Abstract
Background
Evidence on small intestinal bacterial overgrowth (SIBO) in patients with chronic pancreatitis (CP) is conflicting.
Aim
The purpose of this study was to perform a systematic review and meta-analysis on the prevalence of SIBO in CP and to examine the relationship of SIBO with symptoms and nutritional status.
Methods
Case-control and cross-sectional studies investigating SIBO in CP patients were analysed. The prevalence of positive tests was pooled across studies, and the rate of positivity between CP cases and controls was calculated.
Results
In nine studies containing 336 CP patients, the pooled prevalence of SIBO was 36% (95% confidence interval (CI) 17–60%) with considerable heterogeneity (I2 = 91%). A sensitivity analysis excluding studies employing lactulose breath test gave a pooled prevalence of 21.7% (95% CI 12.7–34.5%) with lower heterogeneity (I2 = 56%). The odds ratio for a positive test in CP vs controls was 4.1 (95% CI 1.6–10.4) (I2 = 59.7%). The relationship between symptoms and SIBO in CP patients varied across studies, and the treatment of SIBO was associated with clinical improvement.
Conclusions
One-third of CP patients have SIBO, with a significantly increased risk over controls, although results are heterogeneous, and studies carry several limitations. The impact of SIBO and its treatment in CP patients deserve further investigation.
Keywords: Chronic pancreatitis, small intestinal bacterial overgrowth, symptoms, pancreatic exocrine insufficiency, nutrition
Background
Chronic pancreatitis (CP) is a challenging disease, characterised by inflammatory and destructive changes of the pancreas, which can cause significant symptoms, malnutrition, reduced quality of life and life-threatening complications.1 Most patients with CP experience symptoms such as bloating, diarrhoea, cramping and abdominal pain.2 As some of these symptoms are related with pancreatic exocrine insufficiency (PEI), the therapeutic options for the medical treatment of CP include the administration of pancreatic enzyme replacement therapy (PERT)2 which leads to a significant reduction of bloating and diarrhoea.3,4 When symptoms do not improve with PERT, it has been suggested that there is a need to rule out other possible causes, including small intestinal bacterial overgrowth (SIBO).5,6
SIBO is a condition in which the increased bacterial load in the small bowel results in excessive fermentation and inflammation, leading to a variety of clinical complaints ranging from mild, non-specific symptoms such as abdominal pain, bloating, and flatulence, to more severe manifestations such as malabsorption and weight loss.7 In patients with CP, a number of factors such as fat malabsorption, diabetic neuropathy, use of drugs that affect motility or use of proton pump inhibitors, alcohol intake and surgical procedures can favour the occurrence of SIBO. In clinical practice, glucose or lactulose hydrogen breath tests (GHBT and LHBT) are the most commonly employed tests for the diagnosis of SIBO, with GHBT being considered more accurate than LHBT.8,9
The reported rate of SIBO in CP patients is extremely variable, due to the heterogeneity and several limitations of published studies.10–12 Moreover, as the clinical manifestation and signs of SIBO can be attributed to CP itself and, as there is no evidence suggesting that SIBO might complicate only CP cases unresponsive to PERT, whether all CP patients should be tested for SIBO, irrespective of symptoms, is uncertain. We, therefore, aimed at conducting a systematic review and meta-analysis on the prevalence of SIBO in CP patients. We also aimed at examining whether the presence of SIBO was related to symptoms and nutritional status in CP patients.
Methods
Search strategy and study selection
A computerised literature search of MEDLINE and of the Cochrane database did not identify any previous publication related to systematic review on CP and SIBO. In our search for original studies, we performed a MEDLINE search (until 25 August 2015). Specific search terms were: ‘(chronic pancreatitis) AND (breath tests OR small intestine OR bacterial infections OR bacterial overgrowth OR lactulose hydrogen OR glucose hydrogen)’. The methodology was developed from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.13 There were no language restrictions and abstracts of the articles identified were assessed for appropriateness to the study question, and all potentially relevant articles were obtained and evaluated in detail. Hand-searching of the reference lists of all the included articles was performed to help retrieving further potentially relevant studies.
Inclusion/exclusion criteria
Studies were considered if they met the following criteria: (a) age > 18 years; (b) inclusion of patients with a presumed diagnosis of CP (either according to clinico-radiological data, physician’s opinion, questionnaire, or meeting specific diagnostic criteria); (c) investigation of SIBO by means of any of the following tests or of their combination: LHBT, GHBT, xylose or sucrose hydrogen breath tests (BTs), or jejunal aspirate and culture. (d) either a cross-sectional or case-control design. Studies were excluded if they were available as abstract only. We also excluded: (a) case reports; (b) papers reporting pancreatic disorders different from CP. Two independent reviewers (LA and MS) carried out study identification, selection and discussed disagreements with a third reviewer (GC). Excluded studies and the reasons for exclusion were recorded.
Data extraction and quality assessment
Two reviewers (LA and MS) extracted data from each study onto a Microsoft Excel spreadsheet (XP Professional Edition; Microsoft Corp., Redmond, Washington, USA). From each of the studies that met the eligibility criteria, the following clinical data were extracted: country of origin and number of participating centers, test used to detect SIBO and criteria used to define a positive test, diagnostic criteria used to define CP, source of controls (for case-control studies), and whether studies excluded individuals that received surgery before enrollment. The presence of symptoms, of nutritional deficiencies and their relation to the diagnosis of SIBO were also recorded, as well as the effect of treatment.
The quality of the studies was evaluated independently by two reviewers (SS and GC) using the Newcastle–Ottawa Scale. The specific scales for both case-control and cross-sectional studies14,15 award a maximum of 10 points to each study, with scores for items such as the definition of the sample, ascertainment and representativeness of the exposure and statistical analysis. Studies with a score ≥6 were considered as high quality studies.
Data synthesis and statistical analysis
The proportion of individuals with CP with a positive test for SIBO was combined for both cross-sectional and case-control studies, to give a pooled prevalence in all individuals meeting diagnostic criteria for CP. In addition, for case-control studies, data were pooled for both cases and controls, and the prevalence of a positive test for SIBO, regardless of the type of test used, was compared between the two groups to calculate an odds ratio (OR) and 95% confidence interval (CI).
A meta-analysis of all eligible studies identified was then performed with the software package Comprehensive Meta-Analysis (Biostat, Englewood, New Jersey, USA) using a random-effects model.16,17 In addition to within-study variance, the random-effects model considers heterogeneity among studies and gives estimates that are more conservative. We preferred the random-effects model because we believe that the relevant variation in the risk is most likely a consequence of inter-study differences. The quantity of heterogeneity was assessed by means of the I2 value.18 The I2 quantity describes the percentage of total variation across studies that is caused by heterogeneity and not by chance. We considered an I2 value of 25% or lower as trivial heterogeneity, and an I2 value of 75% or higher as considerable heterogeneity. Publication bias was assessed using the Begg and Mazumdar test. A p-value <0.05 was accepted as statistically significant. Before performing the analysis, we developed the following a priori hypotheses to examine whether this had any effect on the prevalence or odds of SIBO and to explore reasons for any heterogeneity observed: (a) employed method to diagnose SIBO (GHBT vs LHBT); (b) inclusion of CP patients that received previous surgery which can cause SIBO per se; (c) quality of the study (quality score >6/12 or ≤6/12).
Results
Search results and study selection
A total of 1604 references were identified by the MEDLINE search (Figure 1). After evaluation, 1566 studies were excluded, as they were not related to the study topic. This resulted in 38 studies that were examined in more detail, 29 of which were considered as potentially appropriate for inclusion in the review. However, 20 of them did not fulfill all inclusion criteria. Finally, nine studies10–12,19–24 remained for qualitative analysis and quantitative synthesis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of assessment of studies identified in the systematic review.
Study characteristics and quality assessment
The descriptive characteristics of the nine included studies are shown in Table 1. Six studies were from Europe, two from Asia (India and South Korea) and one from South America (Chile). English was the language of seven papers whilst one was in written in Spanish19 and one in Russian21 There were only single centre studies. The number of enrolled patients in each study ranged between 1111 and 102.21 Overall, 336 patients with a diagnosis of CP were investigated for SIBO. The rate of male patients ranged between 34%21 and 100%,11 and the rate of alcoholic aetiology of CP ranged between 32.3%22 and 100%.11 The rate of patients with PEI was reported in all but one paper24 and ranged from 31.7%22 to 100%,11,19,20 while the rate of diabetes ranged from 14.3%20 to 66.6%,24 being not reported in one study.21 In three of the nine studies, there were CP patients who had previously undergone gastrointestinal surgery,10,19,20 which can be a cause of SIBO per se.
Table 1.
Study demographics, population size and characteristics
| First author (reference) | Country | Patients (n) | Sex (male %) | Age (years) | Alcoholic aetiology (%) | PEI | Diabetes mellitus (%) | Pancreatic calcifications (%) | Previous surgery | PERT (%) | Controls (n) | Controls characteristics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lembcke23 | Germany | 20 | NR | NR | 90% | 95% | 40% | 65% | 0% | NR | – | – |
| Casellas10 | Spain | 15 | 11 (73.3%) | Mean 51 | 73.3% | 100% | 66.6% | 80% | 66% | 100% | 39 | Immunodeficiency, gastroduodenal surgery |
| Trespi20 | Italy | 35 | 26 (74.2%) | Mean 53 | 54.3% | 100% | 14.3% | 51.4% | 31.4% | 100% | 61 | Gastric resection |
| Madsen11 | Denmark | 11 | 11 (100%) | Median 46 | 100% | 100% | 45% | NR | 0% | 90% | 11 | Healthy |
| Mancilla19 | Chile | 14 | 11 (78.5%) | Mean 49 | 50% | 100% | 28.5% | 64% | 21.4% | 64% | 14 | Healthy |
| Grigor'eva21 | Russia | 102 | 35 (34.3%) | Mean 55 | NR | 70.6% | NR | NR | 0% | NR | – | – |
| Kumar22 | India | 68 | 48 (70.6%) | Mean 33.6 | 32.3% | 31.7% | 35% | 66% | NR | 66.2% | 74 | Healthy |
| Signoretti12 | Italy | 43 | 24 (55.8%) | Mean 54 | 39.5% | 39.5% | 41.8% | NR | 0% | 62.8% | 43 | Unspecific GI complaints |
| Kim24 | South Korea | 36 | 28 (77.8%) | Mean 52.3 | 78.9% | NR | 66.6% | 47.2% | 0% | 52% | 49 | Healthy |
GI: gastrointestinal; NR: not reported; PEI: pancreatic exocrine insufficiency; PERT: pancreatic enzyme replacement therapy.
Seven studies had a case-control design10–12,19,20,22,24 and the other two were cross-sectional studies,21,23 with some differences regarding the selection of controls. As regards the methods employed to determine the diagnosis of SIBO (see Table 2), there were no studies employing jejunal or duodenal aspirate. Six studies employed glucose as substrate of BT10–12,20,22,23 whilst three studies used lactulose.19,21,24 In a single study,23 SIBO was evaluated by GHBT, but also by 14 C-cholyl-glycine BT; however, as this last test is far less commonly employed and standardised, we decided to analyse only data obtained with glucose BT from that study. In one paper24 not only the excretion of hydrogen (H2) in the breath sample, but also that of methane (CH4) was measured. Furthermore, there was wide heterogeneity regarding BT protocol in terms of doses of substrate, duration of the examination, sampling intervals and cut-off for the diagnosis of SIBO (see Table 2). As far as regards the quality of the included studies, five of the seven case-control studies and one of the two cross-sectional studies were scored as ‘high quality’ (see Supplementary Material, Table 1).
Table 2.
Methods and protocols for the diagnosis of small intestine bacterial overgrowth (SIBO) employed in the evaluated studies
| First author (reference) | Test | Patients (n) | Dose of substrate | Duration of the test | Sample intervals | Cut-off for the diagnosis of SIBO |
|---|---|---|---|---|---|---|
| Lembcke23 | GHBT/14C-CGBT | 12/20 | 50 g | NR | NR | H2 > 20 ppm compared to baseline |
| Casellas10 | GHBT | 15 | 50 g/250 ml | 180 min | 15 min | H2 > 10 ppm compared to baseline |
| Trespi20 | GHBT | 35 | 50 g/250 ml | 180 min | 30 min | H2 > 20 ppm compared to baseline |
| Madsen11 | GHBT | 11 | NR | 6 h | 15 min | H2 ≥ 12 ppm compared to baseline |
| Mancilla19 | LHBT | 14 | 25 mg | 180 min | 10 min | H2 > 20 ppm compared to baseline or >22 in 60 min |
| Grigor'eva21 | LHBT | 102 | NR | NR | NR | H2 ≥ 20 ppm compared to baseline |
| Kumar22 | GHBT | 68 | 100 g/200 ml | 180 min | 15 min | H2 ≥ 12 ppm compared to baseline |
| Signoretti12 | GHBT | 43 | 50 g/250 ml | 120 min | 20 min | H2 ≥ 12 ppm compared to baseline or >20 ppm at baseline |
| Kim24 | LHBT | 36 | 10 g | 180 min | 15 min | H2 ≥ 20 ppm or CH4 ≥ 10 ppm compared to baseline or H2 ≥ 20 ppm or ≥CH410 ppm within 90 min after lactulose load |
14C-CGBT: 14C-cholylglycine breath test; CH4: methane; GHBT: glucose H2 breath test; H2: hydrogen; LHBT: lactulose H2 breath test; NR: not reported; ppm: parts per million.
Prevalence of SIBO in patients with CP
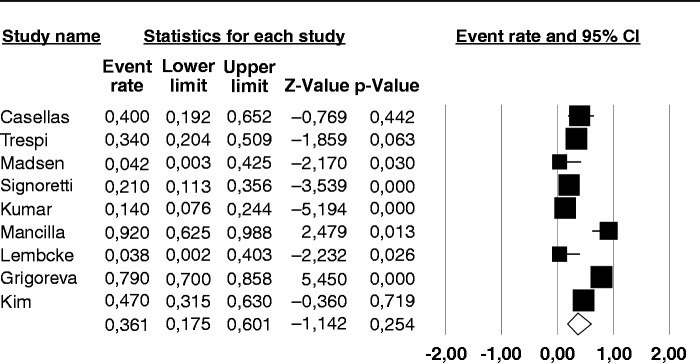
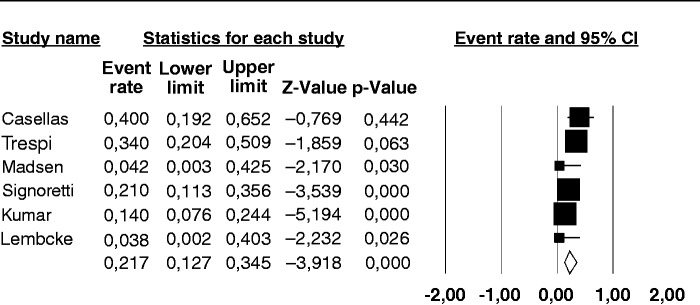
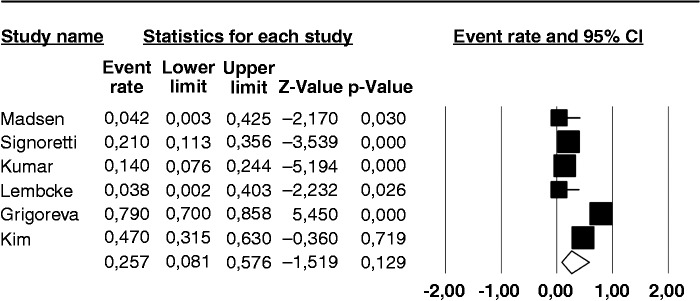
The prevalence of a positive BT for SIBO in the nine individual studies ranged from 14–92%, with a pooled prevalence of 36% (95% CI 17–60%) (Figure 2). There was considerable heterogeneity (I2 = 91%). No publication bias was found (Begg and Mazudmar Kendall’s tau = −0.13; p = 0.6).We repeated the analysis based on our a priori hypotheses considering a number of covariates. When we considered the six studies employing GHBT (Figure 3), the pooled prevalence of SIBO was 21.7% (95% CI 12.7–34.5%) with a lower heterogeneity (I2 = 56%). The same analysis on the three studies using lactulose as substrate for BT, gave a much higher prevalence of 73.3% (95% CI 67.4–90.6%) with considerable heterogeneity (I2 = 86%). Six of the examined studies did not include CP patients that had received surgery, which can be a cause of SIBO per se. The pooled prevalence of SIBO for these studies was slightly lower than that observed in the entire set of studies, being 25.7% (95% CI 8.1–57.6%) (Figure 4) albeit still with considerable heterogeneity (I2 = 94%). In the three studies that included operated patients, the pooled estimate rate was as high as 54.1% (95% CI 23.2–82.1%) with an I2 = 77%. As far as regards the quality of the studies, six of them were classified as of ‘high quality’ and the pooled prevalence of SIBO for these studies was 41.5% (95% CI 15.6–73.2%) with an I2 = 94%. In the three studies with a lower quality score, the pooled estimate rate was as high as 31.4% (95% CI 16.1–52.2%) with an I2 = 40%.
Figure 2.
Forest plot of the pooled prevalence of small intestine bacterial overgrowth in chronic pancreatitis in all the nine included studies. Random-effects model demonstrating a pooled prevalence of 36% (95% confidence interval (CI) 17–60%) with considerable heterogeneity (I2 = 91%).
Figure 3.
Forest plot of the pooled prevalence of small intestine bacterial overgrowth in chronic pancreatitis in the six studies employing glucose as substrate for the hydrogen breath test. Random-effects model demonstrating a pooled prevalence of 21.7% (95% confidence interval (CI) 12.7–34.5%) with reduced heterogeneity (I2 = 56%).
Figure 4.
Forest plot of the pooled prevalence of small intestine bacterial overgrowth in chronic pancreatitis in the six studies that did not include patients who previously received surgery. Random-effects model demonstrating a pooled prevalence a 25.7% (95% confidence interval (CI) 8.1–57.6%) with considerable heterogeneity (I2 = 94%).
Prevalence of SIBO in patients with CP as compared with controls
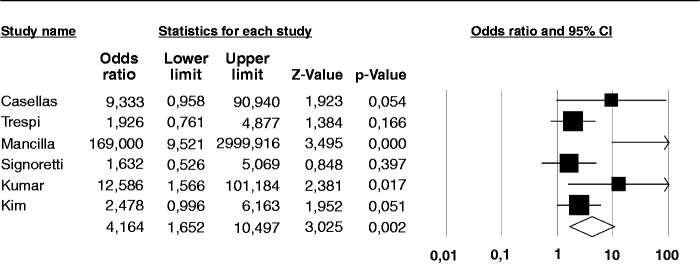
There were seven case-control studies, containing 222 CP patients and 291 controls. One of the studies11 did not report any diagnosis of SIBO among either cases or controls. Therefore, the analysis comparing cases and controls was performed on the remaining six studies, examining 211 cases and 280 controls. Four of these studies employed GHBT, and two LHBT (see details in Table 1). Four studies defined the controls as healthy individuals,11,19,20,22,24 one study as subjects with non-specific, non-chronic gastrointestinal complaints,12 whilst two studies10,20 enrolled individuals with previous history of gastric/duodenal resection20 and/or affected by primary immunodeficiency as controls.10 The odds of a positive test for SIBO in CP patients compared with controls was 4.1 (95% CI 1.6–10.4) (Figure 5), with I2 = 59.7%. No publication bias was found (Begg and Mazudmar Kendall’s tau = 0.4; p = 0.2). The odds of a positive test for SIBO in CP patients when considering only the studies that employed GHBT was 2.8 (95% CI 1.2–6.8) with a lower heterogeneity (I2 = 32%).
Figure 5.
Forest plot of the summary odds ratio (OR) for chronic pancreatitis and the risk of small intestine bacterial overgrowth (SIBO) compared to controls. Random effects model demonstrating and OR of 4.1 (95% confidence interval (CI) 1.6–10.4) for a positive test.
Relation between symptoms and nutritional status and SIBO, and response to treatment
Among the nine studies included in this meta-analysis, five12,20–22,24 evaluated the differences between CP patients with or without SIBO in terms of symptoms and/or nutritional parameters. In the study by Trespi and colleagues,20 patients with SIBO more frequently complained diarrhoea as compared to those without (73% vs 9%, p = 0.001). Grigor’eva et al.21 reported that CP patients with SIBO, when compared to those without, had a higher prevalence of pain caused by bowel dysfunction (80.3% vs 45.2%; p < 0.001) and duodenal hypertension (71.8% vs 41.9%; p < 0.01). The rates of diarrhoea (67.6% vs 41.9%; p < 0.05), flatulence (84.5% vs 41.9%; p < 0.001), nausea (57.7% vs 35.9%; p < 0.05), and feeling of bitter taste in the mouth (69% vs 45.2%; p < 0.05) were also significantly higher in CP patients with SIBO.
Kim et al.24 also reported that CP patients with SIBO were more often symptomatic, with significantly higher symptom scores for hard stool, flatus and bloating. Patients with production of both H2 and CH4 seemed to experience more symptoms. In the same study, there were no differences between CP patients with or without SIBO in terms of nutritional parameters. Kumar et al.22 and Signoretti et al.12 instead, found no difference between patients with or without SIBO in terms of symptoms. Signoretti et al.12 reported that Vitamin D level showed a trend close to significance for being lower in CP patients with SIBO as compared to those without SIBO (15.9 ng/ml vs 25.2 ng/ml, p = 0.08).
Only three of the nine studies included in this meta-analysis evaluated the response to treatment and symptom modification afterwards. Casellas et al.10 treated five CP patients with SIBO with doxycycline or metronidazole, with a clinical response in two of them, as watery diarrhoea disappeared in both. In the study by Kumar et al.,22 33% of CP patients with PEI and persistent diarrhoea had SIBO, and were then treated with rifaximin with improvement of the diarrhoea, although resolution of SIBO by repeated GHBT was not documented. Trespi et al.20 treated CP patients both either with or without SIBO, with rifaximin. All patients with initial diagnosis of SIBO turned SIBO-negative and had disappearance of diarrhoea, while patients that had diarrhoea not associated with SIBO had no response (p = 0.03).
Discussion
To our knowledge, this is the first meta-analysis that investigated the prevalence of SIBO in patients with CP. In the present study, data from nine publications were analysed, resulting in a pooled prevalence of SIBO of 36% in CP patients, with a range between 14–92% and considerable heterogeneity. The prevalence of SIBO seems to depend on the type of test used, with higher prevalence with LHBT than with GHBT, and on the inclusion criteria for CP patients, with higher positivity rates in studies including patients who previously underwent surgery. When the prevalence of a positive test for SIBO was compared between CP cases and controls, regardless of the test used, there was a statistically significant increased risk for a positive test result in CP patients. Furthermore, the relationship between the presence and severity of symptoms and the diagnosis of SIBO in CP patients seemed to vary across the different studies. A higher rate of symptoms in patients with SIBO was reported in only three of the five studies that examined this aspect, but no data suggested that SIBO is more common in patients not responding to PERT. On the other hand, the treatment of SIBO with antibiotics in CP patients seemed associated with clinical improvement.
The strengths of the present study include an exhaustive literature search, rigorous statistical methods, and pooling of data to allow synthesis of all the available published evidence examining the yield of testing for SIBO in CP. The most obvious weaknesses of the study, as with many systematic review and meta-analysis, arise from the available evidence. Only a limited number of studies, with relatively small sample size were identified. All included studies were cross-sectional or case-control studies conducted in tertiary care centres and might therefore be subject to a spectrum bias and include only more advanced cases. Furthermore, all the included studies employed BTs as diagnostic procedure for SIBO and none of them jejunal aspirate and culture, which is often considered the gold standard for the diagnosis.25 However, jejunal aspirate and culture might not diagnose SIBO occurring in the distal part of the small intestine.26
The findings of this meta-analysis suggest that about one-third of CP patients are affected by SIBO. As we anticipated that there might have been considerable heterogeneity for this analysis, we decided to carry out all analyses using the random-effects model, to provide the most conservative estimates, and to perform sensitivity analysis for some variables. The substrate used for the BT was found to be a significant factor affecting both the rate of SIBO, as this is higher in studies employing LHBT compared with GHBT, and heterogeneity, which was lower when only studies with GHBT were analysed (see Figures 2 and 3). These results are in keeping with previous data27 and with guidelines and expert opinions that suggest employing glucose and not lactulose as substrate for H2 BT for the diagnosis of SIBO.8,9 Interestingly, when an expert working team8 analysed the available literature to evaluate the accuracy of breath testing with either lactulose and glucose as compared with jejunal aspirate, the reported median sensitivity and specificity were of 62.5% and 81.8% for GHBT and 52.5% and 85.7% for LHBT, with a diagnostic accuracy of 71.7% for glucose and 55.1% for lactulose. This finding seemed similar in patients with different disorders. A more recent study28 compared GHBT and LHBT to upper gut aspirate culture in 80 patients with irritable bowel syndrome, and found that BT employing glucose had a higher accuracy (sensitivity 27%, specificity 100%), compared with lactulose BTs considering either double peaks (sensitivity 0%, specificity 98%) or early peak (sensitivity 33%, specificity 65%). However, the sensitivity of the GHBT remains relatively low.
Not surprisingly, the rate of positive tests for SIBO in CP patients was also higher in studies that included patients that had previously undergone surgical procedures. It is well known that resective surgery of the gastrointestinal tract can cause SIBO,29 and such patients should be tested for this condition, especially if symptomatic, independently from their diagnosis of CP. A third factor that we considered for sensitivity analysis was the quality of the studies. However, most studies we have been able to analyse had a high quality score, and quality did not seem to influence the rate of SIBO or to determine heterogeneity.
Data from the present study suggest that the risk of SIBO is four times higher in patients with CP as compared with controls. This estimate is slightly lower when taking in consideration only studies that employed glucose as substrate for H2 BT. The reasons for the development of SIBO in CP patients might include malabsorption of nutrients, previous surgery, use of alcohol,30 of drugs such as proton pump inhibitors31 or pain-killers affecting motility, and diabetic neuropathy.32 At any rate, it is currently suggested to test CP patients for SIBO only in cases with PEI not responding to high doses of pancreatic enzymes.6,33 Interestingly, when we analysed data regarding symptoms and their relation with the presence of SIBO, it was difficult to reach a straightforward conclusion, as only some of the studies suggested that CP patients with SIBO have more symptoms. Furthermore, there is no evidence suggesting that SIBO is more common in patients with CP and PEI who do not respond to high doses of PERT, as no studies had a design of this kind.
As far as regards the treatment of SIBO, few studies evaluated the effect of therapy, with data suggesting that, as in other disorders,34 treatment with antibiotics such as rifaximin might be effective in eradicating SIBO and reducing symptoms.
In conclusion, the present study demonstrates that SIBO is a rather frequent condition in patients with CP. The results might suggest that all CP patients should be screened for SIBO, irrespective of symptoms, as clinical complaints due to SIBO might be confused with those related with unresponsive steatorrhoea, possibly leading to a pointless increased dosage of PERT that might result in increased costs and reduced compliance. The studies evaluated in this meta-analysis differ in terms of study design and study population definition. Therefore, we foresee the need for larger studies, employing more rigorous and standardised methods, such as jejunal aspirate culture and GHBT and CH4 breath test. These studies should be conducted in an homogeneous setting of patients with CP and symptoms either under PERT or not, to clarify the role of SIBO in CP, and the effect of its treatment in terms of symptomatic response and possible beneficial effects on nutritional parameters.
Supplementary Material
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lévy P, Domínguez-Muñoz E, Imrie C, et al. Epidemiology of chronic pancreatitis: Burden of the disease and consequences. United European Gastroenterol J 2014; 2: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton F, Alkaade S, Collins D, et al. Use and perceived effectiveness of non-analgesic medical therapies for chronic pancreatitis in the United States. Aliment Pharmacol Ther 2011; 33: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frulloni L, Falconi M, Gabbrielli A, et al. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis 2010; 42: S381–S406. [DOI] [PubMed] [Google Scholar]
- 4.Gubergrits N, Malecka-Panas E, Lehman GA, et al. A 6-month, open-label clinical trial of pancrelipase delayed-release capsules (Creon) in patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery. Aliment Pharmacol Ther 2011; 33: 1152–1161. [DOI] [PubMed] [Google Scholar]
- 5.Martínez J, Abad-González A, Aparicio JR, et al. The Spanish Pancreatic Club recommendations for the diagnosis and treatment of chronic pancreatitis: Part 1 (diagnosis). Pancreatology 2013; 13: 8–17. [DOI] [PubMed] [Google Scholar]
- 6.Pezzilli R, Andriulli A, Bassi C, et al. Exocrine Pancreatic Insufficiency collaborative (EPIc) Group. Exocrine pancreatic insufficiency in adults: A shared position statement of the Italian Association for the Study of the Pancreas. World J Gastroenterol 2013 28; 19: 7930–7946. [DOI] [PMC free article] [PubMed]
- 7.Grace E, Shaw C, Whelan K, et al. Review article: Small intestinal bacterial overgrowth–prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther 2013; 38: 674–688. [DOI] [PubMed] [Google Scholar]
- 8.Gasbarrini A, Corazza GR, Gasbarrini G, et al. 1st Rome H2-Breath Testing Consensus Conference Working Group. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment Pharmacol Ther 2009; 29: 1–49. [DOI] [PubMed] [Google Scholar]
- 9.Saad RJ, Chey WD. Breath testing for small intestinal bacterial overgrowth: Maximizing test accuracy. Clin Gastroenterol Hepatol 2014; 12: 1964–1972. [DOI] [PubMed] [Google Scholar]
- 10.Casellas F, Guarner L, Vaquero E, et al. Hydrogen breath test with glucose in exocrine pancreatic insufficiency. Pancreas 1998; 16: 481–486. [DOI] [PubMed] [Google Scholar]
- 11.Madsen JL, Graff J, Philipsen EK, et al. Bile acid malabsorption or disturbed intestinal permeability in patients treated with enzyme substitution for exocrine pancreatic insufficiency is not caused by bacterial overgrowth. Pancreas 2003; 26: 130–133. [DOI] [PubMed] [Google Scholar]
- 12.Signoretti M, Stigliano S, Valente R, et al. Small intestinal bacterial overgrowth in patients with chronic pancreatitis. J Clin Gastroenterol 2014; 48: S52–S55. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009; 6: e1000097–e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moga C, Guo B, et al. Development of a quality appraisal tool for case series studies using a modified Delphi technique, Edmonton, AB: Institute of Health Economics, 2012. [Google Scholar]
- 15.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses, www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 18 April 2012).
- 16.Sutton AJ. Methods for meta-analysis in medical research, New York, NY: John Wiley, 2000. [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.Mancilla AC, Madrid SAM, Hurtado HC, et al. Small intestine bacterial overgrowth in patients with chronic pancreatitis. Rev Med Chil 2008; 136: 976–980. [PubMed] [Google Scholar]
- 20.Trespi E, Ferrieri A. Intestinal bacterial overgrowth during chronic pancreatitis. Curr Med Res Opin 1999; 15: 47–52. [DOI] [PubMed] [Google Scholar]
- 21.Grigor'eva IuV, Iakovenko ÉP, Volosheˇnikova TV, et al. The clinical manifestations and duodenal mucosa in the patients with chronic pancreatitis and bacterial overgrowth in the small intestine. Eksp Klin Gastroenterol 2010; 11: 29–34. [PubMed] [Google Scholar]
- 22.Kumar K, Ghoshal UC, Srivastava D, et al. Small intestinal bacterial overgrowth is common both among patients with alcoholic and idiopathic chronic pancreatitis. Pancreatology 2014; 14: 280–283. [DOI] [PubMed] [Google Scholar]
- 23.Lembcke B, Kraus B, Lankisch PG. Small intestinal function in chronic relapsing pancreatitis. Hepatogastroenterology 1985; 32: 149–151. [PubMed] [Google Scholar]
- 24.Kim DB, Paik CN, Sung HJ, et al. Breath hydrogen and methane are associated with intestinal symptoms in patients with chronic pancreatitis. Pancreatology 2015; 15: 514–518. [DOI] [PubMed] [Google Scholar]
- 25.Posserud I, Stotzer PO, Björnsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007; 56: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin HC. Small intestinal bacterial overgrowth: A framework for understanding irritable bowel syndrome. JAMA 2004; 292: 852–858. [DOI] [PubMed] [Google Scholar]
- 27.Rana SV, Sharma S, Kaur J, et al. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth inpatients with irritable bowel syndrome. Digestion 2012; 85: 243–247. [DOI] [PubMed] [Google Scholar]
- 28.Ghoshal UC, Srivastava D, Ghoshal U, et al. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur J Gastroenterol Hepatol 2014; 26: 753–760. [DOI] [PubMed] [Google Scholar]
- 29.Petrone P, Sarkisyan G, Fernández M, et al. Small intestinal bacterial overgrowth in patients with lower gastrointestinal symptoms and a history of previous abdominal surgery. Arch Surg 2011; 146: 444–447. [DOI] [PubMed] [Google Scholar]
- 30.Gabbard SL, Lacy BE, Levine GM, et al. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci 2014; 59: 638–644. [DOI] [PubMed] [Google Scholar]
- 31.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: A meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 483–490. [DOI] [PubMed] [Google Scholar]
- 32.Ojetti V, Pitocco D, Scarpellini E, et al. Small bowel bacterial overgrowth and type 1 diabetes. Eur Rev Med Pharmacol Sci 2009; 13: 419–423. [PubMed] [Google Scholar]
- 33.Domínguez-Muñoz JE. Chronic pancreatitis and persistent steatorrhea: What is the correct dose of enzymes? Clin Gastroenterol Hepatol 2011; 9: 541–546. [DOI] [PubMed] [Google Scholar]
- 34.Shah SC, Day LW, Somsouk M, et al. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2013; 38: 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.