Abstract
Analysis of endogenous metabolites in biological samples may lead to the identification of biomarkers in metabolomics studies. To achieve accurate sample analysis, a combined method of continuous quick sampling and extraction is required for online compound detection. Solid-phase microextraction (SPME) integrates sampling, extraction and concentration into a single solvent-free step for chemical analysis. SPME has a number of advantages, including simplicity, high sensitivity and a relatively non-invasive nature. In this article, we reviewed SPME technology in in vitro and in vivo analyses of metabolites after the ingestion of herbal medicines, foods and pharmaceutical agents. The metabolites of microorganisms in dietary supplements and in the gastrointestinal tract will also be examined. As a promising technology in biomedical and pharmaceutical research, SPME and its future applications will depend on advances in analytical technologies and material science.
Keywords: Solid-phase microextraction, endogenous metabolite, metabolomics, pharmaceuticals, medical diagnosis, microorganisms
1. Introduction
Metabolomics systematically investigates the biochemical processes that produce endogenous metabolites in a given organism. Detecting metabolites in biological samples is critical for interpreting health status, medical diagnostics, disease conditions and treatment outcomes [1,2]. For example, cancer is characterized by the abnormal growth of malignant cells beyond their natural boundaries. A successful search for cancer-related biomarkers would contribute to the early diagnosis of malignancy [2]. Early stage melanoma can be detected by volatile organic compounds (VOCs) in the skin [3].
The key technical difficulty to achieving online accurate sample analysis is the establishment of a continuous combined sampling and extraction method [1,4,5]. SPME has a number of advantages: (1) the small sampling needle is relatively nonintrusive and is cost-effective, (2) the coated material on the needle can be diverse, allowing for a wide range of applications, and (3) the two-step integration method for sampling and sample preparation is convenient and time-efficient. SPME also reduces potential extraction or operational errors. Thus, the use of this stable apparatus can achieve identical analytical results with minimal injury to laboratory animals [6-9].
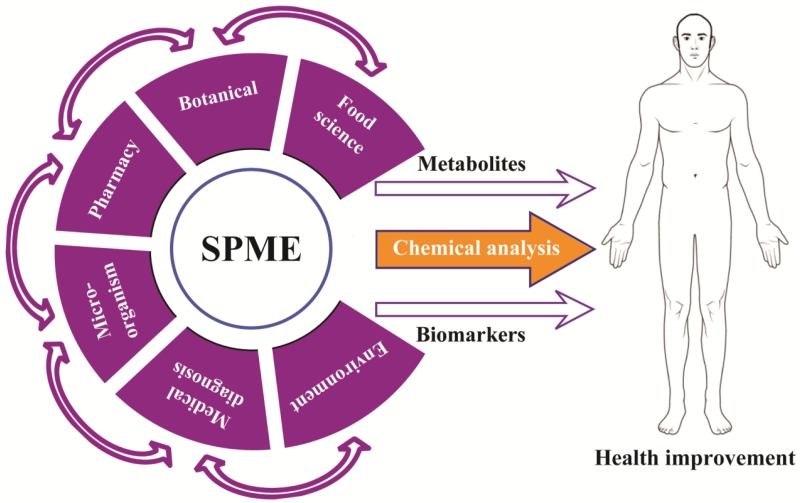
In this article, we will describe the SPME methodology for the analysis of metabolites after the ingestion of herbal medicines, foods and other nutrients, and pharmaceuticals. Then the application of SPME in pharmaceutical research and medical diagnosis will be reviewed in detail. The metabolites of microorganisms in dietary supplements and in the gastrointestinal tract will also be examined (Fig. 1).
Fig. 1.
Application of solid-phase microextraction (SPME) technology. This technique has been used in food science research, botanical component analysis, and pharmaceutical studies. In addition, the SPME has also been reported in research related to microorganisms, environmental factors, and medical diagnosis. The small endogenous molecules detected using SPME investigations are critical for metabolomic analysis and biomarker identification to improve human health.
2. Solid-phase microextraction (SPME)
2.1 Conventional sample extractions and their limitations
The first extraction technique was liquid-liquid extraction (LLE), a time-consuming, labor-intensive and multi-stage operation [4]. During the concentration of solution, LLE can introduce errors or lose its specificity for analyzing VOCs. To overcome these limitations, solid-phase extraction (SPE) was introduced [10]. SPE, however, also needs a sample concentration step, in which VOCs could be lost. Other common problems of SPE include material aggregation, percolation, and channeling formation [10,11]. To overcome the limitations inherent in LLE and SPE, SPME was introduced by Arthur and Pawliszyn in 1989 [12].
2.2 Advantages of SPME and its potential development
SPME integrates sampling, extraction, concentration and sample introduction into a single, solvent-free step [13,14]. SPME saves preparation time by producing a concentrated extract, applicable for direct MS analysis with high accuracy and low operational and disposal cost [4,15,16]. The advantages of SPME have become well-recognized, and many research articles, book chapters and books on the technology have been published [1,17].
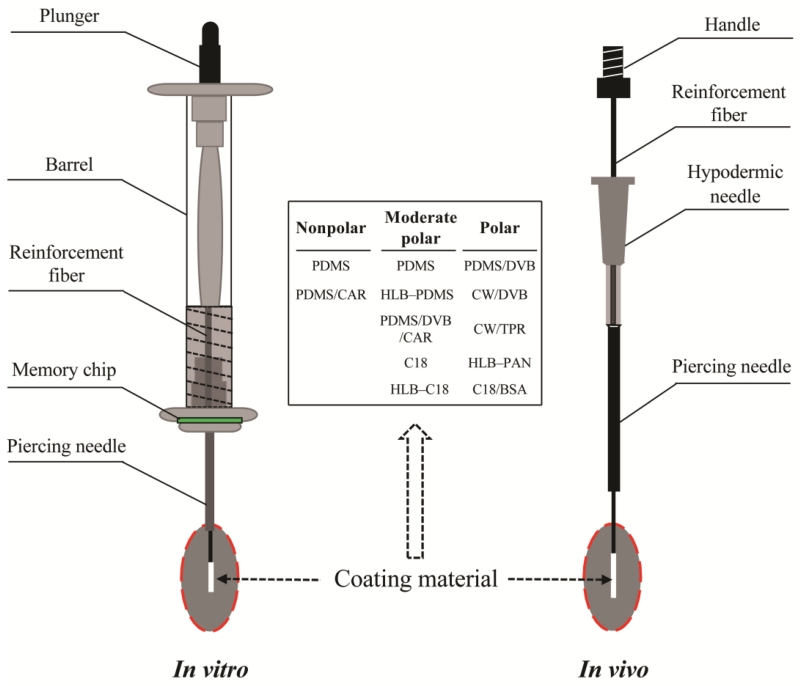
A key driving force of the development of SPME is the progress in the coating material technology [18]. Fig. 2 is a schematic diagram of the in vitro and in vivo SPME needle apparatuses. The commonly used needle-coating chemicals are polydimethylsiloxane (PDMS), carboxen (CAR), hydrophilic lipophilic balanced (HLB), divinylbenzene (DVB), octadecylsilyl derivatized silica column packing material (C18), carbowax (CW), templated resin (TPR), polyacrylonitrile (PAN), benzenesulfonic acid (BSA) [1,4,15]. Coating chemicals with different thicknesses and polarities are now commercially available [4,16]. For example, SPME coating fibers can be characterized with a DNA aptamer for selective enrichment of a low abundance protein from diluted human plasma, and with antibody-linked immunoaffinity sorbents for diagnosing methicillin-resistant Staphylococcus aureus [19,20].
Fig. 2.
Schematic diagram of the in vitro and in vivo SPME needle apparatuses for metabolite analysis. The tips of the needles can be covered by various coating materials. PDMS, polydimethylsiloxane; CAR, carboxen; HLB, hydrophilic lipophilic balanced; DVB, divinylbenzene; C18, octadecylsilyl derivatized silica column packing material; CW, carbowax; TPR, templated resin; PAN, polyacrylonitrile; BSA, benzenesulfonic acid.
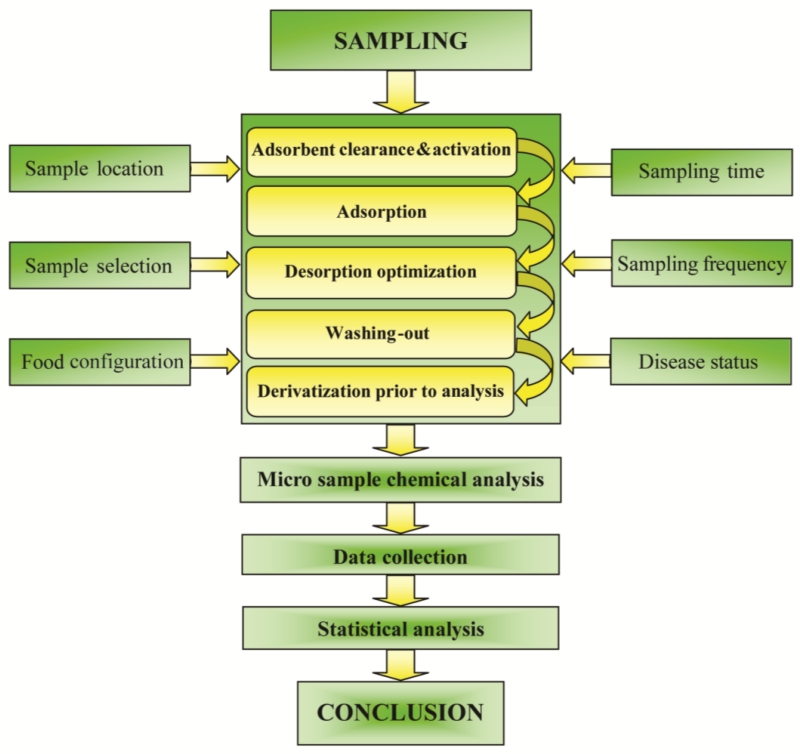
Fig. 3 is a flow chart of the analytical process using SPME as sample preparation technology. The coating is cleaned firstly to remove contaminants, which prevents a high background in the chromatogram. Subsequently, absorption, desorption optimization, a washing-out process and derivatization are performed [4,21]. The sampling processes can be affected by several factors, such as sample location, selection and time [1,4,22]. Data collection and statistical analysis are continuously implemented until a conclusion is obtained [1,4]. During the analysis process, SPME can be matched with GC or LC chromatography methods and MS or UV detection methods [21,23,24]. For instance, the detection of VOCs in the blood stream using SPME-GC/MS provides accurate results, and metabolic biomarkers could be found to screen colorectal cancer patients [25]. The sensitive, selective and reproducible in-tube SPME-LC/UV can also be used to analyze lidocaine and its metabolites in human plasma for anesthesiology research [26]. SPME can be considered a new sample preparation method for global metabolomics studies from living biological samples, supported by newly developed analytical techniques [27].
Fig. 3.
Flow chart of the analytical process using SPME as sample preparation technology. The sampling is influenced by six factors, which are listed on the right and left.
3. SPME applications for metabolite analysis
3.1 In vitro analysis
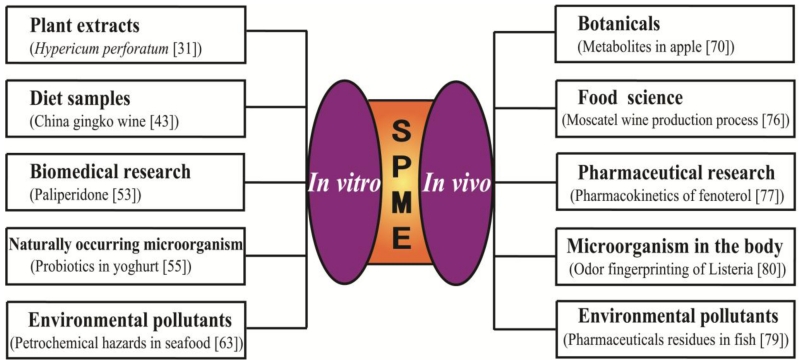
To establish a reliable SPME method, many in vitro studies were first conducted using test samples from botanicals, food, pharmaceuticals, microorganisms, and environmental pollutants. After stable in vitro sample collections and extractions were achieved, SPME was subsequently applied to in vivo experiments (Fig. 4).
Fig. 4.
Different investigations using SPME technology. A representative study in each category is selected.
3.1.1 Plant extracts
Botanical extracts from the liverwort Scapania aspera and citrus essential oil vapor from Peucedanum cervaria are good candidates for SPME in vitro exploration [28-30]. These extracts can be detected by SPME coupled with GC/MS. Extracts of Hypericum perforatum, sunflower oil, Dracaena draco leaf, and Ficus carica were analyzed for their VOCs by head space-SPME (HS-SPME) coupled with GC-ion trap (IT)-MS. The data showed that these extracts are promising antioxidant agents. When the antioxidant effects of these botanicals were evaluated by several chemical assays in vitro, the results showed that DPPH, nitric oxide and superoxide radicals were inhibited in a concentration-dependent manner [31-33].
Other researchers have discovered the existence of anti-malarial VOC components in the Plinia corrocampanensis leaf [34]. The hypoglycemic and hypolipidemic effects of Pelargonium graveolens were studied for their potential application in obesity, diabetes, and metabolic syndrome [35]. The essential oil was detected by HS-SPME-GC/MS.
The composition of volatiles in micropropagated and field-grown botanicals from the islands of Tuscany were identified using HS-SPME with GC/MS. These botanicals have the same aromatic flavor and produce massive materials [36]. In a separate study, designed to detect the activation of purified compounds, data were obtained from Plectranthus ornatus. The researchers focused on the effects of different concentrations of 2,4-dichlorophenoxyacetic acid and 1-naphthaleneacetic acid on the induction of callus and the production of VOCs. The VOCs were detected by HS-SPME followed with GC/MS [37]. Artemisia umbelliformis, a protected species, was also studied with SPME, and only a very small amount of the fresh botanical was needed [38].
The influence of growth regulators on biomass production and the volatile profile of the botanicals of Thymus vulgaris were also studied in vitro with SPME-GC [39]. In another study, five different banana cultivars were distinguished by HS-SPME combined with one-dimensional GC/MS [40].
3.1.2 Diet samples
Eucalyptus essential oil as a natural food preservative was studied using SPME-GC/MS. This essential oil was used for fruit juice preservation against food-spoiling yeast [41]. The effects of kefir culture entrapped in casein and in whey protein as starter cultures for the production of feta-type cheese were also evaluated by SPME-GC/MS. The researchers reported that the VOCs of the different cheese types depended on the nature of the starter culture. When they used kefir culture as the starter, the products showed a soft, fine taste with improved quality [42].
The antioxidant properties of a Chinese gingko wine were accessed using SPME-GC/MS. The results indicated that the total phenol content of gingko wine was 456 mg/L gallic acid equivalents. The antioxidant capacity was higher than that of typical Chinese liquors [43].
Olive oils from two geographic areas were identified and differentiated. The VOCs of the monovarietal virgin olive oils were detected by HS-SPME. The results indicated that the volatile formation was affected by both genetic factors and agronomic conditions [44]. The VOCs in berries were considered at three developmental stages of Vitis vinifera, and were exposed (or not) to UV-B both in vitro and in field experiments. Of the VOCs that were detected by HS-SPME-GC/MS, 10 VOCs were found at all developmental stages and were found to affect wine flavor [45]. HS-SPME-GC/MS was also used to characterize three coffee monoterpene synthases to help improve the quality control of various types of coffee [46].
3.1.3 Biomedical research
To advance our understanding of the uptake, transportation, and transformation dynamics of compounds for pharmaceutical research, SPME was used for compound measurement [47]. Transport of chlorpromazine was investigated in a Caco-2 cell permeability assay. SPME detected free chlorpromazine concentration, and a precise evaluation was made of the Caco-2 cell model [48]. Another study introduced SPME to determine the free concentration of chlorpromazine in aqueous samples containing albumin [49]. Binding studies of carbamazepine were conducted in vitro with SPME and LC/UV [50].
For an in vitro metabolism study, a high-throughput bioanalytical method was applied using 96-blade thin-film SPME and LC/MS/MS for the study of selected compounds and their main metabolites [51]. A special SPME with a new type of β-cyclodextrin-modified nanocellulose as a sorbent material showed a wide linear fluorimetric response against danofloxacin. This special SPME’s recognition has been proven to be highly selective and efficient against this metabolite and other fluoroquinolones [52]. In a stereo-selective study of fungal biotransformation, SPME determined whether fungi could biotransform risperidone into its active metabolite [53]. Previous sample collection and complicated processing after biotransformation may be associated with errors, and these errors can be avoided by applying SPME technology, which is advantageous due to its simplicity.
3.1.4 Naturally occurring microorganisms
The applications and dynamics of lactic acid bacteria for the four-season production of Vastedda-like cheese were investigated. SPME with GC/MS detected the VOCs of the experimental cheeses, obtained with raw milk and inoculated with single and multiple combinations of lactococci [54].
Preculturing of Lactobacillus rhamnosus and Bifidobacterium animalis sub-sp. lactis BB12 under sublethal stress conditions for survival and metabolite formation was investigated in set-yoghurt. 35 volatile and 43 non-volatile polar metabolites were identified by SPME-GC/MS and proton nuclear magnetic spectroscopy (SPME-H1NMR). These data contribute to the possibility of placing stress-adapted probiotics in a food-carrier environment [55]. The effects of probiotics and prebiotics on the metabolic profile of human microbiota have been analyzed with sampling from human feces by SPME-GC/MS [56].
The identification of VOCs produced by Cladosporium cladosporioides, which could accelerate botanical growth, was detected by SPME-GC/MS. Promotion of fungal VOC-mediated botanical growth requires in-depth study for application to large scale crops, particularly those grown under greenhouse conditions [57]. The VOCs in solid-state and submerge-cultured Antrodia camphorate were also accessed by HS-SPME-GC/MS [58]. The starters of yoghurt are critical to the quality of product. Thus, the discovery of different proteolytic strains of Streptococcus thermophilus in the production of set-yoghurt should allow for better control of the quality of yoghurt starters [59].
3.1.5 Environmental pollutants
Indoor molds produce microbial VOCs (MVOCs). MVOCs have been detected with automated HS-SPME and GC/MS analysis. MVOCs produced by malt extract agar, plasterboard, and wallpaper have been compared [60]. In soil, the bioavailability of estrogen-like endocrine-disrupting compounds contributed to the assessment of risk to the environment using thin film geometry SPME (TF-SPME) [61]. With respect to water pollution, petroleum-related substances from refineries and refinery effluents in water were studied with biologically based SPME methods [62]. The technique also can be used to screen environmental petrochemical contamination in seafood [63].
3.1.6 Medical diagnosis from biological samples
TF-SPME coupled to LC/MS/MS analysis was developed to measure bile acids in fluid samples from the bronchoalveolar lavage. Thus, metabolites may provide additional information about the occurrence and severity of gastric reflux/aspiration in lung patients, which will lead to a more accurate diagnosis [64]. Cocaethylene (CE) is a metabolite formed during alcohol and cocaine co-consumption. CE in hair is a biomarker indicating chronic alcohol consumption among individuals who have consumed cocaine. HS-SPME coupled with GC/MS analysis is able to reveal CE as biomarker. Specificity for chronic excessive alcohol consumption was high among cocaine users and other drug addicts [65].
Another study used SPME to collect headspace vapors from methicillin-sensitive Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) grown in vitro in liquid growth medium. The collected molecules were separated and identified by GC/MS. The data distinguished the two strains and provided the foundation for a biomarker library to identify specific bacterial infections [66]. Penicillin-binding protein 2a also can be detected by antibody-linked immuno-affinity SPME sorbents. The established immuno-affinity platform is expected to provide insights into the development of a specific, sensitive, accurate and practical assay for diagnosing MRSA [20].
HS-SPME greatly facilitated the identification of VOCs in human feces for diagnosis and health implications [67]. After investigating the effect of certain organophosphorus pesticides on breast cancer risk, other researchers concluded that endocrine-disrupting chemicals alter normal functioning. In vitro experiments were conducted utilizing HS-SPME combined with HPLC. The results revealed that chlorpyrifos binds to one class of sites on sex hormones [68].
Results of SPME technology from in vitro samples are shown in Table 1. Only after accurate SPME analysis is achieved with in vitro samples can the new technique be applied to in vivo samples.
Table 1.
Selected reports using the SPME technology in in vitro sample analysis
| Study subject | Fiber coating | Thickness (μm) | Sample (g), part | Condition (°C/min) |
Analytical method |
Ref. | |
|---|---|---|---|---|---|---|---|
| Plant Extract | |||||||
| Pinene, sabinene | Peucedanum cervaria | PDMS | 30 | 2.0, fruit | RT/30 | GC/MS | [30] |
| Aldehydes | Hypericum perforatum | PDMS; PDMS/CAR | 75; 100 | Extract | 40/20 | GC/ECD | [31] |
| VOCs, carotenoid | Dracaena draco | PDMS/DVB | 65 | 0.1, leaf | 45/20 | GC/MS | [32] |
| Sesquiterpene, monoterpenes | Ficuscarica | PDMS/DVB | 65 | Fruit | 40/60 | GC/MS | [33] |
| VOCs | Plinia cerrocampanensis | DVB/CAR/PDMS | 30; 50 | 0.5, leaf | 49/14 | GC/MS | [34] |
| VOCs | Pelargonium graveolens | PDMS/DVB | 65 | 0.1/0.2, leaf | RT/2 | GC/MS | [35] |
| Terpinyl acetate, monoterpenes | Plectranthus spp. | PDMS | 100 | 1.0, callus | 60/20 | GC/MS | [37] |
| Terpinene, cymene, thymol | Thymus vulgaris | PDMS/DVB | 65 | 0.2, leaf | 60/15 | GC/MS | [39] |
| Volatile ethyl esters | Dwarf Cavendish banana | PDMS/DVB | 65 | 0.5, fruit | 50/60 | GC/MS | [40] |
| Diet Sample | |||||||
| Cineole, limonene, pinene, terpinene | Eucalyptus | PDMS/DVB | 65 | 5.0, essential oil | RT/10 | GC/MS | [41] |
| Ester, alcohol, acid, hydrocarbon | Gingko wine | PDMS /CAR | 75 | 5.0 ml, wine | 50/45 | GC/MS | [43] |
| Monoterpene, aldehyde, alcohol | Vitis vinifera grapevine | DVB/CAR/PDMS | 30; 50 | 5.0, grape berry | 40/40 | GC/MS | [45] |
| Metabolite | Fecal microbiota | PDMS/CAR | 85 | 3.0, stool/urine | 45/40 | GC/MS | [82] |
| Biomedical Research | |||||||
| Chlorpromazine | Aqueous containing albumin | PA | 30 | 0.2, solution | RT/420 | LC/UV | [49] |
| Carbamazepine | Albumin or mouse plasma | C18 | 1.8 ml, plasma | RT/50 | LC/NMR | [50] | |
| Risperidone/9-OH risperidone | Cunninghamella fungal | C18 | 45 | 2.0 ml, supernatant | RT/30 | LC/MS | [53] |
| Naturally-occurring Microorganisms | |||||||
| Acid, alcohol, aldehyde, ester | Vastedda-like cheese | DBV/CAR/PDMS | 10.0, cheese | 60/30 | GC/MS | [54] | |
| Metabolites | Stool sample | CAR/PDMS | 85 | 3.0, feces | 45/40 | GC/MS | [56] |
| Pinene, caryophyllene | Tobacco | PDMS/DVB | 65 | 6.0 ml, culture | 25/30 | GC/MS | [57] |
| VOCs: alcohol, heterocycle, etc. | Yoghurt | Carboxen/PDMS | 75 | 3.0 ml, culture | RT/10 | GC/NMR | [59] |
| Environmental Pollutants | |||||||
| MVOCs: methylpropanol, etc. | Indoor mold | DVB/CAR/PDMS | 30; 50 | Fresh culture | 35/30 | GC/MS | [60] |
| Alkylbenzene, tetralin, naphthalene | Seafood | PDMS; PDMS/DVB | 65; 100 | 20.0, seafood | 65/3 | GC/MS | [63] |
| Sample for Medical Diagnosis | |||||||
| Penicillin binding protein 2a | PBP2a extraction solution | Immuno-SPME | 25.0, extraction | RT/720 | LC/MS | [20] | |
| Biomarker library | Staphylococcus aureus | CW/DVB; CAR/PDMS | 70;75 | 5.0 ml, culture | RT/10-480 | GC/MS | [66] |
| Chlorpyrfos, sex hormone | CPS and hormone solution | PA-HF | 400 | Solution | 42/30 | LC/UV | [68] |
Abbreviations: Apt-PANCMA-aptamer functionalized poly (acrylonitiile-co-maleic acid); CAR-Carboxen; HLB-hydrophilic lipophilic balanced particles; MVOCs-microbial volatile organic compounds; PA-polyacrylate; PDMS-polydimethylsiloxane; RT-room tempreture; VOCs-volatile organic compounds.
3.2 In vivo analysis
3.2.1 Botanicals
The health benefits of herbs and edible plants are remarkable, and botanical materials have been used as preventative measures or as treatments for disease for thousands of years. Thus, the composition of botanical matrixes from living plants has gained more attention in recent years. With the rapid development of technology, botanicals and their metabolites can be determined using SPME coupled with other advanced analytical methods.
Orchids are one example of botanicals that can be analyzed using SPME coupled methods. When SPME was applied to Italian grown orchids, HS-SPME-GC/MS proved a suitable technique for distinguishing the volatile fingerprint of different orchid species [69]. One study tested whether SPME could uncover the fingerprints of volatile and semi-volatile metabolites in complex samples. With SPME-comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC/GC/TOF-MS), 399 metabolites of apples were identified [70]. These results confirm that metabolites can be characterized in botanical samples in vivo.
3.2.2 Food science
SPME is compatible with methods for analyzing food samples. For rapid food analysis and cleanliness monitoring, the volatile metabolites in farmed and wild European sea bass have been accurately distinguished by SPME followed by GC/MS. These data increased our knowledge of the quality of raw and processed sea bass. For example, the off-flavor components in live fish were detected by SPME, and the detection limit was as low as 0.12ng/g for geosmin and 0.21 ng/g for 2-methylisoborneol, which are far below human sensory thresholds [71,72]. Similarly, the uptake and elimination of organic pesticides in fish muscle were traced by SPME plus GC/MS [73].
One in vivo experiment showed that SPME was reliable for detecting changes in the volatile fingerprint of Achillea collina, changes separately induced by an infestation of aphids, mechanical damage, or jasmonic acid. Differences were clear between control, infested, damaged and jasmonic-treated plants [74]. The responses of plants to external stimuli are traceable and of value in food science. In another example, the biosynthesis of sesquiterpenes in the grape berry exocarp of Vitis vinifera was evidence for transport of farnesyl diphosphate precursors from plastids to the cytosol. The method used was HS-SPME-GC/MS [75]. This SPME has also been applied in the deep processing of grapes. Notable changes in some aroma compounds of Moscatel sparking-wine were detected in the production process. Data showed the 75 compounds were co-eluted [76]. Even extremely small differences in the components could be detected.
3.2.3 Pharmaceutical research and development
Most pharmaceuticals are synthetic or semi-synthetic compounds. The absorption, distribution, metabolism and excretion of pharmaceutical compounds have been studied with SPME. The technology has also contributed to drug discovery. The anti-microbial potential of extracts of the liverwort Scapania aspera was investigated, and the chemical composition of the extracts was determined by SPME-GC/MS. The experimental results suggested that S. aspera contains natural anti-microbial agents [28].
The diffusion-based calibration interface model of SPME has been proposed for the analysis of the pharmacokinetics of selected drugs. For in vivo SPME sampling, this model has several advantages over other kinetic calibration models: (1) it does not require the addition of a standard into the sample matrix, (2) it eliminates the need to pre-load a standard onto the SPME extraction phase, and (3) the calibration constant can be calculated [77]. The in vivo detection of drugs and their metabolites can contribute to pharmaceutical research. A SPME probe inserted directly into the peripheral vein of a living animal can monitor and quantify the concentration of drugs and their metabolites [1].
A space-resolved SPME technique was used to study the tissue-specific bio-concentration of pharmaceutical agents in live fish, which is critical for monitoring antibiotic abuse. SPME needles were segmented and coated with novel fibers to detect specific pharmaceutical residues in fish dorsal epaxial muscles and adipose tissues with repeat in vivo sampling of tissues. Precision was acceptable [78,79]. As material science and analytical technology further develop, the accuracy of this technique will be further improved.
3.2.4 Microorganisms in the body
Metabolomics studies the anabolic and catabolic pathways of bacteria and fungi and the dynamics of their metabolism. Metabolites with low molecular weights can be qualitatively and quantitatively analyzed. VOCs fingerprinting of Listeria monocytogenes was recognized by SPME-GC/MS and E-nose in pure culture medium. Analysis of the VOCs fingerprint of microorganisms has the potential to become routine in microbiology studies [80].
Aliphatic amides were applied to detect Helicobacter pylori using SPME coupled with GC/MS. Propionic and butyric acid were the biomarkers for H. pylori after incubation with the corresponding amides. SPME also detected the acids and verified their hepatic stability. The sensitivity of detection of both acids was in amounts as low as 0.8 μg [81].
Human gastrointestinal microbiota have become the subject of extensive research in recent years. Fecal microbiota excreted by 30 healthy volunteers after treatment with Khorasan wheat was detected by SPME-GC/MS. The data verified the anti-inflammatory effect and counteraction of oxidative stress by Khorasan-based cereal foods [82].
3.2.5 Analysis of environmental pollutants
Environmental pollution is becoming a serious problem in the world, so effective sampling methods may strengthen environmental governance. In vivo sampling of organic contaminants in fish with SPME improved the sensitivity and extraction kinetics of the determination of trace pharmaceutical pollutants in fish tissue. A novel thin film micro-extraction configuration based on C18 thin film was introduced [83]. Another study developed and improved SPME for the sampling of pharmaceuticals in fish tissue. SPME with a PDMS extraction phase was a robust tool and was simpler than the traditional device. The new device is a platform for rapid sampling of carbamazepine, diazepam, and nordiazepam in fish muscle with acceptable precision [83].
The quantitative evaluation of (+)-Δ3-carene metabolites from the living larvae of Spodoptera litura was possible with HS-SPME. The method was sensitive enough to quantitate the (+)-Δ3-carene metabolites released from the Spodoptera larvae [84]. The medication lindane, widely used to treat agricultural pests, was evaluated for its toxicity, persistence and tendency to bioaccumulate in terrestrial and aquatic ecosystems. In the study, lindane was extracted by SPME and identified by GC/MS. Its metabolite, 1,3,4,5,6-pentachloro-cyclohexene, contributed to the in situ bioremediation of this pollutant [85]. In the common reed, in vivo sampling was performed on a site highly polluted with methyl tertiary butyl ether. SPME fiber was directly introduced into the aerenchyma of the botanical stem. This method seems feasible for the screening of VOCs in wetlands [86].
3.2.6 Disease diagnosis
Supported by many studies, SPME is a reliable technology for early diagnosis. Rapid breath analysis was performed by SPME in cystic fibrosis patients, and the analytical data helped the diagnosis of the disease in the near future [87]. TF-SPME combines sampling and sample preparation into a single step. In vivo sampling using TF-SPME coupled with GC and LC for 5 minutes revealed a wide range of analytes with different physical and chemical properties [7]. HS-SPME sampling for VOCs in humans significantly reduced background signal intensity, and resulted in reproducible analysis. The method can be used to detect the biomarkers of garlic intake and alcohol ingestion [88].
During surgery, metabolites can be collected directly with SPME for biochemical analysis and for biomarker identification [89]. SPME coatings functionalized with a DNA aptamer can selectively enrich aspecific proteins from diluted human plasma. This SPME was successfully applied to detect thrombin in human plasma [19]. When SPME was used to screen for VOCs, the results suggested a possible cause of death from a rare case of captan ingestion [90]. With convenient sample preparation [91], SPME could be a valuable tool for the early diagnosis of cardiovascular, oncologic and neurodegenerative illnesses. Selected reports that used SPME for in vivo sample analysis are shown in Table 2.
Table 2.
Selected reports using the SPME technology in in vivo sample analysis
| Study subject | Fiber coating | Thickness (μm) | Sample (g), part | Condition (°C/min) |
Analytical method |
Ref. | |
|---|---|---|---|---|---|---|---|
| Botanical | |||||||
| Hydrocarbon, aldehyde, furan, etc. | Ophrys sphegodes | DVB/CAR/PDMS | 50; 30 | Flowering plant | 280/180 | GC/MS | [69] |
| Food Science | |||||||
| Geosmin, 2-methyl isoborneol | Rainbow trout | PDMS | 65 | 4.0, tissue | RT/30 | GC/MS | [72] |
| Pesticide Residue | Tilapias, Pomfrets | PDMS | 44; 165 | Epaxial muscle | RT/10,20 | GC/LC/MS | [73] |
| Precursor of sesquiterpene | Vitis vinifera | PA | 85 | 1×10−4 , precursor | 60/10 | GC/MS | [75] |
| Monoterpene, ester, alcohol | Moscatel sparkling wine | PDMS/DVB | 65 | 2.0 ml, wine | 40/10 | GC/GC/MS | [76] |
| Pharmacy Research & Development | |||||||
| Methoxy fenoterol/fenoterol | Rat | C18/Cyanopropyl | Organ | RT /4 | LC/MS | [77] | |
| Drug residues | Rainbow trout | PDMS | 330 | Organ | RT /10 | LC/MS | [78] |
| Mefenamic acid/fluoxetine | Fish | PS@PDA-GA | 100 | Organ | RT /10 | LC/MS | [79] |
| Microorganisms in the Body | |||||||
| Propionic / butyric acid | H. pylori reference strain | CW/DVB | 25.0 ml, fresh culture | 37/5 | GC/MS | [81] | |
| Analysis of Environmental Pollutants | |||||||
| Fluoxetine, venlafaxine, sertraline, etc. | Rainbow trout, Fathead minnow | C18 | 45 | Epaxial muscle | RT/1440 | LC/MS | [83] |
| (+)-Δ3-carene metabolite | Spodoptera litura | DVB/CAR/PDMS | 50; 30 | 0.5, larvae | 25/30 | GC/MS | [84] |
| Pentachloro cyclohexene | Hymeniacidon perlevis | PDMS | 100 | 15.0, seawater | 50/30 | GC/MS | [85] |
| Methyl tert-butyl ether | Phragmites australis | CAR/PDMS | 85 | Plant sampling | RT/120 | GC/MS | [86] |
| Disease Diagnosis | |||||||
| Endogenous steroid | Human saliva | PDMS/HLB/C18 | 65 | 1.0 ml, saliva | RT/5 | GC/MS | [7] |
| Thrombin | Human plasma | Apt-PANCMA | 2.0 ml, plasma | RT/60 | LC/MS | [19] | |
| VOCs | Human exhaled breath | CAR/PDMS | 75 | 100.0 ml, EB | RT/10 | GC/MS | [87] |
| DMSO, AMS, allyl-mercaptan | Skin | PDMS | 254 | Skin | 40/60 | GC/MS | [88] |
| Methylprednisolone | Male Yorkshire pig | C18/BA | 45 | Organ | RT/20,30 | LC/MS | [89] |
| Captan and its metabolites | Human viscera content | PDMS | 100 | 1.0 ml, blood/GI | 40/20 | GC/MS | [90] |
Abbreviations: Apt-PANCMA-aptamer functionalized poly (acrylonitiile-co-maleic acid); BA-benzenesulfonic acid; CAR-Carboxen; EB-exhaled breath; HLB-hydrophilic lipophilic balanced particles; MVOCs-microbial volatile organic compounds; PA-polyacrylate; PDMS-polydimethylsiloxane; RT-room tempreture; VOCs-volatile organic compounds.
4. Conclusion and outlook
Sample collection is critical to the determination of various compounds and their metabolites in different matrices. In the last few years, there has been a notable increase in the application of SPME. This new technology is sensitive, selective, simple, reproducible and relatively noninvasive. Thus, SPME is a well-established technique for the study of the chemical composition of botanicals, food and pharmaceuticals, all of which could be affected by microorganisms and the environmental factors. SPME is particularly useful for medical diagnosis because its tissue damage is minimal. The application of SPME to the diagnosis of human diseases, however, is still in its early stage. In the future, the application of SPME in metabolite analysis will depend on advances in specific analytical technologies and material science. It is anticipated that the sensitivity and accuracy of the detection and analysis of non-volatile compounds from minute samples will be increased. SPME has a promising future in biomedical and pharmaceutical research.
Acknowledgement
This work was supported by the Fundamental and Frontier Research Fund of Chongqing under Grant cstc2014jcyjA10108; Fundamental Research Funds for the Central Universities under Grant CQDXWL-2014-Z007; Special Fund for Basic Scientific Research of Central Colleges, Chongqing University under Grant No. 201310611045 and Fundamental Research Funds for the Central Universities under Grant CQDXWL-2012-031; Tang Foundations. We thank Sally Kozlik for editing the manuscript.
References
- [1].Lord HL, Zhang X, Musteata FM, Vuckovic D, Pawliszyn J. In vivo solid-phase microextraction for monitoring intravenous concentrations of drugs and metabolites. Nat. Protoc. 2011;6:896–924. doi: 10.1038/nprot.2011.329. [DOI] [PubMed] [Google Scholar]
- [2].Wulfkuhle JD, Liatta LA, Petricoin EF. Proteomic applications for the early detection of cancer. Nat. Rev. Cancer. 2003;3:267–275. doi: 10.1038/nrc1043. [DOI] [PubMed] [Google Scholar]
- [3].Abaffy T, Duncan R, Riemer DD, Tietje O, Elgart G, Milikowski C, et al. Differential volatile signatures from skin, naevi and melanoma: a novel approach to detect a pathological process. BMC Bioinformatics. 2015;16:158–170. doi: 10.1371/journal.pone.0013813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vas G, Vékey K. Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 2004;39:233–254. doi: 10.1002/jms.606. [DOI] [PubMed] [Google Scholar]
- [5].Attari SG, Bahrami A, Shahna FG, Heidari M. Solid-phase microextraction fiber development for sampling and analysis of volatile organohalogen compounds in air. JEHSE. 2014;12:123–131. doi: 10.1186/s40201-014-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vuckovic D, de Lannoy I, Gien B, Yang Y, Musteata FM, Shirey R, et al. In vivo solid-phase microextraction for single rodent pharmacokinetics studies of carbamazepine and carbamazepine-10,11-epoxide in mice. J. Chromatogr. A. 2011;1218:3367–3375. doi: 10.1016/j.chroma.2010.07.060. [DOI] [PubMed] [Google Scholar]
- [7].Bessonneau V, Boyaci E, Maciazek-Jurczyk M, Pawliszyn J. In vivo solid phase microextraction sampling of human saliva for non-invasive and on-site monitoring. Anal. Chim. Acta. 2015;856:35–45. doi: 10.1016/j.aca.2014.11.029. [DOI] [PubMed] [Google Scholar]
- [8].Pereira J, Silva CL, Perestrelo R, Gonçalves J, Alves V, Câmara JS. Re-exploring the high-throughput potential of microextraction techniques, SPME and MEPS, as powerful strategies for medical diagnostic purposes. Innovative approaches, recent applications and future trends. Anal. Bioanal. Chem. 2014;406:2101–2122. doi: 10.1007/s00216-013-7527-4. [DOI] [PubMed] [Google Scholar]
- [9].Zhang X, Oakes KD, Wang S, Servos MR, Cui S, Pawliszyn J. In vivo sampling of environmental organic contaminants in fish by solid-phase microextraction. Trends Anal. Chem. 2012;32:31–39. [Google Scholar]
- [10].Żwir-Ferenc A, Biziuk M. Solid phase extraction technique - Trends, opportunities and applications. Polish J. Environ. Stud. 2006;5:677–690. [Google Scholar]
- [11].Ekström S, Wallman L, Hök D, Marko-Varga G, Laurell T. Miniaturized solid-phase extraction and sample preparation for MALDI MS using a microfabricated integrated selective enrichment target. J. Proteome. Res. 2006;5:1071–1081. doi: 10.1021/pr050434z. [DOI] [PubMed] [Google Scholar]
- [12].Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990;62:2145–2148. [Google Scholar]
- [13].Belardi R, Pawliszyn J. The application of chemically modified fused silica fibres in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Pollut. Res. J. Can. 1989;24:179. [Google Scholar]
- [14].Kataoka H, Lord HL, Pawliszyn J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A. 2000;880:35–62. doi: 10.1016/s0021-9673(00)00309-5. [DOI] [PubMed] [Google Scholar]
- [15].Pawliszyn J. SPME commercial devices and fibre coatings. In: Pawliszyn J, editor. Handbook of Solid Phase Microextraction. Chemical Industry Press; 2009. [Google Scholar]
- [16].Hinshaw JV, Serveron C. Solid-Phase Microextraction, LC·GC. GC Connections; Europe: Dec, 2003. pp. 2–5. [Google Scholar]
- [17].Hook GL, Kimm GL, Hall T, Smith PA. Solid-phase microextraction (SPME) for rapid field sampling and analysis by gas chromatography-mass spectrometry (GC-MS) Trends Anal. Chem. 2002;21:534–543. [Google Scholar]
- [18].Tong Y, Bohm S, Song M. Graphene based materials and their composites as coatings. Austin J. Nanomed Nanotechnol. 2013;1:01–16. [Google Scholar]
- [19].Du F, Alam MN, Pawliszyn J. Aptamer-functionalized solid phase microextraction-liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin. Anal. Chim. Acta. 2014;845:45–52. doi: 10.1016/j.aca.2014.08.018. [DOI] [PubMed] [Google Scholar]
- [20].Liu Y, Lord H, Jurczyk MM, Jolly S, Hussain MA, Pawliszyn J. Development of an immunoaffinity solid phase microextraction method for the identification of penicillin binding protein 2a. J. Chromatogr. A. 2014;1364:64–73. doi: 10.1016/j.chroma.2014.08.042. [DOI] [PubMed] [Google Scholar]
- [21].Pavlović DM, Babić S, Horvat AJM, Kaštelan-Macan M. Sample preparation in analysis of pharmaceuticals. Trends Anal. Chem. 2007;26:1062–1075. [Google Scholar]
- [22].Stephen JT. Comparison and Integration of Analytical Methods for the Characterization of Vanilla Chemistry, Proquest. Umi Dissertation Publishing; 2012. pp. 86–92. [Google Scholar]
- [23].Mohammad MM, Rana S, Fatma B, Mohamed AR. Solid phase microextraction and related techniques for drugs in biological samples. J. Anal. Methods Chem. 2014;2014:1–24. doi: 10.1155/2014/921350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lao YM, Jiang JG, Yan L. Application of metabonomic analytical techniques in the modernization and toxicology research of traditional Chinese medicine. Br. J. Pharmacol. 2009;157:1128–1141. doi: 10.1111/j.1476-5381.2009.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang C, Li P, Lian A, Sun B, Wang X, Guo L, et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol. Ther. 2014;15:200–206. doi: 10.4161/cbt.26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Caris JA, Silva BJ, Moisés EC, Lanchote VL, Queiroz ME. Automated analysis of lidocaine and its metabolite in plasma by in-tube solid-phase microextraction coupled with LC-UV for pharmacokinetic study. J. Sep. Sci. 2012;35:734–741. doi: 10.1002/jssc.201100872. [DOI] [PubMed] [Google Scholar]
- [27].Vuckovic D, Lannoy ID, Gien B, Shirey RE, Sidisky LM, Dutta S, et al. In vivo solid-phase microextraction: capturing the elusive portion of metabolome. Angew. Chem. Int. Ed. Engl. 2011;50:5344–5348. doi: 10.1002/anie.201006715. [DOI] [PubMed] [Google Scholar]
- [28].Bukvicki DR, Tyagi AK, Gottardi DG, Veljic MM, Jankovic SM, Guerzoni ME, et al. Assessment of the chemical composition and in vitro antimicrobial potential of extracts of the liverwort Scapaniaaspera. Nat. Prod. Commun. 2013;8:1313–1316. [PubMed] [Google Scholar]
- [29].Phillips CA, Gkatzionis K, Laird K, Score J, Kant A, Fielder MD. Identification and quantification of the antimicrobial components of a citrus essential oil vapor. Nat. Prod. Commun. 2012;7:103–107. [PubMed] [Google Scholar]
- [30].Skalicka-Wozniak K, Los R, Glowniak K, Malm A. Volatile compounds in fruits of Peucedanum cervaria(Lap.) L. Chem. Biodivers. 2009;6:1087–1092. doi: 10.1002/cbdv.200800236. [DOI] [PubMed] [Google Scholar]
- [31].Gioti EM, Fiamegos YC, Skalkos DC, Stalikas CD. Improved method for the in vitro assessment of antioxidant activity of botanical extracts by headspace solid-phase microextraction and gas chromatography-electron capture detection. J. Chromatogr. A. 2007;1152:150–155. doi: 10.1016/j.chroma.2007.01.124. [DOI] [PubMed] [Google Scholar]
- [32].Santosa RP, Mendes LS, Silva BM, de Pinho PG, Valentão P, Andrade PB. Phytochemical profiles and inhibitory effect on free radical-induced human erythrocyte damage of Dracaena draco leaf: a potential novel antioxidant agent. Food Chem. 2011;124:927–934. [Google Scholar]
- [33].Oliveira AP, Silva LR, Ferreres F, de Guedes PP, Valentão P, Silva BM, et al. Chemical assessment and in vitro antioxidant capacity of Ficus carica Latex. J. Agric. Food Chem. 2010;58:3393–3398. doi: 10.1021/jf9039759. [DOI] [PubMed] [Google Scholar]
- [34].Durant AA, Rodríguez C, Herrera L, Almanza A, Santana AI, Spadafora C. Anti-malarial activity and HS-SPME-GC-MS chemical profiling of Plinia cerrocampanensis leaf essential oil. Malar J. 2014;13:18–27. doi: 10.1186/1475-2875-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Afifi FU, Kasabri V, Abu-Dahab R, Abaza IM. Chemical composition and in vitro studies of the essential oil and aqueous extract of Pelargonium graveolens growing in Jordan for hypoglycaemic and hypolipidemic properties. European J. Med. Plants. 2014;4:220–233. [Google Scholar]
- [36].Pistelli L, Noccioli C, Angiolillo FD’, Pistelli L. Composition of volatile in micropropagated and field grown aromatic botanicals from Tuscany Islands. Acta. Biochim. Pol. 2013;60:43–50. [PubMed] [Google Scholar]
- [37].Passinho-Soares HC, Meira PR, David JP, Mesquita PR, do Vale AE, de F, Rodrigues M. Volatile organic compounds obtained by in vitro callus cultivation of Plectranthus ornatus Codd. (Lamiaceae) Molecules. 2013;18:10320–10333. doi: 10.3390/molecules180910320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Reale S, Pace L, D’Archivio AA, De Angelis F, Marcozzi G. Volatiles fingerprint of Artemisia umbelliformis subsp. eriantha by headspace-solid phase microextraction GC-MS. Nat. Prod.Res. 2014;28:61–66. doi: 10.1080/14786419.2013.825912. [DOI] [PubMed] [Google Scholar]
- [39].Affonso VR, Bizzo HR, Lage CLS, Sato A. Influence of growth regulators in biomass production and volatile profile of in vitro botanicals of Thymus vulgaris L. J. Agric. Food Chem. 2009;57:6392–6395. doi: 10.1021/jf900816c. [DOI] [PubMed] [Google Scholar]
- [40].Pontes M, Pereira J, Câmara JS. Dynamic headspace solid-phase microextraction combined with one-dimensional gas chromatography-mass spectrometry as a powerful tool to differentiate banana cultivars based on their volatile metabolite profile. Food Chem. 2012;134:2509–2520. doi: 10.1016/j.foodchem.2012.04.087. [DOI] [PubMed] [Google Scholar]
- [41].Tyagi AK, Bukvicki D, Gottardi D, Tabanelli G, Montanari C, Malik A, et al. Eucalyptus essential oil as a natural food preservative: in vivo and in vitro antiyeast potential. Biomed. Res. Int. 2014;2014:969143. doi: 10.1155/2014/969143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dimitrellou D, Kandylis P, Kourkoutas Y, Koutinas AA, Kanellaki M. Cheese production using Kefir culture entrapped in milk proteins. Appl. Biochem. Biotechnol. 2015;176:213–230. doi: 10.1007/s12010-015-1568-4. [DOI] [PubMed] [Google Scholar]
- [43].Wang X, Xie K, Zhuang H, Ye R, Fang Z, Feng T. Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem. 2015;182:41–46. doi: 10.1016/j.foodchem.2015.02.120. [DOI] [PubMed] [Google Scholar]
- [44].Mansour AB, Gargouri B, Flamini G, Bouaziz M. Effect of agricultural sites on differentiation between Chemlali and Neb Jmel olive oils. J. Oleo. Sci. 2015;64:381–392. doi: 10.5650/jos.ess14204. [DOI] [PubMed] [Google Scholar]
- [45].Gil M, Bottini R, Berli F, Pontin M, Silva MF, Piccoli P. Volatile organic compounds characterized from grapevine (Vitisvinifera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry. 2013;96:148–157. doi: 10.1016/j.phytochem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- [46].Terra L, Lonzarich V, Asquini E, Navarini L, Graziosi G, Suggi Liverani F, et al. Functional characterization of three Coffea arabica L. monoterpene synthases: insights into the enzymatic machinery of coffee aroma. Phytochemistry. 2013;89:6–14. doi: 10.1016/j.phytochem.2013.01.005. [DOI] [PubMed] [Google Scholar]
- [47].Zhang X, Oakes KD, Hoque ME, Luong D, Taheri-Nia S, Lee C. Depth-profiling of environmental pharmaceuticals in biological tissue by solid-phase microextraction. Anal. Chem. 2012;84:6956–6962. doi: 10.1021/ac3004659. [DOI] [PubMed] [Google Scholar]
- [48].Broeders JJ, Eijkeren JCV, Blaauboer BJ, Hermens JL. Transport of chlorpromazine in the Caco-2 cell permeability assay: a kinetic study. Chem. Res. Toxicol. 2012;25:1442–1451. doi: 10.1021/tx300221k. [DOI] [PubMed] [Google Scholar]
- [49].Broeders JJ, Blaauboer BJ, Hermens JL. Development of a negligible depletion-solid phase microextraction method to determine the free concentration of chlorpromazine in aqueous samples containing albumin. J. Chromatogr. A. 2011;1218:8529–8535. doi: 10.1016/j.chroma.2011.09.064. [DOI] [PubMed] [Google Scholar]
- [50].Bojko B, Vuckovic D, Pawliszyn J. Comparison of solid phase microextraction versus spectroscopic techniques for binding studies of carbamazepine. J. Pharm. Biomed. Anal. 2012;66:91–99. doi: 10.1016/j.jpba.2012.03.005. [DOI] [PubMed] [Google Scholar]
- [51].Simões RA, Bonato PS, Mirnaghi FS, Bojko B, Pawliszyn J. Bioanalytical method for in vitro metabolism study of repaglinide using 96-blade thin-film solid-phase microextraction and LC-MS/MS. Bioanalysis. 2015;7:65–77. doi: 10.4155/bio.14.203. [DOI] [PubMed] [Google Scholar]
- [52].Ruiz-Palomero C, Soriano ML, Valcárcel M. β-Cyclodextrin decorated nanocellulose: a smart approach towards the selective fluorimetric determination of danofloxacin in milk samples. Analyst. 2015;140:3431–3438. doi: 10.1039/c4an01967a. [DOI] [PubMed] [Google Scholar]
- [53].Bocato MZ, Simões RA, Calixto LA, de Gaitani CM, Pupo MT, de Oliveira AR. Solid phase microextraction and LC-MS/MS for the determination of paliperidone after stereoselective fungal biotransformation of risperidone. Anal. Chim. Acta. 2012;742:80–89. doi: 10.1016/j.aca.2012.05.056. [DOI] [PubMed] [Google Scholar]
- [54].Gaglio R, Scatassa ML, Cruciata M, Miraglia V, Corona O, Di Gerlando R. In vivo application and dynamics of lactic acid bacteria for the four-season production of Vastedda-like cheese. Int. J. Food Microbiol. 2014;177:37–48. doi: 10.1016/j.ijfoodmicro.2014.02.007. [DOI] [PubMed] [Google Scholar]
- [55].Settachaimongkon S, van Valenberg HJ, Winata V, Wang X, Nout MJ, van Hooijdonk TC, et al. Effect of sublethal preculturing on the survival of probiotics and metabolite formation in set-yoghurt. Food Microbiol. 2015;49:104–115. doi: 10.1016/j.fm.2015.01.011. [DOI] [PubMed] [Google Scholar]
- [56].Vitali B, Ndagijimana M, Maccaferri S, Biagi E, Guerzoni ME, Brigidi P. An in vitro evaluation of the effect of probiotics and prebiotics on the metabolic profile of human microbiota. Anaerobe. 2012;18:386–391. doi: 10.1016/j.anaerobe.2012.04.014. [DOI] [PubMed] [Google Scholar]
- [57].Paul D, Park KS. Identification of volatiles produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes botanical growth. Sensors. 2013;13:13969–13977. doi: 10.3390/s131013969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].He Z, Lu ZM, Xu HY, Shi JS, Xu ZH. Analyze on volatile compounds of Antrodia camphorata using HS-SPME-GC-MS. Zhong Yao Cai. 2011;34:1722–1725. [PubMed] [Google Scholar]
- [59].Settachaimongkon S, Nout MJ, Antunes-Fernandes EC, Hettinga KA, Vervoort JM, van Hooijdonk TC, et al. Influence of different proteolytic strains of Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. bulgaricus on the metabolite profile of set-yoghurt. Int. J. Food Microbiol. 2014;177:29–36. doi: 10.1016/j.ijfoodmicro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- [60].van Lancker F, Adams A, Delmulle B, de Saeger S, Moretti A, van Peteghem C, et al. Use of headspace SPME-GC-MS for the analysis of the volatiles produced by indoor molds grown on different substrates. J. Environ. Monit. 2008;10:1127–1133. doi: 10.1039/b808608g. [DOI] [PubMed] [Google Scholar]
- [61].Engler KN, Lemley AT. Development of an in vitro thin-film solid-phase microextraction method to determine the bioavailability of xenoestrogens in soil. Environ. Toxicol. Chem. 2013;32:1962–1968. doi: 10.1002/etc.2292. [DOI] [PubMed] [Google Scholar]
- [62].Comber MH, Girling A, den Haan KH, Whale G. Oil refinery experience with the assessment of refinery effluents and receiving waters using biologically-based methods. Integr. Environ. Assess. Manag. 2015;9999:1–13. doi: 10.1002/ieam.1639. [DOI] [PubMed] [Google Scholar]
- [63].Bencsath FA, Benner RA, jr, Abraham A, Wang Y, el Said KR, Jester EL, et al. Screening for petrochemical contamination in seafood by headspace solid-phase microextraction gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015;407:4079–4090. doi: 10.1007/s00216-015-8624-3. [DOI] [PubMed] [Google Scholar]
- [64].Bessonneau V, Bojko B, Azad A, Keshavjee S, Azad S, Pawliszyn J. Determination of bronchoalveolar lavage bile acids by solid phase microextraction liquid chromatography-tandem mass spectrometry in combination with metabolite profiling: comparison with enzymatic assay. J. Chromatogr. A. 2014;1367:33–38. doi: 10.1016/j.chroma.2014.09.061. [DOI] [PubMed] [Google Scholar]
- [65].Natekar A, Motok I, Walasek P, Rao C, Clare-Fasullo G, Koren G. Cocaethylene as a biomarker to predict heavy alcohol exposure among cocaine users. J. Popul. Ther. Clin. Pharmacol. 2012;19:e466–472. [PubMed] [Google Scholar]
- [66].Jia B, Sohnlein B, Mortelmans K, Coggiola M, Oser H. Distinguishing methicillin-resistant and sensitive Staphylococcus aureus using volatile headspace metabolites. IEEE Sensors J. 2010;10:71–75. [Google Scholar]
- [67].Dixon E, Clubb C, Pittman S, Ammann L, Rasheed Z, Kazmi N, et al. Couch RD. Solid-phase microextraction and the human fecal VOC metabolome. PLoS One. 2011;6:e18471. doi: 10.1371/journal.pone.0018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Farhadi K, Tahmasebi R, Biparva P, Maleki R. In vitro study of the binding between chlorpyrfos and sex hormones using headspace solid-phase microextraction combined with high-performance liquid chromatography: a new aspect of pesticides and breast cancer risk. Hum. Exp. Toxicol. 2015;34:819–827. doi: 10.1177/0960327114559990. [DOI] [PubMed] [Google Scholar]
- [69].Manzo A, Panseri S, Vagge I, Giorgi A. Volatile fingerprint of Italian populations of orchids using solid phase microextraction and gas chromatography coupled with mass spectrometry. Molecules. 2014;19:7913–7936. doi: 10.3390/molecules19067913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Risticevic S, DeEll JR, Pawliszyn J. Solid phase microextraction coupled with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for high-resolution metabolite profiling in apples: implementation of structured separations for optimization of sample preparation procedure in complex samples. J. Chromatogr. A. 2012;1251:208–218. doi: 10.1016/j.chroma.2012.06.052. [DOI] [PubMed] [Google Scholar]
- [71].Vidal NP, Manzanos MJ, Goicoechea E, Guillén MD. Farmed and wild sea bass (Dicentrarchus labrax) volatile metabolites. A comparative study by SPME-GC/MS. J. Sci. Food Agric. 2015:7201. doi: 10.1002/jsfa.7201. [DOI] [PubMed] [Google Scholar]
- [72].Bai Z, Pilote A, Sarker PK, Vandenberg G, Pawliszyn J. In vivo solid-phase microextraction with in vitro calibration: determination of off-flavor components in live fish. Anal. Chem. 2013;85:2328–2332. doi: 10.1021/ac3033245. [DOI] [PubMed] [Google Scholar]
- [73].Xu JQ, Luo JP, Ruan JW, Zhu F, Luan TG, Liu H, et al. In Vivo Tracing Uptake and Elimination of Organic Pesticides in Fish Muscle. Environ. Sci. Technol. 2014;48:8012–8020. doi: 10.1021/es5009032. [DOI] [PubMed] [Google Scholar]
- [74].Giorgi A, Manzo A, Nanayakkara N. Nanayakkarawasam Masachchige Chandrika, Giupponi L, Cocucci M, Panseri S. Effect of biotic and abiotic stresses on volatile emission of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2015;29:1695–1672. doi: 10.1080/14786419.2014.997725. [DOI] [PubMed] [Google Scholar]
- [75].May B, Lange BM, Wüst M. Biosynthesis of sesquiterpenes in grape berry exocarp of Vitisvinifera L.: evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry. 2013;95:135–144. doi: 10.1016/j.phytochem.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Soares RD, Welke JE, Nicolli KP, Zanus M, Caramão EB, Manfroi V, et al. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015;183:291–304. doi: 10.1016/j.foodchem.2015.03.013. [DOI] [PubMed] [Google Scholar]
- [77].Yeung JC, de Lannoy I, Gien B, Vuckovic D, Yang Y, Bojko B. Semi-automated in vivo solid-phase microextraction sampling and the diffusion-based interface calibration model to determine the pharmacokinetics of methox and fenoterol in rats. Anal. Chim. Acta. 2012;742:37–44. doi: 10.1016/j.aca.2012.01.034. [DOI] [PubMed] [Google Scholar]
- [78].Zhang X, Oakes KD, Cui S, Bragg L, Servos MR, Pawliszyn J. Tissue-specific in vivo bioconcentration of pharmaceuticals in rainbow trout (Oncorhynchus mykiss) using space-resolved solid-phase microextraction. Environ. Sci. Technol. 2010;44:3417–3422. doi: 10.1021/es903064t. [DOI] [PubMed] [Google Scholar]
- [79].Xu J, Huang S, Wu R, Jiang R, Zhu F, Wang J, et al. Bioinspired polydopamine sheathed nanofibers for high-efficient in vivo solid-phase microextraction of pharmaceuticals in fish muscle. Anal. Chem. 2015;87:3453–3459. doi: 10.1021/ac5048357. [DOI] [PubMed] [Google Scholar]
- [80].Yu YX, Sun XH, Liu Y, Pan YJ, Zhao Y. Odor fingerprinting of Listeria monocytogenes recognized by SPME-GC-MS and E-nose. Can. J. Microbiol. 2014;61:367–372. doi: 10.1139/cjm-2014-0652. [DOI] [PubMed] [Google Scholar]
- [81].Ferreira JA, Dias E, Rocha SM, Coimbra MA. Process for detecting Helicobacter pylori using aliphatic amides. Anal. Bioanal. Chem. 2011;401:1889–1898. doi: 10.1007/s00216-011-5259-x. [DOI] [PubMed] [Google Scholar]
- [82].Saa DT, Turroni S, Serrazanetti DI, Rampelli S, Maccaferri S, Candela M, et al. Impact of Kamut Khorasan on gut microbiota and metabolome in healthy volunteers. Food Res. Int. 2014;63:227–232. [Google Scholar]
- [83].Togunde OP, Lord H, Oakes KD, Servos MR, Pawliszyn J. Development and evaluation of a new in vivo solid-phase microextraction sampler. J. Sep. Sci. 2013;36:219–223. doi: 10.1002/jssc.201200839. [DOI] [PubMed] [Google Scholar]
- [84].Miyazawa M, Koutari S. Quantitative evaluation of (+)-Δ3-carene metabolites from living larvae of Spodoptera litura by headspace solid-phase microextraction. J. Oleo Sci. 2012;61:65–68. doi: 10.5650/jos.61.65. [DOI] [PubMed] [Google Scholar]
- [85].Aresta A, Nonnis Marzano C, Lopane C, Corriero G, Longo C, Zambonin C, et al. Analytical investigations on the lindane bioremediation capability of the demosponge Hymeniacidon perlevis. Mar. Pollut. Bull. 2015;90:143–149. doi: 10.1016/j.marpolbul.2014.11.003. [DOI] [PubMed] [Google Scholar]
- [86].Reiche N, Mothes F, Fiedler P, Borsdorf HA. solid-phase microextraction method for the in vivo sampling of MTBE in common reed (Phragmites australis) Environ. Monit. Assess. 2013;185:7133–7144. doi: 10.1007/s10661-013-3089-3. [DOI] [PubMed] [Google Scholar]
- [87].Kramer R, Sauer-Heilborn A, Welte T, Guzman CA, Höfle MG, Abraham WR. A rapid method for breath analysis in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:745–751. doi: 10.1007/s10096-014-2286-5. [DOI] [PubMed] [Google Scholar]
- [88].Jiang R, Cudjoe E, Bojko B, Abaffy T, Pawliszyn J. A non-invasive method for in vivo skin volatile compounds sampling. Anal. Chim. Acta. 2013;804:111–119. doi: 10.1016/j.aca.2013.09.056. [DOI] [PubMed] [Google Scholar]
- [89].Bojko B, Gorynski K, Gomez-Rios GA, Knaak JM, Machuca T, Cudjoe E, et al. Low invasive in vivo tissue sampling for monitoring biomarkers and drugs during surgery. Lab. Invest. 2014;94:586–594. doi: 10.1038/labinvest.2014.44. [DOI] [PubMed] [Google Scholar]
- [90].Gottzein AK, Musshoff F, Madea B. Systematic toxicological analysis revealing a rare case of captan ingestion. J. Forensic.Sci. 2013;58:1099–1103. doi: 10.1111/1556-4029.12154. [DOI] [PubMed] [Google Scholar]
- [91].Silva C, Cavaco C, Perestrelo R, Pereira J, Câmara JS. Microextraction by packed sorbent (MEPS) and solid-phase microextraction (SPME) as sample preparation procedures for the metabolomic profiling of urine. Metabolites. 2014;4:71–97. doi: 10.3390/metabo4010071. [DOI] [PMC free article] [PubMed] [Google Scholar]