Graphical abstract
Keywords: Fish protein hydrolysates, Salmon backbones, Antioxidant, ACE-inhibition, Glucose transport inhibition
Highlights
-
•
Defatted bulk fish protein hydrolysates (FPH) exhibit many bioactivities.
-
•
Defatted FPH inhibit lipid oxidation.
-
•
Defatted FPH reduce blood pressure and glucose uptake into enterocyte.
-
•
Trypsin, Bromelain + Papain and Protamex lead to the most bioactive hydrolysates.
-
•
Prolonged hydrolysis gives higher bioactivities.
Abstract
Bioactivities of bulk fish protein hydrolysates (FPH) from defatted salmon backbones obtained with eight different commercial enzymes and their combinations were tested. All FPH showed antioxidative activity in vitro. DPPH scavenging activity increased, while iron chelating ability decreased with increasing time of hydrolysis. All FPH showed ACE inhibiting effect which depended on type of enzyme and increased with time of hydrolysis. The highest effect was found for FPH produced with Trypsin. Bromelain + Papain hydrolysates reduced the uptake of radiolabelled glucose into CaCo-2 cells, a model of human enterocytes, indicating a potential antidiabetic effect of FPH. FPH obtained by Trypsin, Bromelain + Papain and Protamex showed the highest ACE inhibitory, cellular glucose transporter (GLUT/SGLT) inhibitory and in vitro antioxidative activities, respectively. Correlation was observed between the measured bioactivities, degree of hydrolysis and molecular weight profiles, supporting prolonged hydrolysis to obtain high bioactivities.
1. Introduction
One third of Norwegian farmed salmon (370 600 ton from total 1 370 500 ton) was considered as rest raw materials in 2015. Viscera and trimmings accounted for about 40% of the rest raw materials, while heads and backbones made up about 8% each [1]. Profitable utilization of fish rest raw materials is an important topic for both research and fish industry. Protein hydrolysates rich in bioactive compounds represent promising ingredients for food and industrial applications. Fish protein hydrolysates (FPH) have been reported to exhibit good functional [2], [3] and nutritional properties [3], [4]. In addition, the hydrolysates exhibit bioactive properties such as antioxidative, antihypertensive, anti-thrombotic and immunomodulatory activities [5]. Bioactive peptides can be produced by endogenous enzymes or using commercial enzymes [6]. In regard to a successful commercialization bulk production is more viable, due to dramatically lowered costs compared to fractionated purified alternatives.
Antioxidative properties could be used to prolong shelf life of supplemented products. Hydrolysates are reported to inhibit radical formation by inhibiting the transition metal catalysed production of free radicals from hydroperoxides [7]. Moreover, through hydrogen donation, free radicals can be directly scavenged by 3–20 amino acids peptides [8]. However, mechanism for hydrolysates cannot be generalised as it can be dependent on several factors like composition, configuration of proteins, the oxidation system (emulsions, other additives presence etc) involved, therefore indicating the need to test the antioxidativity of novel FPH.
Antihypertensive actions of protein hydrolysates are mainly attributed to their inhibition of Angiotensin converting enzyme (ACE), which is a key target for existing pharmaceuticals such as PeptACE®, Vasotensin® [9]. However, these come with side effects and naturally derived ACE inhibitors are desirable to address these problems. These novel ingredients could translate into substantial economic benefits through the reduction of morbidity and mortality from cardiovascular diseases caused by chronic hypertension [12].
Secondary dietary ingredients, such as flavonoids [10] and chromium dinicocysteinate [11] have been shown to limit glucose uptake into intestinal cells through inhibition of the glucose transporter proteins GLUT1, GLUT2 and SGLT1 [10]. Due to potential structural similarities it is feasible to investigate such functions of FPH. Subsequently developed nutraceuticals could be utilized to remedy blood glucose spikes associated with high glycaemic foods. A reduced glycaemic index will contribute to regulated blood glucose levels in the prevention and treatment of diabetes and cardiovascular diseases [12], [13], addressing the obesity and diabetes “epidemic’ and therefore huge commercial and socio-economic potential.
The main aim of this work was to screen for bioactivities in bulk hydrolysates obtained from defatted salmon backbones. The antioxidative properties of FPH were studied to evaluate their potential as natural preservatives or functional ingredients in food products. In addition, the antihypertensive (ACE-inhibitory) and antidiabetic (intestinal glucose transport inhibitory) effects were studied to assess the hydrolysates suitability of the produced as health promoting agents.
2. Materials and methods
2.1. Raw material
Fresh salmon backbones were obtained from a local fish market (Trondheim, Norway) on the same day as the fish was hand filleted. The backbones were kept on ice before processing. The backbones were minced in a HOBART mincer (model AE 200) with holes of 10 mm (in order to follow most accurately the possible industrial mincing process). The minced material was packed in plastic bags (approx. 1 kg per unit) and vacuum packed. This was performed in a cold room at 10 °C and completed in 1 h. The vacuum packed minced raw material were placed into warm water bath for 20 min in order to warm up the raw material to 40 °C (to avoid a large degree of denaturation), then kept at stable temperature for 5 min, then material centrifuged at 2250 × g for 15 min. Then oil fraction was separated by pipetting and the mixture of stick water and sediments (defatted material) were used for hydrolysis.
2.2. Chemicals
The chemicals and solvents used in this study were of analytical or chromatography grade and were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany), Merck KGaA (Darmstadt, Germany) or Fluka Chemie (Buchs, Germany). The following abbreviations for chemicals are used in the text: MES = 2-(N-morpholino)ethanesulfonic acid, ferrozine = 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acids)-1,2,4-triazine, DPPH = 2,2-Diphenyl-1-picrylhydrazyl radical. For glucose uptake experiments, [3H]-methyl-d-glucose (80.0 Ci/mmol) was purchased from PerkinElmer. Human intestinal tumor cells CaCo-2E were purchased from American Type Culture Collection, ATCC. Chemicals were purchased from Sigma and designated with the following abbreviations: DMEM = Dulbecco’s Modified Eagle’s Medium, FBS = Fetal Bovine Serum, HEPES = N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid), CHAPS = 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate hydrate.
2.3. Enzymes
Based on a literature survey with focus on obtained bioactivities (antihypertensivity, antioxidativity and other) [6], [14] and our earlier experience, eight different proteolytic enzymes were selected for enzymatic hydrolysis (Table 1). Corolase® PP and Corolase® 7089 (both from AB Enzymes GmbH), Protamex® (Novozymes A/S), Papain FG and Bromelain 400 GDU/g (both from Enzybel), Trypsin (Sigma Aldrich), Protex 6L (Genecor) and Seabzyme L 200 (Specialty Enzymes & Biotechnologies) were received from the producer.
Table 1.
Enzymes used for the production of salmon backbones hydrolysates.
| Enzyme | Manufacturer | Source | Main activities | Abbreviation |
|---|---|---|---|---|
| Corolase® PP | AB Enzymes GmbH | Pig pancreas glands | Endopeptidase, amino- & carboxypeptidase | C-PP |
| Corolase® 7089 | AB Enzymes GmbH | Bacillus subtilis | Metalloendopeptidase, subtilis neutral proteinase | C-7089 |
| Protamex® | Novozymes A/S | Bacillus licheniformis, Bacillus amyloliquefaciens | Serine endopeptidase, metalloendopeptidase |
Pr |
| Papain 100TU/mg | Enzybel | Carica papaya | Cysteine endoprotease, broad specificity | P |
| Bromelain 400 GDU/g | Enzybel | Ananas comosus | Cysteine endoproteases, broad specificity | Br |
| Protex 6L | Genencor | Bacillus licheniformis | Alkaline serine endopeptidase | P-6L |
| Seabzyme L 200 | Speciality Enzymes & Biotechnologies | Carica papaya | Endoprotease | Sz |
| Trypsin | Sigma-Aldrich | Bovine pancreas | Serine endoprotease | T |
2.4. Enzymatic hydrolysis
The mixture of stick water and sediments after oil separation was used as a raw material for hydrolysis. The hydrolysis was performed in a 4L closed glass vessel placed in a water bath (52 °C) and stirred with a marine impeller (100–150 rpm) in order to ensure homogeneity of the mixture during the whole hydrolysis. Warm (approx. 50 °C) distilled water (1:1 of raw material mass) was added. The enzymatic hydrolysis was started when the temperature of the mixture had reached 50 °C by adding enzyme dosed at 0.1% (w/w of raw material mixture). In the case of combination of enzymes, each enzyme was added at 0.05% + 0.05% (w/w) of raw material mixture. The hydrolysis proceeded for 120 min followed by enzyme inactivation by microwave heating for 5 min at temperature >90 °C. The mixtures were cooled down to room temperature, placed in 50 mL graduated centrifuge tubes (NUNC™) and centrifuged at 6500 × g for 10 min. After centrifugation, the tubes were put upright in a freezer (–80 °C) and all fractions: oil, emulsion, fish protein hydrolysate (FPH) and sediments were separated by cutting the frozen content of the tubes. The FPH fractions were freeze-dried. Two control hydrolyses were performed using Protamex, which is a common industrially used enzyme, as follows: Control 1 (C1): Hydrolysis with raw material without initial separation of oil; Control 2 (C2): Hydrolysis of raw material after initial separation of oil and without addition of water. All hydrolysis, inactivation and separation steps were done as described above. In order to investigate the changes during hydrolysis, representative samples from each hydrolysis were taken at 0 (stick water, taken before addition of commercial enzymes), 20, 40, 60 and 120 min of hydrolysis.
2.5. Composition of hydrolysates
Moisture content was determined gravimetrically after drying for 24 h at 105 °C until constant weight was achieved. Ash content was determined according to AOAC [15]. Total nitrogen (N) was determined by CHN-S/N elemental analyser 1106 (Costech Instruments ECS 4010 CHNSO Analysator) and crude protein was estimated by multiplying total N by a factor of 5.8 [16], [17]. The measurements were performed in four parallels. The Bligh and Dyer [18] method was used for extraction of lipids. The analyses were performed in duplicates.
2.6. Degree of hydrolysis
The degree of hydrolysis (DH) was evaluated as the proportion (%) of α-amino nitrogen with respect to the total N in the sample [19]. Analyses were performed in duplicate.
2.7. Molecular weight distribution
Molecular weight distribution of the hydrolysates was analyzed using gel filtration on a FPLC system. Hydrolysate powder (0.1 g) was dissolved in acetate buffer (4 mL, 0.05 M, pH5). The sample (100 μL) was separated on a Superdex™ Peptide 10/300 GL column, which separates peptides with a molecular weight range from 7000 to 100 Da. The standards used were Cytochrome c (Mw 12400), Aprotinin (Mw 6500), Vit B12 (Mw 1355) and Hippuryl L phenylalanine (Mw 326).
2.8. Determination of free amino acid content (HPLC)
The content of free amino acids in the FPH was determined according to Osnes and Mohr [20]. Protein was precipitated in 2.5% sulfosalicylic acid and the supernatant was diluted using doubly distilled water. The samples were analysed using reversed phase High Pressure Liquid Chromatography (HPLC) (SIL-9A Auto Injector, LC-9A Liquid Chromatograph, RF-530 Fluorescence HPLC Monitor, Shimazdu).
2.9. Metal chelating ability
Ability to chelate Fe2+ was determined as described by Klompong and others [21] with some modifications. One millilitre of protein solution (10 mg/mL) was mixed with 3.7 mL of water. The mixture was reacted with 0.1 mL of 2 mM FeCl2 and incubated at room temperature for 20 min with 0.2 mL of 5 mM 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acids)-1,2,4-triazine (ferrozine). The absorbance was read at 562 nm. The control was prepared in the same manner except that water was used instead of sample. In order to eliminate the absorbance of protein itself, the absorbance of the sample, prepared in the same manner except that water was used instead of the iron solution, was read. Chelating activity (CA, %) was calculated as follows:
2.10. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical scavenging was determined as described by Thiansilakul and others [22] with a slight modification. Proteins were dissolved in water at 0.25% concentration. 1.5 mL of protein solutions were mixed with 1.5 mL of 0.15 mM DPPH in 96% ethanol and allowed to stand at room temperature in the dark for 30 min. The absorbance was measured at 517 nm. In the blank distilled water was used instead of the sample. For the control sample ethanol was used instead of the DPPH ethanol solution. The radical scavenging ability (RSA, %) was calculated as follows:
2.11. Investigation of ACE-inhibiting activity
The ACE-inhibiting activity was investigated by a method based on Vermeirssen and others. [23], as modified by Geirsdottir and others [24] and Hansen [25]. The method is based on the reaction between the enzyme ACE and the substrate FAPGG (N[-3(2-Furyl)acryloyl]-Phe-Gly-Gly) to furylacryloylphenylalanine (FAP) and glycylglycine (GG). This reaction can be inhibited by bioactive peptides found in e.g. FPH. The following reagents were used solution of angiotensin-converting enzyme (Sigma Aldrich), 0.2U/mL (denoted ACE), substrate: 0.5 mM N[-3-(2-Furyl)acryloyl]-Phe-Gly–Gly in 50 mM Tris-HCl buffer containing 300 mM NaCl at pH 7.5 (denoted FAPGG), inhibitor: different concentrations of FPH (freeze dried powder) dissolved in 0.1 M NaCl. As standards, different concentrations of Ala-Tyr (0.1, 0.75 and 1.5 mg/mL) and Val-Trp (0.001, 0.01 and 0.1 mg/mL) were used. The ACE-inhibiting activity of the standards was measured in the same way as described for the hydrolysates. The ACE inhibiting activity (ACEI) of the FPHs was calculated based on the following equation:
| ACEI(%) = (1 − Δsample/Δcontrol) × 100 |
Δsample is the slope of the development of absorbance over time for the reaction mixture containing the inhibitor FPH. Δcontrol is the slope for the corresponding control sample containing distilled water instead of inhibitor solution.
As the assays are performed with three to four different inhibitor concentrations (each concentration was analysed in three parallels), the concentration of the protein hydrolysate needed to inhibit the ACE by 50% (IC50) was determined. The uncertainty was calculated based on equations for error propagation (Gauss error propagation law). Bromelain + Papain hydrolysates obtained after 0, 20, 40, 60 and 120 min hydrolysis, Protamex hydrolysates without defatting and Trypsin hydrolysates on defatted raw materials after 40, 60 and 120 min hydrolysis were analysed for ACE inhibiting effect. Hydrolysates with all other enzymes were used for ACE inhibiting effect test after 120 min hydrolysis.
2.12. Investigation of cellular glucose uptake inhibiting activity
The hydrolysates obtained from 60 min treatment of salmon backbones with Protamex, Seabzyme, Protex 6L, Corolase 7089 and Corolase PP, and from 0, 20, 60 and 120 min treatment with Bromelain + Papain were investigated for their ability to inhibit cellular glucose uptake. Human intestinal tumor cells were cultured in a humidified incubator in an atmosphere of 5% CO2 − 95% air (v/v) at 37 °C. The cells were grown in DMEM (17.5 mM glucose) supplemented with 10% heat-inactivated FBS, and subcultured at confluence by Trypsin treatment.
For glucose transport experiments, cells were seeded onto 96-well plates and grown to confluence. Cells were preincubated with Krebs buffer (glucose 5 mM, HEPES 30 mM, NaCl 130 mM, KH2PO4 4 mM, MgSO4-7H2O 1 mM, CaCl2-2H2O 1 mM, pH 7.4) for 30 min at 37 °C. Transport experiments were initiated by replacing the buffer with Krebs buffer without glucose, containing [3H]-methyl-d-glucose (0.125 μM) and FPH (4 mg/mL). Transport experiments were continued for 15 min at 25 °C and terminated with the addition of ice-cold Krebs buffer. Cells were washed twice with ice-cold Krebs buffer and lysed with 60 μL of NaOH (0.1 M)/CHAPS (10 mg/mL). Concentration effect of the FPH was determined in a separate set of experiments where the transport buffer was supplemented with 1, 4 or 8 mg/mL FPH. 45 μL aliquots of cell lysates were added to 5 mL of scintillation cocktail and the [3H]-methyl-d-glucose concentration was quantified by scintillation counting. Protein content of the remaining cell lysates was determined using DC Protein Assay Kit (Bio-Rad, USA). The uptake of radiolabelled glucose into CaCo-2E cells was expressed as fmol glucose and normalized to the protein content of the cell lysates. Viability of the intestinal Caco-2E cells and validity of the assay was demonstrated by linear uptake rates of radiolabelled glucose over 15 min in the absence of FPH. All data represent means of at least two experimental sets in which the results were consistent. Each of the experimental sets consisted of three parallel transport experiments (n = 6).
2.13. Statistical analysis
Statistical analysis was carried out by general linear model multivariate analysis using SPSS software (version 12.0.1, SPSS Inc., USA). Level of significance was set at p < 0.05 and assessed by Tukey’s test.
3. Results and discussion
3.1. Yield and composition of FPH
Efficiency of enzymatic hydrolysis was evaluated based on the amount of dry material which ends up in fish protein hydrolysate (FPH). Table 2 shows that the first 20 min of hydrolysis led to the most significant increase in FPH yield. Trypsin (T) and Corolase PP (C-PP) increased the yield by more than 100% during the first 20 min of hydrolysis. Solubilisation by other enzymes was also the most intensive during first 20 min and gave 60–70% increase in FPH yield. Further hydrolysis up to 120 min led to slower increase of FPH yield. After 120 min hydrolysis the same tendency was observed: Trypsin, Corolase PP and mixture of Bromelain + Papain (BrP) yielded the highest amount of FPH −12.1, 11.5 and 11.6 g dry FPH/100 g raw material respectively. Four other enzymes (Protamex, Seabzyme, Protex 6L and Corolase 7089) gave similar yield of dried FPH after 120 min hydrolysis. The three of these four last enzymes (except Seabzyme) originate from microorganisms, while the best yields were obtained from digestive or fruits enzymes. Similar results were reported by Aspmo and others [26] where 1 h hydrolysis of cod viscera with Papain yielded higher amounts of hydrolysates compared to hydrolysis with Protamex and Neutrase.
Table 2.
Yield of FPH as a function of hydrolysis time for all studied enzymes and enzyme mixtures as well as for control hydrolysis with Protamex. Values are presented as g dry FPH from 100 g raw material. C-PP: Corolase PP, T: Trypsin, P-6L: Protex 6L, C-7089: Corolase 7089, BrP: Bromelain + Papain, Sz: Seabzyme, Pr: Protamex, Pr-T: Protamex with raw material without thermally separated oil before hydrolysis: control 1-C1, Pr-W: hydrolysis with Protamex without addition of water: control 2-C2. n.a.: not analysed.
| Hydrolysis time, min | C-PP | T | P-6L | C-7089 | BrP | Sz | Pr | Pr-T C1 |
Pr-W C2 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 4.1 ± 0.2 | 5.5 | 2.2 | ||||||
| 20 | 9.4 | 8.3 | 7.1 | 6.7 | 7.1 | 6.6 | 7.1 | n.a. | 3.8 |
| 40 | 9.5 | 10.0 | 7.5 | 7.8 | n.a | 7.6 | 8.2 | n.a. | 4.3 |
| 60 | 10.1 | 10.1 | 8.0 | 8.6 | 7.5 | n.a. | 8.6 | n.a | 4.7 |
| 120 | 11.5 | 12.1 | 8.5 | 9.3 | 11.6 | 9.9 | 9.4 | 11.5 | 3.4 |
Composition of hydrolysates obtained after 120 min hydrolysis is presented in Table 3. The dried hydrolysates contained 83–86% protein, 6.7–12.4% ash and 2.2–18.6% lipids. The initial separation of oil from the raw material before hydrolysis was found to be necessary with respect to the amount of lipids in FPH: Hydrolysates obtained from minced salmon backbones without initial separation of oil (C1) contained significantly more lipids (18.6%) compared to all hydrolysates (2.2-6.2% lipids) obtained after hydrolysis of defatted raw material.
Table 3.
Composition of FPH. Values are presented as average of the measurements (% of dry meterial) with standard deviation of the mean.
| Water ± 0.0% |
Ash ± 0.1% |
Lipids ± 0.0% |
Proteins ± 0.2% |
|||||
|---|---|---|---|---|---|---|---|---|
| Hydrolysis time | 0 | 120 | 0 | 120 | 0 | 120 | 0 | 120 |
| Enzyme | ||||||||
| C-PP | 10.6 | 6.6 | 11.9 | 6.0 | 5.1 | 2.1 | 73.8 | 83.3 |
| T | 7.8 | 3.1 | 11.5 | 6.1 | 2.2 | 1.7 | 79.0 | 85.8 |
| P-6L | 6.9 | 6.2 | 12.4 | 6.7 | 2.9 | 0.8 | 73.8 | 83.8 |
| C-7089 | 10.6 | 4.2 | 11.9 | 5.9 | 5.1 | 1.3 | 73.8 | 83.4 |
| BrP | 7.8 | 3.8 | 11.5 | 6.8 | 2.2 | 1.1 | 79.0 | 83.3 |
| Sz | 6.9 | 8.9 | 12.4 | 6.7 | 2.9 | 1.6 | 73.8 | 82.9 |
| Pr | 8.6 | 3.6 | 10.8 | 6.7 | 6.2 | 4.4 | 73.8 | 83.4 |
| Pr-T: C1 | 5.3 | 5.9 | 6.7 | 5.7 | 18.6 | 9.7 | 73.8 | 78.0 |
| Pr-W: C2 | 8.6 | 4.4 | 10.8 | 6.3 | 6.2 | 1.7 | 73.8 | 83.2 |
3.2. Molecular weight distribution, degree of hydrolysis and free amino acids
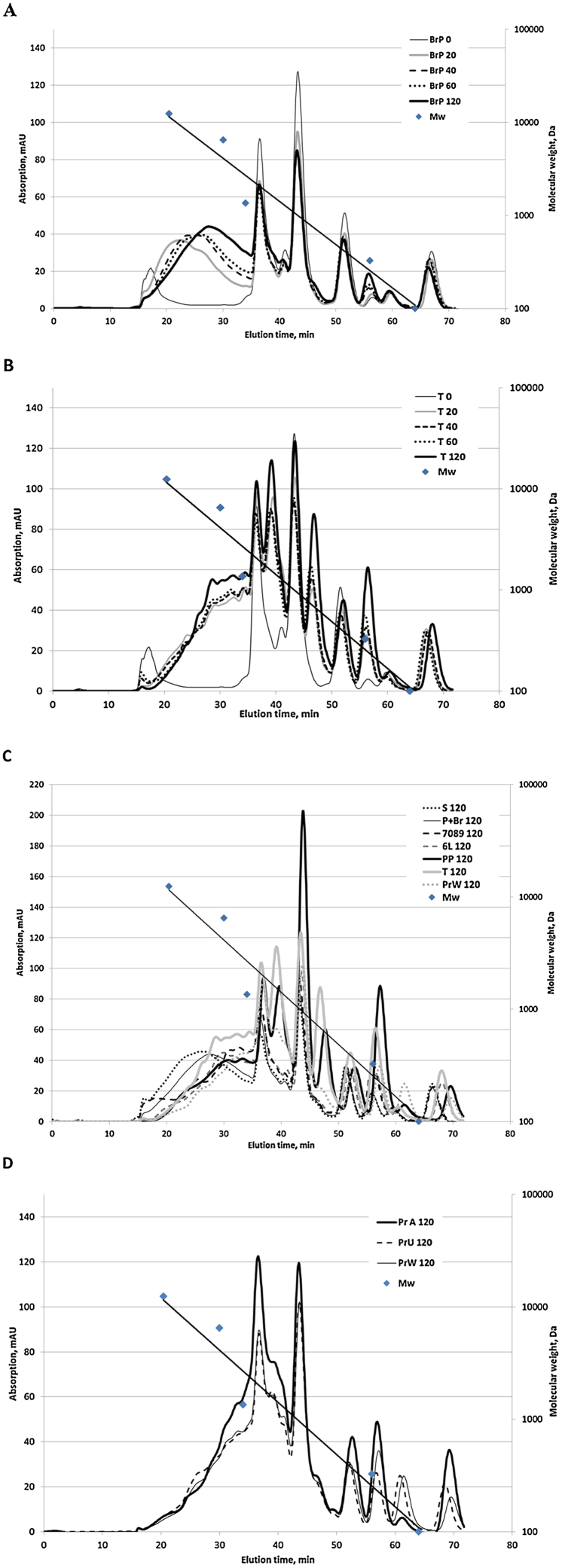
The molecular weight distribution of the FPH reflects the results from FPH yield calculations. Already after 20 min of hydrolysis the amount of large > 10000 Da components started to decrease (Fig. 1A and B). The subsequent hydrolysis increased the amount of smaller (200–500 Da) peptides, but changes were not significant.
Fig. 1.
Molecular weight distribution profiles of the hydrolysates obtained after different hydrolysis time: 20, 40, 60 and 120 min. Hydrolysis with: A) mixture of Bromelain + Papain (BrP); B) Trypsin (T); C) hydrolysates after 120 min hydrolysis; D) Protamex (Pr) after 120 min hydrolysis. Pr-T 120 −hydrolysates obtained without thermal separation of oil, Pr-W 120: hydrolysates of defatted salmon backbones without addition of water, Pr 120: hydrolysates of defatted salmon backbones. Samples “0” stick water sample before the addition of commercial enzymes.
Based on molecular weight distribution, FPH after 120 min of hydrolysis can be divided into three groups (Fig. 1C). Hydrolysates obtained with Trypsin and Corolase PP can be ascribed to the first group: these hydrolysates contained similar amount of peptides with Mw > 2500 Da. Only these two hydrolysates had distinctive peaks representing peptides with molecular weight approx. 1400–1600 and 650–700 Da, containing 12 and 5 amino acids, respectively. Additionally, the hydrolysates after Corolase PP hydrolysis had high yield (Table 2) and degree of hydrolysis (Table 4). The higher amount of free amino acids correlated well with the higher DH (Fig. 1C, Fig. 2). The second group covers hydrolysates after 120 min hydrolysis produced with plant enzymes Seabzyme and with a mixture of Bromelain + Papain. Compared to the other samples, these hydrolysates had more peptides consisting of 42–47 amino acids with molecular size approx. 5500–6500 Da. These two hydrolysates had the lowest degree of hydrolysis (Table 4). Both the DH and yield was slightly higher for the Bromelain + Papain hydrolysate, which also had higher content of free amino acids compared to Seabzyme hydrolysates. Hydrolysates obtained by microbial enzymes (Protex 6L, Protamex and Corolase 7089) belong to the third group. These hydrolysates also have similar DH. Unlike other samples in this group, the Protamex hydrolysate gave a distinctive peak at approx. 140 Da, corresponding to free amino acids. Variation in the hydrolysate molecular weight profiles can be explained by differences in substrate specificity and activity of the studied enzymes at the selected conditions. The size and terminal sequences are important factors affecting peptide bioactivity. Small tri- and dipeptides are less prone to gastrointestinal hydrolysis and thus are expected to be more stable candidates for exerting physiological effects in vivo [27].
Table 4.
Degree of hydrolysis of FPH obtained by use of different enzymes as a function of hydrolysis time, %, n.a.: not analysed. Values are presented as average of the measurements with standard deviation.
| Hydrolysis time, min | C-PP | T | P-6L | C-7089 | BrP | Sz | Pr | Pr-W | Pr-T |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 13.4 ± 0.5 | 8.4 ± 0.1 | |||||||
| 20 | 17.9 ± 0.5 | 14.2 ± 1.8 | 14.5 ± 0.2 | 14.3 ± 0.1 | 13.4 ± 0.1 | 13.0 ± 0.0 | 15.3 ± 0.3 | 15.0 ± 0.1 | n.a |
| 120 | 22.1 ± 0.4 | 18.1 ± 0.2 | 18.2 ± 1.4 | 18.3 ± 0.2 | 16.8 ± 0.1 | 17.1 ± 1.5 | 20.9 ± 0.5 | 21.8 ± 0.7 | 22.6 ± 0.2 |
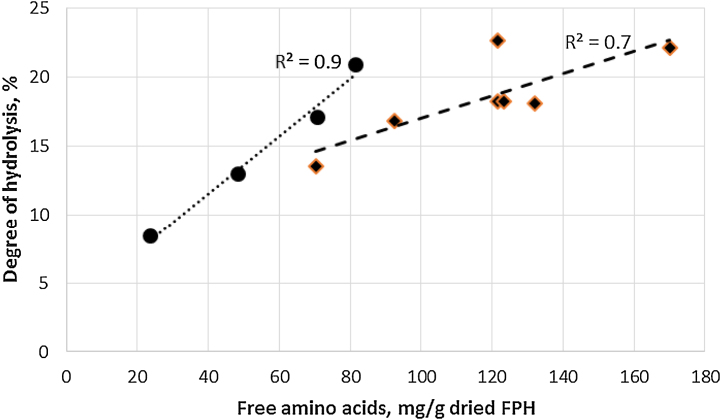
Fig. 2.
Correlation between degree of hydrolysis and amount of free amino acids in the dried FPH after 120 min hydrolysis using different commercial enzymes.
Pre-treatment (thermal separation of oil) and different process conditions (amount of added water) did not significantly influence the molecular size profile of hydrolysates obtained by the use of the same enzyme (Fig. 1D). However, the Protamex hydrolysates produced without thermal pre-treatment (Pr-T) had slightly higher DH than those obtained after pre-treatment (Pr, Pr-W) (Table 4). Thermal pre-treatment of Pr and Pr-W samples may have led to partial inactivation of endogenous enzymes, resulting in lower DH than in Pr-T.
Amount of free amino acids varied from 24 to 170 mg/100 g dry FPH and correlated with degree of hydrolysis (Table 4 and Fig. 2). For some hydrolysates increase in degree of hydrolysis was influenced by increase in the amount of free amino acids, while for others production of short-chain peptides led to higher degree of hydrolysis. Short-chain peptides have been associated with higher bioactivity such as antioxidativity, positive effects for cardiovascular systems [14], [28], and may also be more efficiently absorbed from the digestive system than free amino acids or intact proteins [29]. Therefore, partial hydrolysis using endoproteases is more desirable than the use of proteases giving a high release of amino acids.
3.3. DPPH radical scavenging
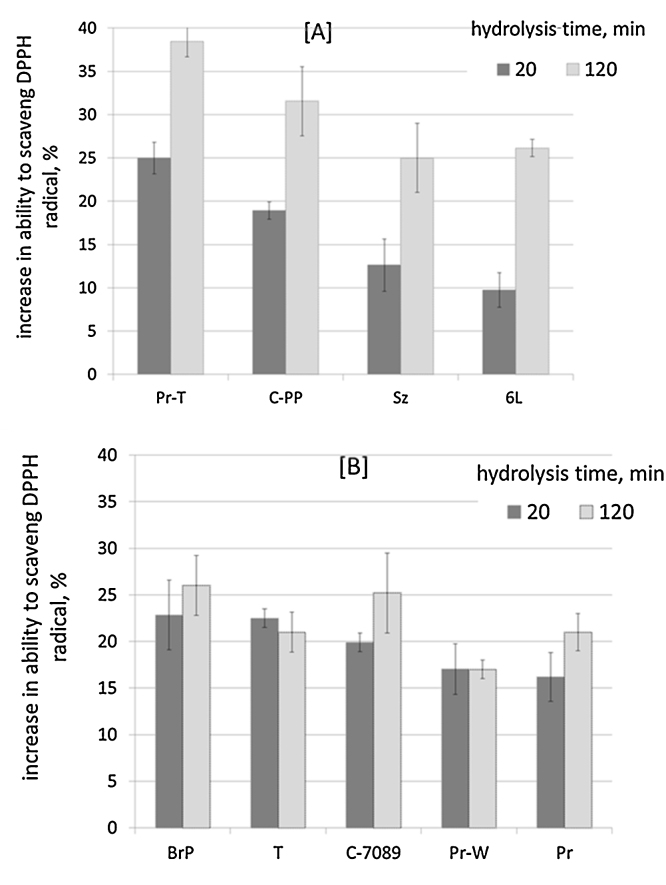
All studied FPH showed antioxidative DPPH radical scavenging ability. All (n = 5) zero samples (stick water before addition of commercial enzymes) showed ∼50% DPPH scavenging effect. Hydrolysis for 20 min led to increased DPPH scavenging effect by 10–25% compared to the DPPH activity of the proteins without hydrolysis (Fig. 3). The highest DPPH scavenging ability was shown in FPH obtained using a mixture of Bromelain + Papain (BrP) and Trypsin (T). FPH with the lowest DPPH scavenging ability were made using Seabzyme (Sz) and Protex 6L (P-6L).
Fig. 3.
Increase in DPPH scavenging ability of proteins from salmon backbones compared to DPPH activity of proteins before hydrolysis as effect of hydrolysis time. Samples: Pr-T: hydrolysis with Protamex (without separation of oil before hydrolysis); C-PP: Coralase PP, Sz: Seabzyme, P-6L: Protex 6L, BrP: Bromelain + Papain mixture, T: Trypsin, C-7089: Coralase 7089, Pr-W: Protamex without addition of water and Pr: Protamex.
By hydrolysing salmon backbones further for 120 min, DPPH scavenging ability of FPH slightly increased for hydrolysates obtained with Protamex (without thermal separation of oil before hydrolysis: Pr-T), Corolase PP (C-PP), Seabzyme (Sz) and Protex 6L (P-6L) (Fig. 3A). However, prolonging the time of hydrolysis (from 20 to 120 min) using Bromelain + Papain mixture (BrP), Trypsin (T), Corolase 7089 (C-7089), and Protamex with and without water (Pr, Pr-W) did not increase DPPH radical scavenging ability (Fig. 3B).
The FPH with the highest scavenging activity were obtained after 120 min hydrolysis using Protamex (without separation of oil before hydrolysis, Pr-T, 38 ± 0.5% increase) and Corolase PP (32 ± 3% increase). These samples also showed the highest degree of hydrolysis (Table 4). Hydrolysis of salmon backbones with Protex 6L or Seabzyme increased radical scavenging activity by 25–26% in comparison to unhydrolysed protein. However, degree of hydrolysis for FPH produced by Protex 6L and Seabzyme were measured 18.2% and 17.1%, respectively. The effect of both enzyme type used for hydrolysis and degree of hydrolysis of the protein hydrolysate was also obtained in other studies [30]. This shows that degree of hydrolysis and size of produced peptides cannot alone predict the DPPH scavenging ability.
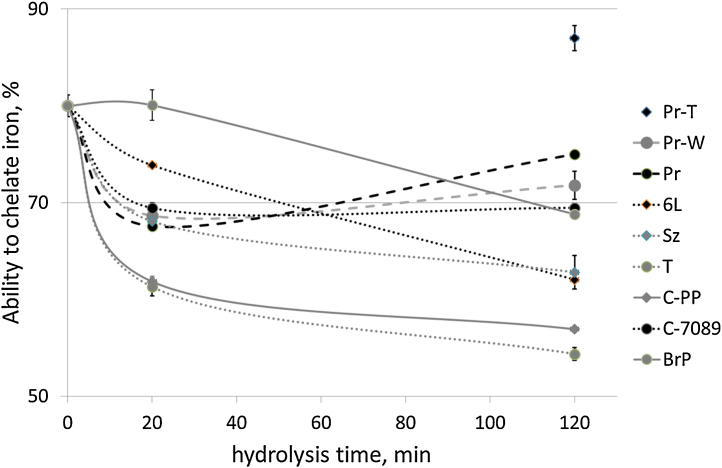
3.4. Iron chelation
Iron chelating ability of the FPH varied from 54 to 87% (Fig. 4). However, the hydrolysates were less effective chelators than casein from milk, which chelated iron by 100% at the same concentration. The water soluble fraction (stick water) obtained from salmon backbones at time 0 showed an iron chelating ability of 80%. Hydrolysis for 20 and 120 min slightly reduced the proteins ability to chelate iron. FPH produced with mixture of Bromelain + Papain (BrP) after 20 min of hydrolysis exhibited the best iron chelating ability. These results indicate that larger peptides have better ability to chelate iron and this property weakens when the peptide size is reduced with hydrolysis time. This is opposite to the DPPH scavenging ability that increased after 20 min of hydrolysis compared to 0 time hydrolysis sample. During hydrolysis formation of smaller peptides increased their ability to scavenge radicals. However, metal chelating ability of proteins is also dependent on the amino acid composition, sequence and protein configuration [7]. Slightly reduced metal chelating ability with increased degree of hydrolysis was also observed by Thiansilakul and others [31] hydrolysing round scad muscle with Flavourzyme. When hydrolysing salmon backbones, changes in the conformation and structure of water soluble peptides or properties of other components may have slightly reduced ability to chelate iron.
Fig. 4.
Ability to chelate iron as a function of hydrolysis time for proteins from salmon backbones. Sample: C-PP: Coralase PP, T: Trypsin, P-6L: Protex 6L, C-7089: Corolase 7089, BrP: Bromelain + Papain, Sz: Seabzyme, Pr: Protamex, Pr-T: Protamex without thermally separated oil before hydrolysis, Pr-W: hydrolysis of deffated salmon backbones with Protamex without addition of water.
Indirect antioxidative activity tests are useful for evaluation and screening of possible antioxidants, but it is also advisable to test the antioxidants in the systems containing lipids. However, very complex systems with transport limitations, containing other additives affecting the oxidation kinetics, could mask the real effect of the compound of interest on the oxidation. The conflicting results from the DPPH radical scavenging and iron chelating ability show that different protein properties are responsible for different antioxidativity mechanisms.
3.5. ACE inhibition
3.5.1. Effect of enzymes used
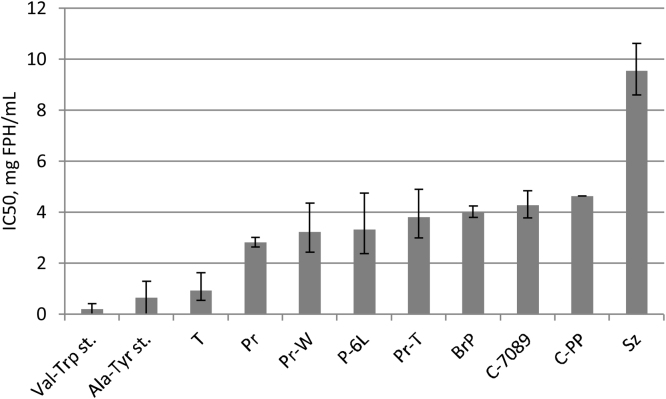
All FPH produced using nine different enzyme combinations with more than 20 min of hydrolysis showed potential ACE-inhibiting activity in vitro. Among the studied enzymes, Trypsin was the most efficient enzyme for production of ACE-inhibiting peptides after 120 min hydrolysis (Fig. 5). Inhibitory activity of the hydrolysates showed an increase with hydrolysis time. Based on their ACE-inhibiting activity after 120 min of hydrolysis, FPHs can be divided into three groups. The first group covers the Trypsin hydrolysate, having similar IC50 value as the standards (Val-Trp and Ala-Tyr) which have documented ACE-inhibiting activity effect [32], [33]. These samples had the best ACE-inhibiting activity with IC50 values of 0.2–0.9 mg/mL (Fig. 5). Most of the hydrolysates fell into the second group with IC50 values of 2.8–4.6 mg/mL. Hydrolysates obtained with Seabzyme belongs to the third group and appears to be the least efficient, displaying an IC50 value 2–3 times larger than the hydrolysates in group 2.
Fig. 5.
The IC50 values of FPHs produced by enzymatic hydrolysis using different commercial enzymes as indicated in the horizontal axis. Hydrolysis time: 120 min.
The differences in IC50 values indicate that ACE-inhibiting effect may vary due to several factors. The ACE-inhibiting activity of the peptides primarily depends on peptide size and amino acid sequence [34]. As the raw material used for the production of FPHs was the same, the differences in IC50 are expected to be due to the enzymes’ different abilities to hydrolyse the raw material into ACE-inhibiting peptides. These may be due to differences in affinity for the substrate, enzyme specificity and differences in optimal conditions.
Trypsin is a major protease in the enzymatic breakdown of food protein and contributes to production of peptides with an average size of 14 amino acids [35], which was confirmed by FPLC analysis (Fig. 1C). This represents a molecular weight that is within the reported optimal range for peptides showing high ACE inhibiting efficiency: 2–20 amino acids [36], [37]. These small peptides most likely account for the high ACE-inhibitory activity of Trypsin hydrolysates. Molecular weight distribution of hydrolysates produced by Trypsin after 120 min hydrolysis (Fig. 1C) shows that these hydrolysates had a different peptide molecular weight profile compared to FPH produced by other enzymes. Hydrolysates after Corolase PP hydrolysis also contained peptides with a similar molecular weight as the hydrolysates from Trypsin hydrolysis, however, ACE-inhibiting activity was lower compared to Trypsin hydrolysates. It is known that Trypsin exclusively cleaves peptide chains at the carboxyl side of arginine or lysine [38], which influences the molecular weight and terminal sequences of the produced peptides. Specifically, Trypsin hydrolysates showed peaks which represent peptides in the range up to 1200 Da. Corolase PP seems to produce similar size peptides. In comparison to the Trypsin hydrolysates, Corolase PP hydrolysates contained higher amount of free amino acids: 170 mg/g FPH (Corolase PP) compared to 132 mg/g FPH (Trypsin), which do not show ACE-inhibiting effects. In addition to the different composition of peptides, this could also be a reason for the lower ACE – inhibitory effects of Corolase PP hydrolysates compared to Trypsin hydrolysates.
Most proteases used for enzymatic hydrolysis work as endopeptidases by cleaving peptide bonds within the protein and generate peptides of various sizes. All enzymes, expect Trypsin and Seabzyme, produced FPHs with similar ACE-inhibiting activity. All FPHs contain larger peptides as well as peptides in the range of 2–20 amino acids (Fig. 1C) which is often associated with ACE-inhibition [39]. Several peptides contribute to the ACE inhibition, but to a different extent. Thus, the overall inhibitory effect of the FPH depends on the relative proportion of active peptides. All the studied enzymes are commercial mixtures and contain several proteases, and are expected to cleave a wide variety of peptide bonds. Even if the FPHs have similar IC50 values, the constituent peptides are not necessarily the same.
Seabzyme had the lowest IC50 value after 120 min hydrolysis (9.5 mg FPH/mL). This difference may be due to larger peptides with higher molecular weights and absence of C-terminal sequences fit for inhibition of ACE. The molecular weight distribution profile of hydrolysate produced with Seabzyme (Fig. 1C) indicates that this hydrolysate contains a large amount of larger peptides and lower amounts of small peptides in the range of 2–20 amino acids, which could explain the low ACE inhibitory effect. The Bromelain + Papain hydrolysate has a much stronger ACE inhibitory effect than the Seabzyme hydrolysate. Compared to the Bromelain + Papain hydrolysate the Seabzyme hydrolysate has a molecular weight distribution that is slightly shifted towards larger peptides (to the left). FPH produced with both enzymes had the lowest degree of hydrolysis (Table 4). These results indicate that the hydrolysate contained higher amount of larger peptides and this could play an important role for the lower ACE inhibitory activity.
3.5.2. Effect of hydrolysis time
The FPHs obtained before addition of commercial enzymes (stick water, or, point 0) did not show any ACE inhibiting effect. These hydrolysates are called stick water fractions since the proteins have not been exposed to hydrolysis by commercial enzymes. The process of heating the raw material (0 °C) and water mixture to 50 °C takes around 30 min. The endogenous enzymes present in the raw material are active during this period and can therefore act on the proteins, but they do not seem to cleave the protein into peptides with ACE-inhibitory activities. The molecular weight distribution of stick water is shown in Fig. 1B: point 0. The characteristic feature of this molecular weight distribution is several peaks in the chromatogram representing relatively small peptides with molecular weight smaller than 1000 Da (Fig. 1). These peaks are also found in all 120 min hydrolysates. Since the stick water does not show any ACE-inhibiting activity and all the 120 min FPHs do, this commonly shared mixture of peptides cannot explain the ACE-inhibiting effect of the 120 FPHs.
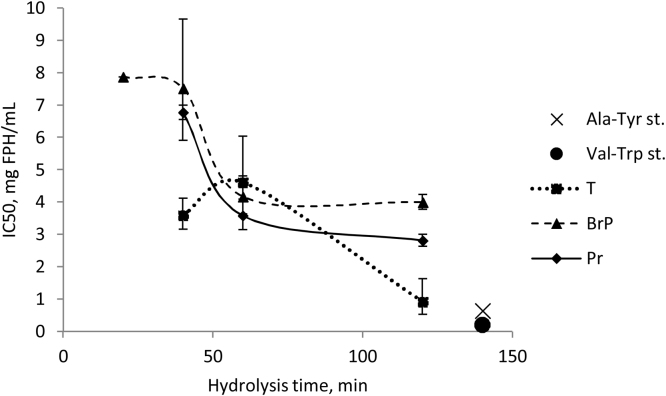
Hydrolysis for 20, 40, 60 and 120 min yielded hydrolysates which all showed ACE-inhibiting effects in vitro. For hydrolysates obtained with Protamex and a mixture of Bromelain + Papain, the largest ACE-inhibiting effect was observed after 120 mins hydrolysis, whereas hydrolysis from 60 min till 120 min did not significantly affect the IC50 value. Conversely, for Trypsin the IC50 value for hydrolysate after 120 min was significantly lower than after 60 min (Fig. 6).
Fig. 6.
The IC50 values of FPHs produced by enzymatic hydrolysis using different commercial enzymes (Trypsin (T), mixture of Bromelain + Papain (BrP) and Protamex (P)) as a function of hydrolysis time. Ala-Tyr st. and Val-Try st. – standards used as controls.
3.6. Inhibition of radiolabelled glucose uptake into CaCo-2E enterocyte monolayers
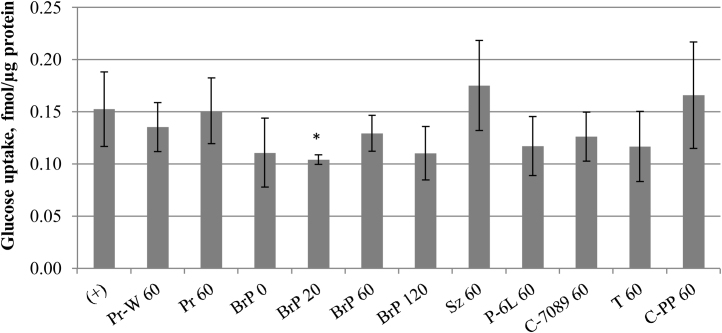
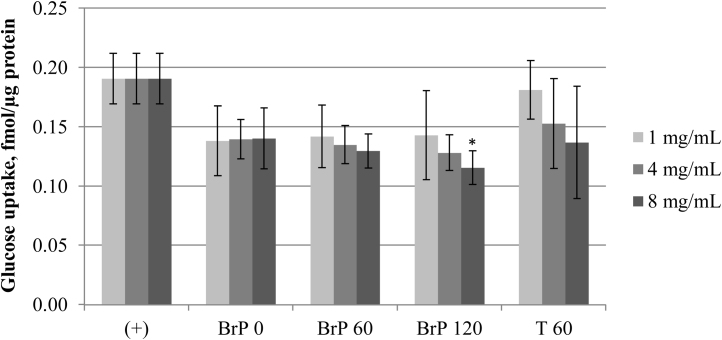
The ability of FPH to reduce cellular glucose uptake was determined in a cell model of human enterocytes. Significant reduction of radiolabelled glucose uptake into CaCo-2E cell monolayers was observed in presence of the hydrolysate treated for 20-min with Bromelain + Papain (BrP 20). Compared to inhibitor-free uptake, 4 mg/mL of BrP 20 reduced the intracellular glucose accumulation by 32% (Fig. 7; p < 0.05). Hydrolysates from longer Bromelain + Papain treatments (BrP 60, BrP 120) showed similar glucose uptake reductions, but the inhibitions were not statistically significant. Hydrolysates produced by other enzymes did not show significant inhibition (Fig. 7). Dose escalation with the Bromelain + Papain hydrolysates proved their effectiveness compared to Trypsin hydrolysates or inhibitor-free uptake (Fig. 8). The BrP 120 showed concentration dependent reduction of radiolabelled glucose accumulation, where the maximum reduction of 39% was observed at 8 mg/mL concentration (Fig. 8). Protein hydrolysates from marine and plant sources have previously been reported to cause antidiabetic effects through inhibition of intestinal digestive enzymes such as α-amylase [40], or inhibition of apoptosis in pancreatic cells [41]. The present study expands the concept that dietary bioactive compounds can blunt blood sugar peaks by inhibiting intestinal glucose absorption [42]. The CaCo-2E cells used in this study highly express both of the main intestinal glucose transporter types, SGLTs and GLUTs, which are inhibited by certain phenolics [43], [44]. Similar to phenolics, specific peptides in the FPH might be hydrophobic in nature to bind the glucose transporter proteins and cause a non-competitive inhibition. The exact mechanism and kinetics remain to be determined in future experiments. The investigated FPH concentrations of 1–8 mg/mL reflect the realistic concentration of peptides in the small intestine [45], providing the rationale for further studies of FPH in vivo.
Fig. 7.
Effect of FPHs on glucose uptake into CaCo-2E cells. 100% glucose uptake (+) was determined in absence of FPHs. Hydrolysis with: Protamex (Pr 60), Protamex without addition of water (Pr-W 60), Seabzyme (Sz 60), Protex 6L (P-6L 60), Corolase 7089 (C-7089 60), Trypsin (T 60) and Corolase PP (C-PP 60) for 60 min, and Bromelain + Papain for 0 min (BrP 0), 20 min (BrP 20), 60 min (BrP 60) and 120 min (BrP 120). Error bars represent standard deviations of 2–9 independent experiments, each consisting of 2–3 replicates (N = 5-26). Statistical significance (p < 0.05) is indicated as “*”.
Fig. 8.
Effect of Bromelain + Papain and Trypsin hydrolysate concentration on glucose uptake into CaCo-2E cells. 100% glucose uptake (+) was determined in absence of FPH. Hydrolysis with: Bromelain + Papain for 0 min (BrP 0), 60 min (BrP 60) and 120 min (BrP 120), and Trypsin for 60 min (T 60). Error bars represent standard deviations of 2–9 experiments, each consisting of 2–3 replicates (N = 5-26). Statistical significance (p < 0.05) is indicated as “*”.
Hydrolysates which were found to possess significant glucose transport inhibiting activity were derived from Bromelain + Papain hydrolysis. Distinct peptide profile produced by the cysteine endoproteases Bromelain and Papain in comparison to the serine endoproteases Trypsin, Protamex, Protex and Corolase 7089 likely explains the different inhibitory activities of the hydrolysates. During Bromelain + Papain hydrolysis the relative proportion of 250–300 Da peptides in the hydrolysates increased while the proportion of other peptides decreased (Fig. 1A), suggesting that these dipeptides could have a role in the regulation of glucose uptake. 300 Da dipeptides were also found in the other hydrolysates (Fig. 1C) but their amino acid composition, which most probably determines the inhibitory activity, was expected to vary depending on the enzyme used.
4. Conclusions
The hydrolysis of thermally defatted salmon backbones yielded fish protein hydrolysates with bioactive properties. All studied FPH showed potential antioxidative properties in the form of DPPH and iron chelating ability. As opposed to the DPPH scavenging ability, intact proteins and larger peptides appeared to have better ability to chelate iron than smaller peptides. FPH also showed ACE-inhibiting effect in vitro. The ACE-inhibiting activity depended on the commercial enzyme used and time of hydrolysis, which determined the peptide sequence and molecular weight. 120-min hydrolysis with Trypsin generated FPH with the highest ACE-inhibiting activity (IC50 = 0.92 mg FPH/mL), which was most probably related to a high content of <1200 Da peptides in this hydrolysate. FPH obtained after hydrolysis with Bromelain + Papain showed inhibitory effects on cellular glucose transport, with max. 39% reduction of glucose uptake into intestinal CaCo-2E cells. Fish protein hydrolysates obtained by Trypsin, Bromelain + Papain and Protamex treatment showed the highest ACE inhibitory, GLUT/SGLT inhibitory and in vitro antioxidative activities, respectively. Correlation was observed in the measured bioactivities, degree of hydrolysis and molecular weight profiles, supporting prolonged hydrolysis of at least 120 min for increased DPPH radical scavenging and ACE-inhibiting activity.
5. Conflict of interest
None.
Acknowledgments
The research leading to these results has received funding from the European Community’s Seventh Framework Programme FP7/2007-2013 under grant agreement No. 289170–APROPOS.
References
- 1.Richardsen R., Nystøyl R., Strandheim G., Viken A. SINTEF Fisheries and Aquaculture; Trondheim: 2015. Analyse marint restråstoff, 2014: Analyse av tilgang og anvendelse for marint restråstoff i Norge. (Report no. A 26863) [Google Scholar]
- 2.Kristinsson H.G. Aquatic food protein hydrolysates. In: Shahidi F., editor. Maximising the Value of Marine By-Products. Woodhead Publishing Ltd; 2007. [Google Scholar]
- 3.Shahidi F., Han X.Q., Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–293. [Google Scholar]
- 4.Slizyte R., Dauksas E., Falch E., Storro I., Rustad T. Characteristics of protein fractions generated from hydrolysed cod (Gadus morhua) by-products. Process Biochem. 2005;40:2021–2033. [Google Scholar]
- 5.Kim S.K., Mendis E. Bioactive compounds from marine processing byproducts – a review. Food Res. Int. 2006;39:383–393. [Google Scholar]
- 6.Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J. Food Sci. 2012;77:11–24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 7.Elias R.J., Kellerby S.S., Decker E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008;48:430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- 8.Ngo D.H., Wijesekara I., Vo T.S., Ta Q.V., Kim S.K. Marine food-derived functional ingredients as potential antioxidants in the food industry: an overview. Food Res. Int. 2011;44:523–529. [Google Scholar]
- 9.Li G.H., Le G.W., Liu H., Shi Y.H. Mung-bean protein hydrolysates obtained with alcalase exhibit angiotensin I-converting enzyme inhibitory activity. Int. J. Food Sci. Technol. 2005;11:281–287. [Google Scholar]
- 10.Song J., Kwon O., Chen S.L., Daruwala R., Eck P., Park J.B., Levine M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J. Biol. Chem. 2002;277:15252–15260. doi: 10.1074/jbc.M110496200. [DOI] [PubMed] [Google Scholar]
- 11.Jain S.K., Croad J.L., Velusamy T., Rains J.L., Bull R. Chromium dinicocysteinate supplementation can lower blood glucose, CRP, MCP-1, ICAM-1, creatinine, apparently mediated by elevated blood vitamin C and adiponectin and inhibition of NF kappa B, Akt, and Glut-2 in livers of zucker diabetic fatty rats. Mol. Nutr. Food Res. 2010;54:1371–1380. doi: 10.1002/mnfr.200900177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloomgarden Z.T. Diabetes and cardiovascular disease. Diabetes Care. 2011;34:24–30. doi: 10.2337/dc11-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone M.T., Veves A. 2nd ed. Humana Press; Totowa, NJ: 2005. Diabetes and Cardiovascular Disease. [Google Scholar]
- 14.Opheim M., Slizyte R., Sterten H., Provan F., Larssen E., Kjos N.P. Hydrolysis of Atlantic salmon (Salmo salar) rest raw materials-Effect of raw material and processing on composition, nutritional value, and potential bioactive peptides in the hydrolysates. Process Biochem. 2015;50:1247–1257. [Google Scholar]
- 15.AOAC . Association of Official Analytic Chemists; Washington DC, USA: 1990. Official Methods of Analysis. [Google Scholar]
- 16.Sosulski F.W., Imafidon G.I. Amino-acid-composition and nitrogen-to-protein conversion factors for animal and plant foods. J. Agric. Food Chem. 1990;38:1351–1356. [Google Scholar]
- 17.Gnaiger E., Bitterlich G. Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stochiometric concept. Oecologia. 1984;62:289–298. doi: 10.1007/BF00384259. [DOI] [PubMed] [Google Scholar]
- 18.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Taylor W.H. Formol titration: an evaluation of its various modifications. Analyst. 1957;82:488–498. [Google Scholar]
- 20.Osnes K.K., Mohr V. Peptide hydrolases of Antarctic krill, Euphausia superba. Comp. Biochem. Physiol. 1985;82B:599–606. [Google Scholar]
- 21.Klompong V., Benjakul S., Kantachote D., Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. [Google Scholar]
- 22.Thiansilakul Y., Benjakul S., Shahidi F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J. Food Biochem. 2007;31:266–287. [Google Scholar]
- 23.Vermeirssen V., Van Camp J., Verstraete W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J. Agric. Food Chem. 2002;51:75–87. doi: 10.1016/s0165-022x(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 24.Geirsdottir M., Sigurgisladottir S., Hamaguchi P.Y., Thorkelsson G., Johannsson R., Kristinsson H.G. Kristjansson MM: Enzymatic hydrolysis of blue whiting (Micromesistius poutassou); functional and bioactive properties. J. Food Sci. 2011;76:14–20. doi: 10.1111/j.1750-3841.2010.01877.x. [DOI] [PubMed] [Google Scholar]
- 25.Hansen E. Norwegian University of Science and Technology, Department of Biotechnology; Trondheim: 2011. Bioaktive peptider i fisk: Med fokus på silderogn (Bioactive peptides in fish: with focus on herring roe) [Google Scholar]
- 26.Aspmo S.I., Horn S.J., Eijsink V.G.H. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 2005;40:1957–1966. [Google Scholar]
- 27.Gomez-Ruiz J.A., Ramos M., Recio I. Identification of novel angiotensin-converting enzyme-inhibitory peptides from ovine milk proteins by CE-MS and chromatographic techniques. Electrophoresis. 2007;28:4202–4211. doi: 10.1002/elps.200700324. [DOI] [PubMed] [Google Scholar]
- 28.Wu H.C., Chen H.M., Shiau C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36:949–957. [Google Scholar]
- 29.Aachary A.A., Thiyam U. A Pursuit of the functional nutritional and bioactive properties of canola proteins and peptides. Crit. Rev. Food Sci. Nutr. 2012;52:965–979. doi: 10.1080/10408398.2010.516033. [DOI] [PubMed] [Google Scholar]
- 30.Bougatef A., Nedjar-Arroume N., Manni L., Ravallec R., Barkia A., Guillochon D., Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010;118:559–565. [Google Scholar]
- 31.Thiansilakul Y., Benjakul S., Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103:1385–1394. [Google Scholar]
- 32.Sato M., Hosokawa T., Yamaguchi T., Nakano T., Muramoto K., Kahara T., Funayama K., Kobayashi A., Nakano T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002;50:6245–6252. doi: 10.1021/jf020482t. [DOI] [PubMed] [Google Scholar]
- 33.Lin F., Chen L.A., Liang R., Zhang Z.F., Wang J.B., Cai M.Y., Yong L. Pilot-scale production of low molecular weight peptides from corn wet milling byproducts and the antihypertensive effects in vivo and in vitro. Food Chem. 2011;124:801–807. [Google Scholar]
- 34.Murray B.A., FitzGerald R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: biochemistry, bioactivity and production. Curr. Pharm. Des. 2007;13:773–791. doi: 10.2174/138161207780363068. [DOI] [PubMed] [Google Scholar]
- 35.Burkhart J.M., Schumbrutzki C., Wortelkamp S., Sickmann A., Zahedi R.P. Systematic and quantitative comparison of digest efficiency and specificity reveals the impact of trypsin quality on MS-based proteomics. J. Proteomics. 2012;75:1454–1462. doi: 10.1016/j.jprot.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Wilson J., Hayes M., Carney B. Angiotensin-I-converting enzyme and prolyl endopeptidase inhibitory peptides from natural sources with a focus on marine processing by-products. Food Chem. 2011;129:235–244. doi: 10.1016/j.foodchem.2011.04.081. [DOI] [PubMed] [Google Scholar]
- 37.Li G.H., Le G.W., Shi Y.H., Shrestha S. Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004;24:469–486. [Google Scholar]
- 38.Olsen J.V., Ong S.E., Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteomics. 2004;3:608–614. doi: 10.1074/mcp.T400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Ryan J.T., Ross R.P., Bolton D., Fitzgerald G.F., Stanton C. Bioactive peptides from muscle sources: meat and fish. Nutrients. 2011;3:765–791. doi: 10.3390/nu3090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alu'datt M.H., Ereifej K., Abu-Zaiton A., Alrababah M., Almajwal A., Rababah T., Yang W. Anti-oxidant, anti-diabetic, and anti-hypertensive effects of extracted phenolics and hydrolyzed peptides from barley protein fractions. Int. J. Food Prop. 2012;15:781–795. [Google Scholar]
- 41.Huang F.J., Wu W.T. Study on the antidiabetic mechanism of a shark liver peptide, S-8300, in alloxan-induced diabetes. J. Pharm. Pharmacol. 2009;61:789–794. doi: 10.1211/jpp/61.06.0012. [DOI] [PubMed] [Google Scholar]
- 42.Kwon O., Eck P., Chen S.L., Corpe C.P., Lee J.H., Kruhlak M., Levine M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007;21:366–377. doi: 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- 43.Mahraoui L., Rodolosse A., Barbat A., Dussaulx E., Zweibaum A., Rousset M., Brot-Laroche E. Presence and differential expression of Sglt1, Glut1, Glut2, Glut3 and Glut5 hexose-transporter messenger-RNAs in Caco-2 cell clones in relation to cell-growth and glucose consumption. Biochem. J. 1994;298:629–633. doi: 10.1042/bj2980629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrell T.L., Ellam S.L., Forelli T., Williamson G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: interactions with SGLT1 and GLUT2 transporters. Biofactors. 2013;39:448–456. doi: 10.1002/biof.1090. [DOI] [PubMed] [Google Scholar]
- 45.Nixon S.E., Mawer G.E. The digestion and absorption of protein in man. Br. J. Nutr. 1970:241–258. doi: 10.1079/bjn19700024. [DOI] [PubMed] [Google Scholar]