Abstract
In low-income-countries, screening for hepatitis C virus (HCV) infection is often based on rapid tests (RT). Their lower sensitivity compared to enzyme immunoassay (EIA) suggests that newer HCV Antigen/Antibody (Ag/Ab) combination assays might have a role in such countries. To test this idea, 1998 blood donors were tested at the University Teaching Hospital blood bank in Yaoundé, Cameroon simultaneously with a RT (HCV rapid test, Human Diagnostics, Berlin, Germany) according to standard practice (S1) and with an Ag/Ab assay (Monolisa HCV Ag/Ab Ultra, Biorad, France) (S2). All discordant, borderline and reactive samples were submitted to confirmatory testing by immunoblot and/or HCV-RNA. Of the 86 (4.3%) samples positive with one or both strategies, 29 were confirmed negative, 37 positive and 20 were false positive or resolved infection. There was a significant difference in test sensitivity (p = 0.01) between S1 (70.3%) and S2 (91.9%) but not in test specificity (99.4% and 98.6%, respectively). The benefit of the Ag/Ab assay in the detection of recent HCV seronegative infections could not be evaluated since no Antigen-only donations were identified. However, better Ag/Ab test sensitivity compared to RT supports the implementation of these newer immunoassays for HCV screening in the African blood bank setting.
Keywords: HCV, Rapid tests, Ag/Ab combination assay, Blood donors, Africa
1. Introduction
The control of hepatitis C virus (HCV) infection is important in Africa because of its high prevalence and serious complications such as cirrhosis or hepatocellular carcinoma. In order to prevent transfusion-related transmission of the virus many African countries perform anti-HCV antibody (Ab) screening of blood donations (Buseri et al., 2009; Nagalo et al., 2011; Tagny et al., 2009). In the University Teaching Hospital blood bank of Yaoundé, Cameroon, where HCV Ab was screened with rapid tests (RT), HCV Ab seroprevalences were 4.8% in 2003, 3.9% in 2005, and 3.9% in 2009 (Mbanya and Tayou, 2005; Tagny et al., 2010). Since RTs have been reported less sensitive than EIAs (Desbois et al., 2008; Scheiblauer et al., 2006) a significant number of HCV-infected blood products might be not identified in a high prevalence context. In addition, as Ab screening precludes the detection of the preseroconversion early phase of infection, improved transfusion safety could be attained by detection of viral capsid antigen (Ag) or RNA. Whereas the systematic use of nucleic acid testing (NAT) is rare in African blood banks due to cost and infrastructure, the detection of HCV Ag can be considered as a cost-effective approach to reduce the residual risk of transfusion-related HCV. Studies in Europe have reported that the simultaneous detection of the HCV capsid Ag and Ab by an Ag/Ab combination assays could be an effective alternative to diagnose early HCV infection among blood donors (Ansaldi et al., 2001; Courouce et al., 2000; Icardi et al., 2001; Laperche et al., 2005a, 2005b; Nolte, 1997; Schnuriger et al., 2006). To estimate the benefit of this strategy in a high prevalence African setting, we replicated our previous approach for HIV Ag/Ab testing (Tagny et al., 2011). The current compared the performance of HCV RT versus HCV Ag/Ab detection and also determined the prevalence of confirmed HCV infection in Cameroonian blood donors.
2. Material and methods
2.1. Blood donors
A total of 1998 consecutive allogenic blood donors who gave blood from the 1 March 2011 to 31 March 2012 at the University Teaching Hospital blood bank of Yaoundé were included in this cross sectional study. Ethical approval for the study was obtained from the Cameroon National Ethics Committee. All blood donors who met donation criteria of the University Teaching Hospital blood bank and gave signed informed consent were included. Their mean age was 29.4 ± 8.0 years and 16.7% [95% IC: 15.7–18.4] were female. Repeat blood donors represented 9.5% [95% IC: 8.3–10.9] of the study population and gave only one donation during the study period.
For each donor, 0.5–1 ml of anticoagulated plasma was aliquoted into small tubes; one aliquot was immediately used for the screening and the remaining vials were stored at −80 °C for further investigations.
2.2. Laboratory methods
Blood testing was performed at the University Teaching Hospital blood bank in Yaoundé which has participated in several studies of viral testing (Laperche et al., 2009, 2013). Before starting the study, a panel of 25 known HCV Ab positive sera and 25 negative samples was tested with the HCV Ag/Ab assay to evaluate the performance of technicians and equipment. The results were in accordance with those expected (data not shown).
All 1998 donors were tested simultaneously for HCV markers with two screening strategies. The first strategy (S1) included a RT used for routine blood donor screening (Hexagon HCV, Human Diagnostics, Berlin, Germany). This assay is based on capsid, NS3, NS4 and NS5 recombinant proteins to detect antibodies. A sample with negative RT results was considered as S1 negative. Initially reactive samples were retested in duplicate with the same assay. If two of three cumulative replicates were positive, the sample was considered as S1 reactive; if only one of three replicates was positive it was considered negative with S1.
The second strategy (S2) included a HCV Ag/Ab combination assay (Monolisa HCV Ag-Ab Ultra, BioRad, Marnes La Coquette, France) based on the combination of an indirect test for qualitative determination of antibodies (with one capsid peptide, two NS3 recombinant proteins and one NS4 recombinant protein) and a sandwich test for HCV capsid Ag (with an anti-capsid monoclonal antibody). Samples were screened in singlicate with this assay. A result was considered as S2 reactive when the sample-to-cut-off (s/co) ratio was superior or equal to 1 and S2 borderline when this ratio was between 0.8 and 0.99. The 10% gray zone recommended by the manufacturer was increased to 20% to investigate samples which could have been collected in the early Ab negative window phase.
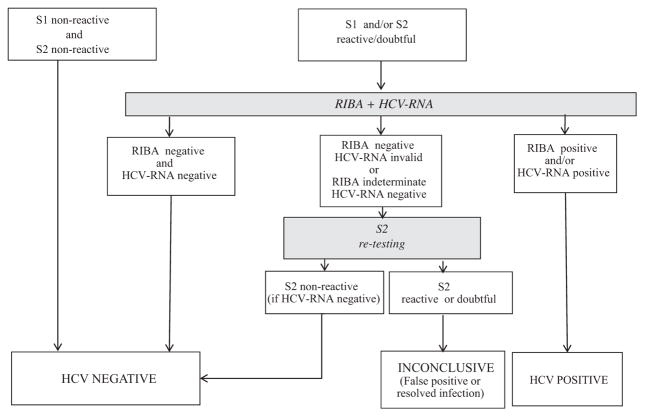
The algorithm used to classify samples is given in Fig. 1. Briefly, samples negative on both S1 and S2 were considered as negative and no further investigations were undertaken. In order to confirm that reactivity obtained with one or both of the two assays was due to the presence of HCV Ab (or Ag for the Ag/Ab combination assay), stored aliquots of reactive samples were shipped frozen to the National Reference Center (NRC), Paris, France, where they were subjected to confirmatory testing with HCV immunoblot (RIBA3 HCV, Ortho Clinical Diagnostics, Issy les Moulineaux, France) and HCV-RNA quantitation with Cobas TaqMan HCV (Roche molecular diagnostics, Meylan, France, limit of quantitation 25 copies/ml). Samples were considered definitively positive when RIBA and/or PCR gave positive results and negative when these two tests were negative. In the other cases, samples when available in a sufficient volume were retested in singlicate with Monolisa HCV Ag–Ab Ultra. If they were non-reactive they were classified as negative, if they were reactive or borderline they were considered as inconclusive due to the impossibility to differentiate false positive results and resolved infection.
Fig. 1.
Screening algorithm and classification of the 1998 tested blood donations according to results obtained with confirmatory investigations. The number of samples included in each category were divided according to groups (g1–g4) to which they belonged after the screening. S1: Hexagon HCV, Human Diagnostics, Berlin, Germany; S2: Monolisa HCV Ag–Ab Ultra, BioRad, Marnes La Coquette, France; RIBA: RIBA 3, Ortho Clinical Diagnostics, Issy les Moulineaux, France; HCV-RNA: Cobas TaqMan HCV, Roche Diagnostic, Meylan, France
To investigate the viral diversity on viremic donors, HCV genotype was determined in HCV-RNA positive samples, by sequencing a part of the NS5b gene. This region was amplified from the Cobas Taq Man RNA extraction by a PCR protocol designed to detect phylogenetically diverse HCV genotypes and described elsewhere (Laperche et al., 2005c; Morice et al., 2001).
The sensitivity for each strategy was calculated as the percentage of positive results obtained among the confirmed positive samples and the specificity as the percentage of negative results obtained among confirmed negative samples. Window period infections were defined as antigen and RNA positive but antibody negative. The prevalence of hepatitis C infection was calculated by dividing the number of confirmed positive samples by the number of samples tested, both overall and according to age, gender, and donor type and donation frequency. Statistical analysis was performed using the chi-square test to compare proportions or the T-test to compare means. Differences were considered significant when p < 0.05.
3. Results
Among the 1998 tested samples, 1912 (95.7%) were non-reactive on both S1 and S2. The remaining 86 (4.3%) were classified into four groups (Table 1): group 1 included 38 samples (44.2%) reactive with both assays; group 2 included 25 samples (29.1%) reactive only with S2; group 3 included 19 samples (22.1%) non-reactive on S1 and borderline on S2; and group 4 included 4 samples reactive only with S1. These 86 samples were further tested in Paris as shown in Fig. 1, and the results are shown in Table 1. A total of 37 (43%) samples were HCV RIBA positive; of these 14 were HCV-RNA negative, 14 were HCV-RNA positive, and 9 had insufficient volume for RNA testing. Of the remaining 49 samples, 29 (33.7%) were classified as negative and 20 (23.3%) could not be classified. This last category included 11 samples that were HCV-RNA negative with non-interpretable RIBA (presence of reaction in the fusion-protein-band); 7 samples that were HCV-RNA negative with isolated RIBA reactivity; and 2 samples that were negative RIBA with invalid HCV-RNA results. Of the 82 samples that were initially reactive or borderline with Ag/Ab assay, 34 (41.5%) were confirmed positive, 20 (24.4%) could not be classified, and 28 (34.1%) were negative (Table 2). Of the 44 samples in these two last categories which could be retested with Monolisa Ag/Ab HCV Ultra, 18 (40.9%) were negative. The Ag/Ab assay mean signal to cutoff (s/c) ratios of these 82 samples were 3.6 ± 1.9, 1.32 ± 0.59 and 1.41 ± 0.98 (p < 10−4), for positive, negative and unclassified samples, respectively. HCV-RNA positive and negative samples had mean s/co ratios at 4.4 ± 1.43 and 1.91 ± 1.96 respectively (p = 0.05). No HCV-RNA positive but antibody negative (window period) infections were identified.
Table 1.
Results of confirmatory testing according to the screening results obtained with S1 and S2.
| Confirmatory result | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| S1/S2 | n | Positive
|
Negative
|
Inconclusive
|
||||
| n | % | n | % | n | % | |||
| Group 1 | R/R | 38 | 23 | 60.5 | 11 | 29.0 | 4 | 10.5 |
| Group 2 | NR/R | 25 | 8 | 32.0 | 9 | 36.0 | 8 | 32.0 |
| Group 3 | NR/bor | 19 | 3 | 15.8 | 8 | 42.1 | 8 | 42.1 |
| Group 4 | R/NR | 4a | 3 | 75.0 | 1 | 25.0 | 0 | 25.0 |
| Total | 86 | 37b | 43.0 | 29c | 33.7 | 20d | 23.3 | |
R, reactive; NR, non-reactive; bor, borderline.
3 of them were reactive with S2 when retested and positive with RIBA, 1 was non-reactive with S2 when retested, and negative with RIBA and HCV-RNA.
37 RIBA positive; 14 HCV-RNA positive, 14 HCV-RNA negative, 9 HCV-RNA not tested.
29 RIBA negative and HCV-RNA negative.
11 non-interpretable RIBA (reactions on the fusion-protein-band) and HCV-RNA negative samples,7 isolated reactivities HCV-RNA negative samples, 2 RIBA negative and HCV-RNA invalid results.
Table 2.
Performance of the two strategies S1 and S2.
| Status of samples after confirmatory investigations
|
Performance scoresa
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive
|
Negative
|
Inconclusive
|
Sensitivityb | Specificityc | PPV | NPV | |||||||
|
N = 37
|
N = 1941
|
N = 20
|
% (95% CI) | % (95% CI) | |||||||||
| R | NR | Bor | R | NR | Bor | R | NR | Bor | |||||
| S1 | 26 | 11 | 0 | 12 | 1929 | 0 | 4 | 16 | 0 | 70.3 (55.6–85.0) | 99.4 (99.1–99.7) | 68.4 | 99.4 |
| S2 | 31 | 3 | 3 | 20 | 1913 | 8 | 12 | 0 | 8 | 91.9 (83.1–100) | 98.6 (98.1–99.1) | 54.8 | 99.8 |
R, reactive; NR, non-reactive; bor, borderline.
Calculated excluding inconclusive samples.
Positive and borderline results in 37 positive samples.
Negative results in 1941 NR samples.
The sensitivities of S1 and S2 compared to confirmatory testing (n = 37 true positives) were 70.3% (95% CI: 55.6–85.0%) and 91.9% (95% CI: 83.1–100%), respectively. Excluding indeterminate results, specificities were 99.4% (95% CI: 99.1–99.7%) and 98.6.0% (95% CI: 98.1–99.1%), positive predictive values were 68.4% and 63.0% and negative predictive values were 99.4% and 99.9% for S1 and S2, respectively (Table 2). There was a statistically significant difference between S1 and S2 for sensitivity (p = 0.01) but not for specificity.
Among the 14 HCV viremic subjects, 6 were HCV-RNA positive but with non-quantifiable viral loads (<25 UI/ml), and 8 ranged from 30 to 360,000 IU/ml (median 1460 IU/ml). The genotype could be determined for only three donors because eight had a viral load too low for sequencing and three samples had no remaining specimen for sequencing. The genotypes were 1, 1l and 2.
The prevalence of HCV markers in Cameroonian blood donors was 1.9% (95% CI: 1.27–2.47%) or 2.8% (95% CI: 2.12–3.58%) depending on whether the 20 inconclusive samples were considered as positive. Among the 37 confirmed positive subjects, 36 (97.3%) were first time blood donors and 33 (89.2%) were male. HCV prevalence was 2.0% in males and in 1.2% females (1.2%), a non-significant difference. When compared to non-infected donors (Table 3), con-firmed positive donors were significantly older (35.2 ± 12.0 vs. 29.2 ± 6.2 years, p < 10−4) with most being 40–49 years-old donors. No other differences between demographic groups were identified.
Table 3.
Comparison between demographic characteristics of 37 confirmed HCV positive and 1941 HCV negative donors (the 20 unclassified donors were excluded).
| Negative
|
Confirmed positive
|
p | |||
|---|---|---|---|---|---|
| (n = 1941) | % | (n = 37) | % | ||
| Frequency of donation | |||||
| FTD | 1754 | 90.4 | 36 | 97.3 | NS |
| RBD | 187 | 9.6 | 1 | 2.7 | NS |
| Age group (years) | |||||
| Mean | 29.2 ± 7.9 | 35.2 ± 12 | p < 10−4 | ||
| <20 | 96 | 4.9 | 1 | 2.7 | NS |
| 20–29 | 1096 | 56.5 | 15 | 40.5 | 0.05 |
| 30–39 | 538 | 27.7 | 9 | 24.3 | NS |
| 40–49 | 165 | 8.5 | 8 | 21.6 | 0.01 |
| ≥50 | 46 | 2.4 | 4 | 10.8 | NS |
| Gender | |||||
| Male | 1614 | 83.2 | 33 | 89.2 | NS |
| Female | 327 | 16.8 | 4 | 10.8 | NS |
FTD, first time blood donor; RBD, repeat blood donor; NS, not significant.
4. Discussion
Similarly to what we recently reported for HIV (Tagny et al., 2011), this head-to-head study found that, in a real world setting in a Cameroonian blood center, the sensitivity of the Ag/Ab combination assay was superior to that of rapid tests. As no early or window period infections were identified, the better sensitivity was probably due to an enhanced detection of antibodies by the former assay.
The choice of antigens, the method of their presentation in the assay and genetic heterogeneity of the virus have all been evidenced as factors that could impair detection of HCV Ab by RT (Neville et al., 1997; Simmonds et al., 1993). However, the impact of viral diversity on the detection of HCV antibodies is debated and it seems that false negative results are more likely due to undetected weak antibody reactivity than to genotype-dependent differences (Scheiblauer et al., 2006). This hypothesis could not be explored because of the low number of samples successfully genotyped in the study. Interestingly, one of the two genotyped strains was the 11 strain previously described in Cameroon (Ndjomou et al., 2003; Pasquier et al., 2005).
We also note that although the Ag–Ab assay was more sensitive than RT, its sensitivity was lower than reported previously (Ansaldi et al., 2006; Laperche et al., 2005a, 2005b; Schnuriger et al., 2006). The fact that three S2 false-negative samples were reactive when retested (data not shown) indicates that this lower sensitivity was probably not due to intrinsic characteristics of the assay but rather to failures in test performance.
The specificity of Ag/Ab assay was (non-significantly) weaker than those observed for RT and did not meet the minimal European Union recommendation of 99.5%. In African samples, cross-reactivity with other endemic infections or the higher IgG levels may adversely affect the specificity of immunoassays, as described for HIV (Everett et al., 2010; Simooya et al., 1988) and HTLV-1 infections (Mahieux et al., 2000). This may explain the non-specificity reported here. On the other hand, specificity may have been under-estimated since all initially S2 reactive samples were not retested in duplicate as S1. Indeed, 41% of initially reactive samples which could be analyzed were not reproduced when retested with the same reagent. Repeat testing is known to improve specificity (Choudhury et al., 2011; Piro et al., 2008).
A significant proportion (23.2%) of reactive samples with at least one of the two screening strategies could not be resolved into non-specific reactions or resolved infections. As non-specific reactivity in viral screening is problematic for blood services because of donor management issues and product loss, the issue of the most appropriate confirmatory strategy is raised, especially in countries where, supplemental assays are not usually implemented. Several solutions have been proposed. One could test simultaneously with two assays and exclude donors reactive with both since these subjects have the highest probability to be positive with NAT and/or Immunoblot (Choudhury et al., 2011). Nevertheless, two tests increase the cost of the screening unless a sequential screening strategy is used (Seed et al., 2003). Another solution would be to use the strength of antibody reactivity as predictive marker of true-positive results and viremia in asymptomatic HCV infected people (Acar et al., 2010; Contreras et al., 2010; Kiely et al., 2010; Stramer et al., 2012). Our results, from an admittedly small study, are in accordance with this last report since mean s/co ratios were significantly higher in viremic subjects.
While higher than values observed in low prevalence countries (Zou et al., 2012), the prevalence of HCV markers in Cameroonian blood donors is at least two times lower than what has been reported in earlier studies in Cameroon (Mbanya et al., 2003; Mbanya and Tayou, 2005; Tagny et al., 2009) and three to five times lower of what observed in other sub Saharan Africa reports (6.0% in Nigeria (Buseri et al., 2009), 8.7% in Burkina-Faso (Nagalo et al., 2011), 9.4% in Ghana (Nkrumah et al., 2011)). These discrepancies are probably due to an overestimation of rates in past studies based only upon screening results or to differences in donor selection strategies. The age association with prevalence of HCV markers is in accordance with recent findings in Brazil (de Almeida-Neto et al., 2013) and may be explained by higher HCV transmission and prevalence in the past compared to the present. This hypothesis of lower current incidence is supported by the low proportion (only 50%) of viremic donors observed among confirmed positive subjects in this study.
5. Conclusions
In summary, the enhanced sensitivity of Ag/Ab assay observed here was related to the better Ab detection compared to RT and supports the use of such “combi” tests in the detection of HCV infection, even though rapid test kits are useful in extreme conditions and emergency settings (O’Connell et al., 2013). Unfortunately the study did not perform NAT on all samples and the small number of donors included in this study its short time-frame prevented us from accurately estimating HCV incidence. Thus, the additional value of Ag/Ab assays in the detection of Ab negative but Ag positive primary infection could not be evaluated. However, if one assumes higher HCV incidence is likely in high prevalence African countries, there is a theoretical benefit to using Ag/Ab assays in such settings if NAT implementation is not possible. Of course, this assumes that adequate infrastructure and quality control is in place, especially when no confirmatory investigations are performed.
Acknowledgments
We thank Blood System Research Institute (San Francisco, CA, USA) which provided funding for this study. We also thank Elvige Guekeng, Annie Girault, Christine Portal, Annie Razer, Annick Ndoumba, Félix Feunou for their assistance and Biorad SA (Paris, France) for providing assays.
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have conflict of interest with respect of this manuscript.
References
- Acar A, Kemahli S, Altunay H, Kosan E, Oncul O, Gorenek L, Cavuslu S. The significance of repeat testing in Turkish blood donors screened with HBV, HCV and HIV immunoassays and the importance of S/CO ratios in the interpretation of HCV/HIV screening test results and as a determinant for further confirmatory testing. Transfus Med. 2010;20:152–159. doi: 10.1111/j.1365-3148.2009.00987.x. [DOI] [PubMed] [Google Scholar]
- Ansaldi F, Torre F, Bruzzone BM, Picciotto A, Crovari P, Icardi G. Evaluation of a new hepatitis C virus sequencing assay as a routine method for genotyping. J Med Virol. 2001;63:17–21. [PubMed] [Google Scholar]
- Ansaldi F, Bruzzone B, Testino G, Bassetti M, Gasparini R, Crovari P, Icardi G. Combination hepatitis C virus antigen and antibody immunoassay as a new tool for early diagnosis of infection. J Viral Hepat. 2006;13:5–10. doi: 10.1111/j.1365-2893.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- Buseri FI, Muhibi MA, Jeremiah ZA. Sero-epidemiology of transfusion-transmissible infectious diseases among blood donors in Osogbo, south-west Nigeria. Blood Transfus. 2009;7:293–299. doi: 10.2450/2009.0071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury N, Tulsiani S, Desai P, Shah R, Mathur A, Harimoorthy V. Serial follow-up of repeat voluntary blood donors reactive for anti-HCV ELISA. Asian J Transfus Sci. 2011;5:26–31. doi: 10.4103/0973-6247.75979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras AM, Ochoa-Jimenez RJ, Celis A, Mendez C, Olivares L, Rebolledo CE, Hernandez-Lugo I, Aguirre-Zavala AI, Jimenez-Mendez R, Chung RT. High antibody level: an accurate serologic marker of viremia in asymptomatic people with hepatitis C infection. Transfusion. 2010;50:1335–1343. doi: 10.1111/j.1537-2995.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- Courouce AM, Le Marrec N, Bouchardeau F, Razer A, Maniez M, Laperche S, Simon N. Efficacy of HCV core antigen detection during the preserocon-version period. Transfusion. 2000;40:1198–1202. doi: 10.1046/j.1537-2995.2000.40101198.x. [DOI] [PubMed] [Google Scholar]
- de Almeida-Neto C, Sabino EC, Liu J, Blatyta PF, Mendrone-Junior A, Salles NA, Leao SC, Wright DJ, Basques FV, Ferreira JE, Busch MP, Murphy EL. Prevalence of serologic markers for hepatitis B and C viruses in Brazilian blood donors and incidence and residual risk of transfusion transmission of hepatitis C virus. Transfusion. 2013;53:827–834. doi: 10.1111/j.1537-2995.2012.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois D, Vaghefi P, Savary J, Dussaix E, Roque-Afonso AM. Sensitivity of a rapid immuno-chromatographic test for hepatitis C antibodies detection. J Clin Virol. 2008;41:129–133. doi: 10.1016/j.jcv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Everett DB, Baisely KJ, McNerney R, Hambleton I, Chirwa T, Ross DA, Changalucha J, Watson-Jones D, Helmby H, Dunne DW, Mabey D, Hayes RJ. Association of schistosomiasis with false-positive HIV test results in an African adolescent population. J Clin Microbiol. 2010;48:1570–1577. doi: 10.1128/JCM.02264-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardi G, Ansaldi F, Bruzzone BM, Durando P, Lee S, de Luigi C, Crovari P. Novel approach to reduce the hepatitis C virus (HCV) window period: clinical evaluation of a new enzyme-linked immunosorbent assay for HCV core antigen. J Clin Microbiol. 2001;39:3110–3114. doi: 10.1128/JCM.39.9.3110-3114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely P, Walker K, Parker S, Cheng A. Analysis of sample-to-cutoff ratios on chemiluminescent immunoassays used for blood donor screening highlights the need for serologic confirmatory testing. Transfusion. 2010;50:1344–1351. doi: 10.1111/j.1537-2995.2009.02572.x. [DOI] [PubMed] [Google Scholar]
- Laperche S, Elghouzzi MH, Morel P, Asso-Bonnet M, Le Marrec N, Girault A, Servant-Delmas A, Bouchardeau F, Deschaseaux M, Piquet Y. Is an assay for simultaneous detection of hepatitis C virus core antigen and antibody a valuable alternative to nucleic acid testing? Transfusion. 2005a;45:1965–1972. doi: 10.1111/j.1537-2995.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Laperche S, Le Marrec N, Girault A, Bouchardeau F, Servant-Delmas A, Maniez-Montreuil M, Gallian P, Levayer T, Morel P, Simon N. Simultaneous detection of hepatitis C virus (HCV) core antigen and anti-HCV antibodies improves the early detection of HCV infection. J Clin Microbiol. 2005b;43:3877–3883. doi: 10.1128/JCM.43.8.3877-3883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laperche S, Lunel F, Izopet J, Alain S, Deny P, Duverlie G, Gaudy C, Pawlotsky JM, Plantier JC, Pozzetto B, Thibault V, Tosetti F, Lefrere JJ. Comparison of hepatitis C virus NS5b and 5′ noncoding gene sequencing methods in a multicenter study. J Clin Microbiol. 2005c;43:733–739. doi: 10.1128/JCM.43.2.733-739.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laperche S, Boukatou G, Kouegnigan L, Nebie Y, Boulahi MO, Tagny CT, Yahaya R, Tapko JB, Murphy E, Lefrere JJ. Transfusion safety on the African continent: an international quality control of virus testing in blood banks. Transfusion. 2009;49:1600–1608. doi: 10.1111/j.1537-2995.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- Laperche S on be half of the Francophone African Group for Research in Blood Transfusion. Multinational assessment of blood-borne virus testing and transfusion safety on the African continent. Transfusion. 2013;53:816–826. doi: 10.1111/j.1537-2995.2012.03797.x. [DOI] [PubMed] [Google Scholar]
- Mahieux R, Horal P, Mauclere P, Mercereau-Puijalon O, Guillotte M, Meertens L, Murphy E, Gessain A. Human T-cell lymphotropic virus type 1 gag indeterminate western blot patterns in Central Africa: relationship to Plasmodium falciparum infection. J Clin Microbiol. 2000;38:4049–4057. doi: 10.1128/jcm.38.11.4049-4057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbanya DN, Takam D, Ndumbe PM. Serological findings amongst first-time blood donors in Yaounde, Cameroon: is safe donation a reality or a myth? Transfus Med. 2003;13:267–273. doi: 10.1046/j.1365-3148.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Mbanya DN, Tayou C. Blood safety begins with safe donations: update among blood donors in Yaounde, Cameroon. Transfus Med. 2005;15:395–399. doi: 10.1111/j.1365-3148.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- Morice Y, Roulot D, Grando V, Stirnemann J, Gault E, Jeantils V, Bentata M, Jarrousse B, Lortholary O, Pallier C, Deny P. Phylogenetic analyses confirm the high prevalence of hepatitis C virus (HCV) type 4 in the Seine-Saint-Denis district (France) and indicate seven different HCV-4 subtypes linked to two different epidemiological patterns. J Gen Virol. 2001;82:1001–1012. doi: 10.1099/0022-1317-82-5-1001. [DOI] [PubMed] [Google Scholar]
- Nagalo MB, Sanou M, Bisseye C, Kabore MI, Nebie YK, Kienou K, Kiba A, Dahourou H, Ouattara S, Zongo JD, Simpore J. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis among blood donors in Koudougou (Burkina Faso) in 2009. Blood Transfus. 2011;9:419–424. doi: 10.2450/2011.0112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndjomou J, Pybus OG, Matz B. Phylogenetic analysis of hepatitis C virus isolates indicates a unique pattern of endemic infection in Cameroon. J Gen Virol. 2003;84:2333–2341. doi: 10.1099/vir.0.19240-0. [DOI] [PubMed] [Google Scholar]
- Neville JA, Prescott LE, Bhattacherjee V, Adams N, Pike I, Rodgers B, El-Zayadi A, Hamid S, Dusheiko GM, Saeed AA, Haydon GH, Simmonds P. Antigenic variation of core, NS3, and NS5 proteins among genotypes of hepatitis C virus. J Clin Microbiol. 1997;35:3062–3070. doi: 10.1128/jcm.35.12.3062-3070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkrumah B, Owusu M, Frempong HO, Averu P. Hepatitis B and C viral infections among blood donors from rural Ghana. Ghana Med J. 2011;45:97–100. [PMC free article] [PubMed] [Google Scholar]
- Nolte FS. Laboratory diagnosis of hepatitis C. Immunol Invest. 1997;26:199–207. doi: 10.3109/08820139709048927. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Gates RG, Bautista CT, Imbach M, Eggleston JC, Beardsley SG, Manak MM, Gonzales R, Rentas FJ, Macdonald VW, Cardo LJ, Reiber DT, Stramer SL, Michael NL, Peel SA. Laboratory evaluation of rapid test kits to detect hepatitis C antibody for use in predonation screening in emergency settings. Transfusion. 2013;53:505–517. doi: 10.1111/j.1537-2995.2012.03770.x. [DOI] [PubMed] [Google Scholar]
- Pasquier C, Njouom R, Ayouba A, Dubois M, Sartre MT, Vessiere A, Timba I, Thonnon J, Izopet J, Nerrienet E. Distribution and heterogeneity of hepatitis C genotypes in hepatitis patients in Cameroon. J Med Virol. 2005;77:390–398. doi: 10.1002/jmv.20468. [DOI] [PubMed] [Google Scholar]
- Piro L, Solinas S, Luciani M, Casale A, Bighiani T, Santonocito D, Girelli G. Prospective study of the meaning of indeterminate results of the recombinant immunoblot assay for hepatitis C virus in blood donors. Blood Transfus. 2008;6:107–111. doi: 10.2450/2008.0037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblauer H, El-Nageh M, Nick S, Fields H, Prince A, Diaz S. Evaluation of the performance of 44 assays used in countries with limited resources for the detection of antibodies to hepatitis C virus. Transfusion. 2006;46:708–718. doi: 10.1111/j.1537-2995.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- Schnuriger A, Dominguez S, Valantin MA, Tubiana R, Duvivier C, Ghosn J, Simon A, Katlama C, Thibault V. Early detection of hepatitis C virus infection by use of a new combined antigen-antibody detection assay: potential use for high-risk individuals. J Clin Microbiol. 2006;44:1561–1563. doi: 10.1128/JCM.44.4.1561-1563.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed CR, Margaritis AR, Bolton WV, Kiely P, Parker S, Piscitelli L. Improved efficiency of national HIV, HCV, and HTLV antibody testing algorithms based on sequential screening immunoassays. Transfusion. 2003;43:226–234. doi: 10.1046/j.1537-2995.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- Simmonds P, Rose KA, Graham S, Chan SW, McOmish F, Dow BC, Follett EA, Yap PL, Marsden H. Mapping of serotype-specific, immunodominant epitopes in the NS-4 region of hepatitis C virus (HCV): use of type-specific peptides to serologically differentiate infections with HCV types 1, 2, and 3. J Clin Microbiol. 1993;31:1493–1503. doi: 10.1128/jcm.31.6.1493-1503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simooya OO, Mwendapole RM, Siziya S, Fleming AF. Relation between falciparum malaria and HIV seropositivity in Ndola, Zambia. BMJ. 1988;297:30–31. doi: 10.1136/bmj.297.6640.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer SL, Dodd RY, Brodsky JP. The value of screening signal-to-cutoff ratios for hepatitis C virus antibody confirmation. Transfusion. 2012;53:1497–1500. doi: 10.1111/j.1537-2995.2012.03955.x. [DOI] [PubMed] [Google Scholar]
- Tagny CT, Diarra A, Yahaya R, Hakizimana M, Nguessan A, Mbensa G, Nebie Y, Dahourou H, Mbanya D, Shiboski C, Murphy E, Lefrere JJ. Characteristics of blood donors and donated blood in sub-Saharan Francophone Africa. Transfusion. 2009;49:1592–1599. doi: 10.1111/j.1537-2995.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- Tagny CT, Owusu-Ofori S, Mbanya D, Deneys V. The blood donor in sub-Saharan Africa: a review. Transfus Med. 2010;20:1–10. doi: 10.1111/j.1365-3148.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- Tagny CT, Mbanya D, Leballais L, Murphy E, Lefrere JJ, Laperche S. Reduction of the risk of transfusion-transmitted human immunodeficiency virus (HIV) infection by using an HIV antigen/antibody combination assay in blood donation screening in Cameroon. Transfusion. 2011;51:184–190. doi: 10.1111/j.1537-2995.2010.02782.x. [DOI] [PubMed] [Google Scholar]
- Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogeneic donations. Transfus Med Rev. 2012;26:119–128. doi: 10.1016/j.tmrv.2011.07.007. [DOI] [PubMed] [Google Scholar]