Abstract
Apolipoprotein L-1 (APOL1) high–risk alleles and the glutathione-S-transferase-μ1 (GSTM1) null allele have been shown separately to associate with CKD progression in the African American Study of Kidney Disease and Hypertension (AASK) trial participants. Here, we determined combined effects of GSTM1 null and APOL1 high–risk alleles on clinical outcomes in 682 AASK participants who were classified into four groups by GSTM1 null or active genotype and APOL1 high– or low–risk genotype. We assessed survival differences among these groups by log-rank test and Cox regression adjusted for important clinical variables for time to GFR event (change in GFR of 50% or 25-ml/min per 1.73 m2 decline), incident ESRD, death, or composite outcomes. The groups differed significantly in event-free survival for incident ESRD and composite outcomes (P≤0.001 by log-rank test). Compared with the reference GSTM1 active/APOL1 low–risk group, other groups had these hazard ratios for the composite outcome of incident ESRD and change in GFR: GSTM1 active/APOL1 high–risk hazard ratio, 2.13; 95% confidence interval, 0.76 to 5.90 (P=0.15); GSTM1 null/APOL1 low–risk hazard ratio, 2.05; 95% confidence interval, 1.08 to 3.88 (P=0.03); and GSTM1 null/APOL1 high–risk hazard ratio, 3.0; 95% confidence interval, 1.51 to 5.96 (P=0.002). In conclusion, GSTM1 null and APOL1 high–risk alleles deleteriously affect CKD progression among blacks with hypertension, and subjects with both GSTM1 null and APOL1 high–risk genotypes had highest risk of adverse renal outcomes. Larger cohorts are needed to fully explore interactions of GSTM1 and APOL1 genotypes in other subgroups.
Keywords: AASK (African American Study of Kidney Disease and Hypertension), genetic renal disease, kidney disease
Blacks account for only 13.2% of the general population, but their proportion among those receiving dialysis treatments in the United States in 2011 was 36.8%.1 The incident rate for starting dialysis was 940 per million in blacks, which is 3.4 times greater than the 280 per million found among whites.1 Higher poverty and more limited access to health care among blacks do not entirely account for their higher ESRD incidence rates,2 suggesting that this faster kidney disease progression must be partly caused by genetic factors.
The Apolipoprotein L-1 (APOL1) gene has been identified as being responsible for a significant health disparity in risks of kidney disease among blacks.3,4 Similar to the evolutionary pressures that have been seen in sickle cell disease, the carriers of APOL1 high–risk alleles are protected from Trypanosoma brucei rhodesiense infection, which causes African sleeping sickness, but individuals carrying two copies of renal risk alleles have increased risk for nondiabetic kidney disease.5 Among patients infected with HIV, the odds of developing FSGS and HIV-associated nephropathy are 17 and 29 times higher, respectively, for individuals carrying two high–risk APOL1 alleles compared with those who do not carry these risk alleles.6 Despite the strong effect of these high-risk variants, the mechanism by which they convey increased susceptibility to CKD progression in blacks remains unknown. Although the odds of developing kidney disease are greatly increased among those carrying two APOL1 high–risk alleles, only an estimated 4% will develop FSGS, and only one half of the untreated patients infected with HIV will develop HIV-associated nephropathy, suggesting that other genetic or environmental factors may play a role.6
It has been suggested that systemic or locally produced APOL1 might modulate oxidative stress, which is a common factor in the progression of CKD.7,8 Hence, genetic variants that affect the capacity to handle oxidative stress may alter APOL1 function and renal injury, thereby influencing the outcomes of CKD. In this regard, we reported that the null variant of the antioxidant gene glutathione-S-transferase-μ1 (GSTM1), GSTM1(0), is associated with accelerated disease progression in hypertensive nephrosclerosis.9
The gene product of GSTM1 is the GSTM1 enzyme, which belongs to a superfamily of glutathione-S-transferases that metabolize reactive oxygen species and reactive aldehydes that are end products of lipid peroxidation. Individuals homozygous for the deletion allele GSTM1(0) completely lack expression of the enzyme.10 Higher risks of cardiovascular disease (CVD) and various malignancies are observed among carriers of the null allele.11,12
The deletion allele is common; 50% of whites and 27% of blacks are homozygous carriers.13 In the African American Study of Kidney Disease and Hypertension (AASK) cohort, we found that black individuals who are hypertensive and carry the GSTM1(0) null allele have faster CKD progression compared with those homozygous for the active allele.9 More recently, APOL1 renal risk variants were shown to also be associated with faster decline in kidney function in the AASK.14,15 Thus, we set out to study the influence of the joint effects of APOL1 and GSTM1 genes in the AASK trial participants.
Results
The AASK was a multicenter, randomized clinical trial designed to test the effect of three different antihypertensive medications and two different levels of BP control on the progression of hypertensive kidney disease among blacks with a GFR between 20 and 65 ml/min per 1.73 m2 with no other identified causes of CKD. Primary outcomes were reduction in GFR defined as 50% reduction or an absolute 25-ml/min per 1.73 m2 decline (ΔGFR), reaching dialysis, death, or their composite outcomes. Doubling of the protein-to-creatinine ratio to >0.22 g/g was a secondary outcome in the AASK.
We assessed the survival differences among the four genotype groups by log-rank test and in the Cox regression for the primary outcomes used in the AASK. On the basis of a priori consideration, the Cox regression analyses were performed sequentially to adjust for the risk factors in the following orders. Model 1 adjusted for age; sex; mean arterial BP (MAP); history of CVD; assigned to amlodipine, metoprolol, or ramipril during the randomized treatment phase; and the degree of European ancestry. Model 2 adjusted for the all of the variables used in model 1 and baseline GFR, whereas in model 3, additional adjustment was made for the urine protein-to-creatinine ratio. Because proteinuria can mediate the effect of genotype on the outcome, to avoid overadjustment, we chose model 2 as our final model. The use of model 2 as our final statistical model is in line with the analysis performed in the previous studies addressing the effect of APOL1 alone in the AASK cohort.14,15
Table 1 summarizes the baseline characteristics of 682 subjects who were included in the study. Overall, approximately 79% of the cohort carried the GSTM1 null allele (27% as homozygous carriers and 52% as heterozygous carriers), and 23% had the APOL1 high–risk genotype. The expected genotype frequencies in the general black population are approximately 76% for the GSTM1 null allele, assuming Hardy–Weinberg equilibrium for the GSTM1 null and active alleles, and 13% for the APOL1 high–risk genotype.5,13 At baseline, the APOL1 low–risk groups, regardless of GSTM1 genotype, were older (P=0.03) and had higher MAP (P=0.01). Among the four genotype groups, the GSTM1 null/APOL1 high–risk group had the lowest baseline renal function (P=0.003), despite having the lowest MAP. Over the 5-year follow-up period, of 682 subjects, 12 subjects (1.8%) died before progressing to ESRD, 80 subjects (11.7%) reached incident ESRD, and overall, 147 subjects (21.6%) met the criteria for the composite outcome (ΔGFR, dialysis, or death). The protein-to-creatinine ratio was available for 680 of 682 subjects. During the 5-year follow-up period, 421 of 680 subjects experienced a secondary outcome of doubling of the protein-to-creatinine ratio to a value >0.22.
Table 1.
Baseline characteristics of the four genotype groups in the AASK among all 682 patients included in the study
| GSTM1 Active/APOL1 Low Risk | GSTM1 Active/APOL1 High Risk | GSTM1 Null/APOL1 Low Risk | GSTM1 Null/APOL1 High Risk | P Value | |
|---|---|---|---|---|---|
| N (%) | 113 (16.56) | 30 (4.39) | 411 (60.26) | 128 (18.76) | |
| Age at randomization, yr | 54.45 (10.44) | 51.21 (12.02) | 54.91 (9.97) | 52.13 (11.63) | 0.03 |
| MAP, mmHg | 117.61 (17.68) | 112.06 (11.79) | 114.19 (16.22) | 110.91 (15.74) | 0.01 |
| Protein-to-creatinine ratio, g/g creatinine | 0.20 | 0.50 | 0.25 | 0.49 | <0.001 |
| Baseline creatinine, mg/dl | 1.90 (0.67) | 1.98 (0.60) | 1.92 (0.67) | 2.17 (0.75) | 0.002 |
| GFR, ml/min per 1.73 m2 | 49.39 (14.28) | 46.98 (13.84) | 47.95 (12.99) | 43.55 (13.42) | <0.001 |
| Men, % | 57.52 | 56.67 | 61.31 | 57.03 | NS |
| CAD history, % | 51.33 | 40.00 | 53.77 | 43.75 | NS |
| Ramipril therapy, % | 44.25 | 40.00 | 40.63 | 41.41 | NS |
| Metoprolol therapy, % | 34.51 | 50.00 | 39.90 | 39.06 | NS |
| Amlodipine therapy, % | 21.24 | 10.00 | 19.46 | 19.53 | NS |
| Low–BP goal group, % | 47.79 | 46.67 | 51.34 | 52.34 | NS |
Subjects who had two copies of high–risk APOL1 variants were classified as having the APOL1 high–risk genotype; those with no copies or one copy were categorized as being in the APOL1 low–risk group. Subjects who were homozygous for the GSTM1 active alleles were considered to have the GSTM1 active genotype, whereas those carrying the GSTM1 null allele were classified as having the GSTM1 null genotype. The mean values are followed by SDs in parentheses. We used the chi-squared test for categorical variables and one-way ANOVA for continuous variables. CAD, coronary artery disease.
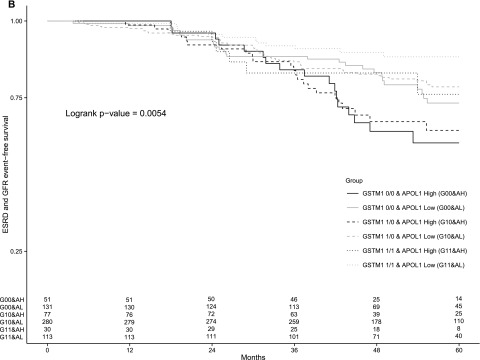
The results of the Kaplan–Meier analysis for the four genotype groups are displayed in Figure 1. The survival curves were not only significantly different among the four groups for the composite outcome of ΔGFR, incident ESRD, or death (Figure 1A), but also, they were also significantly different with regards to the composite outcome of ΔGFR and incident ESRD (Figure 1B) and incident ESRD (Figure 1C). The GSTM1 active/APOL1 low–risk genotype had relatively the best survival from incident ESRD or either of the composite outcomes, whereas the GSTM1 null/APOL1 high–risk group had relatively the lowest survival from incident ESRD or either of the composite outcomes over the 5-year follow-up period. There were only 30 subjects with the GSTM1 active/APOL1 high–risk genotype; thus, no firm conclusion can be made from comparison of the GSTM1 active versus null groups within the APOL1 high–risk group. However, those with APOL1 low–risk genotypes seemed to do worse if they also had the GSTM1 null allele.
Figure 1.
Kaplan–Meier survival curves of the primary outcomes among the four genotype groups. The figure shows the proportion of patients, according to the four genotype groups, among all 682 patients included in the study who were free from (A) the composite outcome of incident ESRD, GFR change, or death; (B) the composite outcome of incident ESRD and GFR change; or (C) incident ESRD alone. GFR event was defined as follows: an absolute 25-ml/min per 1.73 m2 decline or a 50% reduction in the GFR. The numbers of subjects at risk at yearly time points by subgroups are shown at the bottom of each panel.
The results of Cox regression analyses for the four genotype groups are depicted in Table 2. Compared with the GSTM1 active/APOL1 low–risk genotype group as the reference, subjects in GSTM1 null/APOL1 low–risk and GSTM1 null/APOL1 high–risk groups had two and three times higher risk, respectively, to have composite events. For incident ESRD, only the GSTM1 null/APOL1 high–risk group was significantly different from the reference.
Table 2.
Cox regression results for the primary outcome of incident ESRD; the composite outcome of GFR event and incident ESRD; and the composite outcome of GFR event, incident ESRD, and death among the four genotype groups
| ESRD | ESRD and GFR Event | ESRD, GFR Event, and Death | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | HR | 95% CI, P Value | N (%) | HR | 95% CI, P Value | N (%) | HR | 95% CI, P Value | |
| GSTM1 active/APOL1 low risk | 8/113 (7.08) | Reference | 12/113 (10.62) | Reference | 13/113 (11.5) | Reference | |||
| GSTM1 active/APOL1 high risk | 5/30 (16.67) | 3.00 | 0.86 to 10.54, 0.09 | 6/30 (20) | 2.13 | 0.77 to 5.90, 0.16 | 6/30 (20) | 2.09 | 0.76 to 5.71, 0.15 |
| GSTM1 null/APOL1 low risk | 39/411 (9.49) | 1.58 | 0.69 to 3.62, 0.28 | 81/411 (19.71) | 2.05a | 1.09 to 3.88, 0.03a | 86/411 (20.92) | 2.05a | 1.11 to 3.79, 0.02a |
| GSTM1 null/APOL1 high risk | 28/128 (21.88) | 3.17a | 1.32 to 7.62, 0.01a | 40/128 (31.25) | 3.00a | 1.51 to 5.96, 0.002a | 42/128 (32.81) | 3.08a | 1.59 to 5.98, <0.001a |
Numbers and percentages of subjects, HRs for the outcomes, and 95% CIs are shown. Regression analyses were adjusted for the following clinical variables: age; sex; MAP; history of CVD; assigned to amlodipine, metoprolol, or ramipril during the randomized treatment phase; degree of European ancestry; and baseline GFR.
Statistically significant HRs, 95% CIs, and P values.
We then performed a Cox regression analysis for time to doubling of the urinary protein-to-creatinine ratio and found no significant differences among the four genotype groups. We also performed the Cox regression analysis for the four genotype groups using statistical model 3, which adjusts for proteinuria. Compared with the reference GSTM1 active/APOL1 low–risk group, the hazard ratios (HRs) for the composite outcome of incident ESRD and ΔGFR were 1.99 (95% confidence interval [95% CI], 1.03 to 3.84; P=0.04) and 2.01 (95% CI, 1.00 to 4.04; P=0.05) for the GSTM1 null/APOL1 low–risk and the GSTM1 null/APOL1 high–risk groups, respectively. The HR for the GSTM1 active/APOL1 high–risk group was 1.18 (95% CI, 0.41 to 3.38; P=0.75). We note that adjusting for proteinuria reduced the HR from 2.99 to 2.01 for the GSTM1 null/APOL1 high–risk group. This HR is virtually the same as the HR of 1.99 that we see for the GSTM1 null/APOL1 low–risk group in the same analysis, suggesting that the strength of the effect of APOL1 depends on proteinuria and that there is overadjustment when using model 3.
To address the complex relationship between genotypes and outcomes, we also compared Kaplan–Meier survival within genotype subgroups for the composite outcome of ΔGFR, incident ESRD, and death (Supplemental Figure 1). In the APOL1 high–risk genotype subgroup (Supplemental Figure 1A), the event–free survival difference between those with GSTM1 null and GSTM1 active genotypes was not statistically significant, with a log–rank P value of 0.20. However, in the APOL1 low–risk genotype subgroup (Supplemental Figure 1B), those with GSTM1 null genotype had a significantly worse event–free survival compared with those with the GSTM1 active genotype, with a log–rank P value of 0.04. Similarly, in the GSTM1 null genotype subgroup (Supplemental Figure 1C), those with the APOL1 high–risk genotype had a significantly worse event–free survival compared with those with the APOL1 low–risk genotype, with a log–rank P value of 0.003. Finally, no significant difference in event-free survival between those with APOL1 high– and low–risk genotypes was found in the GSTM1 active group (Supplemental Figure 1D), with a log–rank P value of 0.21. Overall, both the GSTM1 null and the APOL1 high–risk genotypes resulted in worse outcomes unless the comparison involved the GSTM1 active/APOL1 high–risk group, which only had 30 participants.
In addition, we performed a gene-gene interaction analysis between the GSTM1 and the APOL1 genes for the composite outcome of ΔGFR, incident ESRD, and death and adjusted for the same set of covariates as those used in model 2. Both the GSTM1 null and the APOL1 high–risk genotypes are significantly associated with faster progression of CKD when analyzed independently. The HRs are 1.87 (P=0.02) and 1.56 (P=0.02) for the GSTM1 null and the APOL1 high–risk genotypes, respectively. However, when their interaction term was added to the Cox regression model, the APOL1 high–risk genotype lost statistical significance. The GSTM1 null genotype alone reaches statistical significance, but the interaction with APOL1 high–risk genotype also failed to reach statistical significance. We postulate that the interaction term must have absorbed some of the effect that was previously attributed to the APOL1 high–risk genotype. Overall, we could not conclusively show or reject a gene-gene interaction.
In the four-group analyses presented above, the GSTM1 null group is defined by carrying the GSTM1(0) null allele [GSTM1(1/0) and GSTM1(0/0)]. We chose a dominant model to determine the effect of GSTM1 on kidney disease outcomes, because in our original study, we had examined the effects of the 0/0 to 1/0 and 1/1 genotypes for GSTM1 on CKD progression in the AASK and found a stepwise effect; GSTM1(1/0) and GSTM1(0/0) groups each had significantly worse outcomes than GSTM1(1/1) (active), whereas the GSTM1(1/0) group was not significantly different compared with the GSTM1(0/0) group, suggesting a dominant effect.9 In addition to the dominant model for GSTM1(0), we also examined its effect using an additive model, which includes the GSTM1(0/0) to GSTM1(1/0) and GSTM1(1/1) genotypes separately and either APOL1 low or high risk, yielding six groups.
The baseline characteristics of the six genotype groups can be found in Table 3. At baseline, the APOL1 low–risk groups, regardless of GSTM1 genotype, had higher MAP (P=0.02). Among the six genotype groups, the GSTM1(0/0) and APOL1 high–risk and the GSTM1(1/0) and APOL1 high–risk groups had the lowest baseline renal function (P=0.02). The APOL1 high–risk groups, regardless of GSTM1 genotype, had significantly higher amounts of proteinuria at baseline (P<0.001).
Table 3.
Baseline characteristics of the six genotype groups in the AASK among all 682 patients included in the study
| GSTM1(1/1)/APOL1 Low Risk | GSTM1(1/1)/APOL1 High Risk | GSTM1(1/0)/APOL1 Low Risk | GSTM1(1/0)/APOL1 High Risk | GSTM1(0/0)/APOL1 Low Risk | GSTM1(0/0)/APOL1 High Risk | P Value | |
|---|---|---|---|---|---|---|---|
| N (%) | 113 (16.56) | 30 (4.39) | 280 (41.05) | 77 (11.29) | 131 (19.20) | 51 (7.47) | |
| Age at randomization, yr | 54.45 (10.44) | 51.21 (12.02) | 54.85 (10.06) | 51.67 (11.38) | 55.6 (9.79) | 52.84 (12.08) | NS |
| MAP, mmHg | 117.61 (17.68) | 112.06 (11.79) | 114.37 (16.38) | 109.13 (14.06) | 113.81 (15.93) | 113.58 (17.80) | 0.02 |
| Protein-to-creatinine ratio, g/g creatinine | 0.2 (0.37) | 0.52 (0.69) | 0.27 (0.47) | 0.49 (0.65) | 0.22 (0.39) | 0.52 (0.64) | <0.001 |
| Baseline creatinine, mg/dl | 1.9 (0.67) | 1.98 (0.6) | 1.93 (0.65) | 2.18 (0.76) | 1.89 (0.7) | 2.15 (0.75) | 0.002 |
| GFR, ml/min per 1.73 m2 | 49.39 (14.28) | 46.98 (13.84) | 47.94 (12.99) | 43.44 (13.24) | 47.97 (13.06) | 43.72 (13.82) | 0.02 |
| Men, % | 57.52 | 56.67 | 61.79 | 61.04 | 60.31 | 50.98 | NS |
| CAD history, % | 51.33 | 40 | 49.29 | 42.86 | 63.36 | 45.1 | 0.03 |
| Ramipril therapy, % | 44.25 | 40 | 39.64 | 40.26 | 42.75 | 43.14 | NS |
| Metoprolol therapy, % | 34.51 | 50 | 40.36 | 37.66 | 38.93 | 41.18 | NS |
| Amlodipine therapy, % | 21.24 | 10 | 20 | 22.08 | 18.32 | 15.69 | NS |
| Low–BP goal group, % | 47.79 | 46.67 | 54.29 | 49.35 | 45.04 | 56.86 | NS |
Subjects who had two copies of high–risk APOL1 variants were classified as having the APOL1 high–risk genotype; those with no copies or one copy were categorized as being in the APOL1 low–risk group. Subjects who were homozygous for the GSTM1 active alleles were considered to have the GSTM1(1/1) genotype, whereas those heterozygotes carrying the GSTM1 null allele were classified as having the GSTM1(1/0) genotype and those homozygous for the GSTM1 null alleles were classified as having the GSTM1(0/0) genotype. The mean values are followed by SDs in parentheses. We used the chi-squared test for categorical variables and one-way ANOVA for continuous variables. CAD, coronary artery disease.
The results of the Kaplan–Meier analysis for the six genotype groups are displayed in Figure 2. The survival curves were not only significantly different from each other for the composite outcome of ΔGFR, incident ESRD, or death (Figure 2A), but they were also significantly different with regards to the composite outcome of ΔGFR and incident ESRD (Figure 2B) and incident ESRD alone (Figure 2C) as well. Mirroring the results seen in the four genotype groups, the GSTM1(1/1) and APOL1 low–risk group had relatively the best survival from incident ESRD or either of the composite outcomes, whereas the GSTM1(0/0) and APOL1 high–risk group had relatively the lowest survival from incident ESRD or either of the composite outcomes in the unadjusted survival analysis. In both those with APOL1 high– and low–risk genotypes, there was a graded separation of the survival curves on the basis of the GSTM1 genotype. Subjects with the GSTM1(1/1) genotype had relatively the best survival, with the relatively worst survival seen in those who had the GSTM1(0/0) genotypes, whereas subjects with the GSTM1(1/0) genotype closely tracked the GSTM1(0/0) group.
Figure 2.
Kaplan–Meier survival curves of the primary outcomes among the six genotype groups. The figure shows the proportion of patients, according to the six genotype groups, among all 682 patients included in the study who were free from (A) the composite outcome of incident ESRD, GFR change, or death; (B) the composite outcome of incident ESRD and GFR change; or (C) incident ESRD alone. GFR event was defined as follows: an absolute 25-ml/min per 1.73 m2 decline or a 50% reduction in the GFR. The numbers of subjects at risk at yearly time points by subgroups are shown at the bottom of each panel.
The results of the Cox regression analyses for the six genotype groups are depicted in Table 4. Compared with the reference GSTM1(1/1) and APOL1 low–risk group, patients who had the APOL1 low–risk genotype and carried the GSTM1 null allele [GSTM1(0/0) and APOL1 low– and GSTM1(1/0) and APOL1 low–risk groups] had approximately two times higher risk to have composite events, whereas those participants who had the APOL1 high–risk genotype and carried the GSTM1 null allele [GSTM1(0/0) and APOL1 high– and GSTM1(1/0) and APOL1 high–risk groups] had three times higher risk to have composite events. For incident ESRD, only the GSTM1(0/0) and APOL1 high– and GSTM1(1/0) and APOL1 high–risk groups were significantly different from the reference group. The four- and six-group comparisons suggest that the GSTM1(0) allele has adverse effects on clinical outcomes, regardless of the use of either the dominant or additive model.
Table 4.
Cox regression results for the primary outcome of incident ESRD; the composite outcome of GFR event and incident ESRD; and the composite outcome of GFR event, incident ESRD, and death among the six genotype groups
| ESRD | ESRD and GFR Event | ESRD, GFR Event, and Death | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | HR | 95% CI, P Value | N (%) | HR | 95% CI, P Value | N (%) | HR | 95% CI, P Value | |
| GSTM1(1/1)/APOL1 low risk | 8/113 (7.07) | Reference | 13/113 (11.50) | Reference | 14/113 (12.38) | Reference | |||
| GSTM1(1/1)/APOL1 high risk | 5/30 (16.66) | 3.00 | 0.86 to 10.55, 0.09 | 6/30 (20.00) | 2.14 | 0.78 to 5.93, 0.15 | 6/30 (20.00) | 2.10 | 0.77 to 5.76, 0.15 |
| GSTM1(1/0)/APOL1 low risk | 26/280 (9.28) | 1.36 | 0.57 to 3.25, 0.49 | 61/280 (21.78) | 2.02a | 1.05 to 3.88, 0.04a | 61/280 (21.78) | 1.94a | 1.03 to 3.64, 0.04a |
| GSTM1(1/0)/APOL1 high risk | 16/77 (20.77) | 2.93a | 1.10 to 7.83, 0.03a | 26/77 (33.76) | 3.15a | 1.48 to 6.67, 0.003a | 26/77 (33.76) | 3.24a | 1.57 to 6.71, 0.002a |
| GSTM1(0/0)/APOL1 low risk | 16/131 (12.21) | 2.17 | 0.83 to 5.71, 0.12 | 33/131 (25.19) | 2.16a | 1.04 to 4.5, 0.04a | 33/131 (25.19) | 2.38a | 1.18 to 4.78, 0.02a |
| GSTM1(0/0)/APOL1 high risk | 13/51 (25.49) | 3.44a | 1.31 to 9.07, 0.01a | 20/51 (39.21) | 2.84a | 1.30 to 6.19, <0.01a | 20/51 (39.21) | 2.94a | 1.39 to 6.22, <0.01a |
Numbers and percentages of subjects, HRs for the outcomes, and 95% CIs are shown. Regression analyses were adjusted for the following clinical variables: age; sex; MAP; history of CVD; assigned to amlodipine, metoprolol, or ramipril during the randomized treatment phase; degree of European ancestry; and baseline GFR.
Statistically significant HRs, 95% CIs, and P values.
Discussion
CKD progression is likely determined by a complex interplay among multiple environmental and genetic factors. Specifically, even with greatly increased odds of developing kidney disease among those carrying two APOL1 high–risk alleles, it is estimated that only approximately 15% of these individuals will develop FSGS or hypertension-attributed ESRD, with some additional fraction developing hypertension-attributed CKD, suggesting that other genetic or environmental factors influence the natural course of the disease.16 To date, the search for disease-modifying variables resulted in the identification of tentative APOL1 interactions with single-nucleotide polymorphisms in the podocin (NPHS2) and serologically defined colon cancer antigen 8 gene and near the bone morphogenetic protein 4 gene as well as JC viruria being associated with lower risk of kidney disease progression in those carrying two APOL1 high–risk alleles.17,18 However, the identification of these disease-modifying variables has not shed light on the pathogenic mechanism of APOL1-associated nephropathy. Here, we show that having no copies or only one copy of the active allele of the GSTM1 gene that modulates oxidative stress seems to amplify the effect with the APOL1 high–risk genotype in kidney disease progression in the AASK. However, we cannot determine whether having two copies of the active allele for GSTM1 would be protective in those with the APOL1 high–risk genotype because of the very small number of subjects in the GSTM1 active/APOL1 high–risk group, which precludes us from drawing firm conclusions regarding the relative differences between the GSTM1 active and null groups within the APOL1 high–risk group. A much larger cohort would be required, because the expected proportion of the GSTM1 active/APOL1 high–risk group is a very small 3% (0.23 GSTM1 active ×0.13 APOL1 high risk).
We performed a gene-gene interaction analysis between the GSTM1 and the APOL1 genes for the composite outcome of GFR event, incident ESRD, and death and could not definitively show or refute a gene-gene interaction. When their interaction term was considered in the Cox regression model, neither the interaction term or the APOL1 high–risk genotype was statistically significant. Only the GSTM1 null genotype remained significant. We postulate that the interaction term must have absorbed some of the effect that was previously attributed to the APOL1 high–risk genotype. Additional studies in larger cohorts are required to investigate the significance of the APOL1 high–risk genotype losing significance after the addition of the interaction term and better determine the interaction between the GSTM1 and APOL1 genes.
We showed earlier that the GSTM1(0) allele is associated with accelerated CKD progression in the AASK cohort.9 This study now shows that the deleterious effect of the GSTM1(0) allele is not confounded by the APOL1 high–risk status. We showed that the GSTM1 enzyme modulates oxidative stress and hence, vascular smooth muscle cell function in vitro.9 In humans, the GSTM1(0) allele has been shown to be associated with higher circulating levels of malondialdehyde, a reactive aldehyde, in patients with epilepsy.19 In patients with HIV, those with the active GSTM1 allele have higher CD4 cell counts, reduced oxidative stress, and lower HIV viral loads compared with those with the GSTM1 (0) allele.20 Here, we show that having no copies or only one copy of the active allele of GSTM1 seems to amplify the effect with the APOL1 high–risk genotype in kidney disease progression in the AASK. Taken together, it is reasonable to speculate that the faster CKD progression observed in those having both the GSTM1 null and the APOL1 high–risk genotype is also attributable to oxidative stress.
We did not observe any association between GSTM1 genotype and progression of proteinuria; however, it is intriguing to note that adjustment for proteinuria diminishes the effect of the APOL1 genotype on the adverse kidney outcomes. Perhaps this can be viewed as a piece of circumstantial evidence that the deleterious effect of the APOL1 genotype on renal outcomes is at least partially mediated by proteinuria.
Our study allows for comparisons between the effects of GSTM1 null and APOL1 high–risk genotypes on CKD progression simultaneously in the same cohort. As previously published, the GSTM1 null allele reaches significance for the composite outcome of incident ESRD and ΔGFR but not for incident ESRD alone,9 whereas the APOL1 high–risk genotype reached statistical significance for incident ESRD alone as well.14 These findings suggest that the APOL1 high–risk allele has a stronger effect on CKD progression among blacks who are hypertensive. Although the observed effect of the GSTM1 null allele may be more modest, its high frequency implicates a potential for a large effect on a population at risk. In this regard, across racial groups, the frequency of the GSTM1 null allele ranges from 0.38 to 0.56 in Indians and from 0.65 to 0.82 in Europeans.10,21 Before our report, the only extensive study examining the frequency of the null allele in blacks that we have found in the literature to date reported the frequency of homozygosity of the null allele in this racial group to be 27% among 479 subjects.13 However, the total frequency of the null allele (homozygous and heterozygous states) was not reported. By Hardy–Weinberg equilibrium, we assume that the frequency of the null allele is 0.519 (0.519×0.519=0.27) in blacks, and therefore, approximately 76% of the general black population are carriers of the null allele in either the homozygous or the heterozygous state, which is in line with the frequencies reported in other populations. We estimated the relative effect of the GSTM1 null and the APOL1 high–risk genotypes on CKD progression on the population level among blacks who are hypertensive using the population–attributable risk fractions (PARs) on the composite outcome of ESRD and ΔGFR adjusted for covariates and time at risk. The PARs were 38.4% (95% CI, 7.9% to 62.4%) and 12% (95% CI, 0.8% to 22.9%) for the GSTM1 null and the APOL1 high–risk genotypes, respectively. Our estimation showed that the GSTM1 null and the APOL1 high–risk genotypes have comparable effects on CKD progression in this population. One also needs to take into account that, although the APOL1 high–risk alleles are specific to those of African ancestry, the GSTM1 null allele is highly prevalent across racial groups.
Our observations generate clinically important questions. First, are the effects of GSTM1(0) and APOL1 high–risk alleles generalizable to CKD attributable to disease processes other than hypertension–associated kidney disease? Second, do GSTM1(0) and APOL1 high–risk alleles have additive effects on the circulating and urinary levels of markers of oxidative stress? Interpretation of our findings is limited to the AASK, a single cohort of subjects with hypertension–associated kidney disease. Additional studies in a similar cohort and those with other kidney diseases with longitudinal follow-up are needed to confirm these findings. The possibility of selection bias is another limitation of our study, because we could ascertain the genotypes of only 682 of the original 1094 AASK participants, although patient baseline data for this study were similar to published data for the entire AASK trial.
In conclusion, the GSTM1 null allele and APOL1 high–risk alleles both have deleterious effects on disease progression among blacks with kidney disease associated with hypertension. Subjects with both the GSTM1(0) and APOL1 high–risk alleles had the highest risk of adverse renal outcomes. Larger cohorts are needed to confirm these findings and further explore the interactions between GSTM1 and APOL1.
Concise Methods
Design, Participants, and Outcomes
We performed a retrospective cohort study among the AASK trial participants. The AASK was a multicenter, randomized clinical trial designed to test the effect of three different antihypertensive medications and two different levels of BP control on the progression of hypertensive kidney disease in a three by two factorial design.22 Briefly, the AASK trial included 1094 blacks ages 18–70 years old with clinical diagnosis of hypertension and an iothalamate GFR between 20 and 65 ml/min per 1.73 m2 with no other identified causes of CKD. Between February of 1995 and September of 1998, participants were randomly assigned to one of two MAP goals (102–107 mmHg [usual; n=554] or ≤92 mmHg [lower; n=540]) and initial treatment with a β-blocker (metoprolol, 50–200 mg/d; n=441), an angiotensin–converting enzyme inhibitor (ramipril, 2.5–10 mg/d; n=436), or a dihydropyridine calcium channel blocker (amlodipine, 5–10 mg/d; n=217). Open-label agents were added as needed to achieve the assigned BP goals. The amlodipine arm was halted early in September of 2000 on the basis of the recommendation of the Data and Safety Monitoring Board (subjects were switched to open-label medications), whereas the other two arms were followed up to September of 2001 as planned. Primary outcomes were reduction in GFR defined as 50% reduction or an absolute 25-ml/min per 1.73 m2 decline (ΔGFR), reaching dialysis, death, or their composite outcomes. Doubling of the protein-to-creatinine ratio to >0.22 g/g was a secondary outcome. Of the 1094 subjects in the AASK, 850 patients consented to provide DNA samples. The institutional review boards of the individual clinical sites had approved use of the DNA samples and clinical data from the AASK trial.
The GSTM1 genotype was successfully determined in 682 of the 693 participants with known APOL1 genotype. Thus, we included in our study 682 of the 1094 original AASK participants.
Genotyping and Classification
GSTM1 genotyping was performed using a real–time PCR method.23 APOL1 genotyping was done by TaqMan assays (Life Technologies, Carlsbad, CA).6 The APOL1 high–risk group was defined by having G1/G1, G1/G2, or G2/G2 genotypes, whereas the GSTM1 null group is defined by carrying the GSTM1(0) null allele [GSTM1(1/0) and GSTM1(0/0)]. The GSTM1 active group, by definition, is homozygous for the active allele [GSTM1(1/1)].
Population Admixture
To adjust for the degree of European ancestry in the AASK and ensure that the AASK participants were comparable in genetic background, our analyses were corrected for population stratification using the multidimensional scaling approach as previously performed.9
Specifically, individual genotypes of a panel of 126 biallelic markers were used to construct an identity by state distance matrix, and the two most significant multidimensional scaling components (C1 and C2) were extracted and used as covariates in regression analyses.24
Statistical Methods
Demographics and baseline risk factors were summarized in proportions and means±SDs as appropriate. Differences among the four and six genotype groups were compared using the chi-squared test for categorical variables and one-way ANOVA for continuous variables. The survival differences among the four and six genotype groups were assessed by log-rank test and in the Cox regression for time to the event of doubling of the protein-to-creatinine ratio, ΔGFR (50% reduction or a 25-ml/min per 1.73 m2 decline), dialysis, death, or their composite outcomes. On the basis of a priori consideration, the Cox regression analyses were performed sequentially to adjust for the risk factors in the following orders. Model 1 adjusted for age; sex; MAP; history of CVD; assigned to amlodipine, metoprolol, or ramipril during the randomized treatment phase; and degree of European ancestry. Model 2 adjusted for the all of the variables used in model 1 and baseline GFR, whereas in model 3, additional adjustment was made for the urine protein-to-creatinine ratio. Because proteinuria can mediate the effect of genotype on the outcome, to avoid overadjustment, we chose model 2 as our final model.
When performing the Cox regression for doubling of the protein-to-creatinine ratio, we adjusted for the same variables as used in our model 2, with the exception of the baseline protein-to-creatinine ratio.
To assess the potential effect of the GSTM1 null and APOL1 high–risk genotypes on the outcomes of interest, the partial PAR was used to estimate the proportion of events that may have been avoided if the participants did not have these risk genotypes.25 The partial PAR and its 95% CI for the GSTM1 null genotype were calculated from a Cox model that was adjusted for the confounding risk factors and presence of the APOL1 high–risk genotype. Likewise, the partial PAR for the APOL1 high–risk genotype was calculated from a Cox model that was adjusted for the confounding factors and the presence of the GSTM1 null genotype. A P value <0.05 was considered to be statistically significant for all statistical results. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the referees and the editors’ contribution to the improvement of our manuscript. We also thank Elizabeth Binns-Roemer for excellent technical assistance.
This work was supported, in part, by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH). This project has been funded, in whole or in part, with federal funds from National Cancer Institute, NIH, Center for Cancer Research contract HHSN26120080001E and in part, by NIH extramural grant R01DK094907-01 (to T.H.L).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, and the mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050487/-/DCSupplemental.
References
- 1.US Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 2.McClellan WM, Newsome BB, McClure LA, Howard G, Volkova N, Audhya P, Warnock DG: Poverty and racial disparities in kidney disease: The REGARDS study. Am J Nephrol 32: 38–46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DJ, Pollak MR: Genetics of kidney failure and the evolving story of APOL1. J Clin Invest 121: 3367–3374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornoni A, Merscher S, Kopp JB: Lipid biology of the podocyte---new perspectives offer new opportunities. Nat Rev Nephrol 10: 379–388, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A: Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler Thromb Vasc Biol 29: 870–876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J, Ma JZ, Zeng Q, Cechova S, Gantz A, Nievergelt C, O’Connor D, Lipkowitz M, Le TH: Loss of GSTM1, a NRF2 target, is associated with accelerated progression of hypertensive kidney disease in the African American Study of Kidney Disease (AASK). Am J Physiol Renal Physiol 304: F348–F355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Board P, Coggan M, Johnston P, Ross V, Suzuki T, Webb G: Genetic heterogeneity of the human glutathione transferases: A complex of gene families. Pharmacol Ther 48: 357–369, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP: Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: A literature-based systematic HuGE review and meta-analysis. Am J Epidemiol 167: 759–774, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Yang M, Zhao J, Xing L, Shi L: Association between GSTM1 null genotype and coronary artery disease risk: A meta-analysis. Med Sci Monit 20: 1550–1555, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P, Bouchardy C, Breskvar K, Brockmoller J, Cascorbi I, Clapper ML, Coutelle C, Daly A, Dell’Omo M, Dolzan V, Dresler CM, Fryer A, Haugen A, Hein DW, Hildesheim A, Hirvonen A, Hsieh LL, Ingelman-Sundberg M, Kalina I, Kang D, Kihara M, Kiyohara C, Kremers P, Lazarus P, Le Marchand L, Lechner MC, van Lieshout EM, London S, Manni JJ, Maugard CM, Morita S, Nazar-Stewart V, Noda K, Oda Y, Parl FF, Pastorelli R, Persson I, Peters WH, Rannug A, Rebbeck T, Risch A, Roelandt L, Romkes M, Ryberg D, Salagovic J, Schoket B, Seidegard J, Shields PG, Sim E, Sinnet D, Strange RC, Stücker I, Sugimura H, To-Figueras J, Vineis P, Yu MC, Taioli E: Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev 10: 1239–1248, 2001 [PubMed] [Google Scholar]
- 14.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ AASK Study Investigators CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dummer PD, Limou S, Rosenberg AZ, Heymann J, Nelson G, Winkler CA, Kopp JB: APOL1 kidney disease risk variants: An evolving landscape. Semin Nephrol 35: 222–236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Divers J, Palmer ND, Lu L, Langefeld CD, Rocco MV, Hicks PJ, Murea M, Ma L, Bowden DW, Freedman BI: Gene-gene interactions in APOL1-associated nephropathy. Nephrol Dial Transplant 29: 587–594, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Divers J, Núñez M, High KP, Murea M, Rocco MV, Ma L, Bowden DW, Hicks PJ, Spainhour M, Ornelles DA, Kleiboeker SB, Duncan K, Langefeld CD, Turner J, Freedman BI: JC polyoma virus interacts with APOL1 in African Americans with nondiabetic nephropathy. Kidney Int 84: 1207–1213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CS, Tsai CS: Enhanced lipid peroxidation in epileptics with null genotype of glutathione S-transferase M1 and intractable seizure. Jpn J Pharmacol 90: 291–294, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Parsons M, Campa A, Lai S, Li Y, Martinez JD, Murillo J, Greer P, Martinez SS, Baum MK: Effect of GSTM1-polymorphism on disease progression and oxidative stress in HIV infection: Modulation by HIV/HCV co-infection and alcohol consumption. J AIDS Clin Res 4: 10002337, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal S, Tripathi G, Khan F, Sharma R, Baburaj VP: Relationship between GSTs gene polymorphism and susceptibility to end stage renal disease among North Indians. Ren Fail 29: 947–953, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Girault I, Lidereau R, Bièche I: Trimodal GSTT1 and GSTM1 genotyping assay by real-time PCR. Int J Biol Markers 20: 81–86, 2005 [PubMed] [Google Scholar]
- 24.Fung MM, Chen Y, Lipkowitz MS, Salem RM, Bhatnagar V, Mahata M, Nievergelt CM, Rao F, Mahata SK, Schork NJ, Brophy VH, O’Connor DT AASK Co-Investigators : Adrenergic beta-1 receptor genetic variation predicts longitudinal rate of GFR decline in hypertensive nephrosclerosis. Nephrol Dial Transplant 24: 3677–3686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E, Wand HC: Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control 18: 571–579, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.