Abstract
Objectives
The metabolic syndrome (MetS) is a cluster of metabolic abnormalities and cardiovascular risk factors that are highly heritable and polygenic. We investigated the association of allelic variants of three candidate genes, rs1799883-FABP2, rs1501299-ADIPOQ and rs5065-ANP with MetS and its components, individually and in combination, using a genetic risk score.
Methods
A cross-sectional study was conducted in 462 Afro-Caribbeans subjects without cardiovascular complications or lipid-lowering medications. Cardiovascular risk factors and MetS components (NCEP-ATPIII criteria) were recorded. The 3 SNPs were genotyped. The genetic risk score was calculated by summing the number of risk alleles at each locus. Logistic regressions were used.
Results
Fifty-eight participants (12.6%) were diabetics and 116 (25.1%) had a MetS. In a dominant model, rs1799883 was associated with hypertriglyceridemia (OR 2.22; P = 0.014) and hypertriglyceridemic waist (HTGW), (P = 0.014) but not significantly with overweight (P = 0.049), abdominal obesity (P = 0.033) and MetS (P = 0.068). In a dominant model, the OR of MetS and HTGW for rs1501299 were 1.80 (P = 0.028) and 2.19 (P = 0.040) respectively. In a recessive model, the OR of hypertriglyceridemia for rs5065 was 1.94 (P = 0.075). The genetic risk score was significantly associated with MetS. Subjects carrying 4–5 risk alleles (18.8%) had a nearly 2.5-fold-increased risk of MetS compared to those carrying 0–1 risk allele (24.3%): OR 2.31; P = 0.025.
Conclusions
This study supports the association of FABP2, ANP and ADIPOQ gene variants with MetS or its components in Afro-Caribbeans and suggests a cumulative genetic influence of theses variants on this syndrome and a potential effect on lipid metabolism.
Introduction
The metabolic syndrome (MetS) is a set of metabolic and cardiovascular risk factors associated with an increased prevalence of diabetes and cardiovascular diseases. The underlying cause of MetS is still unclear but, central obesity, adipose tissue dysregulation and insulin-resistance are considered as key contributors [1]. Each component of MetS has a substantial part of heritability indicating that genetic factors may have an important influence in the pathogenesis of this syndrome. In this line, previous studies demonstrated the association of three candidate gene variants rs1799883 (FABP2), rs1501299 (ADIPOQ) and rs5065 (ANP), separately, with metabolic phenotypes.
The fatty acid binding protein 2 (FABP2) gene is located on the long arm of chromosome 4 and encodes the intestinal FABP2. The G to A transition of codon 54 results in a threonine (Thr) for alanine (Ala) substitution. Associations between FABP2 genetic variants and different metabolic phenotypes have been reported in several studies mostly in Caucasian, American Indian or Asian populations [2–4].
Adiponectin, an adipose tissue-derived cytokine was linked to central obesity and proposed as a major contributor to MetS in addition to insulin resistance [5]. The adiponectin-encoding gene, ADIPOQ, is located on chromosome 3q27 within a region linked to type 2 diabetes mellitus, metabolic syndrome and coronary artery disease [6]. One of the most reported common variant, the rs1501299 (276 G>T) polymorphism, located in intron 2, was reported to be associated with MetS [7].
Several studies have demonstrated that atrial natriuretic peptide (ANP) levels are associated with obesity, metabolic syndrome and its components [8–10]. Interactions between ANP and adiponectin concentrations were also reported [11].
The genetic variant rs5065 of the ANP gene (chromosome 1), which introduces a stop codon that leads to the extension of the ANP peptide from 28 to 30 amino acids, is also one of the most studied ANP variant and has been shown to be associated to diabetic complications and cardiovascular disease [12, 13]. In the French Caribbean Island of Guadeloupe where the majority of the population is Afro-Caribbean (about 85%), there is a high prevalence of hypertension, diabetes, and obesity.
We hypothesized that each of the three variants, rs1799883 (FABP2), rs1501299 (ADIPOQ) and rs5065 (ANP), could be related to key underlying pathological processes of MetS and, that a genetic risk score representing their cumulative effect could be a predictor of MetS-risk in Afro-Caribbeans.
Materials and Methods
Study population
In a cross-sectional study conducted in the island of Guadeloupe, we studied 462 type 2 diabetic and non-diabetic patients from the Department of Diabetology and from the Health centre of the island. All participants were Afro-Caribbeans and were included in the study from 2008 to 2013. The ethnic origin was defined whether the patient defined him/herself and his/her two first-degree relatives as Afro-Caribbean. The exclusion criteria included pregnant women, patients with previous history of kidney or inflammatory disease, previous cardiovascular complications (coronary artery disease, stroke), those treated with lipid-lowering medications and those with another ethnical background. The protocol was approved by the Ethic Committee (South West—Overseas III, Bordeaux, France). All participants gave their written informed consent.
The individuals were interviewed by physicians using a standard questionnaire (S1 and S2 files).
Height and weight were measured with participants standing without shoes and lightly clothed. BMI was calculated as weight divided by height squared (kg/m2). These measurements were made by trained nurses and physicians. Blood pressure was measured according to a standardized protocol with an automatic sphygmomanometer. The retained values were the average of two or more readings. Blood samples were obtained from participants after overnight fasting. Plasma cholesterol and triglyceride concentrations were measured by enzymatic method (Boehringer-Mennheim). All usual blood tests were performed with standardized programs.
Genotyping
Genomic DNA was extracted from peripheral white blood cells by standard methods and stored at –20°C until analysis. Genotyping of the study population was performed using a TaqMan allelic discrimination assay on an ABI PRISM 7900 HT sequence detector according to manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA).
Three single-nucleotide polymorphisms (SNPs) were genotyped: rs1799883, (2445G>A) Ala54Thr of FABP2, rs1501299 (276G>T) of ADIPOQ and rs5065 (A>G, also referred as 2238T>C) of ANP gene.
These polymorphisms were chosen because of different data from the literature: significant associations between rs1799883 and MetS [14], association of rs1501299 with the concomitant presence of MetS and hypertension in a Taiwanese population [15], and association of rs5065 with diabetic complications and cardiovascular disease [12, 13]. In addition, previous studies in relation with cardiometabolic risk were conducted for rs1501299 and rs5065 in our Afro-Caribbean diabetic population with and without history of CAD [16, 17], and in descendants of Indian migrants living in Guadeloupe for rs1799883 [18].
Clinical factors
Overweight was defined as BMI ≥ 25 kg/m2. Dyslipidaemia was defined as having at least one of the following: High-density lipoprotein-cholesterol (HDL-C) concentration < 1.04 mmol/l in men or < 1.29 mmol/l in women, triglyceride concentration ≥ 1.69 mmol/l, Low-density lipoprotein-cholesterol (LDL-C) concentration ≥ 3.40 mmol/l.
The “hypertriglyceridemic waist” phenotype (HTGW), was defined as a waist circumference ≥ 90 cm in men or ≥ 85 cm in women, along with a plasma triglyceride concentration ≥ 2.0 mmol/l in men or ≥ 1.5 mmol/l in women [19, 20].
MetS was diagnosed according to the NCEP/ATP III definition, in patients having at least three of the following five criteria: systolic blood pressure ≥ 130 and/or diastolic blood pressure ≥ 85 mmHg, abdominal obesity (waist circumference > 102 cm in men or > 88 cm in women), hypertriglyceridemia with triglyceride level ≥ 1.69 mmol/L (150 mg/dL), high-density lipoprotein-cholesterol (HDL-C) level < 1.04 mmol/L (40 mg/dL) in men and < 1.29 mmol/L (50 mg/dL) in women, fasting plasma glucose level ≥ 6.1 mmol/L (110 mg/dL) or diabetes [21].
Statistical analysis
Data are presented as numbers (percentages) for categorical variables and as means ± standard deviations (SD) for continuous variables. The chi-squared test and ANCOVA with adjustment for age, sex and diabetes were used to test percentage and mean differences between groups, respectively.
We examined the associations between SNPs, MetS components and MetS. The logistic regression models were tested including each SNP alone with adjustment for age, sex and diabetes. Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated for MetS and for each MetS component.
We used a genetic risk score approach to evaluate the combined effects of SNPs in relation with MetS components and MetS. The genetic risk score (GRS) was calculated by summing the number of risk alleles at each locus. ORs for metabolic syndrome according to the number of risk alleles of the 3 SNPs were also estimated.
The IBM SPSS Statistics software version 21.0 was used for data analyses. All tests were two-sided. A P value < 0.05 was considered to be statistically significant. A correction was applied for multi testing with a significant P value < 0.016.
Results
Characteristics of the study population
Four hundred sixty-two individuals were included in the study. Mean age was 48.9 ± 13.3 years.
Among the participants, 260 (56.3%) were women and 58 (12.6%) were people with diabetes. Overall 116 (25.1%) had MetS with 78 (19.3%) in people without diabetes vs 38 (65.5%) in people with diabetes.
The characteristics and genotype distributions of the study population according to presence or absence of MetS are shown in Table 1.
Table 1. Patients’ characteristics according to metabolic syndrome status.
| Metabolic Syndrome | |||||
|---|---|---|---|---|---|
| Characteristics | NO | YES | P | ||
| Quantitative variables* | |||||
| N | 462 | 346 | 116 | ||
| Age (year) | 462 | 48 ± 13 | 53 ± 13 | < 0.001 | |
| Body mass index (Kg/m2) | 461 | 26 ± 5 | 32 ± 6 | < 0.001 | |
| Waist circumference (cm) | 462 | 87 ± 11 | 101 ± 11 | < 0.001 | |
| Systolic blood pressure (mm Hg) | 462 | 132 ± 18 | 142 ± 16 | < 0.001 | |
| Diastolic blood pressure (mm Hg) | 462 | 81 ± 11 | 87 ± 11 | < 0.001 | |
| HDL-Cholesterol (mmol/L) | 462 | 1.50 ± 0.47 | 1.17 ± 0.39 | < 0.001 | |
| Triglycerides (mmol/L) | 462 | 0.87 ± 0.91 | 1.54 ± 1.41 | < 0.001 | |
| Fasting blood glucose (mmol/L) | 462 | 4.87 ± 1.12 | 5.86 ± 2.54 | < 0.001 | |
| Qualitative variables: | |||||
| N | 462 | 346 | 116 | ||
| Women | 260 | 175 (50.6) | 85 (73.3) | < 0.001 | |
| Men | 202 | 171 (49.4) | 31 (26.7) | ||
| Diabetes | 58 | 20 (5.8) | 38 (32.8) | < 0.001 | |
| Overweight ** | 297 | 195 (56.4) | 102 (88.7) | < 0.001 | |
| Abdominal Obesity | 179 | 78 (22.5) | 101 (87.1) | < 0.001 | |
| Hypertension | 291 | 181 (52.3) | 110 (94.8) | < 0.001 | |
| Low HDL-Cholesterol | 127 | 58 (16.8) | 69 (59.5) | < 0.001 | |
| High Triglycerides | 49 | 21 (6.1) | 28 (24.1) | < 0.001 | |
| High Fasting glucose *** | 113 | 45 (13) | 68 (58.6) | < 0.001 | |
| Hypertriglyceridemic waist | 42 | 10 (2.9) | 32 (27.6) | < 0.001 | |
| Genetic parameters | |||||
| FABP2 rs1799883 | N | 436 | 324 | 112 | |
| GG | 252 | 195 (60.2) | 57 (50.9) | 0.086 | |
| GA / AA | 184 | 129 (39.8) | 55 (49.1) | ||
| ADIPOQ rs1501299 | N | 346 | 116 | 346 | |
| GG | 170 | 136 (42.5) | 34 (30.4) | 0.024 | |
| GT / TT | 262 | 184 (57.5) | 78 (69.5) | ||
| NPPA rs5065 | N | 430 | 323 | 107 | |
| AA | 150 | 108 (33.4) | 42 (39.3) | 0.274 | |
| AG / GG | 280 | 215 (66.6) | 65 (60.7) | ||
* ANCOVA adjusted for age and sex and diabetes. Data are presented as mean ± SD or number (column percentage).
** Overweight, including obese.
*** High fasting glucose, including known diabetes. HDL: high-density lipoprotein.
The following analyses were restricted to the 415 individuals with available data for all the parameters studied including the three SNPs.
Mean levels of all the cardiovascular risk factors (except for HDL-C level) were higher in individuals with MetS than in those without MetS (P < 0.001 for all). Frequencies of overweight, HTGW and all the MetS components were higher in individuals with MetS than in those without MetS (P < 0.001 for all).
The genotype distributions in the overall study population were within the Hardy—Weinberg equilibrium for rs1799883 G>A (57.8% GG, 34.9% GA, 7.3% AA; P = 0.17), rs1501299 G> T (39.4% GG, 46.5% GT, 14.1% TT; P = 0.90) and rs5065 A>G (34.9% AA, 48.6% AG, 16.5% GG; P = 0.90).
The frequencies of ADIPOQ- rs1501299 (GT/TT) and of FABP2- rs1799883 (GA/AA) minor allele carriers in individuals with and without MetS were 69.5% vs 57.5% (P = 0.024) and 49.1% vs 38.9% (P = 0.086), respectively.
Logistic regressions of metabolic syndrome components, metabolic syndrome overweight and HTGW according to genotypes
In Table 2 are presented the adjusted ORs for risk of hypertension, abdominal obesity, Low-HDL cholesterol levels, high triglycerides levels, high fasting blood glucose levels and MetS according to the three SNP genotypes. The regressions were performed separately for each SNP after adjustments for age, sex and diabetes.
Table 2. Logistic regressions of metabolic syndrome components and metabolic syndrome for rs1799883 (FABP2), rs1501299 (ADIPOQ) and rs5065 (ANP) gene polymorphisms.
| Hypertension | Abdominal obesity | Low HDL-Cholesterol | High Triglycerides | High fasting blood Glucose | Metabolic syndrome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | |||||||||||||
| 415 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| rs1799883 (FABP2) | |||||||||||||
| GG | 244 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| GA / AA | 171 | 1.50 (0.97–2.33) | 0.068 | 1.67 (1.04–2.66) | 0.033 | 1.28 (0.82–1.99) | 0.277 | 2.22 (1.18–4.19) | 0.014 | 0.83 (0.48–1.43) | 0.495 | 1.59 (0.97–2.62) | 0.068 |
| rs1501299 (ADIPOQ) | |||||||||||||
| GG | 166 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| GT / TT | 249 | 1.11 (0.71–1.71) | 0.641 | 1.28 (0.80–2.04) | 0.311 | 1.11 (0.71–1.75) | 0.647 | 1.49 (0.77–1.86) | 0.235 | 1.22 (0.74–2.01) | 0.434 | 1.80 (1.07–3.05) | 0.028 |
| rs5065 (ANP) | |||||||||||||
| AG / GG | 270 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| AA | 145 | 1.20 (0.77–1.87) | 0.431 | 1.32 (0.82–2.13) | 0.249 | 1.49 (0.95–2.35) | 0.081 | 1.77 (0.94–3.31) | 0.075 | 0.91 (0.52–1.60) | 0.749 | 1.24 (0.74–2.06) | 0.413 |
ORs were calculated separately for each SNP after adjusting for age, gender and diabetes
In a dominant model, the rare allele of rs1799883 (FABP2) (GA/AA vs GG) was associated with prevalence of high triglycerides levels (OR 2.22; P = 0.014) but not with prevalence of abdominal obesity (OR 1.67; P = 0.033) and MetS (OR 1.59; P = 0.068).
Carriers of the minor allele of rs1799883 had also an increased risk of HTGW phenotype compared to GG homozygotes (P = 0.014), Table 3.
Table 3. Logistic regressions of overweight and hypertriglyceridemic waist for rs1799883 (FABP2), rs1501299 (ADIPOQ) and rs5065 (NPPA) gene polymorphisms.
| Overweight | Hypertriglyceridemic waist | ||||
|---|---|---|---|---|---|
| N | OR (95% CI) | P | OR (95% CI) | P | |
| 415 | |||||
| FABP2 rs1799883 | |||||
| GG | 244 | 1 | 1 | ||
| GA / AA | 171 | 1.53 (1.01–2.33) | 0.049 | 2.32 (1.19–4.52) | 0.014 |
| ADIPOQ rs1501299 | |||||
| GG | 166 | 1 | 1 | ||
| GT / TT | 249 | 1.01 (0.47–1.53) | 0.961 | 2.19 (1.04–4.64) | 0.040 |
| ANP rs5065 | |||||
| AG / GG | 270 | 1 | 1 | ||
| AA | 145 | 1.14 (0.74–1.75) | 0.556 | 1.27 (0.65–2.47) | 0.477 |
ORs were calculated separately for each SNP after adjusting for age, gender and diabetes
In a dominant model, the OR of MetS and HTGW phenotype for the rare allele of rs1501299 (ADIPOQ) (GT/TT vs GG) were 1.80 (P = 0.028) and 2.19 (P = 0.040) respectively, Table 3.
The ORs of Low HDL-C and high triglycerides levels for the most frequent allele of rs5065 (ANP) in a recessive model (AA vs AG/GG) were 1.49 (P = 0.081) and 1.77 (P = 0.075), respectively, Table 2.
Table 4 shows the adjusted ORs of MetS for age, gender, diabetes, rs1799883 (FABP2), rs1501299 (ADIPOQ) and rs5065 (ANP). Age (OR 1.04; P = 0.001), gender (OR 2.95; P < 0.001) and diabetes (OR 5.03; P < 0.001) were associated with an increased risk of MetS. The ORs of MetS for carrying the rare allele (GA + AA) of rs1799883 (P = 0.087), the rare allele (GT + TT) of rs1501299 (P = 0.035) or the frequent allele AA of rs5065 (P = 0.549) did not reach significance.
Table 4. Logistic regression of metabolic syndrome with age, gender, diabetes and the three gene polymorphisms (rs1799883, rs1501299 and rs5065) as covariates.
| OR (95% CI) | P | ||
|---|---|---|---|
| Age (y) | 415 | 1.04 (1.02–1.06) | 0.001 |
| Gender | |||
| Male | 186 | 1 | |
| Female | 229 | 2.98 (1.75–5.08) | <0.001 |
| Diabetes | |||
| No | 372 | 1 | |
| Yes | 43 | 5.03 (2.33–10.9) | <0.001 |
| FABP2 rs1799883 | |||
| GG | 244 | 1 | |
| GA / AA | 171 | 1.55 (0.94–2.57) | 0.087 |
| ADIPOQ rs1501299 | |||
| GG | 166 | 1 | |
| GT / TT | 249 | 1.77 (1.04–3.00) | 0.035 |
| ANP rs5065 | |||
| AG / GG | 270 | 1 | |
| AA | 145 | 1.17 (0.70–1.96) | 0.549 |
Adjusted ORs were calculated for the six covariates included concomitantly in the model.
Relationship between GRS and metabolic syndrome
The risk alleles for MetS were A for rs1799883, T for rs1501299 and A for rs5065. The GRS was calculated for each individual by summing the number of risk allele at each locus (0 if absence of risk allele, 1 if heterozygote, 2 if homozygote for the risk allele). The mean GRS was significantly higher in patients with high triglyceride levels (2.76 vs 2.36 respectively; P = 0.036) and in those with MetS (2.67 vs 2.32 respectively; P = 0.021) (Table 5).
Table 5. Mean genetic risk score, in 415 individuals, according to presence or absence of metabolic syndrome or metabolic syndrome components.
| Metabolic syndrome or metabolic syndrome components | |||||
|---|---|---|---|---|---|
| YES | NO | P | |||
| N | GRS ± SD | N | GRS ± SD | ||
| Metabolic syndrome components | |||||
| Abdominal Obesity | 157 | 2.53 ± 1.29 | 258 | 2.33 ± 1.15 | 0.141 |
| Hypertension | 262 | 2.46 ± 1.21 | 153 | 2.29 ± 1.20 | 0.187 |
| Low HDL-Cholesterol | 118 | 2.49 ± 1.25 | 297 | 2.37 ± 1.19 | 0.368 |
| High Triglycerides | 47 | 2.76 ± 1.06 | 368 | 2.36 ± 1.22 | 0.036 |
| High FBG or diabetes | 105 | 2.47 ± 1.29 | 310 | 2.38 ± 1.18 | 0.617 |
| Metabolic syndrome | 102 | 2.67 ± 1.30 | 313 | 2.32 ± 1.16 | 0.021 |
Data are mean ± SD.
ANCOVA adjusted for age, gender and diabetes
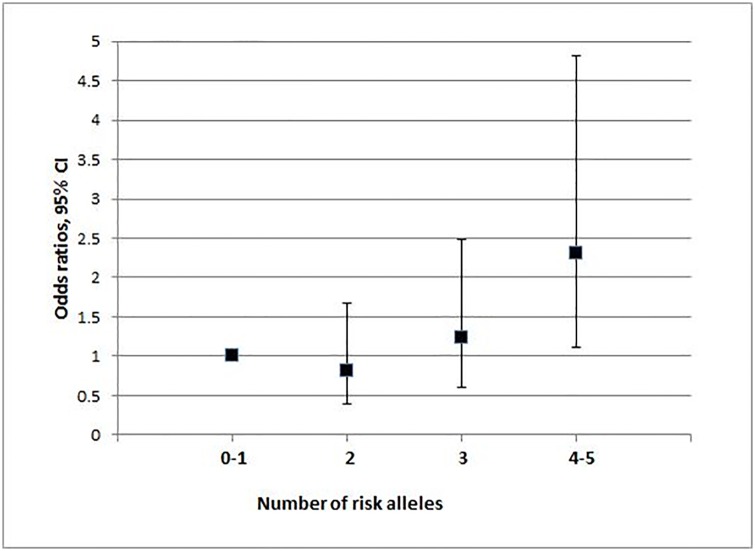
After adjustment for age, gender and diabetes and taking patients with 0–1 risk allele as the reference group (24.3%), the odds ratio (OR) of MetS for patients with 4–5 risk alleles (18.8%) was 2.31 (95% CI 1.11–4.81; P = 0.025) (Fig 1).
Fig 1. Odds ratios for metabolic syndrome according to the number of risk alleles of the 3 SNPs: rs1799883 (Ala54Thr), rs1501299 (276 G>T), rs5065 (2238 T>C).
Number of risk alleles 0–1: reference group. For 2 risk alleles: P = 0.57. For 3 risk alleles: P = 0.50. For 4–5 risk alleles: P = 0.025.
Discussion
In the present study we examined the association of rs1501299 (ADIPOQ), rs1799883 (FABP2) and rs5065 (ANP) variants with MetS-risk in an Afro-Caribbean population. The main finding of our study is that a genetic risk score summarizing the influence of these 3 SNPs, was associated with metabolic syndrome. Our results are in favour of a genetic contribution leading to the development of MetS in this population.
There are strong evidences that ANP, FABP2 and adiponectin play an important role in key metabolic processes and especially in lipids metabolism. The three SNPs investigated in this study are among the most studied variants of their respective genes and two of them (rs5065-ANP and rs1799883-FABP2) affect directly the function of the cognate protein. These SNPs were also selected based on prior knowledge from studies conducted in our population.
The sample from this multi-ethnic population included only people of Afro-Caribbean origin determined if the patient defined him/herself and his/her two first-degree relatives as Afro-Caribbean. We assumed that the combination of these inclusions criteria allowed the selection of a homogeneous and representative sample of the Afro-Caribbean population. We have previously shown in a sample selected by these criteria, from our population, that the distribution of SNP-allele frequencies for 19 SNPs was very close to that of the Afro-Caribbean from Barbados published in 1000 genome [22].
Fatty acid binding protein 2, rs1799883 and metabolic syndrome
FABP2 Ala54Thr polymorphism (rs1799883) was significantly associated with hypertriglyceridemia (OR = 2.22; P = 0.014). A significant association was also noted with hypertriglyceridemic waist phenotype which is known as an optimal screening tool to identify subjects with MetS and increased risk of cardiovascular disease [19].
Fatty acid-binding proteins (FABPs) are members of the super family of small intracellular lipid-binding proteins and intestinal FABP (I-FABP or FABP2) is one of nine different FABPs. FABP2 has a high affinity for saturated and unsaturated long-chain fatty acids, and is widely understood to be involved in the absorption, growth and transport of dietary fatty acids and to increase fat oxidation, which has been shown to reduce insulin action [23].
Among the factors involved in MetS, genetic polymorphisms are often implicated, especially those responsible for metabolism and transport of lipids [24], regulation of blood pressure and regulation of glucose metabolism.
It has been reported that the Ala54Thr polymorphism of FABP2 (rs1799883), has an important effect on postprandial lipids as the threonine-containing protein has a twofold greater affinity for the long chain fatty acids than the alanine-containing protein [23, 25]. As a result, higher fasting plasma levels of cholesterol and TG (especially in postprandial stage) were found in subjects carrying the Thr54 mutation [26]. This excessive level of fatty acids (associated with the Thr54) and their preferential use as a source of energy by skeletal muscle rather than glucose, contribute to an increase in glucose levels, higher basal and glucose-stimulated insulin levels and higher degree of insulin resistance [2, 14, 23]. Significant associations between the FABP2 Ala54Thr polymorphism and MetS components or MetS were reported in some studies [3, 14, 26] whereas some others found no association [27].
In a population of Asian Indian descent living in Guadeloupe, Boullu-Sanchiz et al. reported a significant relation between the FABP2 Ala54Thr polymorphism and type 2 diabetes, that seemed to be related to the metabolic insulin resistance syndrome [18]. Recently a meta-analysis by Liu et al, revealed significant associations for metabolic syndrome and type 2 diabetes, suggesting the implication of FABP2 gene in the pathogenesis of MetS [14]. In our present study, this association with MetS was not significant (OR 1.59; P = 0.068).
Adiponectin, rs1501299 and metabolic syndrome
Low plasma adiponectin levels have been associated with insulin resistance, and increased risk of type 2 diabetes [28, 29]. In the present study, the risk ratio of MetS for carriers of the T allele (GT/TT) of rs1501299 was 1.80; P = 0.028. This lack of significance may be due to a lack of power given the size of our study population. In fact, for an identical OR of 1.80 (Table 2), with a 80% study power, a type 1 error of 5% and a significant p value < 0.016, we should have a sample size of at least 760 subjects (including 190 subjects with MetS).
Both T and G alleles of rs1501299 (ADIPOQ) were reported to be associated with metabolic phenotypes or with adiponectin concentrations. In a meta-analysis for association between ADIPOQ rs1501299 and MetS, most of the studies showed trends of elevated OR for the G allele [7]. But, as in our study population, others showed trends of elevated OR for the T allele [30, 31]. In 1438 Taiwanese subjects, the OR for MetS for GT/TT genotypes (vs GG genotype) was 1.30; P = 0.015 [31]. Ronconi et al found that the rs1501299 TT genotype was associated with a significantly worse metabolic profile and a higher risk for MetS (OR = 1.5 in patients with primary aldosteronism and OR = 1.3 in those with essential hypertension) [30]. These inconsistent results are probably due to different diagnostic criteria for MetS components or MetS but also to ethnic variations and to gene-environment interactions. The rs1501299 (ADIPOQ) has been associated with adiponectin levels in Genome-Wide Association Studies (GWAS) [32, 33] but, the association of rs1501299 with metabolic disorders is not systematically related to differences in plasma adiponectin [34, 35]. The effect of the genetic variant may be independent of the adiponectin circulating levels [36].
ANP, rs5065 and metabolic syndrome
In our study, the associations between the ANP gene variant rs5065 and MetS or MetS components were not significant. Nevertheless, there is increasing evidence for a key role for natriuretic peptides, mainly ANP, in human metabolism and cardio-metabolic disorders. It has been demonstrated that ANP can enhance lipolysis, lipid mobilization and oxidation [37, 38] and can also act indirectly by stimulating the release of adiponectin from adipose tissue [11]. In addition ANP exhibits anti-inflammatory effects which can be of physio-pathological importance in the low chronic inflammation state of adipose tissues, involved in insulin-resistance and cardiovascular complications especially in obese subjects [39].
The ANP gene variant rs5065 has been associated with hypertension, myocardial infarction and stroke [40] but to our knowledge, no study had focused on the association with lipids levels. Some studies indicate that the minor allele of rs5065 variant lead to an altered ANP causing increased permeability and, reduced viability, proliferation and migration, in endothelial cells, that could explain the association of this variant with cardiovascular disease [41, 42]. However, the potential effects of rs5065 on the metabolic properties of the cognate peptide have not been studied and it is still unclear whether rs5065 is associated with a modification of ANP levels or not [43]. Therefore, the functional significance of rs5065 on lipid parameters and metabolic syndrome remains to be elucidated by further investigations.
Association of the GRS with hypertriglyceridemia and metabolic syndrome
The GRS summarizing the influence of the 3 SNPs was associated with metabolic syndrome. Patients with 4–5 risk alleles have nearly 2.5-fold higher risk of MetS compared to those with no or 1 risk allele. In addition, the GRS was significantly higher in subjects having high triglyceride levels or MetS than in the others.
Taken together, our data support the idea that fatty acids and triglycerides regulation/dysregulation plays a crucial role in the development of MetS and reinforce the evidence for a polygenic component in this syndrome. In a systematic review on the most studied SNP-MetS associations, the authors found an association with MetS for SNPs, mostly located in genes involved in lipid metabolism like FTO rs9939609, TCF7L2 rs7903146, APOA5 rs662799, APOC3 rs2854117, IL6 rs1800795, and CETP rs708272 [24].
Increased circulating triglycerides are associated with an atherogenic lipid profile and insulin resistance, resulting in an increased risk for metabolic syndrome diabetes and cardiovascular disease, especially in obese subjects [44]. Hypertriglyceridemia may be the consequence of enhanced liberation of fatty acids from excessive visceral adipose tissue leading to enhanced hepatic synthesis of VLDL [45, 46]. The association of GRS with MetS and hypertriglyceridemia found in our study could reflect the cumulative influence and the interaction of ANP, FABP2 and ADIPOQ genes that are implicated in fatty acid metabolism in different key tissues and cells such as enterocytes, adipocytes, skeletal muscle and liver.
Limitations of this study include its relatively small sample size. In addition, environmental risk factors for MetS such as exercise behaviour or dietary patterns were not taken into account even if they are moderately heritable. However, our study design excluding patients treated with lipid-lowering medications and with previous cardiovascular complications provides a reliable assessment of the association between SNPs, hypertriglyceridemia and MetS in this sample of Afro-Caribbean subjects.
In conclusion, the results of this study indicate that FABP2, ANP and ADIPOQ gene variants are associated with metabolic syndrome or its components in Afro-Caribbeans and suggest a cumulative effect of these variants on the risk of metabolic syndrome and hypertriglyceridemia. Further studies are needed in a large population and other ethnic groups to confirm theses genetic associations and to investigate the underlying physiopathological mechanisms.
Supporting Information
(PDF)
(PDF)
Acknowledgments
We thank all the people who contributed to this study and the “Centre de Ressources Biologiques” of Guadeloupe.
We thank Pr Steve Humphries (from centre for cardiovascular genetics, University College London, UK) for a critical reading of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the University Hospital of Guadeloupe, the “Programme Hospitalier pour la Recherche Clinique (PHRC, France)" and by the “Association pour la Santé Publique et l’Epidémiologie en Guadeloupe (ASPEG)”. There was no additional external funding received for this study.
References
- 1.Bremer AA, Jialal I. Adipose Tissue Dysfunction in Nascent Metabolic Syndrome. Journal of Obesity. 2013;2013:393192 10.1155/2013/393192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu KC, Chuang LM, Yoon C. The A54T polymorphism at the intestinal fatty acid binding protein 2 is associated with insulin resistance in glucose tolerant Caucasians. BMC Genet. 2001;2(7):28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi JR, Cupples LA, Otvos JD, Wilson PW, Schaefer EJ, Ordovas JM. Association of the A/T54 polymorphism in the intestinal fatty acid binding protein with variations in plasma lipids in the Framingham Offspring Study. Atherosclerosis. 2001;159(2):417–24. 10.1016/S0021-9150(01)00517-2 [DOI] [PubMed] [Google Scholar]
- 4.Zhao T, Zhao J, Yang W. Association of the fatty acid-binding protein 2 gene Ala54Thr polymorphism with insulin resistance and blood glucose: a meta-analysis in 13451 subjects. Diabetes Metab Res Rev. 2010;26(5):357–64. 10.1002/dmrr.1085 [DOI] [PubMed] [Google Scholar]
- 5.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clinical science. 2006;110(3):267–78. 10.1042/CS20050182 [DOI] [PubMed] [Google Scholar]
- 6.Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. American journal of human genetics. 2000;67(6):1470–80. 10.1086/316887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao M, Ding D, Huang J, Qu Y, Wang Y, Huang Q. Association of genetic variants in the adiponectin gene with metabolic syndrome: a case-control study and a systematic meta-analysis in the Chinese population. PloS one. 2013;8(4):e58412 10.1371/journal.pone.0058412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan AM, Cheng S, Magnusson M, Larson MG, Newton-Cheh C, McCabe EL, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. The Journal of clinical endocrinology and metabolism. 2011;96(10):3242–9. 10.1210/jc.2011-1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension. 2005;46(4):660–6. 10.1161/01.HYP.0000179575.13739.72 [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115(11):1345–53. 10.1161/CIRCULATIONAHA.106.655142 [DOI] [PubMed] [Google Scholar]
- 11.Birkenfeld AL, Boschmann M, Engeli S, Moro C, Arafat AM, Luft FC, et al. Atrial natriuretic peptide and adiponectin interactions in man. PloS one. 2012;7(8):e43238 10.1371/journal.pone.0043238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruchala M, Ciecwierz D, Wasag B, Targonski R, Dubaniewicz W, Nowak A, et al. Association of the ScaI atrial natriuretic peptide gene polymorphism with nonfatal myocardial infarction and extent of coronary artery disease. American heart journal. 2003;145(1):125–31. 10.1067/mhj.2003.52 [DOI] [PubMed] [Google Scholar]
- 13.Roussel R, Tregouet DA, Hadjadj S, Jeunemaitre X, Marre M. Investigation of the human ANP gene in type 1 diabetic nephropathy: case-control and follow-up studies. Diabetes. 2004;53(5):1394–8. 10.2337/diabetes.53.5.1394 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Wu G, Han L, Zhao K, Qu Y, Xu A, et al. Association of the FABP2 Ala54Thr polymorphism with type 2 diabetes, obesity, and metabolic syndrome: a population-based case-control study and a systematic meta-analysis. Genetics and molecular research: GMR. 2015;14(1):1155–68. 10.4238/2015.February.6.19 [DOI] [PubMed] [Google Scholar]
- 15.Leu HB, Chung CM, Lin SJ, Jong YS, Pan WH, Chen JW. Adiponectin gene polymorphism is selectively associated with the concomitant presence of metabolic syndrome and essential hypertension. PloS one. 2011;6(5):e19999 10.1371/journal.pone.0019999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foucan L, Maimaitiming S, Larifla L, Hedreville S, Deloumeaux J, Joannes MO, et al. Adiponectin gene variants, adiponectin isoforms and cardiometabolic risk in type 2 diabetic patients. Journal of diabetes investigation. 2014;5(2):192–8. 10.1111/jdi.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larifla L, Maimaitiming S, Velayoudom-Cephise FL, Ferdinand S, Blanchet-Deverly A, Benabdallah S, et al. Association of 2238T>C polymorphism of the atrial natriuretic peptide gene with coronary artery disease in Afro-Caribbeans with type 2 diabetes. Am J Hypertens. 2012;25(5):524–7. 10.1038/ajh.2011.233 [DOI] [PubMed] [Google Scholar]
- 18.Boullu-Sanchis S, Lepretre F, Hedelin G, Donnet JP, Schaffer P, Froguel P, et al. Type 2 diabetes mellitus: association study of five candidate genes in an Indian population of Guadeloupe, genetic contribution of FABP2 polymorphism. Diabetes Metab. 1999;25(2):150–6. [PubMed] [Google Scholar]
- 19.Blackburn P, Lemieux I, Lamarche B, Bergeron J, Perron P, Tremblay G, et al. Hypertriglyceridemic waist: a simple clinical phenotype associated with coronary artery disease in women. Metabolism: clinical and experimental. 2012;61(1):56–64. 10.1016/j.metabol.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 20.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102(2):179–84. 10.1161/01.CIR.102.2.179 [DOI] [PubMed] [Google Scholar]
- 21.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106(25):3143 [PubMed] [Google Scholar]
- 22.Larifla L, Beaney KE, Foucan L, Bangou J, Michel CT, Martino J, et al. Influence of Genetic Risk Factors on Coronary Heart Disease Occurrence in Afro-Caribbeans. Can J Cardiol. 2016;32(8):978–85. 10.1016/j.cjca.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baier LJ, Sacchettini JC, Knowler WC, Eads J, Paolisso G, Tataranni PA, et al. An amino acid substitution in the human intestinal fatty acid binding protein is associated with increased fatty acid binding, increased fat oxidation, and insulin resistance. The Journal of clinical investigation. 1995;95(3):1281–7. 10.1172/JCI117778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Povel CM, Boer JM, Reiling E, Feskens EJ. Genetic variants and the metabolic syndrome: a systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2011;12(11):952–67. 10.1111/j.1467-789X.2011.00907.x [DOI] [PubMed] [Google Scholar]
- 25.Baier LJ, Bogardus C, Sacchettini JC. A polymorphism in the human intestinal fatty acid binding protein alters fatty acid transport across Caco-2 cells. J Biol Chem. 1996;271(18):10892–6. [DOI] [PubMed] [Google Scholar]
- 26.Guettier JM, Georgopoulos A, Tsai MY, Radha V, Shanthirani S, Deepa R, et al. Polymorphisms in the fatty acid-binding protein 2 and apolipoprotein C-III genes are associated with the metabolic syndrome and dyslipidemia in a South Indian population. The Journal of clinical endocrinology and metabolism. 2005;90(3):1705–11. 10.1210/jc.2004-1338 [DOI] [PubMed] [Google Scholar]
- 27.Erkkila AT, Lindi V, Lehto S, Pyorala K, Laakso M, Uusitupa MI. Variation in the fatty acid binding protein 2 gene is not associated with markers of metabolic syndrome in patients with coronary heart disease. Nutr Metab Cardiovasc Dis. 2002;12(2):53–9. [PubMed] [Google Scholar]
- 28.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360(9326):57–8. 10.1016/S0140-6736(02)09335-2 [DOI] [PubMed] [Google Scholar]
- 29.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. The Journal of clinical endocrinology and metabolism. 2001;86(5):1930–5. 10.1210/jcem.86.5.7463 [DOI] [PubMed] [Google Scholar]
- 30.Ronconi V, Turchi F, Rilli S, Di Mattia D, Agostinelli L, Boscaro M, et al. Metabolic syndrome in primary aldosteronism and essential hypertension: relationship to adiponectin gene variants. Nutr Metab Cardiovasc Dis. 2010;20(2):93–100. 10.1016/j.numecd.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Yang WS, Yang YC, Chen CL, Wu IL, Lu JY, Lu FH, et al. Adiponectin SNP276 is associated with obesity, the metabolic syndrome, and diabetes in the elderly. The American journal of clinical nutrition. 2007;86(2):509–13. [DOI] [PubMed] [Google Scholar]
- 32.Heid IM, Henneman P, Hicks A, Coassin S, Winkler T, Aulchenko YS, et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis. 2010;208(2):412–20. 10.1016/j.atherosclerosis.2009.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollin TI, Tanner K, O'Connell J R, Ott SH, Damcott CM, Shuldiner AR, et al. Linkage of plasma adiponectin levels to 3q27 explained by association with variation in the APM1 gene. Diabetes. 2005;54(1):268–74. 10.2337/diabetes.54.1.268 [DOI] [PubMed] [Google Scholar]
- 34.Heid IM, Wagner SA, Gohlke H, Iglseder B, Mueller JC, Cip P, et al. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes. 2006;55(2):375–84. 10.2337/diabetes.55.02.06.db05-0747 [DOI] [PubMed] [Google Scholar]
- 35.Gable DR, Hurel SJ, Humphries SE. Adiponectin and its gene variants as risk factors for insulin resistance, the metabolic syndrome and cardiovascular disease. Atherosclerosis. 2006;188(2):231–44. 10.1016/j.atherosclerosis.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 36.Qi L, Li T, Rimm E, Zhang C, Rifai N, Hunter D, et al. The +276 polymorphism of the APM1 gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes. 2005;54(5):1607–10. 10.2337/diabetes.54.5.1607 [DOI] [PubMed] [Google Scholar]
- 37.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14(10):1345–51. 10.1096/fj.14.10.1345 [DOI] [PubMed] [Google Scholar]
- 38.Sengenes C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M, et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278(49):48617–26. 10.1074/jbc.M303713200 [DOI] [PubMed] [Google Scholar]
- 39.Moro C, Klimcakova E, Lolmede K, Berlan M, Lafontan M, Stich V, et al. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia. 2007;50(5):1038–47. 10.1007/s00125-007-0614-3 [DOI] [PubMed] [Google Scholar]
- 40.Vassalle C, Andreassi MG. Genetic polymorphisms of the natriuretic peptide system in the pathogenesis of cardiovascular disease: what lies on the horizon? Clin Chem. 2009;55(5):878–87. 10.1373/clinchem.2008.120832 [DOI] [PubMed] [Google Scholar]
- 41.Barbato E, Bartunek J, Mangiacapra F, Sciarretta S, Stanzione R, Delrue L, et al. Influence of rs5065 atrial natriuretic peptide gene variant on coronary artery disease. J Am Coll Cardiol. 2012;59(20):1763–70. 10.1016/j.jacc.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 42.Scarpino S, Marchitti S, Stanzione R, Evangelista A, Di Castro S, Savoia C, et al. Reactive oxygen species-mediated effects on vascular remodeling induced by human atrial natriuretic peptide T2238C molecular variant in endothelial cells in vitro. J Hypertens. 2009;27(9):1804–13. 10.1097/HJH.0b013e32832d229f [DOI] [PubMed] [Google Scholar]
- 43.Kato N, Sugiyama T, Morita H, Nabika T, Kurihara H, Yamori Y, et al. Genetic analysis of the atrial natriuretic peptide gene in essential hypertension. Clinical science. 2000;98(3):251–8. 10.1042/CS19990220 [DOI] [PubMed] [Google Scholar]
- 44.Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta. 2006;368(1–2):1–19. 10.1016/j.cca.2005.12.026 [DOI] [PubMed] [Google Scholar]
- 45.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–65. 10.1007/s00125-005-0125-z [DOI] [PubMed] [Google Scholar]
- 46.Lewis GF, Xiao C, Hegele RA. Hypertriglyceridemia in the genomic era: a new paradigm. Endocr Rev. 2015;36(1):131–47. 10.1210/er.2014-1062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper.