Abstract
Spitz tumors represent a group of melanocytic neoplasms that typically affects young individuals. Microscopically the lesions are composed of cytologically distinct spindle and epithelioid melanocytes, with a range in the architectural display or the cells, their nuclear features, and secondary epidermal or stromal changes. Recently, kinase fusions have been documented in a subset of Spitz tumors, but there is limited information on the clinical and pathologic features associated with those lesions. Here, we report a series of 17 patients (9 male, 8 female) with spitzoid neoplasms showing ALK fusions (5 Spitz nevi and 12 atypical Spitz tumors). The patients’ ages ranged from 2 years to 35 years (mean = 17; median = 16). Most lesions were located on the lower extremities and presented clinically as polypoid nodules. All tumors were compound melanocytic proliferations with a predominant intradermal growth. Tumor thickness ranged from 1.1 to 6 mm (mean = 2.9 mm; median = 2.5 mm). The most characteristic histopathologic feature of the tumors (seen in all but two lesions) was a plexiform dermal growth of intersecting fascicles of fusiform melanocytes. All but two tumors were amelanotic. All tumors were strongly immunoreactive for ALK. The ALK rearrangements were confirmed in all cases by fluorescence in situ hybridization (FISH) and the fusion partner was determined by quantitative polymerase chain reaction as TPM3 (tropomyosin 3) in 11 cases and DCTN1 (dynactin 1) in 6 cases. None of the eight tumors, which were analyzed by FISH for copy number changes of 6p, 6q, 9p, or 11q met criteria for melanoma. Two patients underwent a sentinel lymph node biopsy, and in both cases melanocytes nests were found in the subcapsular sinus of the node. Array comparative genomic hybridization of these two tumors revealed no chromosomal gains or losses. In conclusion, our study revealed that Spitz nevi/tumors with ALK rearrangement show a characteristic plexiform morphology and that ALK immunohistochemistry and FISH enables the accurate identification of this morphologic and genetic distinct subset of spitzoid neoplasms.
INTRODUCTION
Spitzoid melanocytic neoplasms include Spitz nevi (benign tumors), so-called “atypical Spitz tumors” (tumors of uncertain malignant potential) and “spitzoid” melanomas (malignant tumors, with metastasizing and lethal potential[1]). They remain a problem area in Dermatopathology because of the difficulty in distinguishing the relatively more common indolent lesions from the rare metastasizing and lethal spitzoid melanoma. However, significant progress has been made over the past decade regarding cytogenetic and/or molecular alterations of these tumors.[2-7] A number of subsets of Spitz tumors have been identified with distinct, clinical, pathologic, and genetic features. They include the HRAS-mutant sclerosing Spitz nevi[2], the BAP1loss Spitz tumors[7-9], and the spitzoid melanomas with homozygous deletions of p16[6].
An important milestone in our understanding of Spitz tumors is the recent discovery that some of them harbor gene fusions involving receptor tyrosine kinases ALK, ROS1, NTRK1, and RET or the serine-threonine kinase BRAF.[10] Such gene rearrangements were found in 72 of 140 (51.4%) spitzoid neoplasms. 14 of these 140 (10%) spitzoid neoplasms showed ALK fusions. All kinase fusions were mutually exclusive and occurred only in tumors without HRAS mutations or loss of BAP1. Kinase fusions were detected across the entire spectrum of Spitz lesions (benign Spitz nevi, atypical Spitz tumors, and rare spitzoid melanomas), which suggests that the fusions likely occur early in the pathogenesis of the tumors and are per se not sufficient for malignant transformation. This observation is analogous to that of mutations in oncogenes (such as BRAF, NRAS, GNAQ and GNA11) commonly found in melanocytic neoplasms.[4, 7, 11, 12]
Currently, it is unknown whether or not any of the translocation-associated Spitz tumors have distinct clinical and/or pathologic features. It is also unknown whether the presence or absence of a kinase fusion of a particular type is associated with a more indolent or aggressive clinical course. In this study, we document clinical findings and describe the spectrum of microscopic features associated with Spitz nevi and atypical Spitz tumors carrying ALK fusions.
METHODS
Case Selection
Eight cases were from the personal consultation files of one of the authors (KJB), eight cases from another author (HK), and one case from a third author (LC). Only cases diagnosed as Spitz nevus or “atypical Spitz tumor”, but not tumors reported as or favored to be malignant melanomas were included in this series. 13 of the 17 analyzed tumors were included in the initial study reporting kinase fusions in spitzoid tumors[10]; case 2, 3, 10 and 11 have not been previously reported.
Light Microscopic Analysis
The following histopathologic parameters were recorded: polypoid silhouette, ulceration, epidermal hyperplasia, tumor thickness, anatomic (Clark) level, tumor mitotic rate (number of mitotic figures in dermal or subcutaneous melanocytes per mm2), plexiform growth pattern, inflammation and the presence of melanin pigment.
Immunohistochemistry
Immunohistochemical studies were performed using 5 μm thick unstained sections of formalin-fixed, paraffin-embedded archival material. We used the ALK antibody (clone D5F3) from Cell Signaling (Danvers, MA) on a Discovery Ultra instrument with a multimer/DAB detection system (Ventana Medical Systems, Inc., Tucson, AZ) with appropriate negative and positive controls.
Quantitative Polymerase Chain Reaction and Sanger Sequencing
RNA was extracted from 20μm thick formalin-fixed, paraffin-embedded tissue sections (High Pure miRNA Isolation Kit, Roche) and was reverse-transcribed with random hexamer primers using the Transcriptor First Strand cDNA Synthesis Kit (Roche). The ALK fusions partners were determined by quantitative polymerase chain reaction (qRT-PCR) using breakpoint spanning primers reported previously.[10] Specific PCR amplicons were only detected with the appropriate combination of primers and template, and not with negative controls. The nucleotide sequence of the PCR products was confirmed by Sanger sequencing.
Fluorescence in situ Hybridization (FISH)
Two sets of fluorescence in situ hybridization (FISH) tests were performed. To confirm the ALK fusions, a commercially available break-apart probe was used according to the manufacturer's protocol (Abbott Molecular, Des Plaines, IL). The probes were hybridized on 5μm-thick tissue sections, and the number and localization of the hybridization signals was assessed in a minimum of 100 interphase nuclei with well-delineated contours. At least 50% of neoplastic cells had to show a split signal to report a rearrangement of a kinase.
The so-called “melanoma FISH test” was used in the work-up of some atypical Spitz tumors to identify chromosomal copy number changes typical of melanoma (Abbott Molecular, Des Plaines, IL). In this test, a set of four probes targeting Ras Responsive Element-Binding Protein-1 (Vysis®LSI® RREB1-Spectrum Red), myeloblastosis (Vysis®LSI® MYB-S Gold), cyclin D1 or chromosome 11q (Vysis®LSI® CCND1-Spectrum Green™), and centromeric enumeration probe control for chromosome 6 (Vysis®LSI® CEP6-Spectrum Aqua) was initially used, followed by a probe set assessing aberrations of chromosome 9p (Vysis®LSI® CDKN2A/CEP 9 FISH probe kit). The protocol used for these FISH tests has previously been described.[13-15] A lesion was considered as having a positive FISH result if any of the following criteria were met (1) gain in 6p25(RREB1) relative to CEP6 greater than 55% or (2) gain in 6p25(RREB1) greater than 29% (2) loss in 6q23(MYB) relative to CEP6 greater than 40%, (3) gain in 11q13(CCND1) greater than 38% or homozygous loss of 9p(CDNK2A) in more than 30% of the tumor cells.
aCGH
Tumor and reference DNA were differentially labeled with dCTP-Cy5 and dCTP-Cy3 (GE Healthcare, Piscataway, NJ) using a Bioprime Array CGH Genomic Labeling Kit (Invitrogen, Carlsberg, CA) according to the manufacturer's instructions. Genome-wide analysis of DNA copy number changes was conducted using an oligonucleotide array containing 180k probes according to the manufacturer's protocol (SurePrint G3 Human CGH Microarray Kit, 1×180k, Agilent, Santa Clara, CA). Slides were scanned using Agilent's microarray scanner G2505B and analyzed using Agilent Genomic Workbench.
RESULTS
Clinical Findings
The group of individuals with ALK-fusion Spitz nevi or tumors included nine male and eight female patients. Their ages ranged from 2 - 35 years of age (mean = 17; median = 16). None of them reported a family history of melanoma. None of the clinical lesions were suspected to be melanoma. All were solitary lesions. They were removed as “irritated nevus”, “atypical nevus”, “angioma” or “verruca”. Six lesions were located on the lower extremities, four were from the trunk and buttocks, three were from the head and neck region, and two lesions were located on the upper extremities (Table 1). The anatomic site was not specified in two cases. Five tumors were reported as Spitz nevus and twelve lesions as atypical Spitz tumor. All patients with this diagnosis had a complete excision of the primary tumor with negative margins. Two patients underwent a sentinel lymph node biopsy, and in both cases nests of spitzoid melanocytes were found in the subcapsular sinus of the node. A complete lymph node dissection was not performed. All patients are alive and well with no evidence of disease at last follow-up. The follow-up ranged from 2 months to 4 years.
Table.
Clinical and pathologic features of patients and their tumors
| Patient | Age/ Gender |
Site | Dx | Breslow Thickness |
Clark Level |
TMR | Polypoid | Pigmentation | Ulcer | Plexiform | Inflammation | IHC- ALK |
FISH- ALK |
Fusion Partner |
Cytogenetics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5/M | Chin | CSN | 2.5 | IV | 0 | Yes | + | + | + | − | + | + | TPM3 | − (FISH) |
| 2 | 25/M | Lt Low Leg | CSN | 4 | III | 0 | Yes | − | − | + | − | + | + | TPM3 | − (FISH) |
| 3 | 11/F | Rt Ant Thigh | CSN | 2.5 | IV | 0 | No | + | − | + | Mild | + | + | TPM3 | − (FISH) |
| 4 | 35/M | Lt Ear | AST | 2 | IV | 0 | Yes | − | − | + | Mild | + | + | TPM3 | − (CGH) |
| 5 | 14/M | Rt Buttock | AST | 3.7 | IV | 3 | Yes | − | − | + | Mild | + | + | TPM3 | ND |
| 6 | 2/M | Lt Arm | AST | 4.5 | IV | 3 | Yes | − | − | + | Mild | + | + | TPM3 | ND |
| 7 | 16/F | Lt Buttock | AST | 2 | IV | 1 | Yes | − | − | + | Mild | + | + | TPM3 | ND |
| 8 | 14/F | NA | CSN | 3.5 | IV | 1 | Yes | − | − | + | Mild | + | + | TPM3 | ND |
| 9 | 17/F | Lt Ankle | AST | 6 | IV | 4 | Yes | − | − | + | − | + | + | TMP3 | ND |
| 10 | 23/F | Rt Toe | AST | 2.5 | V | 1 | No | − | − | + | − | + | + | TPM3 | − (FISH) |
| 11 | 19/F | Lt Abdomen | CSN | 1.8 | IV | 0 | Yes | − | − | + | − | + | + | TPM3 | − (FISH) |
| 12 | 14/M | Rt Ear | AST | 3 | IV | 1 | Yes | − | + | + | Mild | + | + | DCTN1 | ND |
| 13 | 9/F | Rt Lat.Thigh | AST | 4.4 | V | 2 | Yes | − | − | + | Mild | + | + | DCTN1 | − (CGH) |
| 14 | 28/F | Rt Back | AST | 2.5 | IV | 0 | No | − | − | (+) | Mild | + | + | DCTN1 | ND |
| 15 | 9/M | NA | AST | 1.8 | IV | 1 | Yes | − | − | + | Moderate | + | + | DCTN1 | ND |
| 16 | 19/M | Lt Arm | AST | 1.5 | IV | 0 | Yes | − | − | (+) | Mild | + | + | DCTN1 | ND |
| 17 | 35/M | Lt Leg | AST | 1.1 | IV | 0 | No | − | − | + | Mild | + | + | DCTN1 | − (FISH) |
Abbreviations:
M = male; F = female; Dx = diagnosis; CSN = compound Spitz nevus; AST = atypical Spitz tumor; NA = information not available; ND = not done; − = negative or absent; + = positive or present; (+) only focally present; rt = right; lt = left; TPM3 = tropomyosin 3; DCTN1 = dynactin 1; FISH – fluorescence in situ hybridization; CGH = comparative genomic hybridization
Histopathologic Findings
Histopathologic and immunohistochemical findings are documented in Table 1 and illustrated in Figures 1 - 6. All lesions were compound melanocytic proliferations with both junctional and intradermal components. Tumor thickness of all lesions ranged from 1.1 to 6 mm (mean = 2.9 mm; median = 2.5 mm). One tumor was confined to the epidermis and papillary dermis (Clark level III). Thirteen tumors extended into the reticular dermis (Clark level IV) and two into the superficial subcutis (Clark level V). All but two tumors were amelanotic (no detectable melanin pigment on H&E-stained sections). Epidermal hyperplasia was common (13 of 17 cases). Two lesions were focally ulcerated (excoriated).
Figure 1.
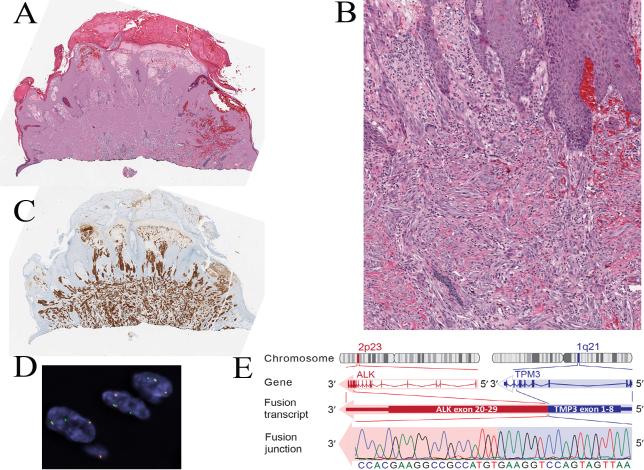
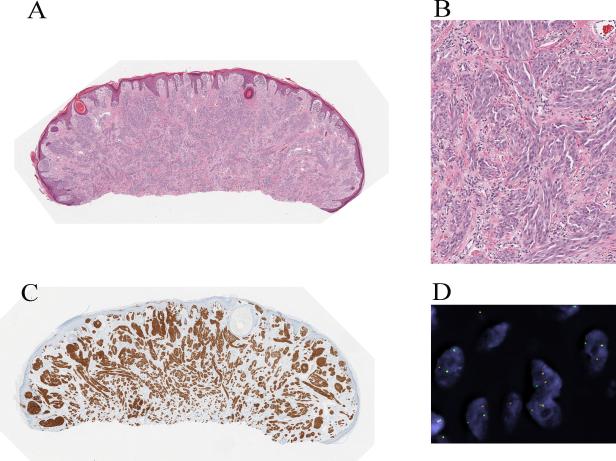
Spitz nevus with a TPM3-ALK fusion from the chin of a 5-year-old boy (case 1). A, Silhouette of an irritated nevus with epidermal hyperplasia and hemorrhagic crust associated with focal ulceration. B, Proliferation of cytologically bland spindle cells (hematoxylin and eosin-stained section). C, The tumor cells are immunoreactive for ALK. D, Fluorescence in situ hybridization (FISH) demonstrates the ALK gene rearrangement by the individual green and orange signals using breakpoint flanking probes. E, TPM3-ALK fusion. ALK is located on chromosome 2p23. Due to genomic rearrangements, exon 1-8 of TPM3 is fused with exon 20-29 of ALK, which contains the tyrosine kinase domain.
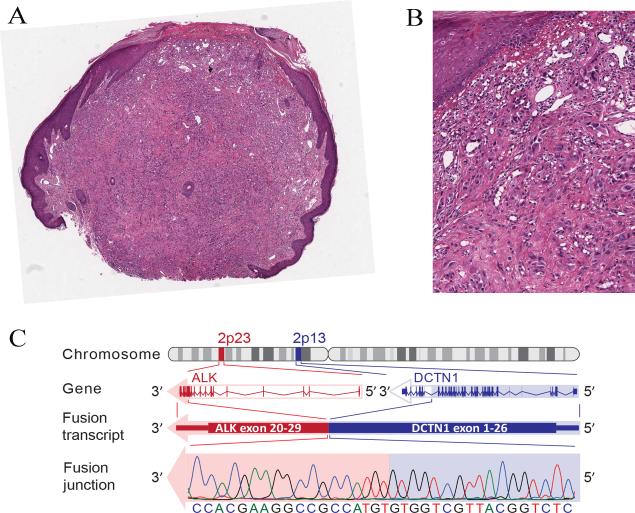
Figure 6.
Compound Spitz tumor with a DCTN1-ALK fusion from the thigh of a 9-year-old girl (case 13). A, Plaque-like intradermal melanocytic proliferation associated with a superficial dermal biopsy-related scar. The lesion shows a plexiform growth of amelanotic spindle cells with bulbous nodular growth into the superficial subcutis (hematoxylin and eosin-stained section). B, The bulbous nodule is composed of a dense proliferation of fusiform melanocytes. C, The melanocytes are immunoreactive for ALK. D, FISH confirms the ALK rearrangement.
Spitzoid melanocytic proliferations with TPM3 (gene for tropomyosin 3) as ALK fusion partner included five Spitz nevi and seven Spitz tumors. Mean and median tumor thickness of lesions with TPM3-ALK fusions was 3.1 mm and 2.5 mm, respectively. All tumors of this series displayed a characteristic plexiform growth pattern of intersecting fascicles of predominantly fusiform melanocytes in the dermis (Figs. 1 - 4, Table 1). The nuclei showed smooth nuclear contours and a slightly vesicular chromatin pattern. Marked pleomorphism was lacking. Two lesions showed prominent dermal sclerosis. Mitoses were absent or rare. Inflammation was absent in five, and mild in six of 11 tumors. One patient with a Spitz tumor harboring a TPM3-ALK fusion underwent a sentinel lymph node (SLN) biopsy. A small cluster of spitzoid melanocytes similar in appearance to the primary tumor was found in the subcapsular sinus of the node (Fig. 4).
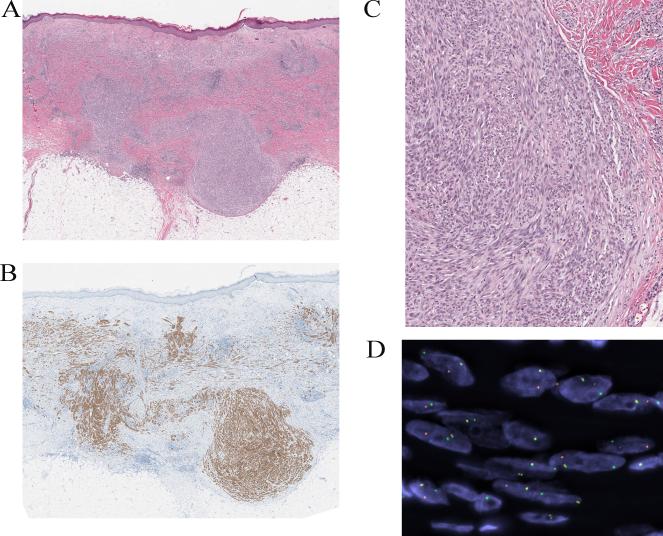
Figure 4.
Compound Spitz tumor with a TPM3-ALK fusion and positive SLN from the ear of a 35-year-old man (case 4). A, Silhouette of a polypoid compound melanocytic proliferation (hematoxylin and eosin-stained section). B, Amelanotic spindle and epithelioid melanocytic proliferation with evidence of maturation. C, The tumor cells are immunoreactive for ALK. D, FISH confirms the ALK rearrangement. E, Spitzoid melanocyte deposits were found in the subcapsular sinus of the sentinel lymph node (immunohistochemical stain for S100 protein).
All spitzoid melanocytic proliferations with DCTN1 (gene for dynactin 1) as ALK fusion partner had been classified as atypical Spitz tumors. Mean and median tumor thickness of lesions with DCTN1-ALK fusions was 2.3 mm and 2.2 mm, respectively. The mitotic rate ranged from 0 to 2/mm2 (mean = 1; median = 1). While five lesions showed a plexiform growth of intersecting fascicles of fusiform melanocytes similar to the Spitz tumors with TPM3-ALK fusions, two tumors were composed of a predominant large epithelioid cell proliferation with enlarged nuclei and nuclear pleomorphism (Fig. 5). These two lesions displayed a plexiform growth pattern only focally. Mild inflammation was observed in five of six tumors, and moderate inflammation in one case. One patient with a Spitz tumor harboring a DCTN1-ALK fusion underwent a sentinel lymph node biopsy with detection of microscopic melanocyte clusters in the subcapsular sinus. This lesion was remarkable for a bulbous nodular growth component in the deep dermis/superficial subcutis (Fig. 6). This lesion was analyzed by array comparative genomic hybridization and no copy number gain or loss was detected.
Figure 5.
Compound Spitz tumor with a DCTN1-ALK fusion from the arm of a 19-year-old man (case 16). A, Polypoid compound melanocytic tumor, focally ulcerated (hematoxylin and eosin-stained section). B, Proliferation of amelanotic predominantly large epithelioid melanocytes with nuclear atypia. C, DCTN1-ALK kinase fusion. ALK is located on chromosome 2p23 and DCTN1 on chromosome 2p13. Due to genomic rearrangements, exon 1-26 of DCTN1 is fused with exon 20 to 29 of ALK, which contains the tyrosine kinase domain. The in-frame junction of the fusion transcript was confirmed with Sanger sequencing.
Immunohistochemical Findings
As implied by the case selection, all lesions were immunohistochemically positive for ALK. Immunoreactivity was strong and homogeneous throughout the entire tumor cell population.
Genetic Findings
Fluorescence in situ hybridization (FISH) with a break-apart probe revealed in all cases an ALK rearrangement. Eight lesions were also analyzed by “Melanoma-FISH” using probes for 6p, 6q, 6cent, 11q, and 9p. None of the lesions met the FISH criteria for melanoma (Table 1). None of the lesions showed homozygous deletions of 9p.
Using qPCR and Sanger sequencing, two types of 5’ fusion partners for ALK were identified: TPM3 (Fig. 1) and DCTN1 (Fig. 5). TPM3-ALK fusions were detected in 11 Spitz lesions (five Spitz nevi and six atypical Spitz tumors). DCTN1-ALK was found in 6 atypical Spitz tumors, and resulted from a balanced translocation between homologous copies of chromosome 2.
Array comparative genomic hybridization (aCGH) was performed retrospectively on lesions from two patients. Both patients had undergone sentinel lymph node biopsy and microscopic clusters of spitzoid melanocyte were detected in the subcapsular sinus of the node. No chromosomal gains or losses were detected by aCGH in both cases.
DISCUSSION
Sophie Spitz reported in 1948 a series of melanocytic tumors (then termed “melanoma of childhood”) composed of spindled or epithelioid melanocytes that developed predominantly in children and adolescents.[16] It subsequently became apparent that these tumors could also arise later in life, and that the majority of these neoplasms behaved in an indolent fashion, which led to the term “Spitz nevus”.[17-19] While criteria have been developed to distinguish Spitz nevi from melanoma, it can at times be very difficult or impossible to make a definitive determination by light microscopic analysis alone, which has led to the category of “borderline” Spitz tumors or spitzoid melanocytic neoplasms of uncertain malignant potential.[1, 17-22]
Given the high stakes of making the correct diagnosis, pathologists have explored the use of ancillary genomic and genetic studies to improve diagnostic accuracy.[2, 3, 23] This effort has not only enriched our understanding of the biology of these tumors, but it has also led to practical applications, such as the increasing utilization of aCGH and/or FISH for improved diagnostic accuracy of spitzoid melanocytic neoplasm.[1, 23, 24] While chromosomal gains or losses cannot be found in most Spitz nevi, as is the case for melanocytic nevi in general, subsets of spitzoid melanocytic neoplasms have been identified with characteristic genomic or genetic aberrations.[2, 25] One example is the sclerosing Spitz nevus, which may carry an increase in 11p and/or a HRAS mutation.[2] Another distinct variant is the large epithelioid Spitz tumor with isolated deletion of 3p and loss of BAP1, which, if present as multiple lesions may suggest a BAP1 germline mutation and be associated with a cancer syndrome.[26]
A recent discovery of cytogenetic abnormalities of spitzoid melanocytic neoplasms is the presence of various types of kinase fusions, involving ROS1, NTRK1, ALK, RET, and BRAF.[10] Current evidence suggests that these fusions occur in a mutually exclusive pattern, do not overlap with HRAS mutations or loss of BAP1, and are found across the biologic spectrum of Spitz lesions. These observations are intriguing as they expand the range of distinct genomic aberrations of spitzoid melanocytic neoplasms.
In this study, we sought to document the morphologic spectrum associated with Spitz nevi/tumors with ALK rearrangements, and to explore a possible association between histopathologic appearance and fusion partner subtype. Spitz nevi/tumors with ALK fusions tended to be polypoid and amelanotic. A plexiform growth of intersecting fascicles of fusiform melanocytes was the most common and characteristic feature. It was seen in all lesions of this series with TPM3-ALK fusions, and in 4 of six tumors with DCTN1-ALK fusions. Lesions with the latter fusion subtype included also tumors with a higher proportion of large epithelioid melanocytes with nuclear pleomorphism. As we report our findings, we want to emphasize that they are preliminary. A larger number of cases will be necessary for a more comprehensive assessment of the association of phenotype with genotype.
An important question is the potential clinical significance of the type of kinase fusion and prognosis. Since the detection of kinase fusions in Spitz tumors is a very recent discovery, there is at this point insufficient knowledge on this issue. While none of the patients of this series experienced a tumor recurrence, the number of cases is too small and currently available follow-up is too short for any meaningful conclusions. On the other hand it may be of interest in this regard to point out that two patients of this series underwent a SLN biopsy after a diagnosis of atypical Spitz tumor was made. Small tumor deposits were found in the SLN of both patients. When retrospectively the primary tumors were analyzed by array CGH, no copy number gains or losses were detected. While clinical follow-up of one patient is less than 1 year, the other patient is alive and well four years after the excision of the primary Spitz tumor and detection of melanocyte deposits in the SLN. That patient had a SLN biopsy only with no subsequent complete lymph node dissection.
In conclusion, herein we present 17 cases of Spitz nevi/tumors with ALK fusions and compare the histopathologic findings with the fusion partner subtype. A plexiform growth pattern of intersecting fascicles of amelanotic spindle cells was found as the most characteristic feature, especially in tumors with TPM3-ALK fusions, but it was not exclusive. Future studies are needed to determine the strength of the association between the light microscopic appearance of a lesion and the type of kinase fusion, and most importantly, to learn more about the biologic and clinical significance of kinase fusions in spitzoid melanocytic neoplasms.
Figure 2.
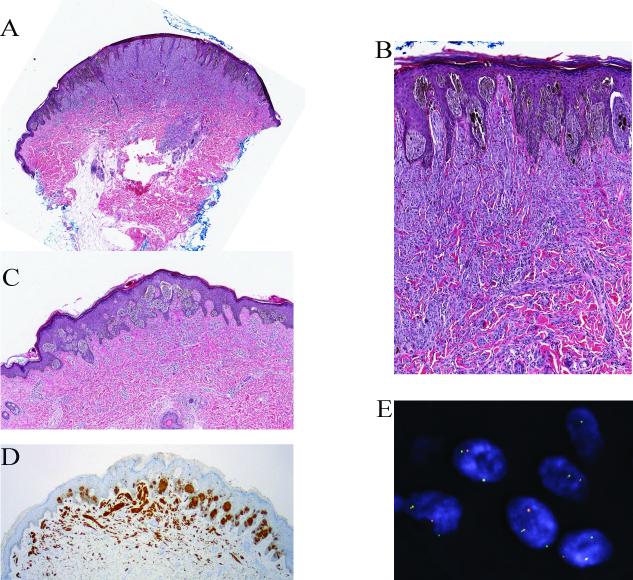
Spitz nevus with a TPM3-ALK fusion from the leg of a 25-year-old man (case 2). A, Silhouette of an amelanotic polypoid compound Spitz nevus (hematoxylin and eosin-stained section) with evidence of maturation. B, Proliferation of cytologically bland spindle and epithelioid melanocytes. C, The tumor cells are positive for ALK. D, FISH confirms the ALK rearrangements using breakpoint flanking probes by the individual green and red signals.
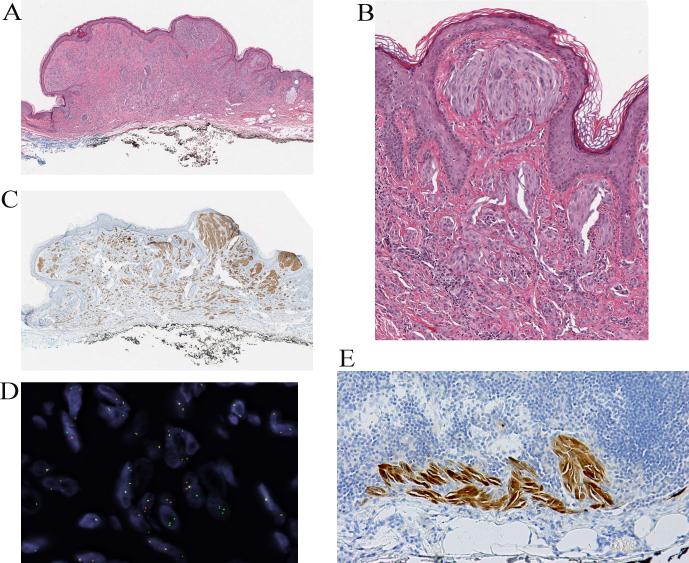
Figure 3.
Partly pigmented compound Spitz nevus with a TPM3-ALK fusion from the thigh of an 11-year-old girl (case 3). A, Wedge-shaped silhouette of a compound spindle cell melanocytic proliferation with epidermal hyperplasia (hematoxylin and eosin-stained section). B, The junctional component shows features of a pigmented spindle cell nevus. C, Deeper section of the lesion, which was adjacent to the section used for immunohistochemistry. The junctional melanocytic proliferation shows a predominant nested pattern and is pigmented. The intradermal melanocytes are amelanotic and display a plexiform growth pattern. D, The tumor cells are positive for ALK in immunohistochemistry. E, FISH confirms the ALK rearrangement.
ACKNOWLEDGEMENTS
This work was funded by grants from the National Institutes of Health P30 CA008748 to MSKCC. Thomas Wiesner is funded the Harry J. Lloyd Trust, the Jubilaeumsfonds of the Oesterreichische Nationalbank, and by a Charles H. Revson Senior Fellowship. The authors thank Dr. Margaret Leversha, Dr. Gouri Nanjangud, Kalyani Chadalavada, and Yuqiang Fang for assisting with FISH. The authors would also like to thank Isla Otap for her assistance in preparing the manuscript.
REFERENCES
- 1.Zedek DC, McCalmont TH. Spitz nevi, atypical spitzoid neoplasms, and spitzoid melanoma. Clin Lab Med. 2011;31(2):311–20. doi: 10.1016/j.cll.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol. 2000;157(3):967–72. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastian BC, et al. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. J Invest Dermatol. 1999;113(6):1065–9. doi: 10.1046/j.1523-1747.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- 4.Fullen DR, et al. BRAF and NRAS mutations in spitzoid melanocytic lesions. Mod Pathol. 2006;19(10):1324–32. doi: 10.1038/modpathol.3800653. [DOI] [PubMed] [Google Scholar]
- 5.Gerami P, Busam KJ. Cytogenetic and mutational analyses of melanocytic tumors. Dermatol Clin. 2012;30(4):555–66. v. doi: 10.1016/j.det.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Gerami P, et al. Risk assessment for atypical spitzoid melanocytic neoplasms using FISH to identify chromosomal copy number aberrations. Am J Surg Pathol. 2013;37(5):676–84. doi: 10.1097/PAS.0b013e3182753de6. [DOI] [PubMed] [Google Scholar]
- 7.Murali R, et al. GNAQ and GNA11 mutations in melanocytomas of the central nervous system. Acta Neuropathol. 2012;123(3):457–9. doi: 10.1007/s00401-012-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busam KJ, et al. Combined BRAF(V600E)-positive melanocytic lesions with large epithelioid cells lacking BAP1 expression and conventional nevomelanocytes. Am J Surg Pathol. 2013;37(2):193–9. doi: 10.1097/PAS.0b013e318263648c. [DOI] [PubMed] [Google Scholar]
- 9.Busam KJ, Wanna M, Wiesner T. Multiple epithelioid Spitz nevi or tumors with loss of BAP1 expression: a clue to a hereditary tumor syndrome. JAMA Dermatol. 2013;149(3):335–9. doi: 10.1001/jamadermatol.2013.1529. [DOI] [PubMed] [Google Scholar]
- 10.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nature Communications. 2014 doi: 10.1038/ncomms4116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Raamsdonk CD, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363(23):2191–9. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk MC, Bernsen MR, Ruiter DJ. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and spitzoid melanoma. Am J Surg Pathol. 2005;29(9):1145–51. doi: 10.1097/01.pas.0000157749.18591.9e. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y, et al. Fluorescence in situ hybridization (FISH) analysis of melanocytic nevi and melanomas: sensitivity, specificity, and lack of association with sentinel node status. Int J Surg Pathol. 2012;20(5):434–40. doi: 10.1177/1066896912445923. [DOI] [PubMed] [Google Scholar]
- 14.Gerami P, et al. Fluorescence in situ hybridization for distinguishing nevoid melanomas from mitotically active nevi. Am J Surg Pathol. 2009;33(12):1783–8. doi: 10.1097/PAS.0b013e3181ba6db6. [DOI] [PubMed] [Google Scholar]
- 15.Gerami P, et al. Fluorescence in situ hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol. 2009;33(8):1146–56. doi: 10.1097/PAS.0b013e3181a1ef36. [DOI] [PubMed] [Google Scholar]
- 16.Spitz S. Melanomas of childhood. Am J Pathol. 1948;24(3):591–609. [PMC free article] [PubMed] [Google Scholar]
- 17.Ackerman AB. Spitz nevus. Am J Dermatopathol. 1997;19(4):419–21. doi: 10.1097/00000372-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Dahlstrom JE, et al. Spitz naevus: diagnostic problems and their management implications. Pathology. 2004;36(5):452–7. doi: 10.1080/00313020412331285318. [DOI] [PubMed] [Google Scholar]
- 19.LeBoit P. Spitz nevus: a look back and a look ahead. Adv Dermatol. 2000;16:81–109. discussion 110. [PubMed] [Google Scholar]
- 20.Barnhill RL. The Spitzoid lesion: rethinking Spitz tumors, atypical variants, ‘Spitzoid melanoma’ and risk assessment. Mod Pathol. 2006;19(Suppl 2):S21–33. doi: 10.1038/modpathol.3800519. [DOI] [PubMed] [Google Scholar]
- 21.Connors RC, Chalet MD, Ackerman AB. Benign juvenile melanoma (Spitz nevus) vs. superficial spreading malignant melanoma: criteria for histologic differentiation. J Dermatol Surg. 1975;1(2):14–5. doi: 10.1111/j.1524-4725.1975.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 22.Spatz A, Barnhill RL. The Spitz tumor 50 years later: revisiting a landmark contribution and unresolved controversy. J Am Acad Dermatol. 1999;40(2 Pt 1):223–8. doi: 10.1016/s0190-9622(99)70192-1. [DOI] [PubMed] [Google Scholar]
- 23.Gerami P, et al. Outcomes of atypical spitz tumors with chromosomal copy number aberrations and conventional melanomas in children. Am J Surg Pathol. 2013;37(9):1387–94. doi: 10.1097/PAS.0b013e31828fc283. [DOI] [PubMed] [Google Scholar]
- 24.McCalmont TH, et al. Molecular-microscopical correlation in dermatopathology. J Cutan Pathol. 2011;38(4):324–6. 323. doi: 10.1111/j.1600-0560.2011.01674_2.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer J, Bastian BC. Distinguishing melanocytic nevi from melanoma by DNA copy number changes: comparative genomic hybridization as a research and diagnostic tool. Dermatol Ther. 2006;19(1):40–9. doi: 10.1111/j.1529-8019.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiesner T, et al. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J Surg Pathol. 2012;36(6):818–30. doi: 10.1097/PAS.0b013e3182498be5. [DOI] [PMC free article] [PubMed] [Google Scholar]