history including drug use was taken.
Variables
H. pylori serology 1989
In the 1989 study serum samples were analysed for anti-H. pylori Immunoglobulin G (IgG) by using the commercially available HM-CAP does not seems to short of anything, it is simply the name of the assay, immunoassay (Enteric Products Inc., Westbury, New York, USA). The assay had a sensitivity of >98% and a specificity of 100%.21,22
H. pylori serology and serology for gastric atrophy 2012
Serum for a test panel on levels of Gastrin-17, pepsinogen I and IgG class antibodies to H. pylori were taken. To detect H. pylori infection, the specific enzyme immunoassay (EIA) test of the panel was used (GastroPanel, Biohit PLC, Helsinki, Finland). The sensitivity and specificity of the IgG enzyme-linked immunosorbent assay (ELISA) assay to detect H. pylori in comparison to culture is 96% and 97%, respectively.23 The whole test panel delineates, in addition, individuals with a normal stomach from those with moderate or severe atrophic corpus gastritis or with an on-going H. pylori infection with a sensitivity and specificity of 95% and 93%, respectively.24
Gastric biopsies for H. pylori status and gastric atrophy 2012
Two biopsies were taken from both the antrum and the corpus for haematoxylin and eosin (H&E) and Warthin Starry (WS for H. pylori) staining, and assessed by the Sydney System25 by two experienced pathologists (MV and LV). Autoimmune gastritis was diagnosed as described by Goncalves et al.26 The pathologist was blinded when doing the histology report, but afterwards the histology for the participants that were H. pylori positive on serology and negative on WS staining on histology was reviewed.
Education
The participants were asked about their level of education which was rated on a five-point scale (1: elementary, 2: comprehensive, 3: secondary, 4: upper secondary, 5: university). The level of education was dichotomised as lower (level 1–3) or higher (level 4 and 5).
Body mass index (BMI)
The BMI was calculated as weight (kg)/(height (m))2.
Tobacco use
Daily smoking and use of snus (smokeless tobacco or moist snuff) was recorded (yes/no). The two forms of tobacco were analysed separately due to potentially different effects on upper gastrointestinal symptoms and diseases.27
Age groups
The results are shown for the age groups in the two samples who were 22–44, 45–55 and 56–80 years old in 1989 and 2011 (Figure 3). The age cut-offs were chosen to investigate the change in H. pylori positivity over time and if the change was consistent with a true birth cohort effect9 as has been postulated for H. pylori positivity.28,29 In order to maximise the number of individuals in each study (1989 and 2012) who could possibly have participated in both studies 23 years apart, we compared the age group 22–56 years old in 1989 (n = 63) with the age group 45–79 years old in 2012 (n = 200) regarding the prevalence of H. pylori positivity, as visualised in Table 1 and Figure 3. Altogether, 32 individuals participated in both studies, i.e. constituted a true birth cohort.
Figure 3.
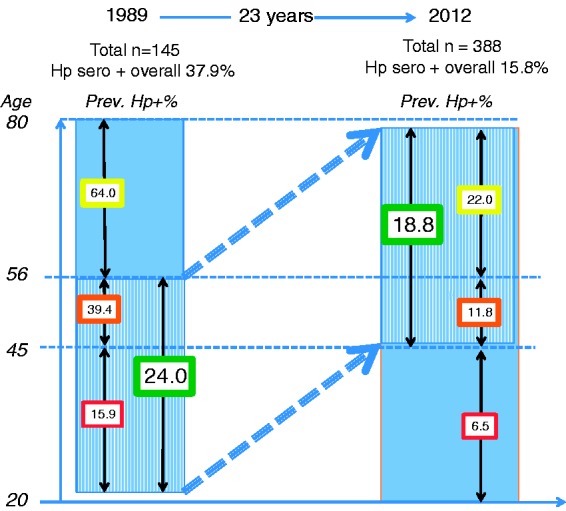
The prevalence (%) of positive Helicobacter pylori (H. pylori) serology in 1989 and 2012 in the three different age groups (20–44, 45–55, 56–80 years old), and the prevalence of positive H. pylori serology in bold figures in the green boxes for the corresponding age ranges 22–56 years in 1989 and 45–79 years 23 years later in 2012 (labelled ‘Aging group’ in Table 2).
Table 1.
Characteristics of participants along the sampling procedure in the 2011/2012 survey
| All Ages | Women % (95% CI) | Mean age (95% CI) | Higher education % (95% CI) | <80 Years of age | Women % (95% CI) | Mean age (95% CI) | Higher education % (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Study population 2011 | 1924 | 51.0 (48.8–53.3) | 54.1a (53.3–54.9) | n.a. | 1757 | 50.2 (47.9–52.5) | 51.1a (50.4–51.9) | 73.8b (68.8–78.7) |
| Responders to ASQ | 1175 | 52.8 (49.0–54.7) | 56.6a (55.7–57.5) | 57.3c (54.4–60.1) | 1073 | 51.7 (48.7–54.7) | 53.9a (53.1–54.8) | 61.0d (58.1–64.0) |
| Available for OEGD | n.a. | n.a. | n.a. | n.a. | 947 | 52.0 (48.8–55.1) | 53.6 (52.6–54.5) | 61.8e (58.6–64.9) |
| OEGD participants 2012 | n.a. | n.a. | n.a. | n.a. | 388 | 52.1 (47.1–57.1) | 54.0 (52.7–55.4) | 62.7f (57.9–67.6) |
ASQ: Abdominal Symptom Questionnaire; CI: confidence interval; OEGD: oesophagogastroduodenoscopy; n.a.: data not available.
The proportion of women, mean age and (when applicable) proportion with higher education among the participants at four of the levels described in Figure 2 for all participants and for those <80 years old as that was an eligibility criterion for upper endoscopy. The statistical analysis are adjusted for age and gender.
p ≤ 0.01; bn = 305; cn = 1151; dn = 1052,; en = 931; fn = 381.
Statistics
Differences in demographic variables between responders to the ASQ and those in the study population who did not respond to the ASQ, and between OEGD participants and participants available for OEGD that did not have an OEGD, were calculated using logistic regression. Logistic regression was also used to test if there was a difference in H. pylori seropositivity between participants that smoked and participants that did not smoke, and between participants that used snus and participants that did not use snus, adjusted for gender, age and level of education.
Change in H. pylori positivity over time was calculated using mixed effect logistic regression with H. pylori positivity as the dependent variable and time as the independent variable, adjusted for age and gender. In further analyses, level of education and BMI were included as independent variables to investigate the association between level of education and BMI and H. pylori positivity, and interaction terms between gender and time and BMI and time were used as independent variables to test if gender or BMI affected the change in H. pylori positivity over time.
All analyses were performed in STATA 13 using a two-sided alpha-level of 0.05 to test for statistical significance.
Ethics
The 1989 study was approved by the Ethical Review Board of the Medical Faculty of Uppsala University (Dnr 1989 §220). Approval for the 2011–2012 study was obtained from the Ethics Committee of Uppsala University on 26 January 2011 (Dnr 2010/443).
Results
H. pylori serology status 1989 and 2012
The overall prevalence of H. pylori positivity on serology was 37.9% in the 1989 survey and 15.5% among those endoscoped (missing data = 2) in 2012 (p < 0.0001, Table 2). Table 2 shows detailed data for the overall prevalence and the prevalence in the three age groups. This is also visualised in Figure 3. The prevalence of H. pylori positivity was statistically significantly lower in 2012 compared to 1989 for all three age groups.
Table 2.
Helicobacter pylori (H. p.) prevalence on serology by age groups in the 1989 and 2012 surveys
| 1989 n | 1989 % H.p.+ | 95% CI | 1989 Mean age | 1989 % Women | 1989 % Higher education | 2012 n | 2012 % H.p.+ | 95% CI | p H.p.+ % | 2012 Mean age | 2012 % Women | 2012 % Higher education | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22–44 | 63 | 15.9 | 6.9–24.9 | 33.6 | 63.5 | 55.6 | 93 | 6.5 | 1.5–11.5 | 0.029 | 35.0 | 47.3 | 95.6 |
| 45–55 | 33 | 39.4 | 22.7–56.1 | 48.3 | 78.8 | 15.2 | 95 | 11.8a | 6.7–19.5 | <0.0002 | 50.5 | 49.5 | 75.5 |
| 56–80 | 50 | 64.0 | 51.0–77.2 | 65.4 | 54.0 | 8.2 | 200 | 22.0 | 16.8–28.2 | <0.0001 | 64.6 | 52.8 | 41.6 |
| Overall | 145 | 37.9 | 23.4–38.3 | 47.7 | 63.4 | 30.3 | 388 | 15.8 | 12.5–19.8 | <0.0001 | 54.0 | 52.1 | 62.7 |
| Aging group b | 96 | 24.0 | 15.5–32.5 | 38.6 | 68.8 | 41.7 | 295 | 18.8 | 14.7–23.6 | 0.134 | 60.0 | 53.6 | 52.3 |
CI: confidence interval.
The number (and proportion of all) individuals and their mean age, proportion of women and proportion with higher education in the three age groups, chosen in order to have as many individuals as possible in the corresponding age ranges (22–56 years in 1989 and 45–79 in 2011, 23 years later, labelled ‘Aging group’).
Missing data on H. pylori serology = 2; bThe two samples, 22–44 years old in 1989 and 56–79 years old in 2012
As shown in Table 2 and Figure 3, the prevalence of H. pylori seropositivity was 24.0% in the 96 individuals within the age range of 20–44 years in 1989, and 18.8% in the 295 individuals with the corresponding age range 23 years later (56–80 in 2012, p = 0.13).
A total of 32 individuals participated in both serology studies (1989 and 2011), thus constituting a true birth cohort. In 1989 when all were ≤56 years old, seven (22%) of these were positive on H. pylori serology and three of those were still positive in 2012. All four that had a seroreversion had been prescribed antibiotics three times or more since 1995. Of the 25 (78%) seronegative participants in 1989, one had a seroconversion over the years. This person had a borderline absorbance value just below the cut off level in 1989, and just above the cut off level in 2012, with the 1989 value likely a false negative.
Effect of time on the prevalence of H. pylori seropositivity
When investigating the effect of time on the prevalence of positive H. pylori serology tests using a random effects logistic regression model including all participants in both survey (145 from 1989 and 386 from 2012 with 32 participants participating in both surveys: 499 participants, 531 observations) and adjusting for age and gender, the odds of H. pylori positivity decreased by 75% per decade (odds ratio (OR): 0.25; 95% confidence interval (CI): 0.11–0.59, p = 0.001). The odds of H. pylori positivity increased by 11% per year of age (OR: 1.11; 95% CI: 1.04–0.18, p = 0.001) but there was no difference in H. pylori positivity between men and women (OR: 0.92; 95% CI: 0.40–2.08).
There were no association between H. pylori positivity and level of education (p = 0.34) or BMI (p = 0.94) tested in a separate analysis (altogether 325 participants, 350 observations, data not shown). In addition, there were no interaction effects of time and gender, level of education or BMI, thus, there were no differences in the reduction in H. pylori positivity between men and women (p = 0.99) or between levels of education (p = 0.90), and BMI was not associated with the reduction in H. pylori positivity (p = 0.44, data not shown).
H. pylori histology status 2012
Out of 379 individuals with histology data, H. pylori was found in 43 individuals (11.3%, 95% CI 8.5–14.0). In the three groups aged 20–45, 46–55 and 56–80 years, respectively, the prevalence was 6.3% (95% CI 2.4–12.4), 8.6% (95% CI 4.4–16.0) and 15.5% (95% CI 11.0–21.1).
Comparing H. pylori status histology and serology 2012
In 376 individuals, the test outcome for H. pylori was available on both histology and serology. Of the 43 individuals where H. pylori was detected on histology, 42 (98%) were positive also on serology. Of the 58 out of the 376 individuals who were seropositive, 16 (28%) had no H. pylori seen on histology. When these cases were reviewed, five of them had an active gastritis at some level in the antrum and/or corpus, most possibly due to bacteria suppressing treatments, which might be indicative of false negative outcome on the WS staining for H. pylori.
Prevalence of corpus atrophy 2012
The prevalence of corpus atrophy assessed by histology was 2.6% (95% CI 1.0–4.8, n = 10, of whom seven had slight atrophy, three moderate atrophy, none severe atrophy, nine out of 388 missing data). Five of the 10 were H. pylori positive on histology. Four were classified as autoimmune gastritis, all negative for H. pylori on histology but two of them were positive for H. pylori on serology. All 10 were 57 years or older, with a median age of 70 years.
The prevalence of corpus atrophy as assessed by serology was 1.8% (95% CI 0.9–3.7, n = 7, H. pylori seropositive n = 4, out of 386 with complete data). All were 57 years or older, with a median age of 69 years.
Five individuals had corpus atrophy on both measurements (on histology four of them had slight and one severe atrophy). Altogether, 3.2% (95% CI 1.8–5.5, n = 12, women n = 6) of the population had corpus atrophy on either histology or serology.
Tobacco use
There were no significant difference between smokers and non-smokers (OR: 0.75; 95% CI: 0.31–1.84, p = 0.53) or those who used snus and those who did not (OR 1.65; 95% CI 0.71–3.84, p = 0.24) in the prevalence of H. pylori seropositivity when controlled for age, gender and level of education.
Discussion
We found that the prevalence of H. pylori-infected adults has significantly decreased over the past two decades in all age groups, and the vast majority of older people do not have gastric corpus atrophy. We also found that H. pylori status did not change significantly over time in participants who were 22–56 years old in 1989 and 45–79 years old in 2012, supporting a birth-cohort effect likely related to acquiring the infection in childhood and not in adulthood.9,28,30 The decreasing trend of infection when comparing these two samples (as shown in Table 2 and Figure 3) is probably explained by some of the adults having had antibiotic treatment for other reasons over the years, as illustrated by the four out of 32 individual who seroreverted over the years.
The overall reduction of odds of H. pylori infection corresponding to 75% per decade is independent of age. Consequently, the 11% increase in H. pylori infection prevalence per year of age reported in the present study is adjusted for the change with time and thus represents the association between age and H. pylori seropositivity at a single time point.
The majority of the few longitudinal studies on H. pylori prevalence report a general decline in H. pylori infection rate. In a Japanese study, 552 adults had their H. pylori serology status analysed twice and 52 (11.2%) out of the 464 (84.1% of all) H. pylori-positive in 1986 had seroreverted by 1997.11 Kosunen et al. showed that the age-adjusted seroprevalence rate declined from 56% to 31% in Finland between 1973 and 1994 (p = 0.001).10 Paired serum samples collected in 1973 and 1994 showed that the individual antibody status remained unaltered over the years in 92% of the cases. In the Norwegian Soerejsa case-control study on dyspepsia in the general population, 272 individuals were tested for H. pylori by culture from biopsies in 1987 and by faeces in 2004. Of the 140 H. pylori-positive individuals in 1987, 39 (28%) were negative in 2004, 19 had been prescribed triple therapy for eradication, and the remaining 20 individuals (14%) may have cleared of H. pylori by other antibiotic treatment.12 In contrast, one Danish population-based cohort study reported a stable prevalence of seropositive of H. pylori infection: 24.7% in 1983 and 24.5% in 1994.31
Two earlier Swedish point prevalence studies from the general population are also of interest. One study from the south of Sweden from the early 1990s, showed that the prevalence of H. pylori seropositivity increased with age and was 7% among those 10–19 years old and 60% among those aged 69 years or older.32 In a study from Northern Sweden from 2000, 33.9% of the participants (mean age 53.5 years) had signs of current infection on either histology or culture, and 43.0% were seropositive for H. pylori. Recently a population-based study over 19 years, also from the Northern Sweden, was published, reporting a decreasing prevalence of the infection with comparable numbers albeit no exact prevalence rates were reported.13
The agreement between serology and histology markers for corpus atrophy in the present study was relatively low. In a previous Swedish study,24 corpus atrophy was found in 7.1% on either histology or serology24 compared to 3.2% in the present study (p < 0.005). In the former study, 46% of those having corpus atrophy had ‘severe’ atrophy reported (data on file), versus none in this study. The analysis was done by the same team of pathologists (Institute of Pathology Klinikum Bayreuth, Germany). The poor agreement between serology and histology in our present study might be because of the low numbers, missed atrophy on histology because it may be patchy, or most likely the absence of any severe gastritis making serology testing less useful.24 Cases with corpus gastritis without persisting or prior H. pylori infection may still occur, as a proportion of those with autoimmune gastritis is independent of H. pylori.33 However, overall our data suggest that stomach health increases continuously over time.
It is apparent that the prevalence of H. pylori infection in Sweden is decreasing and may have declined to under 10% among younger people, at least in parts of Sweden. The rapid decrease is somewhat surprising as socioeconomic status has not improved that much, but antibiotic treatment for other infections besides true eradication cures may be a part of the explanation as 20–25% of all Swedish adults receive an antibiotic prescription annually (no cumulative data available).34,35 The low prevalence rate in the present study is below the level where ‘test & treat’ is currently recommended in the management of younger patients consulting for dyspepsia6,14 although this position has recently been questioned.36
The theory of evolution postulates that there is likely a survival benefit for the host for organisms that thrive inside their body. One theory as to why H. pylori has thrived in the human stomach could be that the hyperacidity in the stomach that occurs with antral H. pylori infection in young and middle aged individuals was beneficial before we began cooking our food and all foods consumed were raw. Most people at that time would have died before corpus infection or hypoacidity, which occur at an older age. There has been speculation on the benefits of being infected in modern times. For example, the lower prevalence of the infection may be a link to the epidemic of obesity,8,37 although we could not find support for an association between BMI and the change in H. pylori infection rate over time. Furthermore, a common hypothesis is that the prevalence of gastroesophageal reflux disease, Barrett’s oesophagus and adenocarcinoma of the distal oesophagus has increased38 as fewer individuals would have H. pylori-induced hypoacidity at older age. Notably, overall mortality may not be increased in those infected; the increased risk of gastric cancer has been reported to be balanced by a decreased risk of stroke and also lung and pancreas cancer.39 Although doubts have recently been raised about the positive health associations of H. pylori infection,40 understanding the natural history of H. pylori infection in the modern era is most likely important for our understanding of human health outcomes.
A strength of the present study is that it is carried out on a representative sample of the general population. The mail survey in 1989 had only minor non-response bias15 and the 2011/2012 mail and OEGD survey had only a minor, probably clinically insignificant, effect of age so that responders were somewhat older than non-responders. The study was repeated in the same population applying an identical sampling technique, and 32 persons participated in both surveys, making calculations on a true birth cohort effect possible, although it would have been desirable to have more participants with repeated data. Our data on reasons for seroconversion in the four of them that seroreverted strongly supports a birth cohort effect as the main explanation for the decrease in H. pylori seropositivity over time, and there is no reason to believe that the rest of the individuals in the youngest group 1989 and oldest group 2012 would have had a different pattern of seroconversion. Another weakness is that we do not have histology data on current infection in the initial cohort, but the prevalence of seropositivity was comparable with the findings in other Swedish studies.13,32,41
Another point is that the stated prevalence of H. pylori by means of WS staining might be underestimated by five cases. This means that the true prevalence on histology might have been up to 12.7% instead of 11.3% due to proton pump inhibitor (PPI), non steroidal anti-inflammatory drug (NSAID), or acetylsalicylic acid (ASA) use (all five used at least one). As the latter figure is given by the standard methodology for histology diagnosis of H. pylori, it is used as the main outcome.
Smoking tobacco or using snus did not affect the prevalence of H. pylori seropositivity. This is not surprising since the vast majority of sufferers get infected in early childhood3 when you do not smoke tobacco or use snus. The number of cases with corpus atrophy was too few for a valid analysis of this issue.
The level of education data in 1989 and 2011 is not completely comparable due to changes in the education system but this did not influence the results, as confirmed by the interaction model where level of education had no impact on H. pylori infection rate change over time. Another potential drawback is the use of different serological kits in the two investigations. The HM-CAP test was not commercially available in 2012 and we used the GastroPanel assay from Biohit, Helsinki in the 2012 cohort. However, as both assays showed excellent and comparable accuracy in Swedish validation studies as shown in the Methods section,22,23 this most probably did not introduce any substantial bias.
To conclude, the stomach is becoming healthier in Europe. The proportion of individuals in the adult population with H. pylori infection has decreased dramatically over the past 23 years, and the prevalence of corpus atrophy is remarkably low. These changes towards a more ‘healthy stomach’ in older age certainly provide health benefits but maybe also new health risks. The falling prevalence of H. pylori may also have important clinical implications for the management of uninvestigated dyspepsia in younger patients.
Acknowledgement
The authors wish to thank Pentti Sipponen for help with interpretation of the GastroPanel data in the 2011/2012 survey.
Funding
The study was supported by Olympus Sverige AB (Box 1816, 171 23 Solna, Sweden), who supplied study equipment. This research received no other specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no affiliation with Olympus Sverige AB, or any other conflicts of interest to declare.
References
- 1.Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007; 445: 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonnenberg A. Review article: Historic changes of Helicobacter pylori-associated diseases. Aliment Pharmacol Ther 2013; 38: 329–342. [DOI] [PubMed] [Google Scholar]
- 3.Blaser MJ. Helicobacter pylori and gastric diseases. Br Med J 1998; 316: 1507–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke CR, Talley NJ, Nelson DK, et al. Helicobacter pylori and dyspepsia: A population-based study of the organism and host. Am J Gastroenterol 2000; 95: 1906–1913. [DOI] [PubMed] [Google Scholar]
- 5.Agréus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol 2012; 47: 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/ Florence Consensus Report. Gut 2012; 61: 646–664. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis 2008; 198: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman KJ, Greenland S, Lash TL. Modern epidemiology, 3rd ed Philadelphia: Lippincott Williams and Wilkins, 2008. [Google Scholar]
- 10.Kosunen TU, Aromaa A, Knekt P, et al. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect 1997; 119: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumagai T, Malaty HM, Graham DY, et al. Acquisition versus loss of Helicobacter pylori infection in Japan: Results from an 8-year birth cohort study. J Infect Dis 1998; 178: 717–721. [DOI] [PubMed] [Google Scholar]
- 12.Asfeldt AM, Steigen SE, Lochen ML, et al. The natural course of Helicobacter pylori infection on endoscopic findings in a population during 17 years of follow-up: The Sorreisa gastrointestinal disorder study. Eur J Epidemiol 2009; 24: 649–658. [DOI] [PubMed] [Google Scholar]
- 13.Song H, Held M, Sandin S, et al. Increase in the prevalence of atrophic gastritis among adults age 35 to 44 years old in northern Sweden between 1990 and 2009. Clin Gastroenterol Hepatol 2015; 13: 1592–1600. [DOI] [PubMed] [Google Scholar]
- 14.Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology 2005; 129: 1756–1780. [DOI] [PubMed] [Google Scholar]
- 15.Agréus L, Svärdsudd K, Nyrén O, et al. Irritable bowel syndrome and dyspepsia in the general population: Overlap and lack of stability over time. Gastroenterology 1995; 109: 671–680. [DOI] [PubMed] [Google Scholar]
- 16.Agréus L, Svärdsudd K, Talley NJ, et al. Natural history of gastroesophageal reflux disease and functional abdominal disorders: A population-based study. Am J Gastroenterol 2001; 96: 2905–2914. [DOI] [PubMed] [Google Scholar]
- 17.Statistics Sweden. Kommunfakta Östhammar 2015, choose “Kommunfakta för enskilda kommuner”, choose “Östhammar 0382”, http://www.scb.se/kommunfakta (Updated 2015).
- 18.Agréus L. The abdominal symptom study. Thesis PhD dissertation, Uppsala University, Sweden, 1993.
- 19.Agréus L, Svärdsudd K, Nyrén O, et al. Reproducibility and validity of a postal questionnaire. The abdominal symptom study. Scand J Prim Health Care 1993; 11: 252–262. [DOI] [PubMed] [Google Scholar]
- 20.Agréus L, Engstrand L, Svärdsudd K, et al. Helicobacter pylori seropositivity among Swedish adults with and without abdominal symptoms. A population-based epidemiologic study. Scand J Gastroenterol 1995; 30: 752–757. [DOI] [PubMed] [Google Scholar]
- 21.Evans DJ, Jr, Evans DG, Graham DY, et al. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology 1989; 96: 1004–1008. [DOI] [PubMed] [Google Scholar]
- 22.Enroth H, Kraaz W, Rohan T, et al. Does the method of Helicobacter pylori detection influence the association with gastric cancer risk? Scand J Gastroenterol 2002; 37: 884–890. [DOI] [PubMed] [Google Scholar]
- 23.Myllyniemi M, Linnala A, Paloheimo L, et al. Improved specificity of Helicobacter pylori IgA/IgG ELISA over other H. pylori tests. European Helicobacter Study Group XXth International workshop on helicobacter and related bacteria in chronic digestive inflammation, Istanbul, Turkey 2007., abstract P116, http://www.abstractsonline.com/viewer/viewAbstractPrintFriendly.asp?CKey={5DA23D91-7E86-46C1-80AD-542BF41D17FE}&SKey={75726FDA-AA32-45BA-8FB0-AABFA6B6F99D}&MKey={0361F100-DB45-4E6C-8E1F-EDE25EA79E31}&AKey={384D2523-AA39-4B08-A120-38A9AB93ADA3} (Updated 2007).
- 24.Storskrubb T, Aro P, Ronkainen J, et al. A negative Helicobacter pylori serology test is more reliable for exclusion of premalignant gastric conditions than a negative test for current H. pylori infection: A report on histology and H. pylori detection in the general adult population. Scand J Gastroenterol 2005; 40: 302–311. [DOI] [PubMed] [Google Scholar]
- 25.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 26.Goncalves C, Oliveira ME, Palha AM, et al. Autoimmune gastritis presenting as iron deficiency anemia in childhood. World J Gastroenterol 2014; 20: 15780–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aro P, Ronkainen J, Storskrubb T, et al. Use of tobacco products and gastrointestinal morbidity: An endoscopic population-based study (the Kalixanda study). Eur J Epidemiol 2010; 25: 741–750. [DOI] [PubMed] [Google Scholar]
- 28.Roosendaal R, Kuipers EJ, Buitenwerf J, et al. Helicobacter pylori and the birth cohort effect: Evidence of a continuous decrease of infection rates in childhood. Am J Gastroenterol 1997; 92: 1480–1482. [PubMed] [Google Scholar]
- 29.Asfeldt AM, Straume B, Steigen SE, et al. Changes in the prevalence of dyspepsia and Helicobacter pylori infection after 17 years: The Sorreisa gastrointestinal disorder study. Eur J Epidemiol 2008; 23: 625–633. [DOI] [PubMed] [Google Scholar]
- 30.Milosavljevic T, Kostic-Milosavljevic M, Krstic M, et al. Epidemiological trends in stomach-related diseases. Dig Dis 2014; 32: 213–216. [DOI] [PubMed] [Google Scholar]
- 31.Rosenstock S, Jørgensen T, Andersen L, et al. Seroconversion and seroreversion in IgG antibodies to Helicobacter pylori: A serology based prospective cohort study. J Epidemiol Community Health 2000; 54: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergenzaun P, Kristinsson KG, Thjodleifsson B, et al. Seroprevalence of Helicobacter pylori in south Sweden and Iceland. Scand J Gastroenterol 1996; 31: 1157–1161. [DOI] [PubMed] [Google Scholar]
- 33.Hershko C, Ronson A, Souroujon M, et al. Variable hematologic presentation of autoimmune gastritis: Age-related progression from iron deficiency to cobalamin depletion. Blood 2006; 107: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 34.The National Corporation of Swedish pharmacies (Apoteksbolaget AB). Svensk Läkemedelsstatistik 1995 (Swedish drug statistics 1995), Stockholm: Apoteksbolaget AB, 1995. [Google Scholar]
- 35.The Swedish eHealth Agency (eHälsomyndigheten). Data on file. eHälsomyndigheten, www.ehalsomyndigheten.se (accessed September 2015).
- 36.Agréus L, Talley NJ and Jones M. Value of the ‘test & treat’ strategy for uninvestigated dyspepsia at low prevalence rates of Helicobacter pylori in the population. Helicobacter. Epub ahead of print 8 September 2015. DOI: 10.1111/hel.12267. [DOI] [PubMed]
- 37.Lender N, Talley NJ, Enck P, et al. Review article: Associations between Helicobacter pylori and obesity–an ecological study. Aliment Pharmacol Ther 2014; 40: 24–31. [DOI] [PubMed] [Google Scholar]
- 38.Sonnenberg A. Effects of environment and lifestyle on gastroesophageal reflux disease. Dig Dis 2011; 29: 229–234. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut 2013; 62: 1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham DY. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148: 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borch K, Jonsson KA, Petersson F, et al. Prevalence of gastroduodenitis and Helicobacter pylori infection in a general population sample: Relations to symptomatology and life-style. Dig Dis Sci 2000; 45: 1322–1329. [DOI] [PubMed] [Google Scholar]