Abstract
The cerebellum represents one of the most morphologically variable structures in the vertebrate brain. To shed light on its evolutionary history, we have examined the molecular anatomy and proliferation of the developing cerebellum of the North American paddlefish, Polyodon spathula. Absence of an external proliferative cerebellar layer and the restriction of Atonal1 expression to the rhombic lip and valvular primordium demonstrate that transit amplification in a cerebellar external germinal layer, a prominent feature of amniote cerebellum development, is absent in paddlefish. Furthermore, expression of Sonic hedgehog, which drives secondary proliferation in the mouse cerebellum, is absent from the paddlefish cerebellum. These data are consistent with what has been observed in zebrafish and suggest that the transit amplification seen in the amniote cerebellum was either lost very early in the ray-finned fish lineage or evolved in the lobe-finned fish lineage. We also suggest that the Atoh1-positive proliferative valvular primordium may represent a synapomorphy (shared derived character) of ray-finned fishes. The topology of valvular primordium development in paddlefish differs significantly from that of zebrafish and correlates with the adult cerebellar form. The distribution of proliferative granule cell precursors in different vertebrate taxa is thus the likely determining factor in cerebellar morphological diversity.
Keywords: Cerebellum, Atonal1, NeuroD1, Sonic hedgehog, proliferation, Polyodon spathula
Introduction
The vertebrate cerebellum is a remarkable neural structure whose synaptic architecture is highly conserved but which varies widely in its size and gross anatomy (Voogd and Glickstein 1998). Despite the similarities in cerebellar circuit organisation across vertebrates, it plays a variety of functional roles, acting principally as a coordinating centre for proprioception in anamniotes (Sherrington 1906; Wingate 2005), having a well described function in mammalian motor learning and coordination (Manto) and also having a significant role in primate cognition (Schmahmann 2010). Within the cerebellar cortex, the highly conserved geometry of the synapses between glutamatergic granule neuron axons and dendrites of GABAergic Purkinje cells indicates that a conserved processing algorithm performed by these cells is key to all these functions (Braitenberg and Atwood 1958; Meek 1992). In contrast, the wide variation in cerebellar size, even between relatively closely related species (Nieuwenhuys 1967; Nieuwenhuys et al. 1998; Sultan and Glickstein 2007; Lisney et al. 2008; Yopak and Montgomery 2008) suggests that functional specialisation relies on exquisite control of relative cell number. Increasing evidence that cerebellar granule cell development, in particular, involves different migratory and proliferative strategies in different taxa (Butts et al. 2011) points to a correlation between mode of granule cell development and cerebellar morphology.
The developmental genetics underlying cerebellar development are understood in some depth (Fig.1A) following detailed study in the chick and the mouse over the last 30 years (Wang and Zoghbi 2001; Hatten and Roussel 2011). The cerebellum develops solely from two germinal zones characterised by the expression of two basic helix-loop-helix (bHLH) transcription factors: a Ptf1a-positive ventricular zone in the rostralmost dorsal hindbrain, which gives rise to GABAergic interneurons, including Purkinje cells (Hoshino et al. 2005); and an Atoh1-positive rhombic lip, which borders the roof plate, gives rise to granule cells (Ben-Arie et al. 1997), and is derived entirely from rostral hindbrain rhombomere 1 (Wingate and Hatten 1999). For the majority of development, expression of Atoh1 is acutely maintained by interactions with the roof plate (Broom et al. 2012). However, in birds and mammals, granule precursor cells acquire the capacity to migrate away from the rhombic lip and form a secondary, transit-amplifying epithelium that is present transiently on the surface of the cerebellum. Mitogenic Sonic hedgehog (Shh)–mediated signals from underlying Ptf1a-derived Purkinje cells (Dahmane and Ruiz-i-Altaba 1999; Wallace 1999; Wechsler-Reya and Scott 1999) precisely regulate a large number of symmetrical divisions that eventually lead to the disappearance of the transient external germinal layer. As granule cells exit mitosis, they down-regulate Atoh1, switch on the bHLH transcription factor gene NeuroD1 (Miyata et al. 1999) and migrate into a deep, internal granule layer, below the layer of Purkinje cell bodies (Fig.1A).
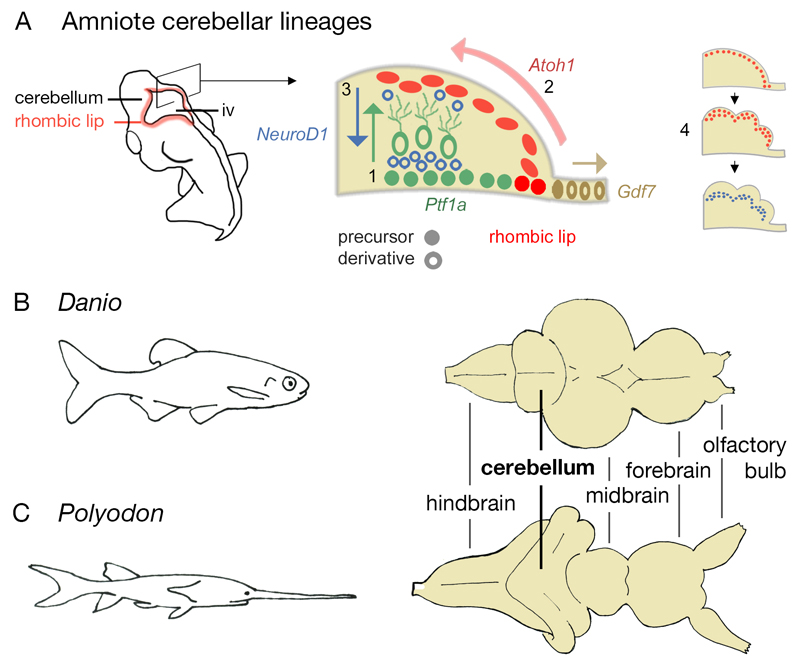
Figure 1. Cerebellar development in amniotes and adult cerebellar form in zebrafish and paddlefish.
Schematic diagram of an amniote embryo (A) showing the location of the rhombic lip and, in section, the distribution of precursors and their derivatives. Three major lineages give rise to Purkinje and other GABAergic neurons (Ptf1a, green), granule cells (Atoh1, red) and roof plate (Gdf7, brown). Purkinje cells migrate radially from the ventricular layer (1). Granule cells migrate tangentially to form a proliferative external germinal layer (2) and then, as NeuroD1-positive post-mitotic neurons, migrate radially to form an internal granule layer (3). Proliferation within the Atoh1-positive external germinal layer (4) leads to transverse foliation of the adult amniote cerebellum (see Fig.6e,f,g). The cerebellum of the zebrafish (Danio rerio) (B) differs from that of a paddlefish (Polyodon spathula) (C, normalised to the same scale as B): the latter displays a ribbon-like cerebellum with a convoluted and extended cerebellar rhombic lip. iv, fourth ventricle.
The historic description of this transient, transit-amplifying stage of granule cell development (Cajal 1894) and its obvious implications for surface area:volume ratio in the cerebellum have led to much speculation and some elegant experimental evidence (Corrales et al. 2006) for it being responsible for the highly folded structure of the cerebellum in birds and mammals (Fig.1A4). However, the existence of this layer has been contested in the zebrafish (a ray-finned teleost fish) and the lesser-spotted dogfish (a shark) (Rodriguez-Moldes et al. 2008; Chaplin et al. 2010; Kani et al. 2010; Wullimann et al. 2011): even if present, its molecular characteristics are very different to that of amniotes. This includes, in the zebrafish, an absence of Shh expression in underlying Purkinje cells (Chaplin, Tendeng, and Wingate 2010; Kani et al. 2010), upon which Atoh1-mediated transit amplification is strictly dependent in mammals (Flora et al. 2009). For teleosts, a separate solution for generating cerebellar granule cells may have arisen in the form of the valvulus, a tongue-like rostral extension of the cerebellum that usually extends beneath the midbrain and, which, in some families such as the weakly electric tapir-fish (Mormyrus), becomes a highly adapted, grossly exaggerated neural structure {Gallant, 2011 #6849}(Nieuwenhuys and Nicholson 1969). In zebrafish, the valvulus primordium represents an enduring stem cell niche (Kaslin et al. 2009) that, in its molecular signature (Chaplin, Tendeng, and Wingate 2010), resembles an extension of larval neurogenesis, rather than transit amplification, mirroring the situation observed in many regions of the zebrafish brain (Kizil et al. 2012). This distinction between extended primary neurogenesis in a ray-finned teleost fish and secondary proliferation in the cerebellum of mammals and birds (a distinction reinforced by the lack of cerebellar Shh in zebrafish) suggests that cerebellum development perhaps followed very different evolutionary trajectories in the actinopterygian (ray-finned) and sarcopterygian (lobe-finned) lineages of bony fishes.
Reconstructing the ancestral organisation of cerebellar development is not appreciably aided by data from chondrichthyans (e.g. sharks and rays). Within this group, granule neurons and their proliferative, Atoh1-positive progenitors (Rodriguez-Moldes et al. 2008; Chaplin, Tendeng, and Wingate 2010) are confined to bilateral midline ridges in the cerebellum, the prominentiae granularis (Nieuwenhuys, ten Donkelaar, and Nicholson 1998), which are continuous with an extended cerebellar rhombic lip. This distinct chondrichthyan cerebellar form is radically different from both amniotes and teleosts. Therefore, to determine the ancestral condition within ray-finned fishes, we examined the molecular anatomy of cerebellar development in the North American paddlefish, Polyodon spathula (Fig.1). This non-teleost ray-finned fish species is a representative of the chondrostean lineage (i.e., sturgeons and paddlefishes): a basal branch (relative to teleosts) of the ray-finned fishes (Hurley et al. 2007; Near et al. 2012). It thus represents an excellent basal in-group with which to reconstruct the likely ancestral ray-finned fish condition. By comparison to a teleost (Fig.1B), the cerebellum of the paddlefish, like other chondrosteans (Nieuwenhuys, ten Donkelaar, and Nicholson 1998), has a distinct ribbon-like form (Fig.1C). Does this divergent structure represent a different mode of granule cell production? By examining gene expression and proliferation across a range of stages of paddlefish development, we demonstrate both the lack of a superficial Atoh1-positive, proliferative layer and the absence of Shh expression in the cerebellum, supporting the argument that a transient, transit amplification step in granule cell development is unique to the sarcopterygian lineage.
Materials and Methods
Gene Cloning
RNA from stage 40-46 paddlefish embryos was extracted with Trizol reagent (Invitrogen). Single-strand cDNA was generated using the Superscript III First Strand Synthesis kit (Invitrogen), as per the manufacturer's instructions. The following primers were used: Atoh1Forward AGCACAGAGGTTATCCACAAAGTC; Atoh1Reverse ACTTTGTAACTCTAGATTTGCCTCC. Standard PCR conditions were used to amplify an approximately 800bp fragment; this fragment was then cloned into the pDrive vector (Qiagen). Clones were isolated and sequenced (Dept of Biochemistry DNA sequencing facility, University of Cambridge) prior to phylogenetic analysis. The GenBank accession number for P. spathula Atoh1 is KC589703.
Phylogenetic Analysis
Phylogenetic analysis of the Atonal family of proteins was performed using an alignment of the bHLH domain constructed in ClustalX and edited in SeaView (Galtier et al. 1996). Neighbour joining and maximum likelihood analysis were implemented in SeaView, with the LG model selected for the likelihood analysis following assessment of model suitability using ProtTest (Abascal et al. 2005). The robustness of the trees were assessed using 1000 bootstrap replicates. Bayesian analysis was conducted using MrBayes version 3.1.2 (Ronquist and Huelsenbeck 2003), using a mixed amino acid model combination, with 2 independent Markov chain Monte Carlo runs of 4 chains. The chains were run for 1 million generations. The burn-in phase was estimated using both likelihood plots and the average standard deviation of split frequencies, and trees within the burn-in were removed from subsequent analysis.
Embryo collection and staining
Polyodon spathula embryos obtained from Osage Catfisheries (Osage Beach, MO, USA) over multiple spawning seasons were fixed in 4% paraformaldehyde at stages 31 to 45 (Bemis and Grande 1992) . Embryos were processed for immunohistochemistry using a polyclonal antibody against phosphohistone H3 (αPH3, Cell Signalling Technology, 1:100) and a fluorescent secondary antibody (goat α-rabbit AlexaFluor-488, Invitrogen, 1:500), or for in situ hybridisation using a standard protocol (Myat et al. 1996), with riboprobes for Atoh1, NeuroD1 (Modrell et al. 2011) and Shh (Davis et al. 2007). Selected embryos were embedded in 20% gelatin and vibratome-sectioned at 50 μm.
Whole mount embryos were photographed on a Zeiss Stemi SV6 microscope and Olympus DP camera. Sections were photographed on a Leica MZFLIII microscope using QCapture Pro 6.0, or a Nikon Eclipse 80i confocal microscope using EZ-C1 3.70 software. Images were compiled in ImageJ version 10.2 and Adobe Photoshop.
Results
The ground plan of granule cell development is determined by patterns of proliferation and bHLH transcription factor expression that define the geometry and identity of proliferative cells and the presence of secondary, transit amplification. The presence of Shh is an additional, potential indicator of secondary proliferation in the cerebellum. We therefore examined these three parameters of cerebellar development in paddlefish embryos from stages 31 to 45, by which point the cerebellum assumes its adult form.
Cell proliferation is confined to the ventricular zone and rhombic lip during cerebellar development
Cells during M-phase of mitosis were identified using an antibody against phosphohistone H3 on paddlefish embryos (Fig.2A). Parasagittal sections through the lateral cerebellum, which encompass the hindbrain, midbrain and lateral cerebellar rhombic lip (Fig.2B-E), are contrasted with sagittal sections through the anterior pole of the cerebellum (Fig.2F-J). Between stages 36 (Fig.2B) and 45 (Fig.2E), there is a dramatic loss of mitotic profiles within the ventricular layer surrounding the fourth ventricle, while proliferation in the midbrain is maintained. At the earliest stage examined (Fig.2F), there is no pronounced difference between the cerebellum, hindbrain and midbrain. However, by comparison to the subsequent decline in proliferation in the ventricular zones of the hindbrain and cerebellum, the cerebellar midline displays numerous mitotic profiles through to stage 45. This suggests that cerebellar proliferation becomes increasingly restricted to the midline anterior pole of the paddlefish cerebellum (Fig.1C). At no point during these phases of cerebellar development is there a superficial layer of proliferative cells on the surface of the growing cerebellar anlagen that would correspond to an external germinal layer.
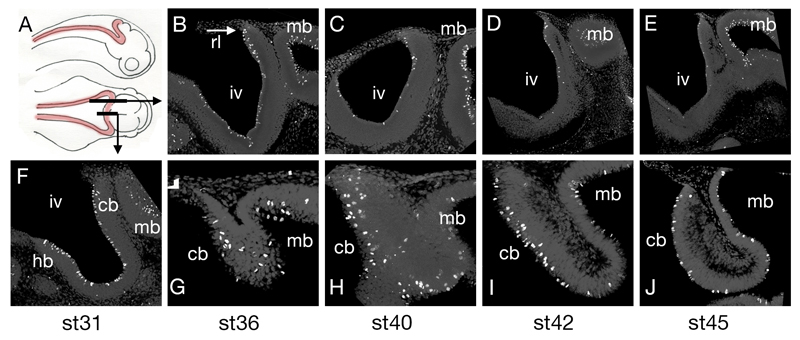
Figure 2. Proliferation in the paddlefish hindbrain.
Schematic diagram (A) of a paddlefish embryo in lateral and dorsal aspect showing the rhombic lip in red and the location of the parasagittal and sagittal sections. Immunostaining for phosphohistone H3 reveals that M-phase cells in the ventricular layer of the fourth ventricle (iv) progressively decline in number between stages 36 (B), 40 (C), 42 (D) and 45 (E), but continue to populate the midbrain (mb) ventricular surface. Sagittal sections at stages 31 (F), 36 (G), 40 (H), 42 (I) and 45(J) showing that proliferation is selectively maintained in the midline cerebellum (cb). Anterior is to the right in all panels.
Abbreviations: cb, cerebellum; hb, hindbrain; iv, fourth ventricle; mb, midbrain; rl, rhombic lip.
Expression of bHLH transcription factor genes in cerebellar granule neurons
In amniotes, granule cell proliferation and maturation are characterised by sequential expression of Atoh1 in the superficial external germinal layer of transit amplifying precursors (Ben-Arie et al. 1997), followed by NeuroD1 in maturing, post-mitotic cells (Miyata, Maeda, and Lee 1999). We therefore investigated the expression of paddlefish orthologues of both Atoh1 (Supplementary Figure 1) and NeuroD1 (Modrell, Buckley, and Baker 2011), in order to determine whether a transit amplification layer forms transiently during paddlefish granule cell maturation. In dorsal view, at stages 31 (Fig.3A) and 36 (Fig.3B), Atoh1 is expressed throughout the rhombic lip of both the cerebellum and hindbrain with the exception of the cerebellar midline. At stage 40, the cerebellar midline becomes Atoh1-positive (Fig.3C) and subsequently becomes the exclusive site of neurogenic gene expression at stage 42, as Atoh1 is down regulated in other parts of the rhombic lip (Fig.3D). By stage 45, expression of Atoh1 deep in the tissue at the cerebellar midline can only be visualised faintly in whole mount embryos (Fig.3E). In lateral view, Atoh1 expression outlines the lateral out-pocketing of the upper rhombic lip at stages 31 (Fig.3F) and 36 (Fig.3G). However, Atoh1 is never expressed in a superficial cerebellar layer covering the pial surface that might correspond to an external germinal zone. We note that between stages 40 and 45 (Fig.3H-J), expression of Atoh1, which is essential for inner ear hair cell development (Driver and Kelley 2009; Maricich et al. 2009), is also seen in the hair cells of lateral line mechanosensory neuromasts and electrosensory ampullary organs, similar to patterns observed for other ampullary organ markers in the paddlefish (Modrell, Buckley, and Baker 2011).
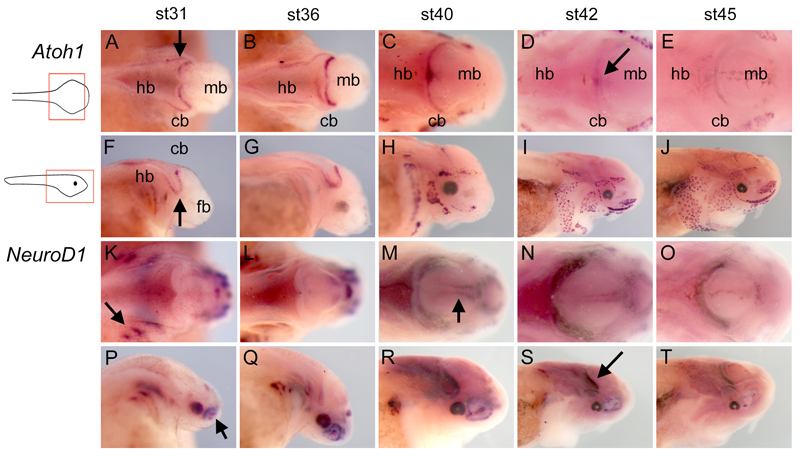
Figure 3. Atoh1 and NeuroD1 expression in wholemount paddlefish embryos.
Whole mount in situ hybridisation for Atoh1 (A–J) and NeuroD1 (K–T) in paddlefish embryos between stages 31 – 45 in dorsal (A–E, K–O) and lateral (F–J, P–T) views. Arrows in A and F indicate the characteristic out-pocketing of the chondrostean cerebellar rhombic lip (Nieuwenhuys, ten Donkelaar, and Nicholson 1998). The arrow in D indicates the refinement of Atoh1 expression to the cerebellum midline at st42. Arrows in K and P, respectively, indicate NeuroD1 expression in the cranial ganglia and olfactory pit. The arrow in M indicates midbrain expression of NeuroD1, corresponding to the position of the mesencephalic trigeminal nucleus (arrow). The arrow in S indicates the position of cerebellar NeuroD1 expression. Abbreviations: cb, cerebellum; fb, forebrain; hb, hindbrain; iv, fourth ventricle; mb, midbrain; rl, rhombic lip.
The expression of NeuroD1 in the presumptive cerebellum is broadly complementary to that of Atoh1. Thus NeuroD1 is absent from the cerebellum at stage 31 (Fig.3K) and expressed in a faint transverse stripe at stage 36 (Fig.3L). In the periphery, NeuroD1 expression is restricted to developing cranial ganglia and the paired sense organs(Modrell, Buckley, and Baker 2011). From stage 40 onwards, as Atoh1 is down regulated, NeuroD1 expression is prominent throughout the cerebellar rhombic lip and in the developing mesencephalic trigeminal nucleus in the midbrain (Fig.3M-O). In lateral view, the rhombic lip is clearly NeuroD1-negative at stages 31 (Fig.3P) and 36 (Fig.3Q). At stages 40-45 (Fig.3R-T), Granule cells form a NeuroD1-positive domain that lies parallel to the rhombic lip, anticipating the structure of the adult paddlefish cerebellum.
The organisation of Atoh1 and NeuroD1 expression at stage 42 is shown in parasagittal and sagittal sections through the cerebellum in Figure 4. The lateral cerebellum is Atoh1-negative (Fig.4A) but strongly expresses NeuroD1 (Fig.4B). In contrast, the midline of the cerebellum strongly expresses Atoh1 (Fig.4C), but is NeuroD1-negative (Fig.4D).
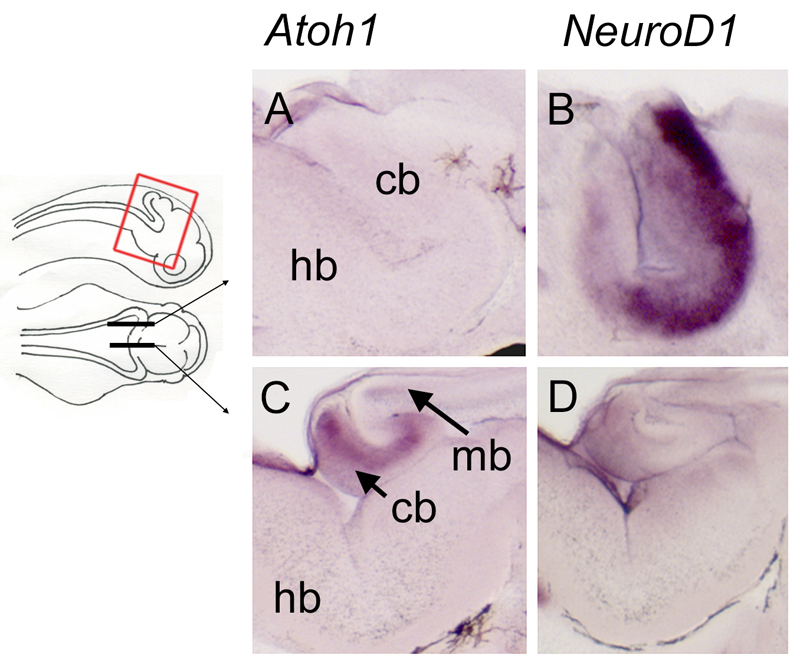
Figure 4. Complementary expression of Atoh1 and NeuroD1 in the medial versus lateral rhombic lip at stage 42.
Sections through the rhombic lip of embryos after in situ hybridisation for Atoh1 and NeuroD1 in parasagittal (A,B) and sagittal (C,D) planes, as indicated on the schematic. In the lateral rhombic lip, Atoh1-positive progenitors are absent (A) but NeuroD1 is strongly expressed (B), consistent with previously reported distributions of granule neurons (Nieuwenhuys 1967). In the medial rhombic lip, i.e., the presumptive valvulus, Atoh1 is strongly expressed (C), while NeuroD1 is reduced (D). Abbreviations: cb, cerebellum; hb, hindbrain; mb, midbrain.
Shh expression in the developing paddlefish
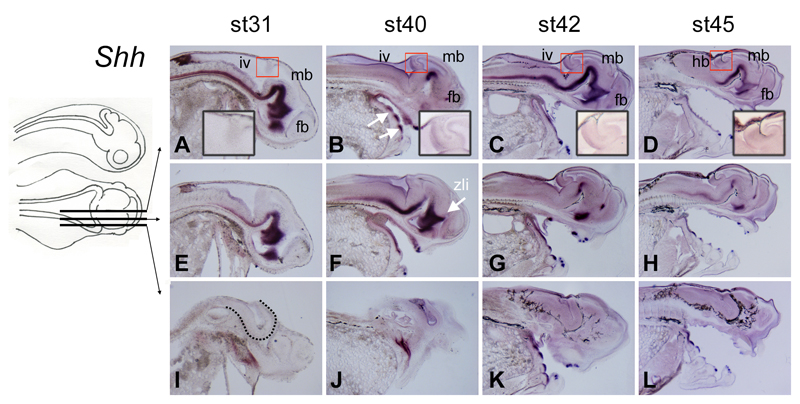
While Shh is expressed in the cerebellum of sharks (Chaplin, Tendeng, and Wingate 2010) and amniotes, it is absent from the cerebellum of zebrafish (Kani et al. 2010) (Chaplin, Tendeng, and Wingate 2010). In paddlefish, at all stages examined (stage 31 through to stage 45), expression of Shh in the central nervous system (CNS) was restricted to the developing ventral midline (Fig.5A-D). There was no expression of Shh within the developing cerebellum in either medial or lateral regions (Fig.5E-L). From stage 40 onwards, as in other gnathostomes (Smith et al. 2009), Shh expression defines the site of developing tooth primordia (arrows in Fig.5B).
Figure 5. Sonic hedgehog is not expressed in the developing paddlefish cerebellum.
Sections through embryos from stages 31-45 after in situ hybridisation for Shh in sagittal (A-D) and parasagittal (E-L) planes, as indicated in the schematic. At all stages examined, Shh expression in the CNS is restricted to the ventral midline, with no expression in the cerebellum (red boxes, shown at higher power in insets). Shh expression in the CNS is lost further laterally (compare E-H with I-L). White arrows in B indicate prominent expression of Shh in tooth buds. The arrow in F indicates the zona limitans intrathalamica (zli), which identifies the mid-point of the diencephalon along the rostrocaudal axis. The dotted line in I demarcates the boundary of the ventral neural tube.
Abbreviations: fb, forebrain; hb, hindbrain; iv, fourth ventricle; mb, midbrain; zli, zona limitans intrathalamica.
Discussion
We examined proliferation and bHLH transcription factor gene expression during cerebellar granule cell development in paddlefish, which together demonstrate an absence of any distinct external germinal layer as defined by the presence of mitotic, Atoh1-positive neurons in a sub-pial layer over the cerebellum (Chaplin, Tendeng, and Wingate 2010).
Sequence of Atoh1 and NeuroD1 expression in granule cell development is highly conserved but geography of expression is not
The sequential activation of first Atoh1 and then NeuroD1 defines the initial steps in the differentiation of granule cells in amniotes (Hatten and Roussel 2011) and zebrafish (Kani et al. 2010). Our observations of paddlefish development suggest a similar sequence in a basal actinopterygian, extending this observation across all bony fish cerebellums examined thus far. In amniotes, the appearance of a transient, external germinal layer is associated with the prolonged duration of Atoh1 expression in migratory derivatives of the rhombic lip. This pronounced Atoh1-positive transit amplification is absent in both zebrafish and shark (Chaplin et al. 2010). In zebrafish, this is correlated with the absence of mitogenic Shh produced by Purkinje cells, on which, in mice, Atoh1-mediated granule cell transit amplification depends (Flora et al. 2009). The lack of both a Shh-expressing domain and an Atoh1–positive external germinal layer in the cerebellum of paddlefish, as well as zebrafish, suggests that the transit amplification seen in amniotes is not a feature of the cerebellum in ray-finned fishes.
Our data suggest that both the origin of granule cells within the rhombic lip in shark, zebrafish (Chaplin, Tendeng, and Wingate 2010; Kani et al. 2010; Wullimann et al. 2011), birds (Broom et al. 2012) and mammals (Ben-Arie et al. 1997) and the simple progression of bHLH transcription factor gene expression that determines their fate, are conserved features of vertebrate cerebellum development. This conservation is paralleled by a synaptic arrangement between granule cells and their Ptf1a–derived Purkinje cell targets that is highly stereotyped and thought to be functionally essential for information processing within the cerebellum (Nieuwenhuys, ten Donkelaar, and Nicholson 1998).
Divergence in proliferation zones and cerebellum structure within actinopterygians
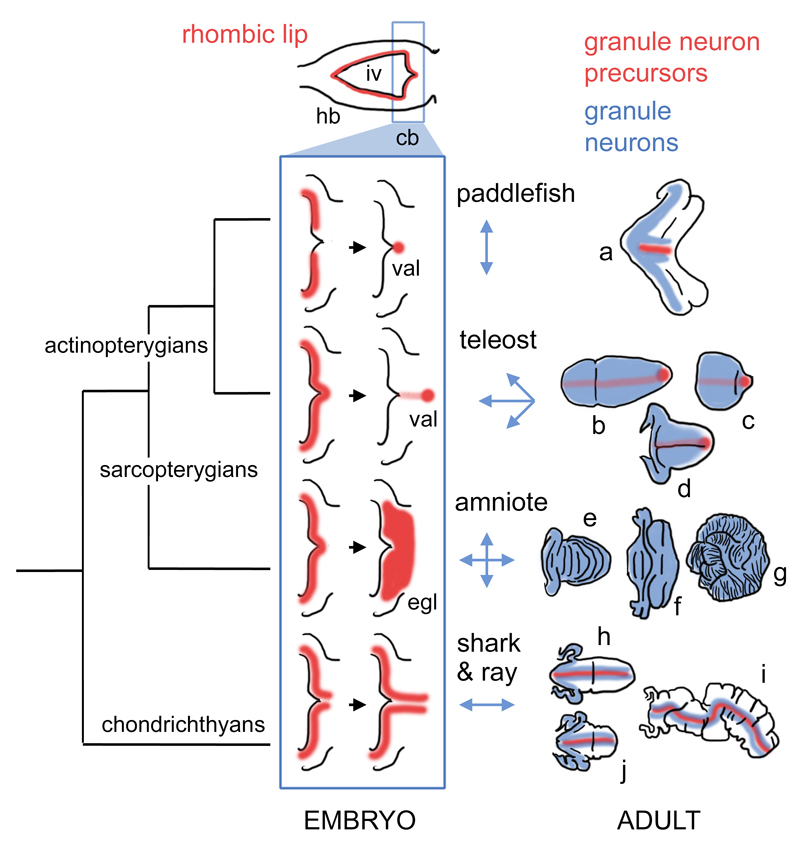
In contrast to the conservation of embryonic origin and molecular specification of cerebellar granule cells, the morphological diversity of the cerebellum across vertebrate taxa is striking (Fig.6). In mammals, for example, there is marked diversity in the degree of cerebellar foliation (Fig.6e,f,g). Here, structural variation appears to relate directly to proliferation within the external germinal layer in response to Shh signalling from underlying Purkinje cells (Corrales et al. 2006). However, this surface area:volume ratio explanation is unlikely to account for either the variation in chondrichthyans (Fig.6h,i,j) (Yopak et al. 2007; Yopak and Montgomery 2008) or actinopterygians (Fig.6a-d) (Nieuwenhuys, ten Donkelaar, and Nicholson 1998), where transit amplification is, respectively, either severely attenuated or absent. How do our results explain the variation between paddlefish and zebrafish cerebellar morphology?
Figure 6. Hypothetical phylogeny of granule neuron proliferative zones in the cerebellum.
Hindbrain development in all vertebrate embryos can be characterised by a phase (top) in which an expanded fourth ventricle roof plate (iv) is bordered by an Atoh1-positive rhombic lip (red) in both the prospective cerebellum (cb) and the rest of the hindbrain (hb). Only the “upper” or cerebellar rhombic lip gives rise to granule cells in taxon-specific ways. We hypothesise that variation in the development of these proliferative zones (blue boxed schematics) constrains the geometry of the expansion of the granule cell layer (blue arrows, right) to produce mature granule cell distributions (blue) in adult, taxon-specific cerebellar morphologies (far right). In actinopterygians, granule cell proliferation becomes confined to a stem cell niche, the valvular primordium (val), which lies at the rostral pole of the cerebellum in teleosts (Kaslin et al. 2009) and borders the roof plate in the paddlefish. In amniotes, granule cell precursors migrate into a transient superficial external germinal layer (egl) and no precursors are retained in the adult cerebellum. In chondrichthyans, granule neuron progenitors are confined to the rhombic lip and on either side of the cerebellar midline (Chaplin, Tendeng, and Wingate 2010). Figures show hypothetical cell distributions in the cerebellar outlines (not shown to the same scale) based on model species for (a) paddlefish (a chondrostean ray-finned fish); (b-d) teleosts: b, remora (a perciform teleost), c, zebrafish (a cypriniform teleost), d, catfish (a siluriform teleost); (e-g) amniotes: e, bird, f, bat, g, giraffe; (h-j) chondrichthyans: h, dogfish (a shark), i, skate, j, stingray.
Abbreviations: cb, cerebellum; egl, external germinal layer; hb, hindbrain; iv, fourth ventricle; val, valvular primordium.
The developing paddlefish cerebellum both resembles and differs from that of the zebrafish cerebellum in key features. In paddlefish, as in zebrafish, the enduring site of Atoh1 expression and granule cell proliferation into late development is the cerebellar midline. This territory is retained into adulthood in the zebrafish (Zupanc et al. 2005), and an intriguing remaining issue is whether the same is true of paddlefish. In zebrafish, this apical proliferative “niche” (Kaslin et al. 2009) has been equated to the valvular primordium (Chaplin, Tendeng, and Wingate 2010; Kani et al. 2010), which gives rise, in the form of the valvulus, to the most variable structural feature of the actinopterygian cerebellum. The presumptive valvular primordium in paddlefish and zebrafish is similar in initial position, proliferative activity and gene expression and yet generates very different cerebellar morphologies (Fig.1B,C). Cerebellar growth in zebrafish results in the valvular primordium becoming distanced from the rhombic lip (Fig.6b-d), which is the site of Atoh1 induction via roof plate-derived Gdf7 (Alder et al. 1996; Broom et al. 2012), whereas in paddlefish, the valvular primordium remains close to the roof plate (Fig.6a).
This change in the location of the valvular primordium may have been enabled by the teleost whole-genome duplication event that resulted in three Atoh1 (Fig.S1) and two Gdf7 paralogues with divergent expression patterns in zebrafish (Chaplin, Tendeng, and Wingate 2010; Kani et al. 2010). The maturing cerebellar rhombic lip of zebrafish is characterised by Gdf6 and Atoh1c expression. In contrast, the apical midline niche is characterised by the coordinated co-expression of Gdf7 and Atoh1a/Atoh1b. Though the paddlefish lineage has undergone an independent whole-genome duplication (Crow et al. 2012), this is likely much more recent, and only a single Atoh1 gene fragment could be isolated. In paddlefish, the cerebellar midline fails to extend such that the valvular primordium remains adjacent to the fourth ventricle roof plate, the likely exclusive source of Gdf signalling. We speculate that this simple topographic constraint on proliferation limits the paddlefish cerebellum to only lateral growth from the midline resulting in an anisomorphic, ribbon-like growth pattern reflected in its adult form (Fig.1C and Fig.6a).
Our results also suggest that the Atoh1-positive proliferative valvular primordium, which comprises a site of granule cell production that is maintained into late development, may represent a significant synapomorphy of actinopterygians. The absence of Shh in the cerebellum of both paddlefish (a basal actinopterygian) and zebrafish (a teleost actinopterygian), and the absence of a superficial germinal layer, argue against the valvular primordium being a direct homologue of the amniote external germinal layer. We propose that cerebellar structural diversity in actinopterygians results from the different proliferative properties of this unique valvular primordium, in terms of both rate and duration, and that this is facilitated to some extent by duplication of the Atoh1 gene, enabling the generation of distinct sub-domains of expression. In paddlefish, the presumptive lack of such sub-domains may “tie” the valvulus to the fourth ventricle roof plate and prevent the extension of the cerebellar midline, resulting in anisomorphic growth.
Supplementary Material
Supplementary figure 1: Phylogenetic analysis of the Atonal gene family in vertebrates. The cloned paddlefish atonal gene robustly groups with other vertebrate Atonal1 genes to the exclusion of the atonal5/7 subfamily (A). The strength of this grouping is enhanced when only a single clade of teleost-specific duplicates (B) is included in the analysis. Support values (bootstrap replicate percentages or posterior probabilities) are shown above and below the subfamily nodes (NJ/ML above; PP below).
Acknowledgements
The authors would like to thank Andrew Gillis for lab assistance and the members of the Wingate and Graham groups at King’s for stimulating discussions. This work was funded by the BBSRC (BB/I021507/1 to R.J.T.W; BB/F00818X/1 to C.V.H.B.), and the Fisheries Society of the British Isles (Small Research Grant to M.S.M.).
References
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21(9):2104–5. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17(3):389–99. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Bemis WE, Grande L. Early development of the acitnopterygian head. 1. External development and staging of the paddlefish Polydon spatula. Journal of Morphology. 1992;213:47–83. doi: 10.1002/jmor.1052130106. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390(6656):169–72. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Atwood RP. Morphological observations on the cerebellar cortex. J Comp Neurol. 1958;109:1–34. doi: 10.1002/cne.901090102. [DOI] [PubMed] [Google Scholar]
- Broom ER, Gilthorpe JD, Butts T, Campo-Paysaa F, Wingate RJT. The roof plate boundary is a bi-directional organiser of dorsal neural tube and choroid plexus development. Development. 2012;139(22):4261–4270. doi: 10.1242/dev.082255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts T, Chaplin N, Wingate RJ. Can clues from evolution unlock the molecular development of the cerebellum? Mol Neurobiol. 2011;43(1):67–76. doi: 10.1007/s12035-010-8160-2. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. In: Les Nouvelles Idées sur la Structure du Système Nerveux chez l'Homme et chez les Vertébrés. 1 ed. Azoulay L, translator. Paris: C. Reinwald & Cie; 1894. [Google Scholar]
- Chaplin N, Tendeng C, Wingate RJ. Absence of an external germinal layer in zebrafish and shark reveals a distinct, anamniote ground plan of cerebellum development. J Neurosci. 2010;30(8):3048–57. doi: 10.1523/JNEUROSCI.6201-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales JD, Blaess S, Mahoney EM, Joyner AL. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development. 2006;133(9):1811–21. doi: 10.1242/dev.02351. [DOI] [PubMed] [Google Scholar]
- Crow KD, Smith CD, Cheng JF, Wagner GP, Amemiya CT. An independent genome duplication inferred from Hox paralogs in the American paddlefish--a representative basal ray-finned fish and important comparative reference. Genome Biol Evol. 2012;4(9):937–53. doi: 10.1093/gbe/evs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Ruiz-i-Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126(14):3089–100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447(7143):473–6. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- Driver EC, Kelley MW. Specification of cell fate in the mammalian cochlea. Birth Defects Res C Embryo Today. 2009;87(3):212–21. doi: 10.1002/bdrc.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora A, Klisch TJ, Schuster G, Zoghbi HY. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science. 2009;326(5958):1424–7. doi: 10.1126/science.1181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;12(6):543–8. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Roussel MF. Development and cancer of the cerebellum. Trends Neurosci. 2011;34(3):134–42. doi: 10.1016/j.tins.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, Watanabe M, et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47(2):201–13. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hurley IA, Mueller RL, Dunn KA, Schmidt EJ, Friedman M, Ho RK, Prince VE, Yang Z, Thomas MG, Coates MI. A new time-scale for ray-finned fish evolution. Proc Biol Sci. 2007;274(1609):489–98. doi: 10.1098/rspb.2006.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kani S, Bae YK, Shimizu T, Tanabe K, Satou C, Parsons MJ, Scott E, Higashijima S, Hibi M. Proneural gene-linked neurogenesis in zebrafish cerebellum. Dev Biol. 2010;343(1-2):1–17. doi: 10.1016/j.ydbio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Geffarth M, Grandel H, Hans S, Brand M. Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J Neurosci. 2009;29(19):6142–53. doi: 10.1523/JNEUROSCI.0072-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72(3):429–61. doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- Lisney TJ, Yopak KE, Montgomery JC, Collin SP. Variation in brain organization and cerebellar foliation in chondrichthyans: batoids. Brain Behav Evol. 2008;72(4):262–82. doi: 10.1159/000171489. [DOI] [PubMed] [Google Scholar]
- Manto M. The cerebellum, cerebellar disorders, and cerebellar research--two centuries of discoveries. Cerebellum. 2008;7(4):505–16. doi: 10.1007/s12311-008-0063-7. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Xia A, Mathes EL, Wang VY, Oghalai JS, Fritzsch B, Zoghbi HY. Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J Neurosci. 2009;29(36):11123–33. doi: 10.1523/JNEUROSCI.2232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek J. Comparative aspects of cerebellar organisation. European Journal of Morphology. 1992;30:37–51. [PubMed] [Google Scholar]
- Miyata T, Maeda T, Lee JE. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 1999;13(13):1647–52. doi: 10.1101/gad.13.13.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Buckley D, Baker CV. Molecular analysis of neurogenic placode development in a basal ray-finned fish. Genesis. 2011;49(4):278–94. doi: 10.1002/dvg.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat A, Henrique D, Ish-Horowicz D, Lewis J. A chick homologue of Serrate and its relationship with Notch and Delta homologues during central neurogenesis. Dev Biol. 1996;174(2):233–47. doi: 10.1006/dbio.1996.0069. [DOI] [PubMed] [Google Scholar]
- Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL. Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sci U S A. 2012;109(34):13698–703. doi: 10.1073/pnas.1206625109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R. Comparative anatomy of the cerebellum. In: Fox C, Snider R, editors. Progress in Brain Research. Amsterdam: Elsevier; 1967. [Google Scholar]
- Nieuwenhuys R, Nicholson C. A survey of the general morphology, the fiber connections, and the possible functional significance of the gigantocerebellum of mormyrid fishes. In: Llinás R, editor. Neurobiology of Cerebellar Evolution and Development. Chicago: American Medical Association; 1969. [Google Scholar]
- Nieuwenhuys R, ten Donkelaar HJ, Nicholson C. The Central Nervous System of Vertebrates. Berlin: Springer-Verlag; 1998. [Google Scholar]
- Rodriguez-Moldes I, Ferreiro-Galve S, Carrera I, Sueiro C, Candal E, Mazan S, Anadon R. Development of the cerebellar body in sharks: spatiotemporal relations of Pax6 expression, cell proliferation and differentiation. Neurosci Lett. 2008;432(2):105–10. doi: 10.1016/j.neulet.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. Original edition. New Haven: Yale University Press; 1906. 1906. [Google Scholar]
- Smith MM, Fraser GJ, Chaplin N, Hobbs C, Graham A. Reiterative pattern of sonic hedgehog expression in the catshark dentition reveals a phylogenetic template for jawed vertebrates. Proc Biol Sci. 2009;276(1660):1225–33. doi: 10.1098/rspb.2008.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan F, Glickstein M. The cerebellum: Comparative and animal studies. Cerebellum. 2007;6(3):168–76. doi: 10.1080/14734220701332486. [DOI] [PubMed] [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21(9):370–5. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9(8):445–8. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2(7):484–91. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Wingate R. Math-Map(ic)s. Neuron. 2005;48(1):1–4. doi: 10.1016/j.neuron.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Wingate RJT, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126(20):4395–404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T, Distel M, Babaryka A, Grothe B, Koster RW. The long adventurous journey of rhombic lip cells in jawed vertebrates: a comparative developmental analysis. Front Neuroanat. 2011;5:27. doi: 10.3389/fnana.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yopak KE, Lisney TJ, Collin SP, Montgomery JC. Variation in brain organization and cerebellar foliation in chondrichthyans: sharks and holocephalans. Brain Behav Evol. 2007;69(4):280–300. doi: 10.1159/000100037. [DOI] [PubMed] [Google Scholar]
- Yopak KE, Montgomery JC. Brain organization and specialization in deep-sea chondrichthyans. Brain Behav Evol. 2008;71(4):287–304. doi: 10.1159/000127048. [DOI] [PubMed] [Google Scholar]
- Zupanc GK, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol. 2005;488(3):290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.