Summary
Large cell transformation of mycosis fungoides (MF-LCT) occurs in 20–50% of advanced MF, and is generally associated with poor prognosis, although some patients have indolent disease. We sought to identify clinicopathological prognostic factors in a large number of patients with MF-LCT. We identified patients with MF-LCT treated between 1991 and 2012 at a referral centre for cutaneous lymphoma. Clinical and pathological records, and histopathological slides were reviewed. Associations of clinicopathological variables with disease-specific survival were analysed. In 51 patients with MF-LCT, factors significantly associated with shorter survival were: age >60 years (25 versus 61 months, p = 0.01), stage III/IV (25 versus 44 months, p = 0.049), high serum lactate dehydrogenase (LDH; 24 versus 53 months, p = 0.007), absent papillary dermal involvement (8 versus 30 months, p = 0.008); follicular mucin at transformation (24 versus 42 months, p = 0.007); and the absence of fibrosis at transformation (21 versus 42 months, p = 0.03). Patients presenting with transformation at diagnosis had better survival than those who started with a small cell phenotype (p = 0.02). Age >60 years was independently associated with poorer survival (HR 5.61, 95%CI 1.17–26.8, p = 0.03), and the presence of fibrosis at transformation was independently associated with improved survival (HR 0.30, 95%CI 0.09–0.97, p = 0.045). In patients with MF-LCT, clinical features (age, stage, serum LDH) are important in assessing prognosis. Additional clinical and pathological features identified in this study may also assist in prognostic stratification. Studies of larger cohorts should be performed to validate the prognostic significance of these features.
Keywords: Cutaneous T-cell lymphoma, large cell transformation, mycosis fungoides, pathology, prognosis, skin, tumour
Introduction
Large cell transformation (LCT) in mycosis fungoides (MF) is the histopathological transformation of neoplastic small lymphocytes to a clonally identical1,2 large cell phenotype, which may occur in 20–55% of advanced MF cases.3,4 Less commonly, patch or plaque stage MF may exhibit large cell morphology de novo.3,5,6 LCT is often a histological marker of poor prognosis, and is associated with mean 5-year survival of less than 20%.3 However, some patients with well-documented LCT have indolent disease with long-term survival.7–10 Given the clinical heterogeneity amongst MF patients who exhibit LCT, it is important to seek additional means of accurately identifying patients who will have an aggressive clinical course, in order to optimise therapeutic strategies.
Features that have been previously shown to predict outcome in patients with transformed MF include advanced stage at transformation,3,5,8–11 increased extent of skin lesions, folliculotropism and CD30 expression.10,12 In this study, we investigated a large set of clinical, histopathological, immunophenotypical and molecular parameters in an attempt to identify features with prognostic utility in MF patients with LCT.
Materials and Methods
Patient selection
This retrospective study was conducted with the approval of the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC). Patients with primary cutaneous T-cell lymphoma with LCT diagnosed between 1991 and 2012 were identified from the MSKCC pathology archive. The database was queried using the following Boolean search parameters: free text including ‘large cell transformation’, ‘large cells’, or ‘progression’ were required, with a diagnosis of ‘mycosis fungoides’ or ‘cutaneous T-cell lymphoma’, or the names of clinicians PM or SH, identifying potential cases. Histological review was performed by a dermatopathologist with expertise in cutaneous lymphoma to confirm cutaneous T-cell lymphoma with features of LCT using the criteria of >25% large cells (>4× the size of a small lymphocyte). Cases in which the key slides showing transformation were not available for review were excluded from the study. Cases which met the above criteria were further clinically confirmed as MF, via detailed chart review and clinical reassessment as well as with integration of immunohistochemical and molecular data in consensus with two clinical cutaneous lymphoma experts (in dermatology and haematology). Staging was performed using the criteria proposed by the ISCL (International Society for Cutaneous Lymphoma)/EORTC (European Organization for Research and Treatment of Cancer).13
Clinical and pathological data
Patient charts, clinical images, defining histopathology slides, and molecular data were reviewed. All additional biopsies from the selected patients were reviewed when available, and pathological reports were reviewed when slides were no longer accessible.
Clinical features assessed (Table 1) were: patient age; gender; race/ethnicity; dates and clinical stage (per TNMB classification) at first sign, first diagnosis, first presentation at our institution, first tumour, and first histological documentation of LCT; dates of death/last follow up; patient status; number of skin sites biopsied; number of extracutaneous sites biopsied for lymphoma evaluation; sites of extracutaneous disease; serum lactate dehydrogenase (LDH) and absolute eosinophils prior to and at LCT; evidence of clonal T-cell gene rearrangements in blood and/or tissue samples (including comparison of clones in individual patients where multiple results were available); and medication/treatment history specific to cutaneous T-cell lymphoma (CTCL) and response to therapies.
Table 1. Clinical, histological and immunohistochemical features assessed in patients with LCT.
| Clinical |
|
|
| Age |
| Sex |
| Race/ethnicity |
| Age/stage at first histological documentation of LCT |
| Age of death/last follow-up |
| Number and sites of skin and extracutaneous tissue biopsied for lymphoma |
| Serum LDH prior to and at LCT |
| Absolute and percent eosinophils prior to and at LCT |
| Medication history (no., type, and response to therapy) |
|
|
| Histological |
|
|
| Presence or absence of LCT defined as lymphocytes >4× the size of ‘normal’ lymphocytes comprising ≥25% of the entire atypical infiltrate |
| % of all atypical lymphocytes showing large cell morphology |
| Density of infiltrate: estimated number of cells per HPF, assessed as low, moderate, or high |
| Location of infiltrate: specific involvement of epidermis, papillary dermis, reticular dermis, subcutis, follicular epithelium, or sweat gland epithelium |
| Presence/absence of fibrosis: defined by thickened or wiry collagen bundles in dermis |
| Presence/absence of ulceration: defined by full-thickness erosion of epidermis, including basement membrane |
| Epidermotropism: presence of atypical lymphocytes extending into the epidermis, out of proportion to any visible spongiosis |
| Pautrier microabscesses: aggregates of at least 3 atypical lymphocytes in the epidermis, sometimes visibly associated with Langerhans cells, in the absence of the vase-like shapes more typically seen in spongiotic dermatitis |
| Spongiosis: visible intercellular oedema |
| Epidermal hyperplasia: acanthosis/widening of epidermis beyond that expected for the specified region of the skin |
| Eosinophils: quantified by light microscopy, per 5 HPF |
| Neutrophils: present or absent |
| Langerhans cell hyperplasia: present or absent within the epidermis on H&E stain |
| Follicular mucin: intercellular mucin in follicular epithelium, seen on H&E stain, with or without ‘lake-like’ collections |
| Folliculotropism: presence of atypical lymphocytes extending into the follicular epithelium, out of proportion to spongiosis |
| Histopathological stage: tumour vs plaque vs patch (as previously described) |
| Mitotic rate: number of atypical lymphocytes observed to be in mitosis per 5 HPF |
|
|
| Immunohistochemical |
|
|
| CD3 |
| CD4 |
| CD8 |
| CD7 |
| CD4:CD8 |
| % CD30 of total infiltrate |
| Absolute CD30/5 HPF |
| Dermal vs epidermal predominance of CD30 |
| % Ki-67 |
HPF, high power field; LCT, large cell transformation; LDH, lactate dehydrogenase.
The following pathological features were documented (Table 1): dates and sites of all reviewed biopsies; presence or absence of LCT; % large cells of atypical lymphocytes; density, characterised as mild, moderate or high number of cells per high power field; specific site involvement of infiltrate; presence/absence of fibrosis; ulceration; epidermotropism; spongiosis; epidermal hyperplasia; eosinophils; neutrophils; follicular mucin; folliculotropism; and Langerhans cell hyperplasia. Histopathological stage (tumour versus plaque versus patch) was noted. Mitotic rate and eosinophil number were quantified per 5 high power fields (HPF). Partial immunohistochemical data were available for 47 patients. Immunohistochemical stains assessed included CD3 (40 patients/90 cases); CD4 (41 patients/98 cases); CD7 (29 patients/58 cases); CD8 (40 patients/96 cases); CD4:CD8 ratio (39 patients/87 cases); CD30 (38 patients/105 cases); CD56 (19 patients/26 cases) and Ki-67 (15 patients/26 cases). CD30 localisation (primarily dermis versus epidermis), percent of entire infiltrate, and cell number/5 HPF was assessed in available cases.
Statistical analysis
Analyses were carried out using IBM SPSS Statistics 20 software (IBM Corporation, USA). Associations of clinical and pathological variables with disease-specific survival (DSS) were assessed by the Kaplan–Meier method (differences between survival functions for different strata were assessed with Mantel–Cox log rank tests). DSS was defined as the interval between diagnosis of first LCT and death from CTCL. Patients with no events during follow-up (e.g., no death) were censored. p values of <0.05 were considered to be statistically significant.
Multivariate Cox proportional hazards models including variables that were statistically significant in univariate analyses were explored. Inclusion of some variables (each of which had ≤5 patients in one level) resulted in unstable multivariate models; therefore, they were excluded from the final multivariate model (along with other variables that were closely correlated with one another).
Results
Clinical features
Fifty-one patients (24 females, 27 males) were identified with confirmed LCT (Table 2). The mean age of LCT was 63 (range 25–102) years. LCT occurred prior to cutaneous tumour development (n = 9), concurrently with or following cutaneous tumour development (n = 30), or extracutaneously without eventual cutaneous tumour development (n = 12). At the time of data analysis, 27 patients had died (22 confirmed to have died of disease), and 24 were alive. Most of the patients who died of disease were advanced stage (IIB–IVB) and most (15/27) also had tumours. No patient with early stage disease (IA–IIA) died. Three of the 12 patients without skin tumours at the time of LCT died; they included a 90-year-old woman with lung involvement (stage IVB), a 75-year-old woman with a second (B-cell) nodal lymphoma as well as stage IVA2 (N3) involvement by her cutaneous T-cell lymphoma, and a 56-year-old male with stage IVA2 (N3) MF. Of the remaining nine patients without skin tumours at the time of LCT, seven had patch/plaque disease (one was N1, none of the remaining six had documented nodal disease), and two did not have evidence of skin disease, but had nodal (N2, 1 patient) and blood (IVA1, 1 patient) involvement. The last two patients had had skin disease previously, which was in remission at the time of transformation.
Table 2. Summary of relevant clinical and pathological features of patients with LCT.
| Parameter | Mean, years (range) | |
|---|---|---|
| Age at onset of symptoms | 56 (15–99) | |
| Age at biopsy-confirmed diagnosis of MF | 60 (22–99) | |
| Age at development of tumour-stage MF | 64 (22–102) | |
| Age at LCT of MF | 63 (25–102) | |
|
| ||
| Parameter | n/Total | % |
|
| ||
| Sex | ||
| Female | 24 | 47 |
| Male | 27 | 53 |
| Race | ||
| Causasian | 44 | 88 |
| Black | 3 | 6 |
| Asian | 3 | 6 |
| Number of patients who developed tumour stage disease in skin | 39/51 | 76 |
| Number of patients DOD | 24/51 | 47 |
| Thickest stage = tumour | ||
| Ever | 34/50 | 68 |
| At LCT | 24/42 | 57 |
| Involvement of subcutis | ||
| Ever | 17/47 | 36 |
| At LCT | 11/37 | 30 |
| Ulceration | ||
| Ever | 17/50 | 34 |
| Folliculotropism | ||
| Ever | 35/47 | 74 |
| At LCT | 25/36 | 69 |
| Follicular mucin | 11/47 | 23 |
| At LCT | ||
| Eccrinotropism | ||
| Ever | 15/49 | 31 |
| At LCT | 8/40 | 20 |
| % large cells in skin at diagnosis of LCT | ||
| 100% | 15/42 | 36 |
| 25–75% | 27/42 | 64 |
| Fibrosis | ||
| Ever | 46/50 | 92 |
| At LCT | 33/41 | 80 |
| Vascular prominence | ||
| Ever | 48/50 | 96 |
| At LCT | 34/41 | 83 |
| Epidermal hyperplasia | ||
| Ever | 47/50 | 94 |
| Epidermotropism | ||
| Ever | 45/50 | 90 |
| At LCT | 34/40 | 85 |
| Pautrier microabscesses | ||
| Ever | 37/50 | 74 |
| At LCT | 27/41 | 66 |
| Neutrophils | ||
| Ever | 19/50 | 38 |
| At LCT | 10/41 | 24 |
DOD, dead of disease; Ever, at any time during follow-up period; LCT, large cell transformation; MF, mycosis fungoides.
Pathological features
From the patients with confirmed LCT, 317 biopsies were taken, of which 251 had slides available for review. Patients had an average of four documented skin biopsies performed for evaluation of lymphoma (range 1–13), and an average of 1.4 (0–6) non-skin biopsies for lymphoma which included the lymph nodes, bone marrow, parotid gland, lung, tonsil and bladder. The average number of skin biopsies reviewed per patient for the study was 3.6 (0–13).
Thirty-seven patients had biopsies showing both non-transformed and transformed MF within skin and extracutaneous sites. We largely limited our analysis to biopsies showing large cell transformation, and selected the most infiltrated lesions for review. However, certain features, such as follicular involvement or subcutaneous involvement were recorded if present in any biopsy showing such findings, under the term ‘ever’ (Table 2).
Most patients had moderate to high density infiltrates of atypical lymphocytes and, in many, the density increased after transformation. All 51 patients had >25% of large lymphocytes within the infiltrates. Of 42 patients who showed transformation in the skin, the percentage of large cells in the infiltrates at LCT varied: 15 of 42 patients had 100% large cells (Fig. 1A), while 27 of 42 patients had 25–75% large cells. Thirty-four of 50 patients in whom this variable was evaluable developed tumours as their thickest pathological stage, and in 24 of 42 patients, LCT occurred within tumour-stage MF. Sixteen patients maintained patch/plaque stage MF. Folliculotropism (Fig. 1B), fibrosis (Fig. 1C), vascular prominence, Pautrier microabscesses, neutrophils, eccrinotropism and epidermotropism were commonly found in at least one of the patients' biopsies, although they were less prevalent in transformation biopsies than in other biopsies (Table 2). Fifty percent of biopsies had >3 mitoses per HPF at transformation, while 50% had fewer than 3 mitoses per HPF at transformation.
Fig. 1.
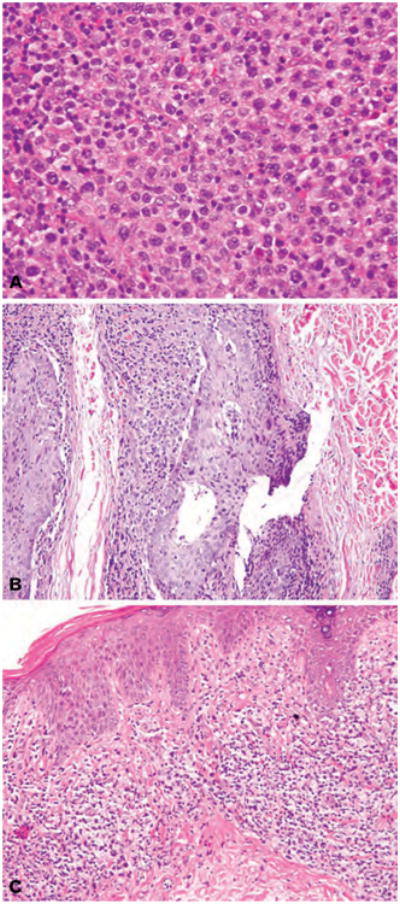
(A) H&E stain. Diffuse dermal infiltrate comprised of 100% large cells (>4× the size of a normal lymphocyte). (B) H&E stain. Hair follicles with folliculotropic infiltrate of small and large lymphocytes, at least 25% of which show large cell transformation, associated with intra-epithelial mucin deposition (follicular mucin). (C) H&E stain. Bandlike (lichenoid) infiltrate of small and large atypical lymphocytes associated with fibrotic, eosinophilic collagen bundles characteristic of chronic disease.
Associations of clinicopathological variables with survival
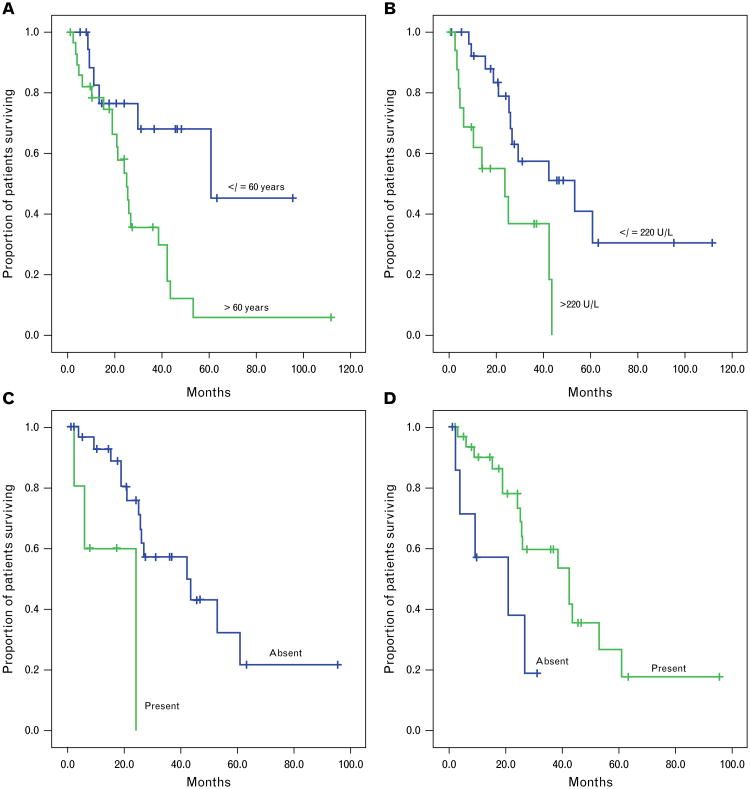
Clinical variables in patients who underwent transformation that showed statistically significant associations with shorter median survival were: age >60 years at transformation (25 versus 61 months, p = 0.01; Fig. 2A); stage II or greater at transformation (25 versus 44 months, p = 0.049); and elevated serum LDH at transformation (24 versus 53 months, p = 0.007; Fig. 2B) (Table 3).
Fig. 2.
Kaplan–Meier curves showing associations of selected clinicopathological variables with disease-specific survival from the date of transformation. (A) Age at transformation (p = 0.01). (B) Serum LDH level at transformation (p = 0.007). (C) Follicular mucin at transformation (p = 0.007). (D) Fibrosis at transformation (p = 0.03).
Table 3. Statistically significant associations of clinical and pathological variables with disease-specific survival from first transformation.
| Variable/Level | n | Univariate analysis* | Multivariate analysis† | ||
|---|---|---|---|---|---|
|
|
|
||||
| Median survival (months) | P value | HR (95%CI) | p value | ||
| Age at transformation | |||||
| ≤60 years | 19 | 61 | 0.01 | 1.00 (ref) | |
| >60 years | 32 | 25 | 5.61 (1.17–26.8) | 0.03 | |
| Stage at transformation | |||||
| I | 11 | 44 | 0.049 | 1.00 (ref) | |
| II-IV | 40 | 25 | 2.56 (0.56–11.78) | 0.23 | |
| Serum LDH at transformation | |||||
| Normal (≤220) | 27 | 53 | 0.007 | 1.00 (ref) | |
| Elevated (>220) | 17 | 24 | 1.96 (0.70–5.50) | 0.20 | |
| Cell density in skin biopsy at transformation‡ | |||||
| Low | 4 | 26 | 0.02 | ||
| Moderate | 22 | 42 | |||
| High | 16 | 24 | |||
| Follicular mucin at transformation‡ | |||||
| No | 31 | 42 | 0.007 | ||
| Yes | 5 | 24 | |||
| Fibrosis at transformation | |||||
| No | 8 | 21 | 0.03 | 1.00 (ref) | |
| Yes | 33 | 42 | 0.30 (0.09–0.97) | 0.045 | |
| Site of predominant CD30 staining of neoplastic lymphocytes‡ | |||||
| Epidermal | 4 | 11 | 0.02 | ||
| Dermal | 34 | 42 | |||
Clinicopathological variables that did not show statistically significant associations with survival are not shown.
Median survival and p values (from Mantel–Cox log rank test of survival distributions) estimated using the Kaplan–Meier method.
Hazard ratio (95% confidence intervals for hazard ratio) and p values derived from multivariate Cox proportional hazards models.
Inclusion of these variables (each of which had ≤5 patients in one level) resulted in unstable multivariate models. Therefore, they were excluded from the final multivariate model which is shown.
Pathological variables correlating with shorter survival were: absent papillary dermal involvement (8 versus 30 months, p = 0.008); presence of subcutaneous involvement at transformation (19 versus 44 months, p = 0.04); follicular mucin at transformation (24 versus 42 months, p = 0.007; Fig. 2C); the absence of epidermal hyperplasia ever (4 versus 39 months, p < 0.001); and the absence of fibrosis at transformation (21 versus 42 months, p = 0.03; Fig. 2D). Predominance of CD30 in the epidermis rather than dermis was associated with poorer survival (11 versus 42 months, p = 0.02).
In a multivariate model including age, stage, serum LDH and fibrosis at transformation, age >60 was independently associated with poorer survival (HR 5.61, 95%CI 1.17–26.8, p = 0.03), and the presence of fibrosis was independently associated with improved survival (HR 0.30, 95%CI 0.09–0.97, p = 0.0045).
Discussion
Mycosis fungoides is the most common variant of primary CTCL, defined as an uncontrolled proliferation of mature, skin-homing cutaneous T-lymphocyte antigen (CTLA)-expressing, CD4+, CD45RO+ helper T-cells. While rare overall, with a yearly incidence in the US of approximately 2000, MF comprises more than 70% of all primary cutaneous lymphomas, approximately 2% of all lymphomas, and is thought to affect at least 30,000 people in the US alone, at any given time.14
MF usually behaves as an indolent lymphoma, but patients with tumour stage (T3) disease exhibit 5-year survival as low as 45%15 and median survival of 35 months.8,9 Prognosis in MF is typically stratified by stage, which is based on extent of body surface area involvement, type of skin lesions, or presence of extracutaneous involvement. In particular, patients with LCT have been noted to have a generally short median survival of 19–36 months.8,9 In our cohort, the mean age of death was found to be 67.4 years (range 31–90). This represents a loss of 10 years from the average life expectancy for a male in the US in 2009 (average life expectancy 75.5, versus 64.9 years in our cohort), and a loss of 9 years for a woman in the US in 2009 (average life expectancy 80.5 versus 71 years in our cohort).
The idea that LCT signified worsening disease was initially, in part, inferred from similar findings in systemic B-cell lymphoma16 and was sustained by the idea that the large lymphocytes were the histological expression of a transition to an independent malignant lymphoma.17 The definition of LCT has evolved over time: at one point, LCT implied a change in classification of a CTCL to a subtype of large cell lymphoma;4 subsequently, the term was defined as requiring >50% large cells18 or a ‘predominance’ of large cells;19 the current minimum criteria for LCT include the presence of >25% large cells or the presence of microscopic aggregates of large cells.3,18
Recent studies have questioned the assertion that LCT is an independent prognostic variable in patients with MF. No difference in survival was noted in three studies of patients with tumour stage (IIB) LCT.7,10,19 Furthermore, the overall median survival of patients with LCT was found to be 99–100 months.7,10 Regardless, it is clear that there is a poorly defined subset of patients with LCT who have adverse outcomes. Our goal in this study was to attempt to identify potential prognostic factors within the group of patients who have LCT.
In our analysis of 51 MF patients with LCT, we found that potential markers of tumour burden, namely, stage at either first sign of disease or at transformation, site of first transformation in lymph nodes (versus skin), and serum LDH>220 were significantly associated with survival. These findings validate previous studies. Salhany et al., Diamandidou et al. and Greer et al. found significant differences in survival between patients with stage I-IIA versus IIB (T3) disease.3,5,9 More recently, Benner et al. found a significant difference between IIB (T3) versus IV (extracutaneous disease), but not between stages IB versus IIB.10 While our patients with extracutaneous transformation (particularly in the lymph nodes) had a significantly poorer survival (21 versus 39 months, p < 0.001), we also saw a trend (p = 0.06) towards poorer survival for patients with stage II versus stage I disease at transformation.
Our finding that follicular mucin at transformation correlated with adverse outcomes (24 versus 42 month survival, p = 0.007) is novel. As folliculotropism was previously found to be associated with poorer survival7,10 and higher risk of disease progression,7 and non-transformed folliculotropic MF is known to have a poorer survival than non-folliculotropic MF, this finding may warrant further examination. While folliculotropism did not correlate significantly with survival in our group, there was a trend to this effect. Interestingly, we found follicular mucin in five of 36 patients at the time of transformation. In a study of all stage MF, Van Doorn et al. found 10.4% of patients to show follicular mucinosis at the time of diagnosis. In this group, follicular mucinosis was independendly associated with MF-related mortality.20
The expression of CD30 (Ki-1), a marker of lymphocyte activation, in some cases of MF-LCT suggests a shift to an activating phenotype. We found a statistically significant correlation between poorer survival and predominant CD30 localisation in the epidermis rather than dermis (11 versus 42 months, p = 0.02). This seems to align with most previous studies which showed that overall CD30 expression in LCT is associated with a better prognosis.6,9,10,21 Conversely, one study of non-transformed MF showed that increased dermal CD30 correlated with a shorter survival. However, in that study, stage was not included in the analysis,12 and CD30 proportion did not correlate with Ki-67, which also related to survival.
Pathological features including lack of papillary dermal involvement, and subcutaneous involvement at LCT as well as lack of epidermal hyperplasia could be surrogates for pathological tumour stage, which although not significant on overall analysis, was significantly associated with survival in our analysis of ‘chronic’ versus ‘dead of disease’ cases. Other apparently significant prognostic features such as age at either first sign, diagnosis or transformation may reflect vulnerability due to increasing senescence of the immune system with advancing age.22,23 Fibrosis at transformation, epidermal hyperplasia, and ulceration could also be related to T-cell subset and cytokine milieu, and may be surrogates for evidence of long-standing disease (fibrosis, hyperplasia) versus rapidly evolving disease (atrophic epidermis, ulceration).
In our patients, differences in mitotic rate (analysed as a continuous variable, or in a binary fashion comparing >3/5 HPF to <3/5 HPF) only trended towards significance; however, this trend is in keeping with a previous report of Ki-67 association with worse outcome in non-transformed MF,12 although others have shown that in MF, Ki-67 seems to correlate with stage.24,25 Mitotic rate in Sezary syndrome (CTCL) has been shown to be generally low.26
As with previous reports,10 we did not find a statistically significant association of percent large cells within tumour tissues and outcomes. However, we did notice a trend toward significance of % of large cells with outcomes, when we looked at this as both a continuous or binary variable (25–50% versus 75–100%). It is difficult, in the absence of multivariate analysis, to interpret the significance of this trend, particularly without controlling for stage.
It has been noted that limitations in studies of potential prognostic features of transformed MF in the past have included small size (median sample: 22 patients),10 and lack of discrimination of Sezary syndrome and stage distinctions, preventing adequate power or capability for multivariate analysis. These are also often performed at large referral centres, which may result in lead-time bias.10 Furthermore, many such studies3,4,8,19,27 report large percentages of tumour stage MF, hovering around 50%, which could bias findings. In multivariate analysis, we found that age >60 years and absence of fibrosis at transformation were independently associated with poorer survival. However, these findings must be tempered by the limitations of our multivariate model, namely: (1) small numbers of patients in the arms of some variables which, when included, resulted in unstable multivariate models; and (2) differences in sample type and size of patient biopsies, some of which did not always capture features of interest, resulting in missing datapoints and the prohibitive exclusion of a majority of cases from the multivariate models. Studies of larger datasets with complete datapoints are required to validate our findings.
Overall, our results indicate that there are associations of poorer outcomes with specific histopathological features in transformed MF. One feature not previously shown to be significant in transformed MF is the presence of follicular mucin at transformation. Other features include high tumour cell density, subcutaneous involvement, and CD30 epidermal versus dermal predominance. Longer survival was associated with the presence of epidermal hyperplasia ever, and dermal fibrosis at transformation. We confirm the findings of others, that a clinically advanced stage at transformation, and older age and higher stage at transformation, and a serum LDH >220 U/L appear to correlate with shorter survival. Further studies involving larger patient cohorts with multivariate analysis should help to better evaluate the prognostic utility of individual features in clinical practice.
Acknowledgments
We are grateful to Drs Klaus Busam and Ahmet Dogan for editorial suggestions and support.
Footnotes
Conflicts of interest and sources of funding: The authors state that there are no conflicts of interest to disclose.
References
- 1.Wolfe JT, Chooback L, Finn DT, et al. Large-cell transformation following detection of minimal residual disease in cutaneous T-cell lymphoma: molecular and in situ analysis of a single neoplastic T-cell clone expressing the identical T-cell receptor. J Clin Oncol. 1995;13:1751–7. doi: 10.1200/JCO.1995.13.7.1751. [DOI] [PubMed] [Google Scholar]
- 2.Wood GS, Bahler DW, Hoppe RT, et al. Transformation of mycosis fungoides: T-cell receptor beta gene analysis demonstrates a common clonal origin for plaque-type mycosis fungoides and CD30+ large-cell lymphoma. J Invest Dermatol. 1993;101:296–300. doi: 10.1111/1523-1747.ep12365416. [DOI] [PubMed] [Google Scholar]
- 3.Salhany KE, Cousar JB, Greer JP, et al. Transformation of cutaneous T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol. 1988;132:265–77. [PMC free article] [PubMed] [Google Scholar]
- 4.Cerroni L, Rieger E, Hodl S, et al. Clinicopathologic and immunologic features associated with transformation of mycosis fungoides to large-cell lymphoma. Am J Surg Pathol. 1992;16:543–52. doi: 10.1097/00000478-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Greer JP, Salhany KE, Cousar JB, et al. Clinical features associated with transformation of cerebriform T-cell lymphoma to a large cell process. Hematol Oncol. 1990;8:215–27. doi: 10.1002/hon.2900080406. [DOI] [PubMed] [Google Scholar]
- 6.Arulogun SO, Prince HM, Ng J, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112:3082–7. doi: 10.1182/blood-2008-05-154609. [DOI] [PubMed] [Google Scholar]
- 7.Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–9. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 8.Diamandidou E, Colome M, Fayad L, et al. Prognostic factor analysis in mycosis fungoides/Sezary syndrome. J Am Acad Dermatol. 1999;40:914–24. doi: 10.1016/s0190-9622(99)70079-4. [DOI] [PubMed] [Google Scholar]
- 9.Diamandidou E, Colome-Grimmer M, Fayad L, et al. Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood. 1998;92:1150–9. [PubMed] [Google Scholar]
- 10.Benner MF, Jansen PM, Vermeer MH, et al. Prognostic factors in transformed mycosis fungoides: a retrospective analysis of 100 cases. Blood. 2012;119:1643–9. doi: 10.1182/blood-2011-08-376319. [DOI] [PubMed] [Google Scholar]
- 11.Vergier B, de Muret A, Beylot-Barry M, et al. Transformation of mycosis fungoides: clinicopathological and prognostic features of 45 cases. French Study Group of Cutaneous Lymphomas. Blood. 2000;95:2212–8. [PubMed] [Google Scholar]
- 12.Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860–8. doi: 10.1097/PAS.0b013e3181bf677d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–22. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 14.Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch Dermatol. 2007;143:854–9. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 15.Zackheim HS, Amin S, Kashani-Sabet M, et al. Prognosis in cutaneous T-cell lymphoma by skin stage: long-term survival in 489 patients. J Am Acad Dermatol. 1999;40:418–25. doi: 10.1016/s0190-9622(99)70491-3. [DOI] [PubMed] [Google Scholar]
- 16.Chan WC, Dekmezian R. Phenotypic changes in large cell transformation of small cell lymphoid malignancies. Cancer. 1986;57:1971–8. doi: 10.1002/1097-0142(19860515)57:10<1971::aid-cncr2820571015>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Braverman IM. Transformation in cutaneous T-cell lymphoma. J Invest Dermatol. 1993;101:249–50. doi: 10.1111/1523-1747.ep12365124. [DOI] [PubMed] [Google Scholar]
- 18.Dmitrovsky E, Matthews MJ, Bunn PA, et al. Cytologic transformation in cutaneous T cell lymphoma: a clinicopathologic entity associated with poor prognosis. J Clin Oncol. 1987;5:208–15. doi: 10.1200/JCO.1987.5.2.208. [DOI] [PubMed] [Google Scholar]
- 19.Vonderheid EC, Tam DW, Johnson WC, et al. Prognostic significance of cytomorphology in the cutaneous T-cell lymphomas. Cancer. 1981;47:119–25. doi: 10.1002/1097-0142(19810101)47:1<119::aid-cncr2820470120>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.van Doorn R, Van Haselen CW, van Voorst Vader PC, et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol. 2000;136:504–10. doi: 10.1001/archderm.136.4.504. [DOI] [PubMed] [Google Scholar]
- 21.Barberio E, Thomas L, Skowron F, et al. Transformed mycosis fungoides: clinicopathological features and outcome. Br J Dermatol. 2007;157:284–9. doi: 10.1111/j.1365-2133.2007.08008.x. [DOI] [PubMed] [Google Scholar]
- 22.Solana R, Tarazona R, Gayoso I, et al. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–41. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000;21:515–21. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 24.Gambichler T, Bischoff S, Bechara FG, et al. Expression of proliferation markers and cell cycle regulators in T cell lymphoproliferative skin disorders. J Dermatol Sci. 2008;49:125–32. doi: 10.1016/j.jdermsci.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Dummer R, Michie SA, Kell D, et al. Expression of bcl-2 protein and Ki-67 nuclear proliferation antigen in benign and malignant cutaneous T-cell infiltrates. J Cutan Pathol. 1995;22(1):11–7. doi: 10.1111/j.1600-0560.1995.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 26.Marti RM, Pujol RM, Servitje O, et al. Sezary syndrome and related variants of classic cutaneous T-cell lymphoma. A descriptive and prognostic clinicopathologic study of 29 cases. Leuk Lymphoma. 2003;44:59–69. doi: 10.1080/1042819021000054652. [DOI] [PubMed] [Google Scholar]
- 27.Vidulich KA, Talpur R, Bassett RL, et al. Overall survival in erythrodermic cutaneous T-cell lymphoma: an analysis of prognostic factors in a cohort of patients with erythrodermic cutaneous T-cell lymphoma. Int J Dermatol. 2009;48:243–52. doi: 10.1111/j.1365-4632.2009.03771.x. [DOI] [PubMed] [Google Scholar]