Abstract
Objective
Anxiety predicts cardiovascular events, though the mechanism remains unclear. We hypothesized that anxious symptoms will correlate with impaired resistance and conduit vessel function in participants aged 55–90 years.
Method
Anxious symptoms were measured with the Symptom Checklist-90-Revised in 89 participants with clinically diagnosed atherosclerotic cardiovascular disease and 54 healthy control participants. Vascular function was measured in conduit arteries using brachial flow-mediated dilatation (FMD) and in forearm resistance vessels (FRV) using intra-arterial drug administration and plethysmography.
Results
Anxious symptoms were not associated with FMD in either group. Participants with atherosclerosis exhibited significant inverse associations of anxious symptoms with FRV dilatation (β for acetylcholine =−0.302, p=0.004). Adjustment for medication, risk factors and depressive symptoms did not alter the association between anxiety and FRV dysfunction, except for BMI (anxiety β=−0.175, p=0.060; BMI β=−0.494, p<0.001). While BMI was more strongly associated with FRV function than anxiety, combined BMI and anxiety accounted for more variance in FRV function than either separately. Control participants showed no association of anxiety with FRV function.
Conclusion
Anxiety is uniquely and substantially related to poorer resistance vessel function (both endothelial and vascular smooth muscle function) in individuals with atherosclerosis. These relationships were independent of medication, depression and cardiovascular risk factors, with the exception of BMI. These findings support the concept that anxiety potentially increases vascular events through worsening of vascular function in atherosclerotic disease.
Keywords: Atherosclerosis, Anxiety, Conduit Arteries, Resistance Vessels, Endothelium Vascular Smooth Muscle, Depression
Introduction
Anxious symptoms, while common, are under-recognized in the elderly (1, 2, 3). Anxiety has been linked to future risk of cardiovascular (CV) events, with a hazard ratio of 1.26 for cardiovascular events in one meta-analysis (4). In a compelling longitudinal study, a formal psychiatric diagnosis of anxiety disorders between the ages of 18–20 was found to significantly predict a two-fold increase in coronary heart disease 37 years later, independent of conventional cardiovascular risk factors at the time of psychiatric diagnosis (5). The relationship between anxiety and vascular disease is also independent of depression (6).
Despite this strong evidence, most clinicians do not routinely consider anxiety when assessing CV risk. Reasons for this include the time needed for a comprehensive psychiatric examination, uncertainty about the type of cardiovascular events that may be associated with anxiety, and the fact that plausible mechanisms for the link have not been identified. Ease of screening has been addressed in some studies using questionnaires to assess sub-clinical anxiety symptoms (7, 8). However, there is still diagnostic uncertainty as to the exact mode of cardiovascular disease that anxiety contributes to, with strong associations with sudden cardiac death and weaker ones with incident coronary atherosclerotic events (4, 7). A recent meta-analysis on anxiety and cardiovascular disease stated that “More research is needed focusing on these possible mechanisms and the impact on different outcomes”.
Given that the exact mode of cardiovascular disease associated with anxiety is still unclear, it is perhaps no surprise that mechanisms underlying the association are unknown. A link between anxiety and atherosclerosis could be due to worsened risk factors [blood pressure (9), lipids (9), glucose (10), obesity (11, 12), and smoking (13)] or be independent of them. For example, obesity has been linked to cardiovascular disease (11) and anxiety (12). An alternative non-atherosclerotic mechanism could be sympathetic activation leading to arrhythmia, increased blood pressure and cardiac dysfunction (14) or decreased heart rate variability (15). The mechanism is important to appreciate the clinical implications. Behavioral interventions and pharmacotherapy can be targeted at anxiety itself, lifestyle, risk factors and/or the sympathetic nervous system.
It is difficult to dissect out these various mechanisms through studies that focus on binary events that occur in a small subset of participants. An alternative approach is to use an intermediate phenotype that provides a quantitative marker of cardiovascular risk relevant to one of the postulated mechanisms above. Measurement of vascular function is one such phenotype and permits assessment of vascular damage in every participant. There have been variable associations reported between anxiety and flow-mediated dilatation (FMD) of conduit arteries using ultrasound (8, 16, 17, 18, 19). However, several of these studies did not specify ischemia site (16) or used upper arm occlusion (17, 18, 19), which results in elevated and insensitive FMD (20). Others did not adjust for baseline brachial artery diameter (16, 17, 18, 19) or stimulus velocity (8, 16, 17, 18, 19), or lacked testing of vascular smooth muscle function using nitroglycerin (8, 16, 17, 18, 19). No prior study has tested the association between anxiety and forearm resistance vessel (FRV) function, which is more reproducible (21, 22) and less subject to operator error than FMD. Importantly, FRV function has been found to be a powerful and independent predictor of cardiovascular events in older participants, with better discriminatory power than FMD (23).
Given the uncertainty regarding the mode and mechanism(s) of the link between anxiety and cardiovascular events, and inconsistent prior work that is difficult to interpret, we explored the association using state-of-the-art methodology. We recruited a high-risk population with a prior diagnosis of atherosclerosis and a healthy comparison cohort who had assessment of both conduit artery function (through rigorous use of FMD) and resistance artery function (through intra-arterial drug administration and plethysmography). We hypothesized that anxious symptoms would show an inverse relationship with vascular function unique to participants with atherosclerosis, independent of standard risk factors.
Methods
Participants
The current study sample was comprised of 89 participants with diagnosed atherosclerosis (37 women, 52 men; mean age = 68, SD = 8) and 54 control participants without atherosclerosis (HC; 40 women, 14 men; mean age = 68, SD = 8) enrolled in an Institutional Review board approved and ongoing study of vascular disease and cognition at the University of Iowa (24). Almost all participants self-identified as white. Two participants (one with atherosclerosis and one without) self-identified as African American. The atherosclerosis cohort was recruited from the University of Iowa Heart and Vascular Center. Potential participants were contact by mail and informed of the ongoing study. A member of the research team contacted those initially interested, as determined by returned letters, via telephone. Following a review of the study procedures, interested participants were invited to enroll in the study and written informed consent was subsequently obtained. Inclusion criteria for the atherosclerosis cohort included: 1) age 55–90 years 2) clinically diagnosed atherosclerotic vascular disease (AVD) with a history of myocardial infarction, angina pectoris, angioplasty, stent placement, or peripheral vascular disease. For clarification, clinically diagnosed AVD is referred to as atherosclerosis, herein. The control cohort consisted of community-dwelling, age-matched volunteers without a diagnosis of atherosclerosis. Exclusion criteria for both cohorts included stroke, coronary artery bypass surgery, carotid endarterectomy, organ transplant, chronic heart failure, dementia, learning disability, schizophrenia, bipolar disorder, other illness that may affect cognitive function, or history of head injury with a loss of consciousness for more than 30 minutes. Briefly, the aforementioned ongoing study from which these participants were drawn is being conducted to determine the longitudinal relationship between vascular function and cognition. The ongoing study began recruiting in 2003. Currently, 54 healthy control participants and 108 participants with atherosclerosis have completed baseline.
During each participant’s initial screening visit, a history and physical examination was performed including a review of current medications. Blood pressure, heart rate, height, weight, electrocardiograph, and a fasting blood sample for lipids, hsCRP and glucose were obtained. Additionally during the screening visit, all participants underwent a neuropsychological battery approximately two hours long, which included assessment of cognition, anxiety and depression.
Anxiety and Depression Assessment
Anxious and depressive symptoms were measured by the Anxiety and Depression subscales of the Symptom Checklist-90-Revised (25) during the screening visit. This self-report questionnaire consists of 90 psychological symptoms. Symptoms assessed by the anxiety subscale include nervousness, trembling, fear, panic, restlessness, and frightening thoughts and is therefore a brief composite measure of psychic and somatic anxiety symptoms. Participants indicate how affected they have been by each symptom over a seven day period, ranging from not at all (0), a little bit (1), moderately (2), quite a bit (3) to extremely (4). While the SCL-90-R is not frequently used for mood assessment in atherosclerotic patients, these SCL-90-R subscales are valid and reproducible measures of anxiety and depression symptoms (25–30). Scores on the SCL-90-R are given as T-scores (based on a non-psychiatric population), with a normative Mean of 50 and a SD of 10. The anxiety subscale of the SCL-90-R has been shown to have excellent sensitivity and specificity in detecting anxiety disorders with an area under the receiver operator curve characteristic of 0.86 compared to a structured clinical interview by a mental health professional (31). The SCL-90-R was administered prior to vascular testing for all participants (mean=11±14 days). The delay between assessments was due to scheduling issues related to the larger ongoing study.
Vascular Function Assessment
Participants fasted for 12 hours prior to vascular studies and were asked to refrain from taking any medication the morning of each visit to avoid acute changes in hemodynamics caused by recent ingestion of medication. Only one participant was taking a drug (clonidine) that could cause rebound hypertension in such a short time, and results were not altered by excluding this participant. Endothelium-dependent FMD was measured by ultrasound-Doppler scan of the left brachial artery using a 10 MHz linear array transducer ultrasound system (Biosound Esaole, Indianapolis, IN) as previously described (20) by a Registered Diagnostic Medical Sonographer & Vascular Technician, whose efforts are solely dedicated to research. Because of limitations with image quality, only 77 participants in the atherosclerosis group and 52 healthy controls had measurement of FMD. After measurement of basal brachial artery diameter and Doppler velocity for five minutes, a blood pressure cuff, placed on the forearm, was inflated to 250 mmHg for five minutes to induce distal ischemia. After the cuff was deflated, velocity and diameter were measured over 2 minutes. Following a 10-minute recovery period, participants received nitroglycerin 400 μg sublingually. The outcome measure for FMD was the percent change of vessel diameter 60–70 seconds post-deflation of the BP cuff. The outcome measure for FMD with nitroglycerin was the percent change of the vessel diameter four – four and half minutes post-deflation of the BP cuff. Measurements were computed offline using automated image analysis. This FMD protocol, involving a dedicated research ultrasonographer, the use of a lower arm occlusive cuff, correction for baseline diameter and velocity, and testing of nitroglycerin responses, was more rigorous than previous FMD protocols (8, 16, 17, 18, 19). In our lab, within subject variability (coefficient of variation on a separate sample of 10 participants studied twice on different days) was 13%.
Forearm blood flow response to local brachial artery administration of vasoactive agents was used to test resistance vessel endothelial function as previously described (32). Briefly, three vasoactive agents were administered at low, medium and high doses. Each dose was administered for 6 minutes. The average response during the last two minutes of each dose was used as the outcome measure. Forearm blood flow was measured in both arms by venous occlusion plethysmography with indium/gallium-in-silastic strain gauges. Nitroprusside (NTP; 1, 3 and 10 μg/min), an endothelium-independent vasodilator that directly donates nitric oxide to vascular smooth muscle, acetylcholine (3, 10 and 30 μg/min), an endothelium-dependent vasodilator and verapamil (10, 30 and 100 μg/min), an endothelium-independent and nitric oxide independent vasodilator, were administered into the left brachial artery through a 27G metal catheter. Blood flow was permitted to return to baseline between administrations of these drugs. The order in which acetylcholine and nitroprusside were administered was randomized to minimize time-dependent confounding. Given its longer lasting effects, verapamil was administered last in all cases. The outcome measure was percent change from baseline in the ratio of blood flow between infused and non-infused arms; use of the ratio halves variability in blood flow responses to infused agents (33).
Statistical Analyses
Statistical analyses included 32 participants who were taking psychopharmaceuticals (21 atherosclerotic participants, 11 control participants). The exclusion of these participants did not affect the main outcome, and inclusion of these participants provides greater generalizablity of the findings. Therefore, all participants were included in the analyses. Given that there was an average of 11 days between the anxiety measurement and vascular measurements, an intraclass correlation (ICC) was conducted on the larger study sample to assess the reliability of the SCL-90-R anxiety sub-scale between baseline and three-year follow-up anxiety scores. Measures of blood vessel function were normally distributed. However, scores on the SCL-90-R Anxiety subscale were positively skewed (skewness=0.655, SD=0.255). However, after reviewing the distribution and the residuals of multivariate analyses of the data, non-transformed data were used for all variables. T-tests were used to test for differences between groups in age, education, vascular function, anxiety, depression and BMI. A chi-squared analysis was used to test for sex differences between groups. Bivariate Pearson’s correlations were calculated between anxious symptoms and the outcome measures of FMD and FRV function in both groups. An interaction effect was then calculated between subjects with and without atherosclerosis. Analyses of FMD and nitroglycerin-mediated brachial dilatation were controlled for baseline diameter and blood velocity on cuff release. All bivariate correlations, for both FMD and FRV, were followed by multiple regressions controlling for sex, age, and smoking. A participant was classified as a smoker if he or she had smoked within the last five years. Given that smoking is known to have acute effects on endothelial function, the main analysis was performed with and without the inclusion of smokers.
Acetylcholine responses are affected by both endothelial and vascular smooth muscle cell changes. Nitroprusside responses are affected by vascular smooth muscle cell sensitivity to nitric oxide. Verapamil responses are affected by general vascular smooth muscle cell dilator capacity. Thus, in order to assess the site of association within resistance vessels, we calculated the acetylcholine/nitroprusside response ratio as an index of endothelial nitric oxide generation independent of vascular smooth muscle sensitivity (24). We also calculated the acetylcholine/verapamil response ratio as an index of overall endothelial vasodilator generation (i.e. including nitric oxide and other dilators) independent of vascular smooth muscle damage. (24).
Follow-up analyses were focused on acetylcholine responses given that this FRV marker had the strongest relationship with anxiety. A reduced regression model including anxious symptoms and response to acetylcholine was compared to models that included covariates for cardiovascular risk factors: hypertension (systolic BP; number of antihypertensives), glucose level, high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), smoking and body mass index (BMI), independently. Depression was also added as a covariate in the regression models as a risk factor and because it has a high comorbidity with anxiety (34). Follow-up multiple regressions also assessed the relationship between depression and vascular function, separate from anxiety. Additionally, whether or not participants were receiving beta-blocker, ACE inhibitor, calcium channel blocker or a statin treatment were included as covariates. When it became evident that two variables, in addition to anxiety (HDL-c and BMI), were associated with FRV response to acetylcholine, a regression model was used to examine the relationship between anxious symptoms and acetylcholine function controlling for HDL-c, and BMI.
Results
Table 1 provides descriptive statistics for demographics, risk factors, vascular function, and anxious and depressive symptoms for the study sample. The intraclass correlation for the SCL-90-R anxiety sub-scores over three years yielded a significant result (ICC=0.690, p<0.001) suggesting that the SCL-90-R anxiety sub-scale is reproducible. Scores for anxiety were in the normal range (quartile scores shown in legend for figure 1). There were no significant differences in age (p=0.59), education (p=0.77), anxiety scores (p=0.067), or in responses to acetylcholine (p=0.59), nitroprusside (p=0.99), or verapamil (p=0.24) between participants with and without atherosclerosis. However, depressive symptoms (p=0.010) and BMI (p=0.006) were significantly higher in the atherosclerotic group. Additionally, there were significantly more women in the HC group than the AVD group (p≤0.001). All participants in the atherosclerotic group were taking at least one antihypertensive medication at time of enrollment (mean number of antihypertensives = 2.4).
Table 1.
Participant Characteristics
| Atherosclerotic Participants |
Healthy Control Participants |
|||
|---|---|---|---|---|
|
| ||||
| Variable | Mean ±SD | N | Mean ±SD | n |
| Age, yrs | 68 ± 8 | 89 | 68 ± 8 | 54 |
| Males, n | 52 | 89 | 14 | 54 |
| CAD, n | 89 | 89 | 0 | 54 |
| PAD, n | 16 | 89 | 0 | 54 |
| Diabetes, % | 28.1 | 89 | 3.7 | 54 |
| Smoking, % | 28.7 | 87 | 2.4 | 54 |
| BMI, kg/m2 | 31 ± 7 | 89 | 28 ± 6 | 54 |
| Systolic BP, mm Hg | 140 ± 20 | 88 | 132 ± 15 | 52 |
| Heart rate, bpm | 61 ± 9 | 88 | 61 ± 11 | 52 |
| Triglycerides, mg/dl | 138.0 ± 67.6 | 89 | 100.4 ± 57.9 | 54 |
| HDL cholesterol, mg/dl | 50.4 ± 15.7 | 89 | 58.8 ± 17.9 | 53 |
| LDL cholesterol, mg/dl | 88.7 ± 29.8 | 88 | 102.7 ± 28.7 | 54 |
| Glucose, mg/dl | 112.8 ± 25.1 | 89 | 93.3 ± 9.2 | 52 |
| hsCRP, mg/dl | 4.4 ± 6.1 | 81 | 3.1 ± 3.8 | 53 |
| Anxiety, SCL-90-R T-score* | 49 ± 10 | 89 | 46 ± 9 | 54 |
| Depression, SCL-90-R T-score* | 56 ± 10 | 89 | 51 ± 10 | 54 |
| Forearm blood flow, ml/min/dl | 30 ± 39 | 78 | 33 ± 34 | 52 |
| FRV acetylcholine 30 response, % | 214 ± 137 | 89 | 225 ± 139 | 52 |
| FRV nitroprusside 10 response, % | 239 ± 125 | 89 | 236 ± 155 | 52 |
| FRV verapamil 100 response, % | 201 ± 102 | 89 | 229 ± 136 | 51 |
| Brachial artery diameter, mm | 4.3 ± 0.8 | 78 | 3.9 ± .8 | 52 |
| Flow-mediated dilatation, % | 6.9 ± 3.8 | 78 | 6.0 ± 3.6 | 52 |
| Nitroglycerin dilatation, % | 10.4 ± 10.9 | 79 | 13.7± 5.7 | 50 |
Note: CAD = coronary artery disease; PAD = peripheral arterial disease; BMI = body mass index; HDL = high density lipoprotein; LDL = low density lipoprotein; FRV = forearm resistance vessel.
normalized to a non-psychiatric population with a mean of 50, and SD of 10.
Figure 1.
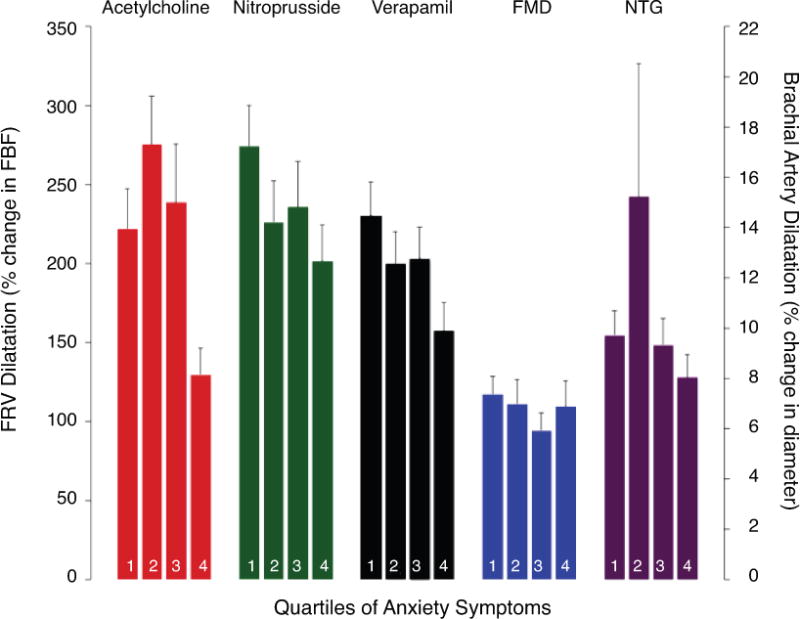
Vascular responses subdivided by quartiles of anxiety symptom score (SCL-90-R mean scores for Q1 = 39; Q2 = 45; Q3 = 52; Q4 = 63). Left vertical axis shows forearm resistance vessel (FRV) function as change (%) in forearm blood flow (FBF) in response to the highest dose of each intra-arterial infusion of acetylcholine (30 μg/min), nitroprusside (10 μg/min) and verapamil (100 μg/min). Right vertical axis shows forearm conduit vessel function, as flow-mediated dilatation (FMD) of the brachial artery. FRV function, but not FMD nor nitroglycerin-mediated FMD (NTG), was significantly reduced in participants with elevated anxiety scores.
There was no apparent relationship between FMD and anxious symptoms on inspection of non-transformed data in the atherosclerotic participants (Figure 1). While there was a significant relationship between anxiety and baseline diameter as predictors of FMD [R=0.329, F(2, 75)=4.558, p=0.014], the sole significant contributor to the model was baseline diameter (β=−0.314, p=0.006); whereas, anxiety was not significantly related (β =−0.065, p=0.56). Controlling for velocity did not alter this non-significant result for anxiety (β =−0.064, p=0.54), nor did controlling for any cardiovascular risk factor. An identical multiple regression analysis excluding smokers revealed no significant relationship between anxious symptoms and FMD, after controlling for baseline diameter (β =−0.112, p=0.39) and velocity (β =−0.097, p=0.46). Anxious symptoms were also not associated with nitroglycerin-mediated dilatation of the brachial artery in atherosclerosis (β =−0.098, p=0.40). When this same regression was applied to non-smokers, the relationship remained non-significant (β =−0.139, p=0.31).
A similar pattern emerged in control participants. Anxiety and baseline diameter were predictive of FMD [R=0.365, F(2, 49)= 3.761, p=0.030); however, baseline diameter remained the sole significant contributor of the relationship (β =−0.386, p=0.008) compared to anxious symptoms (β =0.133, p=0.35). Controlling for velocity did not alter the non-significant relationship of anxiety (β =0.135, p=0.36). Anxious symptoms and baseline diameter were associated with nitroglycerin-mediated dilatation of the brachial artery in the control group [R=0.417, F(2, 47)=4.959, p=0.011). However, like those with atherosclerosis, the baseline diameter was the sole significant contributor to the model (β =−0.367, p=0.013), compared to anxious symptoms (β=−0.108, p=0.45).
In contrast, anxious symptoms were inversely associated with FRV function, as measured by response to acetylcholine, on inspection of non-transformed data by quartiles of anxiety score in atherosclerosis (Figure 1) as well as a continuous measure of anxiety (Table 2). Participants with atherosclerosis exhibited significant negative associations between anxious symptoms and FRV response to the highest doses of acetylcholine (β =−0.302, p=0.004), nitroprusside (β=−0.228, p=0.031), and verapamil (β =−0.285, p=0.007). Anxiety was not associated with the acetylcholine/nitroprusside ratio (r =−0.175; p=0.10) or the acetylcholine/verapamil ratio (r=−0.138; p=0.20) (Figure 2). Figure 2 shows a schematic illustrating the interaction between acetylcholine and components of the resistance vessel (endothelial cell, nitric oxide and vascular smooth muscle) that contribute to vasodilation. When smokers were removed from the analysis, the relationship between response to acetylcholine, nitroprusside, verapamil and anxiety symptoms remained significant (β =−0.371, p=0.003, β =−0.314, p=0.013; β =−0.377, p=0.003, respectively).
Table 2.
Relationships between anxious symptoms and measures of vascular function
| Atherosclerotic Participants | Healthy Control Participants | |||
|---|---|---|---|---|
|
| ||||
| Variable | Standardized β | p-value | Standardized β | p-value |
| FMD | −0.065 | 0.56 | 0.133 | 0.35 |
| NTG | −0.098 | 0.40 | −0.108 | 0.45 |
| Acetylcholine | −0.302 | 0.004 | 0.129 | 0.36 |
| Nitroprusside | −0.228 | 0.031 | 0.180 | 0.20 |
| Verapamil | −0.285 | 0.007 | 0.114 | 0.43 |
Note: Measures of forearm conduit vessel function are flow-mediated dilatation (FMD; % change from baseline) of the brachial artery, as well as the nitroglycerine-mediated (NTG; % change from baseline) response. Measures of forearm resistance vessel function are change (%) in blood flow in response to intra-arterial infusion of acetylcholine (30 μg/min), nitroprusside (10 μg/min) and verapamil (100 μg/min). Standardized beta values represent the relationship between anxiety symptoms and vascular function, not adjusted for age, gender, depression and cardiovascular risk factors. Conduit vessel standardized beta values (FMD and NTG) are adjusted only for baseline brachial artery diameter.
Figure 2.
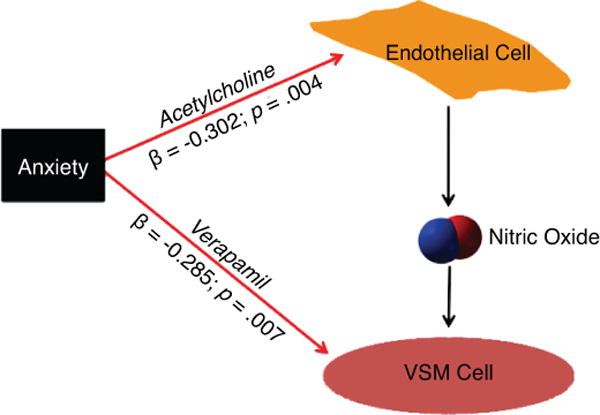
Anxiety has multiple effects on resistance vessels. There is a negative relationship with verapamil responses suggesting impaired vascular smooth muscle (VSM) function in anxiety. This effect probably accounts for the substantial inverse association between anxiety and acetylcholine mediated resistance vessel dilatation. β = standardized betas on linear regression analysis.
Given that anxiety was most strongly correlated with acetylcholine response, follow-up analyses focused solely on response to acetylcholine. Covariates including smoking, age and sex did not significantly alter the relationship between acetylcholine response and anxious symptoms (β=−0.294, p=0.006; β=−0.295, p=0.006; β=−0.338, p=0.001; respectively). Conversely, there was no association in control participants between anxiety and response to acetylcholine (β=0.129, p=0.36), nitroprusside (β =0.180, p=0.20) or verapamil (β=0.114, p=0.43).
Due to the lack of an association in the control group, follow-up analyses focused solely on participants with atherosclerosis. The interaction of other risk factors with the anxiety to FRV function relationship was assessed in multiple regression analyses (Table 3). All models still showed a significant relationship of anxiety with FRV function, with almost no impact of risk factors. The only risk factor that substantively modified the anxiety-FRV association was BMI. BMI itself accounted for 24% of the variance in dilatation to acetylcholine (p<0.001) while anxiety accounted for 3% of variance (p=0.060). Together, anxious symptoms and BMI accounted for 32% of the variance in FRV vascular function. However, anxious symptoms and BMI were significantly associated (r=0.256, p=0.015).
Table 3.
Strength of the Association between Acetylcholine FRV response, Anxiety, Depression and Cardiovascular Risk Factors in Participants with Atherosclerosis
| Risk Factor | Standardized β | p-value | % Reduction in β for anxiety |
|---|---|---|---|
| Anxiety | −0.302 | 0.004 | |
|
| |||
| Glucose | −0.180 | 0.08 | 7.28 |
| HDL-c | 0.265 | 0.009 | 3.31 |
| LDL-c | −0.029 | 0.78 | 0.99 |
| Body Mass Index | −0.494 | <.001 | 42.05 |
| hsCRP | −0.026 | 0.81 | −10.26 |
| Depression | 0.017 | 0.91 | −3.97 |
| Number of Antihypertensives | 0.014 | 0.90 | −0.66 |
| Presence of Beta-blockers | −0.126 | 0.22 | 0.0 |
| Presence of ACE Inhibitors | −0.031 | 0.77 | −0.66 |
| Use of Calcium Channel Blockers | 0.085 | 0.41 | −2.65 |
| Use of Statins | −0.001 | 0.99 | 0.0 |
| Systolic Blood Pressure | 0.008 | 0.48 | −10.26 |
| Smoking | 0.10 | 0.34 | 2.65 |
| Heart Rate | 0.037 | 0.72 | −0.33 |
Note: Beta values are for the relationship between acetylcholine FRV response and anxiety (first row), and between acetylcholine FRV response, anxiety and the other covariates (glucose, HDL, etc.). Beta reductions are expressed as the percent reduction in the standardized β from multivariate regression models; Anxiety remained significantly associated with resistance vessel response to acetylcholine in all models except for BMI where it approached significance; ACH = acetylcholine.
Interestingly, anxious symptoms remained the significant predictor of vascular dysfunction when controlling for depressive symptoms, even though depressive and anxious symptoms were highly correlated (r=0.682, p<0.001). Additional analyses suggested that both anxious and depressive symptoms had a similar relationship with vascular function. The relationship with depressive symptoms and FRV response to acetylcholine approached significance (β =−0.204, p=0.056). Like anxious symptoms, depressive symptoms were not a significant predictor of FMD when controlling for basal diameter (β=−0.072, p=0.52) or with NTG-mediated dilatation (β=−0.017, p=0.88). However, when both anxious and depressive symptoms were entered in a multivariate regression together with FRV response to acetylcholine, anxious symptoms emerged as a significant predictor of vascular dysfunction (β=−.314, p=0.033), whereas depressive symptoms did not (β=0.017, p=0.91).
Bivariate correlations between acetylcholine FRV response and all covariates resulted in four significant associations: anxiety, glucose, HDL-c and BMI (r=−0.302, p=0.004; r=−0.215, p=0.044; r=0.276, p=0.009; r=−0.539, p<0.001, respectively). The subsequent regression with the four significant covariates also yielded a significant result (r=0.585, p<0.001) where BMI emerged as the only unique contributing correlate to the model (β=−0.450, p<0.001) with anxious symptoms trending towards significance (β=−0.179, p=0.054). Given that obesity and anxiety were themselves associated (β=0.256; p=0.015) it is somewhat difficult to dissect out the relative roles of obesity and anxiety.
Concomitant medication use was also assessed in regression analyses. Use of statin, beta-blocker, calcium channel blocker, ACE-inhibitor, and number of anti-hypertensive drugs did not significantly affect the anxiety to FRV function relationship (Table 3). Though, 14% of participants were taking an SSRI, controlling for SSRI use did not significantly alter the relationship between acetylcholine FRV response, anxious symptoms and BMI symptoms [R=0.567, F(3, 85)=13.39, p<0.001).
Compared to a participant with no anxious symptoms (score of 0), a participant answering ‘a little bit’ for all anxiety items or ‘moderately’ on half of them (both raw scores of 10) would exhibit reductions in FRV dilation to acetylcholine of 0.60 and 0.62 standard deviations in men and women, respectively.
Discussion
We assessed the relationship between anxiety and both resistance and conduit vessel function in participants with and without atherosclerosis. To our knowledge, no previous studies have examined the impact of anxiety on vascular function in participants with atherosclerosis, and none have tested resistance vessels. We report four novel findings. First, symptoms of anxiety are substantially related to resistance vessel dysfunction in participants with atherosclerosis. Second, the association between anxiety and impaired FRV function is independent of most conventional risk factors and depression. Third, there is an apparent relationship with obesity, such that about 42% of the effects of anxiety on vascular function are attenuated when controlling for obesity. Fourth, anxiety associates with impairment of vascular smooth muscle function. We observed no association between anxiety and impaired conduit vessel vascular function.
Resistance versus conduit vessels
Our data show a substantial, independent association of anxiety with resistance but not conduit vessel function in atherosclerosis. Prior studies of anxiety only explored conduit vessel function, with varying methodologies and inconsistent results (8, 16, 17, 18, 19). We did not find an association between anxious symptoms and conduit vessel endothelial (FMD) and vascular smooth muscle function (nitroglycerin). We used comprehensive and rigorous methodology (involving a dedicated research ultrasonographer, the use of a lower arm occlusive cuff, correction for baseline diameter and velocity, and tested nitroglycerin responses) and a different population (atherosclerotic patients) than prior studies. There are several reasons why anxiety may have been associated with resistance vessel but not conduit vessel function. First, measurements of resistance vessel function in humans are more reproducible than even a carefully performed FMD (21, 22). Second, FRV function is a powerful and independent predictor of cardiovascular events in older participants, whereas FMD is not (23). Third, FMD is heavily confounded (reduced) by increased arterial stiffness (35, 36). Thus, an interaction of FMD with arterial stiffness might explain why anxiety is not a good predictor of FMD in participants with increased age and atherosclerosis, because both conditions increase arterial stiffness. We also had fewer participants in our FMD than FRV analyses resulting in reduced power in the FMD analyses.
Pathophysiological mechanisms
There has been substantial debate as to the putative pathophysiological mechanisms for the association between anxiety and cardiovascular events. Some have suggested that it is due to direct effects of anxiety-related sympathetic activation leading to sudden cardiac death, hemorrhagic stroke and heart failure. Our finding of a link between anxiety and vascular function in atherosclerosis suggests that direct autonomic effects are not the only explanation, and supports the concept that anxiety may contribute to vascular damage in patients with atherosclerosis. We did not directly measure sympathetic activity. However, the effect of anxiety on vascular function was not attenuated by heart rate, median blood pressure, number of antihypertensives being taken or beta-blocker use, all of which provides evidence against sympathetic involvement. The effects of anxiety on vascular function were also independent of LDL-c, smoking, diabetes, inflammation, depression, age and sex. The significant interaction with atherosclerosis could be due to factors such as genetic predisposition or increased oxidant stress that lead to an exacerbated propensity to vascular damage. Given the current findings, it is possible to speculate that participants without atherosclerosis may have been protected from the deleterious vascular effects of anxiety; however, a temporal relationship cannot be definitively established.
Interestingly, anxiety symptoms were a strong predictor of vascular function independent of depressive symptoms in atherosclerosis. This finding is intriguing because anxiety and depression are often highly correlated (34), as they were in the current study sample. Additionally, depressive symptoms were associated with vascular dysfunction in the current sample. Interestingly, the relationship of depressive symptoms and vascular dysfunction in the current sample was similar to that for anxious symptoms. However, when anxious and depressive symptoms were both entered into the model, anxious symptoms emerged as the sole predictor of vascular dysfunction. This finding may be due to the fact that resistance vessels are highly responsive to autonomic and hormonal changes, which may differ between anxiety and depression.
There was a significant and important interaction between obesity, anxiety and resistance vessel function that has not been previously reported. Both HDL-c and BMI were associated with vascular function. However, when entered into a model together, BMI was the sole significant contributor to vascular function. The association of anxiety with resistance vessel function was attenuated by about 42% (β of anxiety went from −0.302 to −0.175) when BMI was added to the model. Importantly, BMI was significantly associated with anxiety, which makes dissecting the relative effects of obesity and anxiety difficult. Intriguingly, though BMI had an association with vascular function on univariate analysis, the magnitude of this effect diminished when anxiety was added to the model. The mechanism of the interaction of obesity with anxiety-related vascular damage could relate to augmentation of the known sympathetic activation caused by obesity (37), though presumably without changes in heart rate or blood pressure. Alternatively, neurohumoral mediators like serotonin, cortisol, leptin or cannabinoids could be involved. Nevertheless, controlling for SSRI use did not alter the relationship between BMI, anxious symptoms and vascular dysfunction. The interaction with obesity and anxiety in causing vascular damage should be explored further because it may have scientific implications for the pathogenesis of obesity and clinical implications for the prevention of atherosclerosis.
Cellular localization
Dilatation to acetylcholine requires nitric oxide activity and vascular smooth muscle sensitivity. For that reason, we also tested FRV dilatation to nitroprusside (as an index of nitric oxide action) and to verapamil (as an index of vascular smooth muscle dilator sensitivity). We found associations of anxious symptoms with all three FRV dilators, though the effects were more substantial with acetylcholine. However, anxious symptoms were not associated with the acetylcholine/nitroprusside response ratio, which represents endothelial nitric oxide generation independent of vascular smooth muscle sensitivity to nitric oxide. At a minimum, our data suggest that anxiety is associated with impaired vascular smooth muscle dilator function. The mechanisms of these cell specific interactions are still unclear and warrant further exploration.
Limitations
Because we recruited participants with atherosclerosis and controls at different times and through different means, we cannot definitely assess the interaction of anxiety with the presence or absence of atherosclerosis. Though the SCL-90-R anxiety sub-score is a validated measure of anxious symptoms (20–23, 25), it has not been validated for patients with atherosclerosis. Furthermore, there are more sensitive and comprehensive measures that separate state (transient) and trait (chronic) anxiety. The SCL-90-R anxiety sub-score does not specifically delineate between state and trait anxiety, but measures anxiety symptoms within the last seven days. As anxiety symptoms can fluctuate from week to week, we suggest caution when interpreting these results for various anxiety-related constructs and disorders (i.e. Trait Anxiety, Generalized Anxiety Disorder, etc.). Additionally, the SCL-90-R anxiety sub-scale has not been tested for reliability over longer periods of time in older adults with and without atherosclerosis. However, the ICC between baseline and three-year follow-up anxiety scores in the larger study sample suggests that the SCL-90-R anxiety sub-scale is a reliable measure of anxious symptoms over time, in the current study sample. Even so, our use of the SCL-90-R may have biased our results towards null rather than false positive.
A significant number of participants received psychopharmaceuticals though this is not unexpected; exclusion of these participants did not significantly alter the relationship between anxious symptoms and vascular function. Many participants were receiving vasoactive medication, but controlling for these did not alter the magnitude of the association between anxiety and vascular dysfunction. Including patients receiving these different classes of medication improves the generalizability of our findings. Menopausal status may alter the relationship between anxious or depressive symptoms and vascular dysfunction. While it is likely, given the age of our sample (55–90 years), that women were post-menopausal, menopausal status was not obtained, so we cannot exclude an effect of female sex hormones or menopausal changes on vascular function. Given that this is a cross-sectional study, we were unable to confirm a temporal association between anxiety and vascular function.
Clinical relevance
In a prior study, one standard deviation worse resistance vessel response to acetylcholine was associated with 35% more cardiovascular events on five-year follow-up (23). Our data indicate that moderate anxious symptoms are associated with 0.60–0.62 standard deviations worse vascular function in men and women, which would approximate to about 25% more cardiovascular events (23). A meta-analysis indicated that anxiety symptoms are associated with a 26% increase in cardiovascular events (95% confidence interval: 1.15–1.38) (4). Thus, it is possible that a substantial proportion of the effects of anxiety on cardiovascular events are mediated through impaired vascular function. The severity of anxious symptoms in our study was mild to moderate, and not based on formal psychiatric diagnosis of anxiety disorder. Therefore, the association with impaired vascular function is relevant to the general population, and supports better and perhaps more formalized assessment of anxiety in preventive medicine.
Conclusion
To our knowledge, this is the first study assessing the relationship between anxiety levels and resistance vascular function. Our data indicate important and independent relationships between with anxious symptoms and both endothelium and vascular smooth muscle function, and intriguing interactions with obesity and atherosclerosis. The mechanisms of these interactions merit exploration. Future research should explore longitudinal relationships between anxiety, weight change and vascular pathophysiology, including measures of sympathetic activity.
Acknowledgments
The authors thank Christine Sinkey and Robyn Netz whose data collection was a vital contribution to this study.
This research was supported by the following grants to Dr. David J. Moser from the National Institute on Aging: NIA RO1 AG030417-01A2; NIA 1 K23 AG020649-01A1; and to Dr. William G Haynes from the National Heart Lung and Blood Institute: HL14388. This publication was made possible by Grant Number UL1RR024979 from the National Center for Research Resources (NCRR), a part of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CTSA or NIH.
Abbreviations
- AVD
Atherosclerotic Vascular Disease
- SCL-90-R
Symptom Checklist-90-Revised
- FRV
forearm resistance vessels
- FMD
flow-mediated dilatation
- CV
cardiovascular
- HC
healthy control
- BMI
body mass index
- SBP
systolic blood pressure
- LDL-c
low density lipoprotein cholesterol
- HDL-c
high density lipoprotein cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no relationship with industry.
References
- 1.Beekman A, Bremmer M, Deeg D, Van Balkom A, Smit J, De Beurs E, Van Dyck R, Van Tilburg W. Anxiety disorders in later life: a report from the longitudinal aging study Amsterdam. Int J Geriat Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Forsell Y, Winbald B. Feelings of anxiety and associated variables in a very elderly population. Int J Geriat Psychiatry. 1998;14:454–458. doi: 10.1002/(sici)1099-1166(199807)13:7<454::aid-gps795>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Smalbrugge M, Pot A, Jongenelis K, Beekman A, Eefsting J. Prevalence and correlates of anxiety among nursing home patients. J Affect Disorders. 2005;88:145–153. doi: 10.1016/j.jad.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Roest AM, Martens EM, de Jonge P, Denollet J. Anxiety and Risk of Incident Coronary Heart Disease: A Meta-Analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-Onset Depression, Anxiety, and Risk of Subsequent Coronary Heart Disease: 37-Year Follow-Up of 49,321 Young Swedish Men. J Am Coll Cardiol. 2010;56:31–37. doi: 10.1016/j.jacc.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Fiedorowicz JG, He J, Merikangas KR. The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J Psychosom Res. 2011;10:145–154. doi: 10.1016/j.jpsychores.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation. 1994;90:2225–9. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- 8.Harris KF, Matthews KA, Sutton-Tyrrell K, Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65:402–409. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 9.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med. 1997;6(1):43–9. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Barker L, Ford ES, Zhang X, Strine TW, Mokdad AH. Diabetes and anxiety in US adults: findings from the 2006 Behavioral Risk Factor Surveillance System. Diabet Med. 2008 Jul;25(7):878–81. doi: 10.1111/j.1464-5491.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- 11.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an idependent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 12.Baumeister H, Härter M. Mental disorders in patients with obesity in comparision with healthy probands. Int J Obesity. 2007;31:1155–1164. doi: 10.1038/sj.ijo.0803556. [DOI] [PubMed] [Google Scholar]
- 13.McCabe RE, Chudzik SM, Antony MM, Young L, Swinson RP, Zolvensky MJ. Smokingbehaviors across anxiety disorders. J Anxiety Disord. 2004;18(1):7–18. doi: 10.1016/j.janxdis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Lambert E, Dawood T, Straznicky N, Sari C, Schlaich M, Esler M, Lambert G. Association between the sympathetic firing pattern and anxiety level in patients with the metabolic syndrome and elevated blood pressure. J Hypertens. 2010;28(3):543–50. doi: 10.1097/HJH.0b013e3283350ea4. [DOI] [PubMed] [Google Scholar]
- 15.Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, Srinivasan K, Weinberg P. Decreased heart rate variability in panic disorder patients: A study of power-spectral analysis of heart rate. Psychiat Res. 1997;46(1):89–103. doi: 10.1016/0165-1781(93)90011-5. [DOI] [PubMed] [Google Scholar]
- 16.Cooper DC, Milic MS, Tafur JR, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. Adverse impact of mood on Flow-Mediated Dilation. Psychosom Med. 2010;72:122–127. doi: 10.1097/PSY.0b013e3181cdbfc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narita K, Murata T, Hamada T, Takahashi T, Omori M, Suganuma N, Yoshida H, Wada Y. Interactions among higher trait anxiety, sympathetic activity and endothelial function in the elderly. J Psychiat Res. 2007;41:418–427. doi: 10.1016/j.jpsychires.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Narita K, Murata T, Hamada T, Takahashi T, Kosaka H, Yoshida H, Wada Y. Association of trait anxiety and endothelial function observed in elderly males but not in young males. International Psychogeriatr. 2007;19:947–954. doi: 10.1017/S1041610206004571. [DOI] [PubMed] [Google Scholar]
- 19.Narita K, Murata T, Hamada T, Takahashi T, Kosaka H, Yoshida H, Wada Y. Associations between trait anxiety, insulin resistance, and atherosclerosis in the elderly: a pilot cross-sectional study. Psychoneuroendocrinology. 2008;33:305–312. doi: 10.1016/j.psyneuen.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Guthikonda S, Sinkey CA, Haynes WG. What is the most appropriate methodology for detection of conduit artery endothelial dysfunction? Atheroscler Thromb Vasc Biol. 2007;27:1172–1176. doi: 10.1161/ATVBAHA.106.131011. [DOI] [PubMed] [Google Scholar]
- 21.Lind L, Sarabi M, Millgård J. Methodological aspects of the evaluation of endothelium-dependent vasodilation in the human forearm. Clin Physiol. 1998;18:81–87. doi: 10.1046/j.1365-2281.1998.00077.x. [DOI] [PubMed] [Google Scholar]
- 22.Lind L, Hall J, Larsson A, Annuk M, Fellstrom B, Lithell H. Evaluation of endothelium-dependent vasodilation in the human peripheral circulation. Clin Physiol. 2000;20:440–448. doi: 10.1046/j.1365-2281.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 23.Lind L, Berglund L, Larsson A, Sundström J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 24.Moser DJ, Robinson RG, Hynes SM, Reese RL, Arndt S, Paulsen JS, Haynes WG. Neuropsychological performance is associated with vascular function in patients with Atherosclerotic Vascular Disease. Arterioscler Thromb Vasc Biol. 2007;27:141–146. doi: 10.1161/01.ATV.0000250973.93401.2d. [DOI] [PubMed] [Google Scholar]
- 25.Derogatis LR. SCL-90-R: Administration, scoring and procedures manual. Minneapolis, MN: National Computer Systems; 1994. [Google Scholar]
- 26.Koeter M. Validity of the GHW and the SCL anxiety and depression scales: A comparative study. J Affect Disorders. 1992;24:271–280. doi: 10.1016/0165-0327(92)90112-j. [DOI] [PubMed] [Google Scholar]
- 27.Steer R, Ranieri W. Further evidence for the validity of the Back Anxiety Inventory with psychiatric outpatients. J Anxiety Disord. 1993;7:195–205. [Google Scholar]
- 28.Cameron OG, Hudson CJ. Influence of exercise on anxiety level in patients with anxiety levels. Psychosomatics. 1996;27:720–723. doi: 10.1016/S0033-3182(86)72622-4. [DOI] [PubMed] [Google Scholar]
- 29.Clark A, Friedman M. Factor structure and discriminant validity of the SCL-90 in a veteran psychiatric population. J Pers Assess. 1983;47:396–404. doi: 10.1207/s15327752jpa4704_10. [DOI] [PubMed] [Google Scholar]
- 30.Steer R, Clark D, Ranieri W. Symptom dimensions of the SCL-90-R: A test of the tripartite model of anxiety and depression. J Pers Assess. 1994;64:525–536. doi: 10.1207/s15327752jpa6203_12. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz N, Kruse J, Heckrath C, Alberti L, Tress W. Diagnosing mental disorders in primary care: the General Health Questionnaire (GHQ) and the Symptom Check List (SCL-90-R) as screening instruments. Soc Psychiatry Psychiatr Epidemiol. 1999;34:360–366. doi: 10.1007/s001270050156. [DOI] [PubMed] [Google Scholar]
- 32.Guthikonda S, Sinkey C, Barnez T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 33.Haynes WG, Strachan FE, Webb DJ. Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Ciruclation. 1995;92:357–363. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- 34.Lowe B, Spitzer RL, Williams JBW, Mussell M, Schellbert D, Droenke K. Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiat. 2008;30:191–199. doi: 10.1016/j.genhosppsych.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Witte DR, van der Graaf Y, Grobbee DE, Bots ML, SMART Study Group Measurement of flow-mediated dilatation of the brachial artery is affected by local elastic vessel wall properties in high-risk patients. Atherosclerosis. 2005;182:323–330. doi: 10.1016/j.atherosclerosis.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Lind L. Arterial compliance influences the measurement of flow- mediated vasodilation, but not acetylcholine-mediated forearm blood flow: the prospective investigation of the vasculature in Uppsala seniors (PIVUS) study. Atherosclerosis. 2007;190:212–215. doi: 10.1016/j.atherosclerosis.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Correia M, Haynes WG. Does selective leptin resistance cause obesity-related hypertension? Rev Bras Hipertens. 2008;15:189–194. [Google Scholar]


