Abstract
Lactic acidosis (LA) is a marker for mortality in severe malaria, but the mechanisms that lead to LA in the different types of severe malaria and the extent to which LA-associated mortality differs by type of severe malaria are not well described. We assessed the frequency of LA in children admitted to Mulago Hospital, Kampala, Uganda with cerebral malaria (CM, n = 193) or severe malarial anemia (SMA, n = 216). LA was compared to mortality and measures of parasite biomass and sequestration (P. falciparum histidine-rich protein-2 (PfHRP2) concentration, platelet count), and to a measure of systemic tissue oxygen delivery (hemoglobin level). LA was more frequent in children with SMA than CM (SMA, 47.7%, CM, 34.2%, P = 0.006), but mortality was higher in children with CM (13.0%) than SMA (0.5%, P<0.0001). In CM, LA was associated with increased PfHRP2 concentration and decreased platelet count but was not associated with hemoglobin level. In contrast, in SMA, LA was associated with a decreased hemoglobin level, but was not associated with PfHRP2 concentration or platelet count. LA was related to mortality only in CM. In multivariable regression analysis of the effect PfHRP2 and hemoglobin levels on LA and DB, only PfHRP2 level increased risk of LA and DB in CM, while in SMA, elevated hemoglobin strongly decreased risk of LA and DB, and PfHRP2 level modestly increased risk of LA. The study findings suggest that LA in CM is due primarily to parasite sequestration, which currently has no effective adjunctive therapy, while LA in SMA is due primarily to anemia, which is rapidly corrected with blood transfusion. Differing etiologies of LA in CM and SMA may explain why LA is associated with mortality in CM but not SMA.
Introduction
The World Health Organization estimates that in 2015 there were 214 million new cases of malaria and 438,000 deaths [1]. 90% of these deaths occurred in sub-Saharan Africa, 77% were children under the age of 5, and 66% occurred within 24 hours of hospital admission[1, 2]. Lactic acidosis (LA) has been useful as a marker of disease severity in other infectious disease processes such as sepsis [3], and has been associated with mortality in multiple studies of severe malaria [4–7]. Since measurement of LA requires diagnostic equipment not available in many health centers in sub-Saharan Africa, researchers have assessed whether a clinical proxy would provide similar information. Respiratory distress (RD), a summary diagnosis comprised of nasal flaring, chest in-drawing, intercostal recessions, and deep breathing (DB) correlated strongly with LA [8], and studies by English et al investigating each component of RD separately found that DB was the most sensitive and specific indicator of severe metabolic acidosis in children with severe malaria [9]. The observation was confirmed by the Severe Malaria in African Children (SMAC) network, a network comprising more than 14,000 children in 3 countries, which found DB to be one of three clinical signs used in the Lambaréné organ dysfunction score to predict mortality in hospitalized patients with malaria [10].
Studies to date on the associations between LA, DB or RD and mortality have assessed children with severe malaria as a single clinical entity and have not assessed risks in the different types of severe malaria independently. Severe malarial anemia (SMA) is among the most common forms of severe malaria, with an estimated 1.5 to 5 million children affected annually [11]. Reported mortality rates in SMA vary widely from 1.7% [12] to 9% [13], and are consistently lower than the mortality rates for cerebral malaria (CM), which is less common but more often fatal, with an average 18.6% mortality [14]. Diverse etiologies can lead to acidosis in children suffering from different forms of severe malaria, so the reversal of LA requires knowledge of what is causing the LA. Potential contributors to LA in children include local tissue parasite sequestration, with resulting localized ischemia, which may occur in any form of severe malaria, but is likely most pronounced in CM, and systemic impairment of oxygen delivery due to low hemoglobin levels, which is most common in SMA.
To resolve the questions of how LA, RD and DB relate to each other, how they differ in two of the most common forms of severe malaria, how mortality with each factor differs, and which processes contribute to LA, RD and DB in each form of severe malaria, we assessed LA, RD and DB, along with markers of parasite biomass and sequestration and markers of systemic perfusion and oxygen delivery in a cohort of Ugandan children with CM or SMA.
Methods
Study Population
The study was performed at Mulago Hospital, Kampala, Uganda. The primary goal of the study was to assess the presence of neurocognitive impairment in children with CM or SMA, and pathogenesis of disease in these two forms of malaria. Children with CM, or SMA were enrolled if they were between 18 months and 12 years of age. CM was defined as: 1) coma (Blantyre Coma Score [BCS]≤2 or Glasgow Coma Score [GSC]≤8); 2) Plasmodium falciparum on blood smear; 3) no other known cause of coma (e.g., meningitis, a prolonged postictal state or hypoglycemia-associated coma reversed by glucose infusion). SMA was defined as presence of Plasmodium falciparum on blood smear in children with hemoglobin level ≤ 5 g/dL. Hemoglobin was measured by photometry (HemoControl; EKF Diagnostics). Exclusion criteria for all children included: 1) known chronic illness requiring medical care; 2) known developmental delay; or 3) prior history of coma, head trauma, hospitalization for malnutrition, or cerebral palsy. Additional exclusion criteria for children with SMA included 1) impaired consciousness to any degree on physical exam (BCS<5); 2) other clinical evidence of CNS disease; or 3) >1 seizure prior to admission.
Children with CM or SMA were managed according to the Ugandan Ministry of Health treatment guidelines current at the time of the study. These included initial intravenous quinine treatment followed by oral quinine for severe malaria while admitted, artemisinin combination therapy for outpatient follow-up therapy, and blood transfusion for all children with a hemoglobin ≤5 g/dL (which included all children with SMA, including children with concurrent CM and SMA).
Clinical and laboratory assessment
Respiratory distress (RD) was defined as the presence of acidotic breathing, nasal flaring, intercostal recessions, or chest indrawing, and deep breathing (DB) was defined solely by the presence of deep acidotic breathing. Plasma P. falciparum histidine-rich protein-2 (PfHRP2) levels were tested to assess parasite biomass and sequestration [15], and platelet counts measured to assess for platelet contribution to sequestration [16]. To assess systemic perfusion and oxygen delivery, hemoglobin levels and oxygen saturation were assessed.
On admission, venous blood was collected for microscopy and lactate measurement. Blood smears for microscopy were Giemsa stained and read independently by two readers, with discordant results resolved by a third reader. Lactate was measured using a point of care lactate analyzer (Accutrend lactate meter, Roche Diagnostics, Mannheim, Germany). Lactic acidosis was defined as a blood lactate level > 5.0 mmol/L. Plasma PfHRP2 levels were quantified using the commercially available Malaria Ag CELISA kit (Cellabs, Brookvale, Australia). The enzyme linked immunosorbent assays were performed according to the manufacturer’s protocol with optical density (OD) determined at 450 and 620 nm. Plasma was diluted 1:2400 in the kit-provided 1X PBS/Tween buffer (PBST). Samples with results above or below the range of the standard curve were retested at a dilution of 1:24,000 or 1:200, respectively. To assess intra-assay repeatability, 10% of samples (from a mix of study groups) from each assay plate were replicated on subsequent plates. The coefficient of variation for these samples was 19.6%. Total, circulating and sequestered parasite biomass were calculated using a formula adapted from Dondorp et al. [17] and Cunnington et al [18], in which total parasite biomass is 7.3* PfHRP2*(1- (hematocrit*0.01))*weight*(10^7), circulating biomass is parasite density*(10^6)*(0.08*weight), and sequestered parasite biomass is total biomass–circulating biomass.
Data Analysis
Data was entered into a FileMaker Pro 11 database (FileMaker Inc, Santa Clara, CA), and exported to and analyzed in Stata SE 12 (Stata Corporation, College Station, TX). Categorical variables were analyzed using the chi-squared test, and continuous variables were assessed using the Wilcoxon rank sum test. Logistic regression was used to explore the predictive power of LA, RD or DB on mortality. PfHRP2 levels and platelet count were not normally distributed and so were natural log transformed for regression analysis.
Ethical approval
Written informed consent was obtained from parents or guardians of study participants. The Institutional Review Boards for human studies at Makerere University School of Medicine and the University of Minnesota granted ethical approval for the study.
Results
Demographic, clinical and laboratory findings in children with CM compared to children with CM and SMA
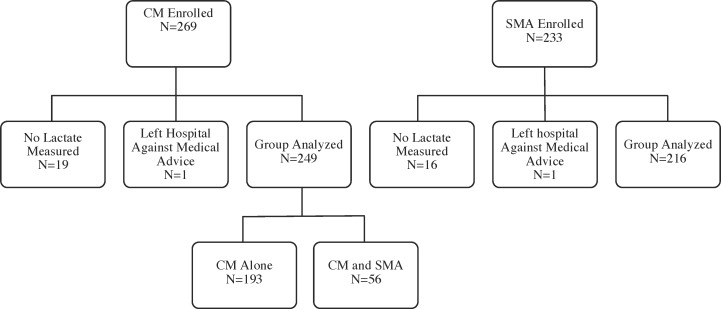
A total of 269 children with CM and 233 children with SMA were enrolled in the study. 1 child with CM and 1 with SMA left the hospital against medical advice, and 19 children with CM and 16 children with SMA did not have lactate level measured. Analysis was done on the remaining 249 children with CM and 216 children with SMA (Fig 1). Children with CM or SMA who had LA measured did not differ from those who did not have LA measured in terms of mortality (CM, 12.4% vs. 15.8%, P = 0.7; SMA, 0.5% vs 0%, P = 1.0) or frequency of deep breathing (CM, 8.8% vs. 10.5%, P = 0.7. SMA, 7.4% vs. 0%, P = 0.6).
Fig 1. Study enrollment and testing for lactic acidosis.
Of the 249 children with CM, 56 had concurrent SMA. Children with CM and concurrent SMA differed from children with CM alone in several aspects: they were significantly younger, and had more frequent respiratory distress and deep breathing, lower hemoglobin levels, higher PfHRP2 levels, and lower peripheral P. falciparum parasite density than children with CM alone (S1 Table). Despite these differences, mortality did not differ between children with CM alone (13.0%) and children with CM and SMA (10.7%). Because we wanted to explore how risk factors differed specifically in CM as compared to SMA, primary analysis was conducted comparing children with CM and no SMA (n = 193) to children with SMA alone (none of whom had CM). The subsequent analyses refer to these groups, but S1–S5 Tables describe the same findings as Tables 1–5 for children with both CM and SMA compared to children with CM alone.
Table 1. Demographic, clinical and laboratory findings children with cerebral malaria alone (CM) or severe malarial anemia alone (SMA) on admission.
| Characteristic or finding | CM | SMA | Pa |
|---|---|---|---|
| N = 193 | N = 216 | ||
| Age in years, mean (SD) | 4.2 (2.0) | 3.4 (1.7) | <0.0001 |
| Sex, N female (%) | 75 (38.9) | 84 (38.9) | 0.9 |
| Mortality, N (%) | 25 (13.0) | 1 (0.5) | <0.0001 |
| Respiratory distress, N (%) | 47 (24.4) | 65 (30.1) | 0.2 |
| Deep breathing, N (%) | 12 (6.2) | 16 (7.4) | 0.6 |
| Lactic acidosisb, N (%) | 66 (34.2) | 103 (47.7) | 0.006 |
| Blood lactate, mmol/L, median (IQR) | 3.8 (2.3, 6.5) | 4.8 (2.9, 8.1) | 0.001 |
| Hemoglobin, g/dL, mean (SD) | 7.8 (1.9) | 3.7 (0.9) | <0.0001 |
| O2 Saturation, median (IQR) | 97 (94, 99) | 98 (95, 99)c | 0.5 |
| O2 Saturation, <92%, N (%) | 19 (9.8) | 15 (6.9)c | 0.3 |
| Platelet count, 109/L, median (IQR) | 56 (35, 103)c | 154 (96, 234)c | <0.0001 |
| PfHRP2, 103ng/mL, median (IQR) | 2,561 (881, 5,148) | 881 (367, 2,581)c | <0.0001 |
| Peripheral blood P falciparum density, parasites/μL, median (IQR) | 53,120 (13,980, 340,770)c | 35,740 (10,100, 156,040)c | 0.005 |
a P-value for continuous variables compared by Students’ t-test if normally distributed and Wilcoxon rank-sum if skewed distribution, and for categorical variables by χ2 or Fisher’s exact test where appropriate
b Lactic acidosis defined as blood lactate > 5.0 mmol/liter
c N differs from total N and is noted in S6 Table
Table 5. Mortality in children with cerebral malaria (CM) or severe malarial anemia (SMA) in the presence or absence of deep breathing and lactic acidosis.
| CM | SMA | |||||
|---|---|---|---|---|---|---|
| N = 193 | N = 216 | |||||
| Finding | N with factor | % with factor | Mortality with factora | N with factor | % with factor | Mortality with factora |
| All children | 193 | - | 25 (13.0) | 216 | - | 1 (0.5) |
| DB | 12 | 6.2 | 6 (50.0) | 16 | 7.4 | 0 |
| LA | 66 | 34.2 | 13 (19.7) | 103 | 47.7 | 0 |
| DB and LA | 12 | 6.2 | 6 (50.0) | 15 | 6.9 | 0 |
| DB without LA | 0 | 0 | 0 | 1 | 0.5 | 0 |
| LA without DB | 54 | 28.0 | 7 (13.0) | 88 | 40.7 | 0 |
| No DB | 181 | 93.8 | 19 (10.5) | 200 | 92.6 | 1 (0.5) |
| No LA | 127 | 65.8 | 12 (9.5) | 113 | 52.3 | 1 (0.9) |
a N (%)
Demographic, clinical and laboratory findings in children with CM as compared to children with SMA
More than 60% of children with CM or SMA were male, and children with CM were significantly older than children with SMA. Mortality was significantly higher in children with CM (13.0%) than in children with SMA (0.5%, P<0.0001, Table 1). DB occurred at similar frequencies in children with CM or SMA, but LA was more common and lactic acid levels were higher in children with SMA than CM (Table 1). Children with CM had higher PfHRP2 levels, higher peripheral P. falciparum parasite density, and lower platelet count (all factors associated with parasite biomass and sequestration) than children with SMA, while children with SMA had lower hemoglobin levels (associated with less systemic tissue oxygen delivery) than children with CM. Children with CM had higher total, circulating and sequestered parasite biomass than children with SMA (Table 2).
Table 2. Parasite biomass.
| CM | SMA | Pa | |
| N = 166 | N = 179 | ||
| Total parasite biomass x 108, median (IQR) | 17,427 (5,747, 37,523) | 7,572 (2,371, 18,214) | <0.0001 |
| Circulating parasite biomass x 108, median (IQR) | 643 (170, 3,814) | 410 (124, 1,676) | 0.004 |
| Sequestered parasite biomass x 108, median (IQR) | 13,717 (4,628, 32,886) | 5,963 (1,546, 16,177) | <0.0001 |
| CM Deep Breathing | CM No Deep Breathing | ||
| N = 9 | N = 157 | ||
| Total parasite biomass x 108, median (IQR) | 44,831 (19,859, 202,395) | 16,610 (5,314, 34,895) | 0.01 |
| Circulating parasite biomass x 108, median (IQR) | 3,534 (868, 7,583) | 588 (169, 3,574) | 0.07 |
| Sequestered parasite biomass x 108, median (IQR) | 32,525 (16,325, 197,712) | 13,273 (4,535, 30,851) | 0.02 |
| CM lactic acidosis | CM No lactic acidosis | ||
| N = 57 | N = 109 | ||
| Total parasite biomass x 108, median (IQR) | 21,460 (11,688, 42,333) | 15,019 (3,792, 32,887) | 0.03 |
| Circulating parasite biomass x 108, median (IQR) | 1,092 (327, 4,721) | 488 (117, 3,271) | 0.05 |
| Sequestered parasite biomass x 108, median (IQR) | 16,325 (8,405, 39,820) | 12,362 (2,487, 30, 851) | 0.05 |
| SMA Deep Breathing | SMA No Deep Breathing | ||
| N = 9 | N = 170 | ||
| Total parasite biomass x 108, median (IQR) | 6,910 (5,238, 32,009) | 7,677 (2,251, 17,364) | 0.3 |
| Circulating parasite biomass x 108, median (IQR) | 869 (378, 2,769) | 397 (119, 1,597) | 0.1 |
| Sequestered parasite biomass x 108, median (IQR) | 6,249 (3,322, 28,502) | 5,892 (1,546, 16,030) | 0.6 |
| SMA lactic acidosis | SMA No lactic acidosis | ||
| N = 75 | N = 104 | ||
| Total parasite biomass x 108, median (IQR) | 8,620 (2,904, 22,886) | 6,260 (2,215, 14,081) | 0.05 |
| Circulating parasite biomass x 108, median (IQR) | 400 (99, 1,731) | 427 (133, 1,636) | 1.0 |
| Sequestered parasite biomass x 108, median (IQR) | 8,001 (2,056, 22,334) | 5,109 (1,471, 12,515) | 0.07 |
| CM Died | CM Survived | ||
| N = 22 | N = 144 | ||
| Total parasite biomass x 108, median (IQR) | 33,493 (13,481, 52,668) | 16,351 (5,208, 32,983) | 0.008 |
| Circulating parasite biomass x 108, median (IQR) | 1,069 (242, 3,240) | 578 (170, 4,111) | 0.8 |
| Sequestered parasite biomass x 108, median (IQR) | 31,957 (12,290, 51,668) | 13,011 (3,197, 29,789) | 0.006 |
a P-value for Wilcoxon rank-sum
Risk factors for deep breathing (DB) and lactic acidosis (LA) in children with CM as compared to children with SMA
Children with CM who had DB or LA had markers of increased parasite biomass and sequestration (higher PfHRP2, lower platelet count) compared to children with CM and no DB or LA, but the groups did not differ in hemoglobin levels (Table 3). In contrast, children with SMA and DB or LA had lower hemoglobin levels than children with SMA and no DB or LA, but the groups did not differ in markers of increased parasite biomass or sequestration (Table 3). Supporting this association, lactate levels were inversely correlated with hemoglobin level in children with SMA (Spearman’s rho = -0.3, P<0.0001) but not in children with CM (Spearman’s rho = -0.07, P = 0.3). Multivariable regression analysis of hemoglobin and log-transformed PfHRP2 showed that for children with CM, only increased PfHRP2 correlated with an increased risk of LA or DB (odds ratio (OR) [95% confidence interval (CI)], LA: PfHRP2, 1.54 [1.20, 1.99], P = 0.001, hemoglobin, 0.97 [0.82, 1.15], P = 0.76; DB: PfHRP2, 2,67 [1.48, 4.82], P = 0.001, hemoglobin, 0.97 [0.67, 1.40], P = 0.86). For children with SMA, elevated hemoglobin strongly decreased risk of LA, but increased PfHRP2 level was less strongly associated with an increased risk of LA (PfHRP2, 1.25 [1.05, 1.48], P = 0.01, hemoglobin, 0.39 [0.28, 0.57], P<0.001), and similarly elevated hemoglobin decreased and elevated PfHRP2 increased the risk of DB, the latter just shy of statistical significance (PfHRP2, 1.45 [0.98, 2.16], P = 0.06, hemoglobin, 0.48 [0.25, 0.90], P = 0.02). In children with both CM and SMA, neither hemoglobin level nor PfHRP2 was significantly associated with LA or DB (OR [95% CI], LA: PfHRP2, 1.27 [0.76, 2.13], P = 0.36, hemoglobin, 0.57 [0.27, 1.21], P = 0.15; DB: PfHRP2, 0.83 [0.46, 1.47], P = 0.52, hemoglobin, 0.92 [0.37, 2.30], P = 0.86)).
Table 3. Levels of disease pathogenesis markers in children with cerebral malaria and severe malaria anemia with vs. without deep breathing or lactic acidosis.
| Cerebral Malaria | ||||
| Deep breathing | No deep breathing | Pa | ||
| N = 12 | N = 181 | |||
| Platelet Count 109/L, median (IQR) | 34 (14, 43)b | 59 (35, 108)b | 0.008 | |
| PfHRP2103ng/ml, median (IQR) | 7,291 (3,550, 19,175) | 2,486 (812, 4,894) | 0.0005 | |
| Hemoglobin, g/dL, mean (SD) | 7.2 (1.5) | 7.8 (2.0) | 0.3 | |
| Peripheral blood P falciparum Densityb | 365,840 (47,880, 789,940)c | 49,765 (13,100, 295,060)c | 0.05 | |
| Lactic acidosis | No lactic acidosis | |||
| N = 66 | N = 127 | |||
| Platelet Count 109/L, median (IQR) | 42 (28, 62)c | 65 (38, 130)c | 0.0003 | |
| PfHRP2 103ng/ml, median (IQR) | 3,335 (1,680, 7,281) | 2,244 (542, 4,507) | 0.0005 | |
| Hemoglobin, g/dL, mean (SD) | 7.5 (2.0) | 7.9 (1.9) | 0.2 | |
| Peripheral blood P falciparum Densityb | 114,240 (29,000, 448,360)c | 42,890 (10,220, 248,210)c | 0.009 | |
| Severe Malaria Anemia | ||||
| Deep breathing | No deep breathing | Pa | ||
| N = 16 | N = 200 | |||
| Platelet Count 109/L, median (IQR) | 175 (118, 347) | 151 (95, 226)b | 0.3 | |
| PfHRP2103ng/ml, median (IQR) | 1,387 (515, 3,264)c | 848 (312, 2,578)c | 0.2 | |
| Hemoglobin, g/dL, mean (SD) | 3.3 (0.9) | 3.8 (0.9) | 0.03 | |
| Peripheral blood P falciparum Densityb | 37,570 (1,506, 256,710) | 35,180 (10,660, 151,760)c | 0.8 | |
| Lactic acidosis | No lactic acidosis | |||
| N = 103 | N = 113 | |||
| Platelet Count 109/L, median (IQR) | 160 (96, 281)c | 148 (94, 224)c | 0.7 | |
| PfHRP2 103ng/ml, median (IQR) | 952 (312, 3,134)c | 865 (371, 2,065)c | 0.2 | |
| Hemoglobin, g/dL, mean (SD) | 3.4 (0.9) | 4.0 (0.7) | <0.0001 | |
| Peripheral blood P falciparum Densityb | 32,983 (3,110, 123,180)c | 41,360 (12,380, 178,020) | 0.3 | |
a P-value for continuous variables compared by Students’ t-test if normally distributed and Wilcoxon rank-sum if skewed distribution, and for categorical variables by χ2 or Fisher’s exact test where appropriate
b Parasites/μL, median (IQR)
c N differs from total N and is noted in S6 Table.
In children with CM, total and sequestered parasite biomass were both higher for children with DB or LA. Circulating biomass was higher in children with LA and showed a trend toward increase in children with DB (P = 0.07, Table 2). In children with SMA, values for total, circulating, and sequestered parasite biomass did not differ significantly in children with vs. without DB, but children with SMA and LA did have higher total parasite biomass than children without LA, and children with SMA and LA also showed a trend toward higher sequestered parasite biomass (P = 0.07, Table 2).
Risk factors for mortality in children with CM
Risk factors for mortality were assessed only in children with CM. (Low mortality in children with SMA precluded analysis of risk factors). A higher proportion of children with CM who died had DB and LA than CM survivors, and children with CM who died showed markers of greater parasite biomass and sequestration (higher PfHRP2 levels, lower platelet counts) than children who survived CM (Table 4). Total and sequestered but not circulating parasite biomass were higher in children with CM who died than in those who survived (Table 2). We further assessed how DB and LA, alone or in combination, related to mortality in children with CM (Table 5). Children with DB (all of whom had LA) had the highest mortality rate (50.0%). Children with LA and no DB had a similar mortality rate to the overall cohort of children with CM (13.0%), and mortality was modestly lower in children without DB (10.2%) or without LA (9.5%). Among children with CM who received a transfusion, transfusion was not associated with a decrease in mortality (data not shown).
Table 4. Demographic, clinical and laboratory findings children with cerebral malaria (CM) who survived vs. died.
| Characteristic or finding | Died | Survived | Pa |
|---|---|---|---|
| N = 25 | N = 168 | ||
| Age in years, mean (SD) | 3.5 (1.7) | 4.3 (2.1) | 0.08 |
| Sex, N (% female) | 10 (40.0) | 65 (38.7) | 0.9 |
| Respiratory distress, N (%) | 9 (36.0) | 38 (22.6) | 0.1 |
| Deep breathing, N (%) | 6 (24.0) | 6 (3.6) | <0.0001 |
| Lactic acidosisb, N (%) | 13 (52.0) | 53 (31.6) | 0.04 |
| Blood lactate, mmol/L, median (IQR) | 5.1 (2.8, 7.8) | 3.7 (2.2, 6.2) | 0.09 |
| Hemoglobin, g/dL, mean (SD) | 8.2 (1.8) | 7.7 (2.0) | 0.3 |
| O2 Saturation, median (IQR) | 96 (93, 98) | 98 (95, 99) | 0.07 |
| O2 Saturation <92%, n (%) | 5 (20.0) | 14 (8.3) | 0.07 |
| Platelet count, 109/L, median (IQR) | 41 (17, 62)c | 60 (35, 110)c | 0.02 |
| PfHRP2, 103ng/mL, median (IQR) | 4,514 (1,598, 7,517) | 2,389 (834, 4,921) | 0.02 |
| Peripheral blood P falciparum densityd, | 83,680 (21,240, 340,770)c | 48,640 (12,920, 368,820)c | 0.5 |
a P-value for continuous variables compared by Students’ t-test if normally distributed and Wilcoxon rank-sum if skewed distribution, and for categorical variables by χ2 or Fisher’s exact test where appropriate
b Lactic acidosis defined as blood lactate > 5.0 mmol/liter
c N differs from total N and is noted in S6 Table
d Parasites/μL, median (IQR)
In a regression model including all factors associated with mortality (DB or LA, PfHRP2 level and platelet count), only DB was significantly associated with mortality (OR 4.3, 95% CI 1.1–17.1). Of note, the one child with SMA who died had neither DB nor LA (Table 3).
Discussion
In the present study, we show that LA occurs at similar frequency in CM and SMA, but correlates with mortality only in CM. We also show that the pathophysiologic correlates of LA are different in the two forms of severe malaria: in CM, LA correlates with increased parasite biomass and decreased platelet count, both factors associated with infected red blood cell sequestration, while in SMA, DB and LA primarily correlate with lower hemoglobin level, a factor associated with impaired tissue oxygen delivery, and correlate to a lesser extent with parasite biomass. The study findings suggest that in CM, LA is associated with greater parasite sequestration, for which there is currently no effective adjunctive therapy, while in SMA, LA is primarily associated with anemia and consequent impaired tissue oxygen delivery, which are rapidly corrected with blood transfusion. The differing primary etiologies of LA in CM and SMA may explain why LA is associated with mortality in CM but not SMA, and suggest that interventions to address LA in children with severe malaria must address the underlying cause of LA, which may differ in the different forms of severe malaria.
Multiple pathophysiologic processes can lead to LA. In studies of lactic acidosis in sepsis, for example, pathways that lead to LA include factors that lead to systemic or regional hypoxia, as well as factors not associated with hypoxia, such as underlying organ disease, drugs and toxins, and inborn errors of metabolism [19]. In the present study, LA appears to be associated with processes leading to systemic hypoxia in SMA and localized hypoxia and ischemia in CM. The present study and others have shown that prompt blood transfusions can reverse the inadequate O2 delivery associated with LA or DB in patients with SMA and will lead to resolution of LA and low mortality rates [20, 21]. The pathophysiology of platelet adhesion and parasite sequestration, however, is not easily reversed. As a result, LA and DB in CM have been associated with a significantly higher mortality rate in multiple studies of African children [7, 22, 23]. In sepsis and other illnesses, interventions specifically addressing the elevated lactate concentration or acidosis, such as administration of dichloroacetate [24] or bicarbonate [25], have not resulted in improved survival, even though in the case of dichloroacetate, acidemia improved. Dichloroacetate decreased mortality in an animal model of severe malaria [26], but the studies in sepsis and the findings of the present study suggest that interventions that can decrease or reverse the underlying cause of LA, presumably the sequestration process in children with CM, are likely to have greater benefit than interventions like dicholoroacetate. Trials of interventions that could decrease sequestration, e.g., low anticoagulant heparin derivatives [27], are therefore urgently needed, as they have the potential to decrease mortality and morbidity in CM.
In the present study, we show that parasite biomass appears to play a role in both CM and SMA, as total and sequestered biomass were higher in children with LA than without LA in both groups. However, thrombocytopenia was associated with LA only in children with CM. Laboratory studies have shown that platelets aid in the cytoadhesion of parasitized red blood cells and could be pathologically important to cerebral malaria [16, 28]. Post mortem studies of children with CM confirm these findings, reporting a significant increase of platelets in the brain microvasculature of children with CM as compared to children without it [29], and involvement of monocytes in platelets in sequestration in children with fatal CM [30]. The association of thrombocytopenia with LA in CM but not SMA in the present study is consistent with a contribution of platelet adhesion to sequestration in the brain and other organs, and supports the idea that sequestration, involving not only sequestered parasites but platelet adhesion and likely leukocyte adhesion (a factor more difficult to measure in peripheral blood samples), leads to LA in CM. Sequestration could also play a role in the LA seen in SMA, but the strong correlation of hemoglobin level with LA in SMA and not CM, and the low mortality in SMA with blood transfusion, both suggest that the primary cause of LA in SMA is a low hemoglobin level and the resulting systemic hypoxia and not sequestration. Children with both CM and SMA did not have significant associations of either hemoglobin level or PfHRP2 level with LA or DB, but the strongest association seen was of elevated hemoglobin with a decreased risk of LA (hemoglobin, OR, 95% CI, 0.57 [0.27, 1.21], P = 0.15), suggesting that children with both CM and SMA, LA is more strongly related to hemoglobin level than parasite biomass, i.e., that LA is driven most by the SMA component in these children.
Studies in malaria have shown a stronger relationship of base deficit than LA with mortality [31], and recent studies have shown the presence of previously unmeasured organic acids are also associated with mortality in Asian adults with severe malaria [32]. In the present study, the presence of DB but not RD was significantly associated with mortality in children with CM (P = <0.0001 and P = 0.1 respectively), confirming the observations of English et al [9] and the Severe Malaria in African Children (SMAC) network [10], that DB is the most useful respiratory sign in predicting mortality in children with malaria. DB emerged as the only independent risk factor for death when included in a multivariable regression model with PfHRP2 and platelet count, suggesting that the clinical manifestation of DB may be an “end pathway” result of the sequestration reflected by high PfHRP2 levels and low platelet counts.
The low mortality rate in children with SMA and LA in the present study, similar to that of a previous study of Ugandan children with SMA and LA (mortality 1.4%), demonstrates the benefit of rapid effective transfusion in SMA. In contrast, the similar mortality rates of children with CM in the present study (13.0%) and in an observational study published twenty years ago (16.8%) [33] show the need for effective adjunctive therapy in CM.
DB/metabolic acidosis alone, even in the absence CM or SMA, is an important form of severe malaria. The present study design did not include children with this form of severe malaria, but an ongoing study by our group in Uganda does include children with DB/metabolic acidosis as well as children with CM and SMA. When this study is completed, we will be able to compare the contribution of parasite biomass and hemoglobin to DB/LA and mortality in the three major forms of severe malaria: CM, SMA and DB/LA without CM or SMA. In this ongoing study, we are also assessing base deficit in addition to LA and DB, as this measure directly assesses acidosis from multiple acids in addition to LA. Our findings suggest LA may not be a good surrogate marker of mortality in studies of severe malaria that include SMA, as LA was common in SMA but did not predict mortality in this condition.
In conclusion, the present study provides evidence that LA and DB are caused by different primary pathogenic mechanisms in CM and SMA. The differences in mechanisms may explain why LA and DB are associated with mortality in CM but not SMA. The study findings suggest that in CM, LA and DB are associated with organ and tissue level sequestration, a process for which adjunctive therapies are currently unavailable, while in SMA, LA and DB are associated with anemia and consequent tissue hypoxia, which can be rapidly corrected by blood transfusion. The study findings support continued investigation of interventions to prevent or reduce parasite sequestration and platelet adhesion in cerebral malaria.
Supporting Information
(XLS)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the children and their parents who participated in this study, and the dedicated study team in Kampala, Uganda whose dedication to both the care of the patients and the study were foundational to the research we conducted. The authors have no associations that would pose conflicts of interest.
Data Availability
All relevant data are within the paper and its supporting information files.
Funding Statement
This work was supported by the grants from the National Institute of Neurologic Disorders and Stroke and the Fogarty International Center (R01 NS05534, D43 NS078280). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. World Malaria Report 2015. Geneva: World Health Organization, 2015. [Google Scholar]
- 2.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376(9753):1647–57. 10.1016/S0140-6736(10)61924-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Critical care medicine. 2015;43(3):567–73. 10.1097/CCM.0000000000000742 . [DOI] [PubMed] [Google Scholar]
- 4.Dzeing-Ella A, Nze Obiang PC, Tchoua R, Planche T, Mboza B, Mbounja M, et al. Severe falciparum malaria in Gabonese children: clinical and laboratory features. Malaria journal. 2005;4:1 10.1186/1475-2875-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mtove G, Nadjm B, Hendriksen IC, Amos B, Muro F, Todd J, et al. Point-of-care measurement of blood lactate in children admitted with febrile illness to an African District Hospital. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53(6):548–54. 10.1093/cid/cir471 . [DOI] [PubMed] [Google Scholar]
- 6.Newton CR, Valim C, Krishna S, Wypij D, Olola C, Agbenyega T, et al. The prognostic value of measures of acid/base balance in pediatric falciparum malaria, compared with other clinical and laboratory parameters. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;41(7):948–57. 10.1086/432941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English M, Sauerwein R, Waruiru C, Mosobo M, Obiero J, Lowe B, et al. Acidosis in severe childhood malaria. QJM: monthly journal of the Association of Physicians. 1997;90(4):263–70. 10.1093/qjmed/90.4.263 . [DOI] [PubMed] [Google Scholar]
- 8.Marsh K, English M, Crawley J, Peshu N. The pathogenesis of severe malaria in African children. Annals of tropical medicine and parasitology. 1996;90(4):395–402. . [DOI] [PubMed] [Google Scholar]
- 9.English M, Waruiru C, Amukoye E, Murphy S, Crawley J, Mwangi I, et al. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. The American journal of tropical medicine and hygiene. 1996;55(5):521–4. . [DOI] [PubMed] [Google Scholar]
- 10.Helbok R, Kendjo E, Issifou S, Lackner P, Newton CR, Kombila M, et al. The Lambarene Organ Dysfunction Score (LODS) is a simple clinical predictor of fatal malaria in African children. The Journal of infectious diseases. 2009;200(12):1834–41. 10.1086/648409 . [DOI] [PubMed] [Google Scholar]
- 11.Breman JG, Holloway CN. Malaria surveillance counts. The American journal of tropical medicine and hygiene. 2007;77(6 Suppl):36–47. . [PubMed] [Google Scholar]
- 12.Dhabangi A, Mworozi E, Lubega IR, Cserti-Gazdewich CM, Maganda A, Dzik WH. The effect of blood storage age on treatment of lactic acidosis by transfusion in children with severe malarial anaemia: a pilot, randomized, controlled trial. Malaria journal. 2013;12:55 10.1186/1475-2875-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton CR, Warn PA, Winstanley PA, Peshu N, Snow RW, Pasvol G, et al. Severe anaemia in children living in a malaria endemic area of Kenya. Tropical medicine & international health: TM & IH. 1997;2(2):165–78. 10.1046/j.1365-3156.1997.d01-238.x . [DOI] [PubMed] [Google Scholar]
- 14.Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacology & therapeutics. 1998;79(1):1–53. 10.1016/s0163-7258(98)00008-4 . [DOI] [PubMed] [Google Scholar]
- 15.Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L, Mtove G, White LJ, Olaosebikan R, et al. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 measurement. PLoS medicine. 2012;9(8):e1001297 10.1371/journal.pmed.1001297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassmer SC, Taylor T, Maclennan CA, Kanjala M, Mukaka M, Molyneux ME, et al. Platelet-induced clumping of Plasmodium falciparum-infected erythrocytes from Malawian patients with cerebral malaria-possible modulation in vivo by thrombocytopenia. The Journal of infectious diseases. 2008;197(1):72–8. 10.1086/523761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS medicine. 2005;2(8):e204 10.1371/journal.pmed.0020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunnington AJ, Bretscher MT, Nogaro SI, Riley EM, Walther M. Comparison of parasite sequestration in uncomplicated and severe childhood Plasmodium falciparum malaria. The Journal of infection. 2013;67(3):220–30. 10.1016/j.jinf.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suetrong B, Walley KR. Lactic Acidosis in Sepsis: It's Not All Anaerobic: Implications for Diagnosis and Management. Chest. 2016;149(1):252–61. 10.1378/chest.15-1703 . [DOI] [PubMed] [Google Scholar]
- 20.English M, Waruiru C, Marsh K. Transfusion for respiratory distress in life-threatening childhood malaria. The American journal of tropical medicine and hygiene. 1996;55(5):525–30. . [DOI] [PubMed] [Google Scholar]
- 21.English M, Muambi B, Mithwani S, Marsh K. Lactic acidosis and oxygen debt in African children with severe anaemia. QJM: monthly journal of the Association of Physicians. 1997;90(9):563–9. 10.1093/qjmed/90.9.563 . [DOI] [PubMed] [Google Scholar]
- 22.Cserti-Gazdewich CM, Dhabangi A, Musoke C, Ssewanyana I, Ddungu H, Nakiboneka-Ssenabulya D, et al. Inter-relationships of cardinal features and outcomes of symptomatic pediatric Plasmodium falciparum MALARIA in 1,933 children in Kampala, Uganda. The American journal of tropical medicine and hygiene. 2013;88(4):747–56. 10.4269/ajtmh.12-0668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awandare GA, Goka B, Boeuf P, Tetteh JK, Kurtzhals JA, Behr C, et al. Increased levels of inflammatory mediators in children with severe Plasmodium falciparum malaria with respiratory distress. The Journal of infectious diseases. 2006;194(10):1438–46. 10.1086/508547 . [DOI] [PubMed] [Google Scholar]
- 24.Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. The New England journal of medicine. 1992;327(22):1564–9. 10.1056/NEJM199211263272204 . [DOI] [PubMed] [Google Scholar]
- 25.Cooper DJ, Walley KR, Wiggs BR, Russell JA. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study. Annals of internal medicine. 1990;112(7):492–8. . [DOI] [PubMed] [Google Scholar]
- 26.Holloway PA, Knox K, Bajaj N, Chapman D, White NJ, O'Brien R, et al. Plasmodium berghei infection: dichloroacetate improves survival in rats with lactic acidosis. Experimental parasitology. 1995;80(4):624–32. 10.1006/expr.1995.1078 . [DOI] [PubMed] [Google Scholar]
- 27.Vogt AM, Pettersson F, Moll K, Jonsson C, Normark J, Ribacke U, et al. Release of sequestered malaria parasites upon injection of a glycosaminoglycan. PLoS pathogens. 2006;2(9):e100 10.1371/journal.ppat.0020100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pain A, Ferguson DJ, Kai O, Urban BC, Lowe B, Marsh K, et al. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1805–10. 10.1073/pnas.98.4.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. The Journal of infectious diseases. 2003;187(3):461–6. 10.1086/367960 . [DOI] [PubMed] [Google Scholar]
- 30.Hochman SE, Madaline TF, Wassmer SC, Mbale E, Choi N, Seydel KB, et al. Fatal Pediatric Cerebral Malaria Is Associated with Intravascular Monocytes and Platelets That Are Increased with HIV Coinfection. mBio. 2015;6(5):e01390–15. 10.1128/mBio.01390-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, et al. Predicting the clinical outcome of severe falciparum malaria in african children: findings from a large randomized trial. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(8):1080–90. 10.1093/cid/cis034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herdman MT, Sriboonvorakul N, Leopold SJ, Douthwaite S, Mohanty S, Hassan MM, et al. The role of previously unmeasured organic acids in the pathogenesis of severe malaria. Critical care. 2015;19:317 10.1186/s13054-015-1023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. The New England journal of medicine. 1995;332(21):1399–404. 10.1056/NEJM199505253322102 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its supporting information files.