Abstract
Like many organs, the kidney stiffens after injury, a process that is increasingly recognized as an important driver of fibrogenesis. Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are related mechanosensory proteins that bind to Smad transcription factors, the canonical mediators of profibrotic TGF-β responses. Here, we investigated the role of YAP/TAZ in the matrix stiffness dependence of fibroblast responses to TGF-β. In contrast to growth on a stiff surface, fibroblast growth on a soft matrix led to YAP/TAZ sequestration in the cytosol and impaired TGF-β–induced Smad2/3 nuclear accumulation and transcriptional activity. YAP knockdown or treatment with verteporfin, a drug that was recently identified as a potent YAP inhibitor, elicited similar changes. Furthermore, verteporfin reduced YAP/TAZ levels and decreased the total cellular levels of Smad2/3 after TGF-β stimulation. Verteporfin treatment of mice subjected to unilateral ureteral obstruction similarly reduced YAP/TAZ levels and nuclear Smad accumulation in the kidney, and attenuated renal fibrosis. Our data suggest that organ stiffening cooperates with TGF-β to induce fibrosis in a YAP/TAZ- and Smad2/3-dependent manner. Interference with this YAP/TAZ and TGF-β/Smad crosstalk likely underlies the antifibrotic activity of verteporfin. Finally, through repurposing of a clinically used drug, we illustrate the therapeutic potential of a novel mechanointerference strategy that blocks TGF-β signaling and renal fibrogenesis.
Keywords: chronic kidney disease, extracellular matrix, fibrosis, TGF-beta, YAP, TAZ
A key step in fibrogenesis is the conversion of quiescent fibroblasts into active myofibroblasts that deposit extracellular matrix (ECM).1 TGF-β is a principal factor driving this activation process.2,3 An important step in TGF-β–induced fibroblast activation is the C-terminal phosphorylation of the profibrotic transcription factors Smad2 and Smad3. Phosphorylated Smad2/3 accumulates in the nucleus, where it drives the expression of TGF-β–sensitive, profibrotic genes.4
After injury, a critical but under-recognized change that occurs in the kidney and other organs is increased tissue stiffness.5–9 Multiple injury–induced processes lead to organ stiffening, including not only the deposition of scar tissue but also, the increased expression of collagen and elastin crosslinking enzymes that stiffen the existing ECM even before scarring occurs.7,8,10 The profibrotic response of fibroblasts to TGF-β is exquisitely mechanosensitive, with fibroblast survival, activation, and collagen expression being dependent on a stiff ECM reminiscent of an injured fibrotic kidney but inhibited by soft healthy kidney–like ECM.5,11–13 How fibroblasts sense and transduce these mechanical cues, however, has largely remained a mystery.
Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are transcription cofactors which promote TGF-β signaling via retaining activated Smad2/3 in the nucleus.14–16 The activity of YAP and TAZ is determined by their localization within the cell, with nuclear protein being active and cytosolic protein being inactive. The localization of YAP/TAZ was recently shown to be regulated by ECM stiffness through modulation of cytoskeletal tension.17 Whereas YAP and TAZ localize to the cytoplasm in cells grown on soft, healthy tissue substrates, a situation characterized by reduced cytoskeletal tension, they localize to the nucleus in cells grown on stiff, injured surfaces.17,18 These findings suggested to us that YAP and TAZ may be critical mechanotransducers that link fibroblast stiffness sensation to regulation of TGF-β responses in kidney fibrosis.
Here, we show that soft ECM inhibits and stiff ECM augments TGF-β–induced profibrotic Smad signaling by controlling Smad2/3 localization in a process that is mediated by YAP and TAZ. Furthermore, treating cells and mice with verteporfin, a small molecule used for many years as a therapy for eye disease with newly described YAP inhibitory activity,19 blocks TGF-β–induced myofibroblast activation. Verteporfin induced a dramatic reduction in YAP and TAZ that was associated not only with reduced Smad2/3 nuclear accumulation, but also diminished Smad2/3 levels after TGF-β stimulation. In vivo, verteporfin reduced renal interstitial YAP/TAZ abundance, a finding that was accompanied by decreased nuclear Smad2/3 accumulation and markers of fibrosis in the kidney. Taken together, our results define a novel mechanosensitive YAP/TAZ–dependent pathway that regulates TGF-β–induced fibroblast Smad signaling. Moreover, through the repurposing of a clinically used small molecule inhibitor, we also describe a novel tool to interfere with the mechanoregulation of TGF-β/Smad signaling, and in the process, identify a promising new antifibrotic treatment strategy for CKD.
Results
TGF-β–Induced Smad Signaling Is Regulated by ECM Stiffness
Although multiple studies have shown that TGF-β–induced fibroblast responses are ECM stiffness dependent,5,11–13,20,21 none have examined whether stiffness regulates the canonical profibrotic Smad signaling pathway. We, therefore, examined the effects of differences in ECM stiffness on various events in the Smad pathway using fibroblasts cultured on fibronectin–coated soft (2 kPa) or stiff (100 kPa) substrates.
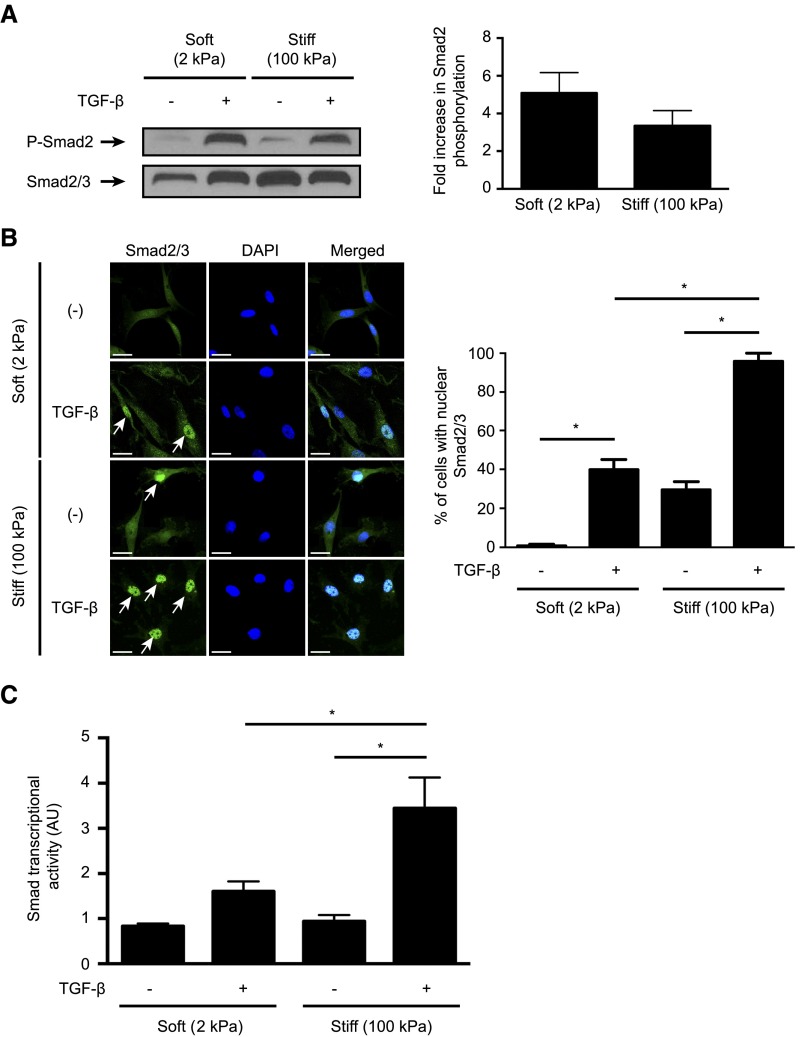
TGF-β induced equivalent and robust Smad2 and Smad3 phosphorylation in both soft and stiff gel–cultured fibroblasts (Figure 1A, Supplemental Figure 1). TGF-β also induced a robust nuclear accumulation of Smad2/3 in fibroblasts grown on stiff gels, with nearly 100% of cells showing predominantly nuclear Smad2/3 staining. This effect, however, was diminished in cells grown on soft gels, with only 40% of TGF-β–stimulated cells exhibiting nuclear Smad2/3 (Figure 1B). Accordingly, TGF-β–induced Smad transcriptional activity in fibroblasts grown on soft gels was reduced in a Smad–dependent luciferase reporter assay22,23 compared with stiff substrate cultures (Figure 1C). These results indicate that ECM stiffness controls subcellular localization of Smad2/3 without affecting TGF-β receptor–mediated Smad phosphorylation. Taken together, our results suggest that intracellular Smad2/3 localization and downstream Smad–driven transcription are mechanically regulated.
Figure 1.
Substrate stiffness modulates TGF-β–induced Smad signaling via regulation of intracellular localization of Smad2 and Smad3. (A and B) NRK49F rat renal fibroblasts cultured on soft (2 kPa) and stiff (100 kPa) fibronectin–coated gels were stimulated with TGF-β followed by analysis of (A) Smad2 phosphorylation and (B) intracellular Smad2/3 localization. In A, quantification of the TGF-β–induced fold increase in Smad2 phosphorylation is presented (n=4 per condition). In B, cells were counterstained with DAPI to identify nuclei (n=3 coverslips per condition). Arrows point to cells with predominantly nuclear Smad2/3 staining. Scale bar, 20 μm. (C) Fibroblasts transfected with a Smad–binding element luciferase reporter and a normalizing Renilla luciferase control were stimulated with TGF-β, and the resultant luminescence was measured (n=6 per condition). One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; P-Smad2, phospho-Smad2. *P<0.05.
YAP and TAZ Localization in Fibroblasts Is Mechanically Regulated
We next set out to determine how the intracellular localization of Smad2/3 is influenced by ECM stiffness. YAP and TAZ are transcription cofactors that interact with and regulate Smad2/3 localization in embryonic stem and epithelial cells after TGF-β stimulation.14,15 Interestingly, the localization of YAP and TAZ in epithelial and endothelial cells was also recently shown to be ECM stiffness regulated.17,18,24,25 To establish whether YAP/TAZ localization is similarly regulated by ECM stiffness in kidney fibroblasts, we generated fibronectin–coated soft substrates (2 kPa) mimicking the mechanics of normal kidney26–28 and stiff substrates (100 kPa) corresponding to injured fibrotic kidney.29 Immunostaining for YAP/TAZ in renal fibroblasts cultured on soft and stiff gels showed diffuse staining in cells grown on soft matrix and predominantly nuclear staining in cells grown on stiff matrix (Supplemental Figure 2), confirming that YAP and TAZ are mechanically regulated in renal fibroblasts.
Verteporfin Inhibits YAP and TAZ Primarily by Inducing a Dramatic Reduction in YAP/TAZ Levels
Because YAP and TAZ are known to promote Smad signaling, the finding that both YAP/TAZ and profibrotic TGF-β/Smad signaling are regulated by stiffness in renal fibroblasts suggested to us that these two pathways may be linked. To address this question, we sought to inhibit YAP/TAZ in cells grown on stiff surfaces, such as fibronectin-coated glass and plastic, that exhibit maximal YAP/TAZ activation, to determine if YAP/TAZ blockade would mimic the TGF-β inhibitory effects of soft matrix.
Verteporfin is a small molecule currently clinically used as an ocular photodynamic therapy acting via reactive oxygen species–dependent, non–YAP/TAZ–related mechanisms.30 This drug was recently shown, in the absence of light activation, to have previously unrecognized and highly potent YAP inhibitory activity.19 It was proposed in that report that verteporfin mediates this inhibition by interfering with the ability of YAP to interact with other proteins by inducing a conformational change.19 However, whether this is the dominant effect of verteporfin is unclear, and whether verteporfin also inhibits TAZ, a closely related homolog of YAP, remains unknown.
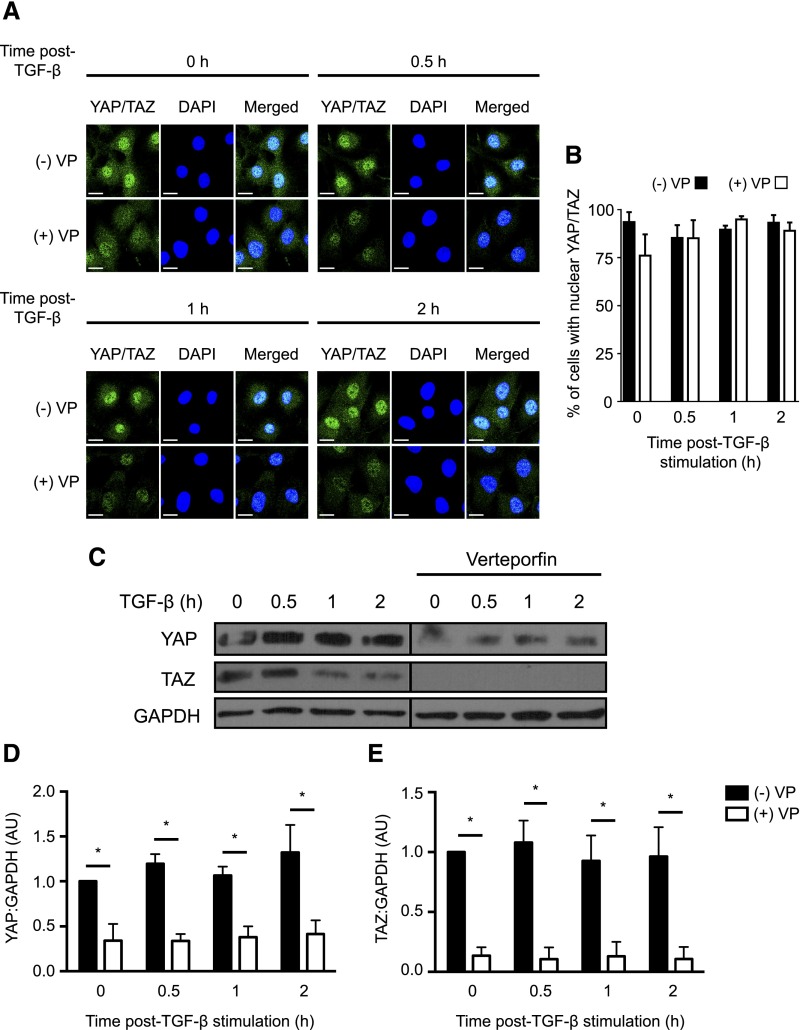
To examine the effects of this drug on YAP and TAZ in fibroblasts, we treated NRK49F cells grown on fibronectin–coated plastic dishes, which provide a stiff matrix environment, with verteporfin in the presence or absence of TGF-β. Because control of subcellular localization is a major YAP/TAZ regulatory mechanism, we first examined the effect of verteporfin on YAP/TAZ distribution in the presence or absence of TGF-β. Although verteporfin did not affect the localization of YAP/TAZ (Figure 2, A and B), it unexpectedly induced a dramatic reduction in YAP/TAZ levels as determined by immunofluorescence staining (Figure 2A) and immunoblotting (Figure 2C). Verteporfin also reduced YAP/TAZ mRNA levels, suggesting that the reduction in YAP/TAZ protein was, in part, because of decreased synthesis (Supplemental Figure 3). Although YAP and TAZ are degraded by the proteasome,31,32 proteasomal inhibition with MG132 did not rescue YAP/TAZ protein levels after verteporfin treatment, suggesting that verteporfin does not enhance YAP/TAZ proteasomal degradation (Supplemental Figure 4).
Figure 2.
Verteporfin treatment results in dramatically reduced levels of YAP and TAZ. NRK49F fibroblasts were treated with or without 250 nmol/L verteporfin for a total of 12 hours. TGF-β (10 ng/ml) was added for the indicated times. In A, after treatment with verteporfin and TGF-β as described above, NRK49F fibroblasts were stained with an antibody directed against YAP/TAZ (green) and counterstained with DAPI (blue) to identify nuclei. Representative images are shown showing loss of whole–cell YAP/TAZ staining even before TGF-β stimulation but no change in the predominantly nuclear localization of YAP/TAZ. Scale bar, 15 μm. (B) The percentage of cells with predominantly nuclear YAP/TAZ staining was quantified at each time point. A minimum of 50 cells was counted per replicate (four replicates per condition). In C, after treatment with verteporfin and TGF-β, lysates were immunoblotted for YAP, TAZ, and GAPDH. Representative blots are shown in C, and quantification of relative levels of (D) YAP (n=3 per condition) and (E) TAZ (n=5 per condition) are also shown. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. *P<0.05.
Consistent with its ability to downregulate YAP/TAZ, treatment with verteporfin resulted in a loss of detectable interactions between YAP/TAZ and TEAD1, a known YAP/TAZ binding partner (Supplemental Figure 5, A and C). Similarly, although TGF-β induced a dramatic increase in YAP/TAZ-Smad2/3 nuclear interactions in NRK49F cells grown on fibronectin-coated glass, this effect was attenuated by verteporfin (Supplemental Figure 5, B and D). Taken together, our results suggest that, in fibroblasts, verteporfin inhibits YAP and TAZ primarily by reducing their abundance, thereby blocking complex formation with transcriptional partners, such as TEAD1 and Smad2/3.
Verteporfin-Induced Reductions in YAP/TAZ Inhibit TGF-β Signaling by Reducing the Nuclear Accumulation and Overall Abundance of Activated Smads
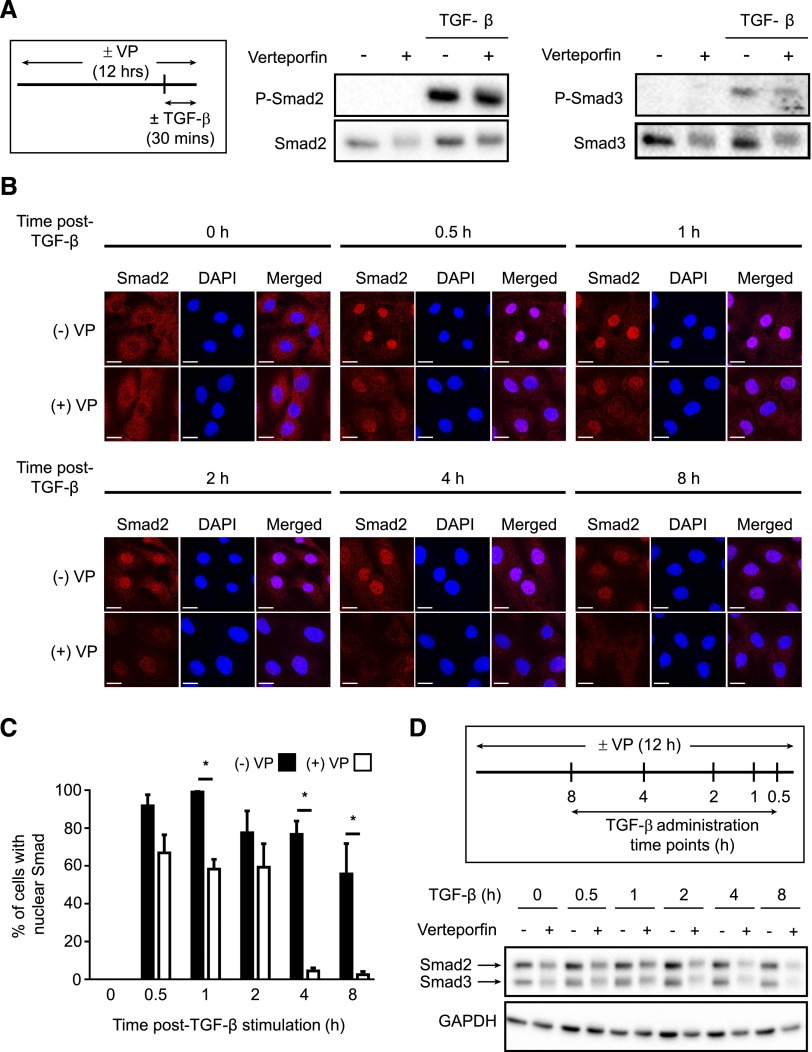
Having shown that verteporfin induces a loss of YAP/TAZ, we next examined the effects of this drug on TGF-β–induced Smad signaling. Verteporfin treatment did not affect the TGF-β–induced rapid phosphorylation of Smad2 or Smad3, because levels of phospho-Smad2 and phospho-Smad3 increased appropriately within 30 minutes of TGF-β stimulation, even with 12 hours of verteporfin treatment (Figure 3A). After phosphorylation, activated Smad2/3 accumulates in the nucleus, an effect that is facilitated by YAP/TAZ–mediated nuclear Smad2/3 retention. As expected, YAP/TAZ reduction induced by 12 hours of verteporfin treatment impaired TGF-β–stimulated Smad nuclear accumulation (Figure 3, B and C). Interestingly, in addition to this effect on nuclear accumulation, treatment with verteporfin also reduced total cellular Smad2 and Smad3 protein. This effect was modest in the absence of TGF-β, a situation in which Smad2/3 is mostly inactive (Figure 3, B and D). Verteporfin-induced reductions in Smad2/3 were particularly enhanced, however, after TGF-β–induced Smad activation, particularly at later time points (2, 4, and 8 hours post–TGF-β) (Figure 3, B and D). Because TGF-β–activated Smads are eventually targeted for breakdown by the proteasome,33,34 we next tested whether the late reduction in TGF-β–activated Smads induced by verteporfin was mediated by enhanced proteasomal degradation. Proteasomal inhibition with MG132 partially blocked the verteporfin-induced reductions in Smad2 and Smad3 that occur after TGF-β stimulation, suggesting that verteporfin does enhance the proteasomal degradation of activated Smad2 and Smad3 in this context (Supplemental Figure 6).
Figure 3.
Verteporfin treatment impairs TGF-β–induced Smad2/3 signaling via reducing the nuclear accumulation and total levels of Smad2/3 protein. (A) NRK49F renal fibroblasts grown in fibronectin–coated plastic dishes were treated with and without 250 nmol/L verteporfin for 12 hours and stimulated with or without TGF-β for 30 minutes. Lysates were immunoblotted for phospho-Smad2, phospho-Smad3, Smad2, or Smad3. Blots shown are representative of three replicates. (B) NRK49F fibroblasts grown on fibronectin-coated coverslips were treated with or without 250 nmol/L verteporfin for a total of 12 hours and 10 ng/ml TGF-β for the indicated times. Cells were stained with an anti-Smad2 antibody (red) and counterstained with DAPI (blue) to identify nuclei. Representative images demonstrate not only reduced nuclear accumulation of Smad2 beginning early post–TGF-β (0.5–1 hours) but also, diminished whole–cell Smad2 levels at later time points (2, 4, and 8 hours). Scale bar, 15 μm. (C) The percentage of cells with predominantly nuclear Smad2 staining was quantified at each time point. A minimum of 50 cells was counted per replicate (four replicates per condition). One-way ANOVA with post hoc Fisher least significant difference was performed. *P<0.05. In D, NRK49F fibroblasts grown on fibronectin-coated plastic were treated with 250 nmol/L verteporfin for a total of 12 hours and 10 ng/ml TGF-β for the indicated times. Verteporfin treatment resulted in a dramatic reduction in Smad2 and Smad3 post–TGF-β, a setting in which Smad2 and Smad3 are primarily in their activated state. P-Smad2, phospho-Smad2; P-Smad3, phospho-Smad3; VP, verteporfin.
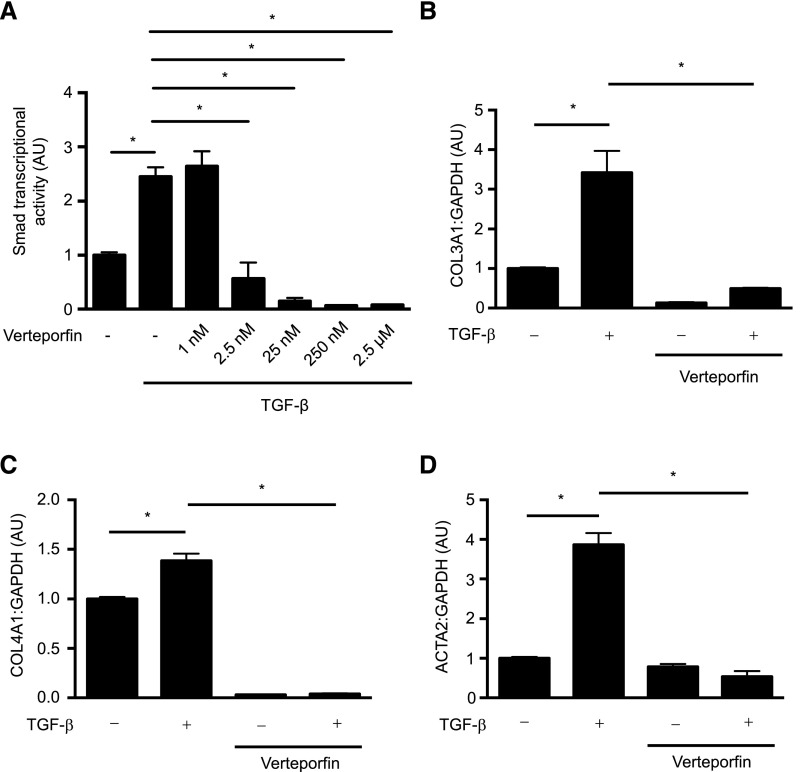
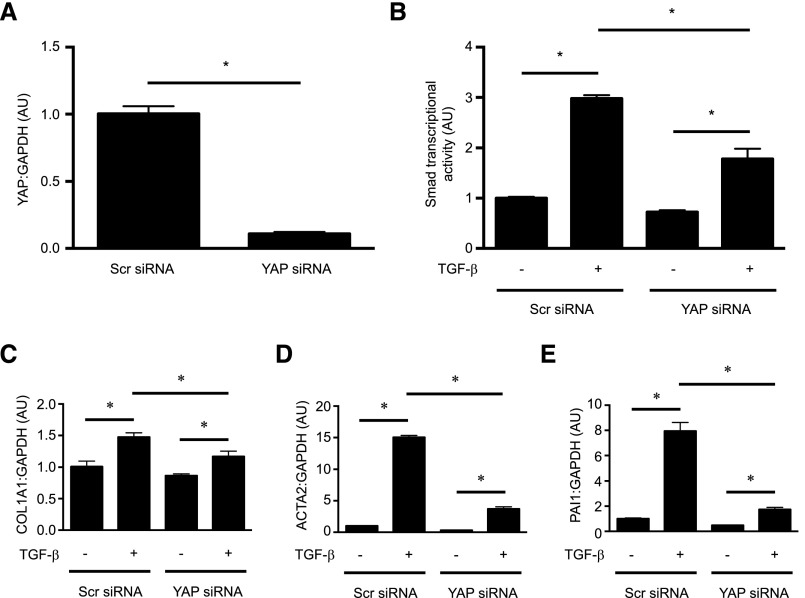
Consistent with its Smad inhibitory effects, verteporfin treatment of NRK49F fibroblasts significantly attenuated late TGF-β signaling events, such as TGF-β–induced Smad3 transcriptional activity (Figure 4A) and the endogenous expression of Smad-inducible genes (Figure 4, B–D). siRNA-mediated knockdown of YAP similarly inhibited TGF-β–induced Smad transcriptional activity as measured by a luciferase reporter system, and also the endogenous expression of Smad target genes, suggesting that the Smad-inhibitory effects of verteporfin are mediated at least in part through YAP (Figure 5).
Figure 4.
Verteporfin treatment attenuates TGF-β/Smad–induced profibrotic gene expression. (A) NRK49F fibroblasts grown in fibronectin–coated plastic dishes were transfected with a Firefly luciferase reporter driven by four tandem Smad–binding elements and then treated with or without 250 nmol/L verteporfin for 4 hours followed by the addition of 10 ng/ml TGF-β for 20 hours. Twenty-four hours post-transfection, Smad3-dependent transcription was assessed using a Smad–dependent luciferase reporter activity assay (n=3 replicates per condition). (B–D) NRK49F cells were treated with or without 250 nmol/L verteporfin for 2 hours followed by stimulation with or without TGF-β for 24 hours. mRNA levels of (B) COL3A1, (C) COL4A1, and (D) ACTA2, normalized to GAPDH, were measured by quantitative RT-PCR. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit. *P<0.05.
Figure 5.
YAP silencing inhibits the TGF-β/Smad–induced increase in profibrotic gene expression. NRK49F renal fibroblasts were transfected with siRNA directed against YAP or a scrambled siRNA. (A) Forty-eight hours post-transfection, knockdown of YAP was confirmed via quantitative RT-PCR. The effects of YAP knockdown on TGF-β–induced Smad–dependent transcription were assessed (B) using a Smad–dependent luciferase reporter activity assay and via measurement of endogenous mRNA levels of the TGF-β/Smad–inducible genes (C) COL1A1, (D) ACTA2, and (E) PAI1. Results are representative of n=3 independent replicates per condition. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; Scr, scrambled. *P<0.05.
In the Setting of Fibrotic Renal Injury, Verteporfin Induces YAP/TAZ Reduction In Vivo
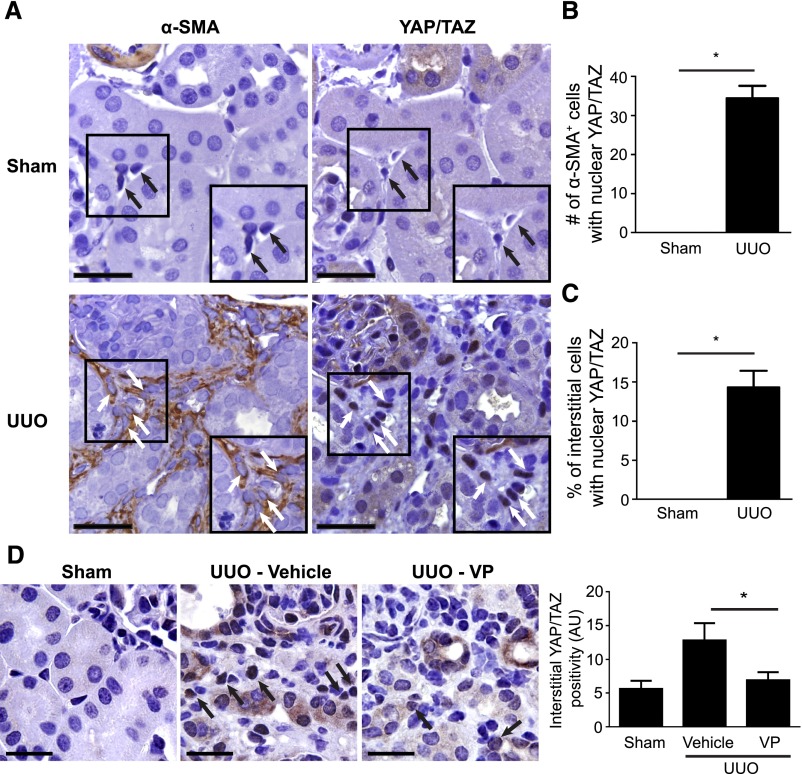
To explore the in vivo relevance of our findings, we first examined the effect of fibrotic injury on YAP/TAZ localization in interstitial fibroblasts, comparing kidneys from mice undergoing (left–sided) unilateral ureteral obstruction (UUO) with those undergoing sham surgery. Seven days after obstructive injury, at a time when fibrosis is developing, we stained serially cut sections of left kidneys for the myofibroblast marker α–smooth muscle actin (α-SMA) and YAP/TAZ. In injured kidneys from UUO mice, interstitial α–SMA+ myofibroblasts showed nuclear YAP/TAZ staining (Figure 6, A and B). In contrast, healthy, soft kidneys from sham-operated controls that were similarly examined showed only rare α-SMA+ cells, none of which showed nuclear YAP/TAZ (Figure 6, A and B). Because only rare interstitial fibroblasts in the healthy kidney express α-SMA, we also quantified the percentage of cells in the interstitium (the location of quiescent fibroblasts) with nuclear YAP/TAZ staining in healthy and injured kidneys, finding that roughly 15% of all interstitial cells in obstructed kidneys and no interstitial cells in healthy kidneys expressed nuclear YAP/TAZ (Figure 6C).
Figure 6.
Verteporfin treatment leads to reduced levels of YAP and TAZ in injured, fibrosing kidneys. (A) Mice undergoing UUO (n=4) or sham surgery (n=3) were euthanized 7 days postprocedure. Shown are representative images of serially cut sections immunostained for (left panels) α-SMA and (right panels) YAP/TAZ from (upper panels) a sham-operated kidney and (lower panels) an obstructed kidney. White arrows identify serially cut interstitial α-SMA+ cells, and black arrows identify serially cut interstitial cells. In UUO kidneys, α-SMA+ cells frequently exhibited nuclear YAP/TAZ staining. In sham-operated kidneys, no serially cut interstitial cells stained for both α-SMA and YAP/TAZ could be found. Scale bar, 80 μm. (B) The number of cortical α-SMA+ cells and (C) the percentage of cortical interstitial cells with nuclear YAP/TAZ staining were quantified; t test was performed. In D, UUO mice were treated with (n=7) or without verteporfin (n=9) beginning immediately after surgery, with sham-operated mice as controls (n=6). Seven days later, reduced interstitial YAP/TAZ staining was seen in verteporfin-treated mice. Black arrows depict YAP/TAZ–stained interstitial cells. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. Scale bar, 40 μm. *P<0.05.
We next tested whether verteporfin treatment of UUO mice would elicit renal YAP/TAZ and Smad inhibitory effects similar to what we had observed in vitro in cultured kidney fibroblasts. Verteporfin significantly reduced interstitial YAP/TAZ staining in UUO mice, a finding that was consistent with our in vitro data (Figure 6D). Furthermore, obstructed kidneys showed significant increases in interstitial α–SMA staining and nuclear accumulation of Smad2/3 compared with sham-operated kidneys, a phenomenon that was not seen when YAP/TAZ levels were diminished with verteporfin treatment (Supplemental Figure 7).
Verteporfin Inhibits Renal Fibrogenesis In Vivo
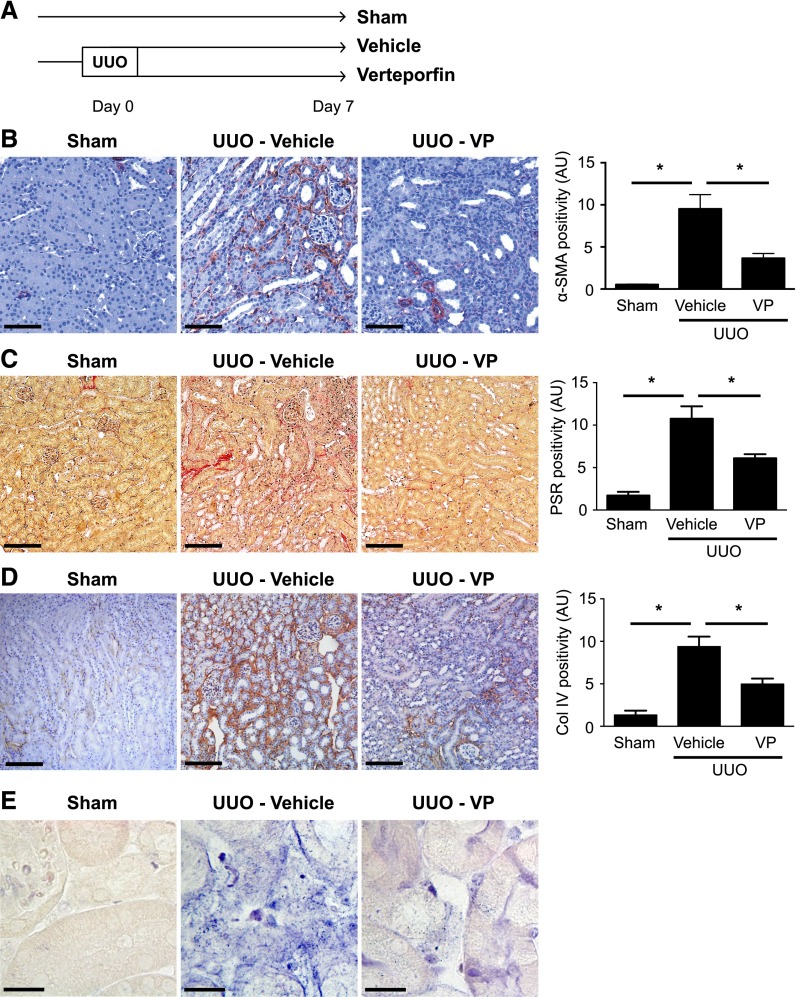
Given its ability to downregulate YAP/TAZ levels and Smad activation in injured kidneys of UUO mice, we next tested whether verteporfin prevents renal fibrogenesis. Mice undergoing UUO were randomized to immediate treatment with verteporfin or vehicle (Figure 7A). Consistent with an inhibitory effect on renal fibroblast TGF-β/Smad signaling, verteporfin resulted in fewer α-SMA+ myofibroblasts 7 days after UUO (Figure 7B), reduced interstitial collagen deposition (Figure 7, C and D), and diminished interstitial expression of CTGF, a known TGF–β/Smad–inducible gene (Figure 7E).
Figure 7.
Verteporfin significantly reduces renal fibrosis and myofibroblast accumulation after UUO. (A) Mice undergoing left-sided UUO were randomized to treatment with verteporfin (n=7) or vehicle (n=9) beginning immediately after surgery. Sham-operated mice served as healthy controls (n=6). Paraffin–embedded kidney tissue sections from verteporfin–treated UUO mice were stained with (B) an α-SMA antibody to identify interstitial myofibroblasts, (C) picrosirius red (PSR) to identify fibrillar collagen, and (D) a type 4 collagen (Col IV) antibody. Representative images from the different treatment groups are shown. Entire kidney sections (excluding the renal pelvis) were digitally analyzed for positive staining. Scale bar, 150 μm. *P<0.05. (E) Paraffin–embedded kidney tissue sections were incubated with a digoxygenin-conjugated probe specific for CTGF mRNA. Blue staining depicts positive riboprobe staining. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. Scale bar, 20 μm.
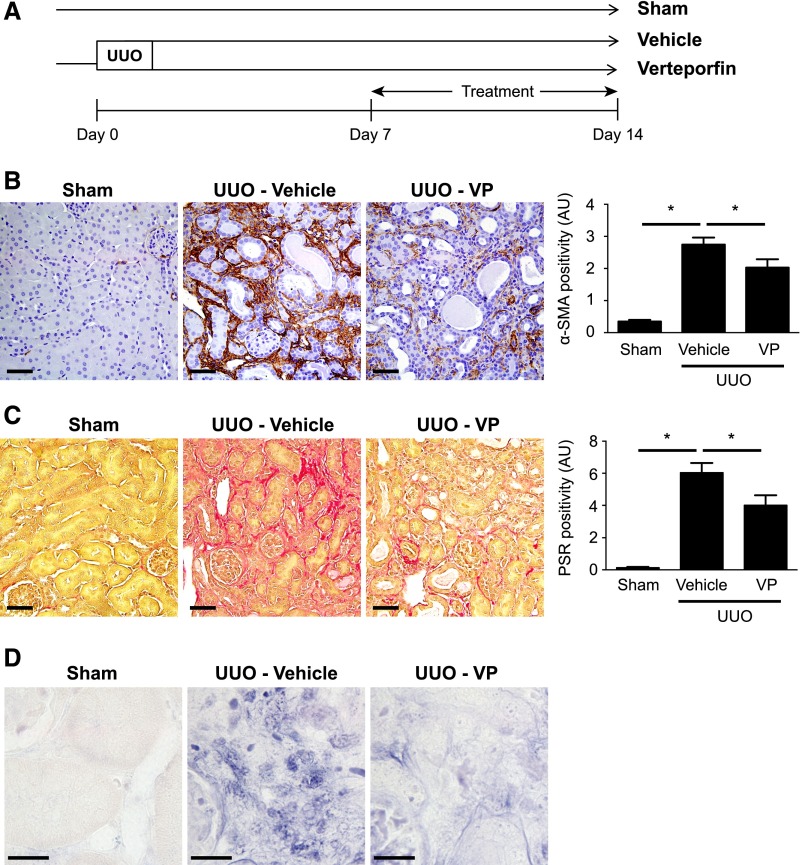
We next tested whether verteporfin inhibits the progression of established fibrosis. Seven days after UUO, at a time point when fibrosis has already developed, mice were randomized to receive intraperitoneal injections of verteporfin or vehicle for 1 week (Figure 8A). Fourteen days after UUO, obstructed kidneys from verteporfin-treated mice again showed fewer α–SMA+ myofibroblasts (Figure 8B), less fibrosis (Figure 8C), and reduced interstitial CTGF expression (Figure 8D). Taken together, our results indicate that verteporfin not only reduces de novo fibrogenesis in the injured kidney, but also prevents the progression of established fibrosis. Moreover, our findings also suggest a previously unrecognized crosstalk between YAP/TAZ- and Smad-dependent pathways that modulates TGF-β–driven fibrogenesis.
Figure 8.
Verteporfin arrests additional progression of established renal fibrosis after UUO. (A) Mice undergoing left-sided UUO were treated with verteporfin (n=10) or vehicle (n=7) beginning 7 days after surgery. Sham-operated mice served as healthy controls (n=4). Paraffin–embedded kidney tissue sections from verteporfin–treated UUO mice were stained with (B) an α-SMA antibody to identify interstitial myofibroblasts and (C) picrosirius red (PSR) to identify fibrillar collagen. Verteporfin treatment attenuated UUO-induced increases in α-SMA and fibrillar collagen staining. Representative images from the different treatment groups are shown. Entire kidney sections (excluding the renal pelvis) were digitally analyzed for positive staining. Scale bar, 50 μm. (D) Paraffin–embedded kidney tissue sections were incubated with a digoxygenin-conjugated probe specific for CTGF mRNA. Blue staining depicts positive riboprobe staining. One-way ANOVA with post hoc Fisher least significant difference was performed. AU, arbitrary unit; VP, verteporfin. Scale bar, 20 μm. *P<0.05.
Discussion
Although recent studies have shown that fibroblasts are influenced by tissue stiffness,5,11–13,20,21 the underlying mechanoregulatory pathways are poorly understood. We show for the first time that TGF-β–induced canonical Smad2/3 signaling is regulated by ECM stiffness via control of Smad2/3 localization and that this mechanoregulation may be dependent on the proteins YAP and TAZ. We further show that verteporfin, a small molecule approved for the treatment of ocular vascular disease with recently described YAP/TAZ inhibitory properties, can block not only TGF-β–induced Smad2/3 signaling in cultured renal fibroblasts, but also, the progression of renal fibrosis in vivo. Verteporfin mediates its antifibrotic effects by multiple mechanisms, ultimately interfering with profibrotic YAP/TAZ-TGF–β/Smad crosstalk in renal fibroblasts.
To date, soft matrix has been shown to inhibit fibroblast-myofibroblast transition primarily through TGF-β–independent pathways, such as via regulation of cyclooxygenase-235 and Rho/ROCK/megakaryoblastic leukemia 1 activity.11,12 These TGF-β–independent fibroblast mechanoregulatory pathways can act in concert with TGF-β, but the mechanisms underlying this mechanochemical synergy remain poorly defined. In this work, we establish a mechanistic link between matrix stiffness, YAP/TAZ, and Smad2/3 signaling in the regulation of fibroblast responses to TGF-β. This effect is consistent with prior evidence of physical interactions between YAP/TAZ and Smad2/3 in the nucleus,14–16 and it is in line with reports from our group and others indicating that YAP and TAZ exhibit profibrotic activity.35–37 Indeed, Speight et al.24 previously showed a role for TAZ in controlling α-SMA expression in injured epithelium as part of epithelial-myofibroblast transition.35 Similarly, recent reports have showed that a stiff environment promotes YAP/TAZ-dependent activation of fibroblasts and hepatic stellate cells, promoting matrix synthesis independent of TGF-β, possibly through the induction of CTGF and PAI1.35,37 Taken together, these reports are in line with our results, suggesting that YAP and TAZ are critical mechanosensitive mediators of TGF-β–dependent and –independent fibrogenesis.
Recognizing the importance of YAP and TAZ, we identified verteporfin as a novel potent inhibitor of experimental fibrosis. Although a previous report had shown that verteporfin induces a conformational change of YAP that affects its binding to other proteins,19 our data indicate that the dominant effect of this drug on YAP and TAZ in fibroblasts is a dramatic reduction in the absolute levels of these two proteins. We show that verteporfin reduces YAP and TAZ mRNA levels, suggesting that decreased synthesis of YAP and TAZ may at least partially explain our findings. However, because recent reports have suggested that verteporfin may also cause protein aggregation through oxidative crosslinking and enhanced degradation,38,39 other effects of this drug may also play a role.
In addition to its effects on YAP and TAZ, verteporfin exerted multiple effects on the profibrotic proteins Smad2 and Smad3. The profibrotic activity of Smad2/3 is regulated at multiple levels, with activation mediated by C-terminal phosphorylation and subsequent nuclear accumulation, and inactivation mediated by dephosphorylation, nuclear export, and degradation.2,3 Although verteporfin did not affect TGF-β–induced Smad2/3 phosphorylation, it did inhibit subsequent Smad nuclear accumulation (Figure 3). This effect was likely caused by reductions in functional YAP/TAZ protein, because YAP and TAZ are known to act as Smad nuclear retention factors.15 Interestingly, we also noted that verteporfin reduced the abundance of Smad2 and Smad3 in fibroblasts, inducing only a modest reduction on its own but a much more dramatic downregulation on coincubation with TGF-β (Figure 3). WW domain–containing proteins, such as YAP and TAZ, compete with Smad ubiquitin ligases for access to activated nuclear Smads after TGF-β stimulation.40,41 It is, therefore, possible that the verteporfin-induced reduction in YAP/TAZ protein may have enhanced the ubiquitination and degradation of TGF-β–activated nuclear Smads. Consistent with this hypothesis, we showed that proteasomal inhibition partially rescued the loss of Smads induced by coincubation with TGF-β and verteporfin (Supplemental Figure 6). Recent reports have also suggested, however, that verteporfin may directly enhance the degradation of certain proteins through oxidative crosslinking and oligomerization.38,39 Thus, verteporfin may reduce Smad2/3 levels via multiple mechanisms, some of which are likely YAP/TAZ dependent, whereas others may be YAP/TAZ independent.
Recognizing the many potential mechanisms by which verteporfin might be altering YAP/TAZ- and Smad-dependent signaling, we took a genetic approach to confirm the importance of YAP/TAZ and TGF-β/Smad crosstalk in the regulation of fibrogenesis. In these experiments, we showed that siRNA-mediated knockdown of YAP reciprocated the effects of verteporfin, in that both strategies diminished TGF-β–induced Smad transcriptional activity and expression of classic profibrotic genes in cultured fibroblasts. Taken together, our results suggest that crosstalk between these two pathways exerts important regulatory effects on fibroblast activation and ECM production. Interestingly, the effects of YAP silencing were not as profound as those of verteporfin, suggesting that verteporfin may also mediate its effects through other non–YAP-dependent mechanisms. Indeed, although prior studies have mostly focused on the profibrotic activity of YAP,36,37 it is possible, for example, that verteporfin-induced reductions in TAZ may also have important effects.
Having shown these novel effects of verteporfin in cultured renal fibroblasts, we next extended our in vitro findings to a mouse model of renal fibrosis. In these studies, we showed that verteporfin treatment of UUO mice reduced YAP/TAZ levels and Smad nuclear accumulation in the injured kidney along with the expression of profibrotic TGF-β/Smad–inducible genes, such as ACTA2, COL3A1, COL4A1, and CTGF. Although the expression of these Smad-inducible genes is regulated by other factors other than Smads,23,42,43 taken together, these data suggest that verteporfin interferes with profibrotic YAP/TAZ-Smad2/3 crosstalk in vitro in cultured fibroblasts and in vivo in the injured mouse kidney, thereby inhibiting fibrogenesis.
Classically thought of as targets of the Hippo pathway, YAP and TAZ are regulated by phosphorylation of serine residues that control not only their intracellular localization but also, their ubiquitination and subsequent degradation.31,32 In 2011, Dupont et al.17 showed that matrix stiffness is an additional regulator of these two proteins. The mechanisms underlying this mechanoregulation remain unclear, although both Hippo-dependent44,45 and Hippo-independent17,18 pathways likely contribute. Whether fibroblast Hippo pathway activity is regulated by matrix stiffness or verteporfin treatment remains an open question that warrants additional exploration.
Irrespective of these ongoing mechanistic studies, our finding that verteporfin is a potent inhibitor of renal fibrogenesis represents an exciting advance. No specific, safe, and effective renal antifibrotic therapies exist, despite the central role that scarring plays in the progression of nearly all forms of CKD.46 A key barrier to the development of effective antifibrotic therapies has been the complexity and redundancy of the profibrotic signaling network. An under-recognized contributor to this complexity is the synergistic interaction that exists between biomechanical and biochemical fibrogenesis regulatory signals. By targeting both classic Smad2/3 and novel mechanosensitive YAP/TAZ profibrotic signaling, we define, using a repurposed drug, a new pharmacologic strategy that disrupts this mechanochemical synergy, thereby inhibiting profibrotic TGF–β signaling and attenuating renal fibrogenesis.
Concise Methods
Detailed methods can be found in Supplemental Material.
Polyacrylamide and Silicone Gel Preparation
Glass coverslips were coated with 2-kPa (soft) or 100-kPa (stiff) polyacrylamide or silicone gels using modified versions of published protocols.47–49 Gels were coated with sterile fibronectin (10 μg/ml; YO Proteins AB, Huddinge, Sweden) to create a uniform, thin layer of ECM protein for cell adhesion.
Cell Culture and Treatments
Immortalized normal rat kidney interstitial fibroblasts (NRK49F) and primary human dermal fibroblasts were purchased from American Type Culture Collection (Manassas, VA) and Lonza (Mississauga, ON, Canada), respectively. After overnight serum deprivation (DMEM and 1% FBS), cells were stimulated with TGF-β (10 ng/ml; R&D Systems, Minneapolis, MN) and/or verteporfin (Sigma-Aldrich, St. Louis, MO) for the indicated times.
Immunofluorescence Staining and Immunoblotting
NRK49F fibroblasts cultured on fibronectin–coated glass coverslips were immunostained with antibodies directed against YAP/TAZ, Smad2, or Smad2/3. After the addition of appropriate secondary antibodies, nuclear counterstaining with DAPI was performed. Confocal microscopic images were obtained, and the percentage of cells with predominantly nuclear YAP/TAZ or Smad2/3 was calculated using a modified published protocol.22 Lysates from NRK49F cells grown in fibronectin-coated dishes were blotted with the following primary antibodies: Smad2, Smad3, Smad2/3, phospho-Smad2, phospho-Smad3, YAP/TAZ, α-tubulin, GAPDH, or β-actin. Quantitative analysis of band intensity was performed using ImageJ (National Institutes of Health, Bethesda, MD).
Quantitative RT-PCR
After treatments as described above, RNA was harvested from NRK49F cells and reverse transcribed, and quantitative RT-PCR was performed to analyze differences in the mRNA levels of ACTA2, COL1A1, COL3A1, COL4A1, GAPDH, PAI1, YAP, and TAZ. Please see Supplemental Table 1 for primer sequences.
Transfection and Luciferase Reporter Assay
Fibroblasts grown in fibronectin–coated plastic wells were transfected with a Firefly luciferase reporter construct driven by tandem Smad-binding element consensus motifs along with a control Renilla luciferase vector constitutively driven by the HSV-thymidine kinase promoter (Promega, Madison, WI). Transfected fibroblasts were stimulated with TGF-β (10 ng/ml), and Firefly and Renilla luminescence was read 24 hours later using a Dual-Glo Luciferase Kit and Luminometer (Promega).23 Smad transcriptional activity was calculated as the Firefly-to-Renilla luminescence ratio.
YAP Silencing
After serum deprivation in DMEM and 2% FBS for 4 hours, NRK49F fibroblasts cultured in fibronectin–coated plastic wells were transfected with 10 nmol/L siRNA directed against YAP1 or a scrambled siRNA. Forty-eight hours after transfection, YAP silencing was confirmed via quantitative RT-PCR, and cells were used for experiments.
Proximity Ligation Assay
NRK49F fibroblasts grown on fibronectin–coated glass coverslips were treated with or without verteporfin (250 nmol/L) for 2 hours followed by TGF-β (10 ng/ml) for 30 minutes. Cells were then subjected to Duolink proximity ligation assays as per the manufacturer’s instructions (Olink Bioscience, Uppsala, Sweden).50 Briefly, cells were incubated with mouse anti–human YAP/TAZ and rabbit anti–human Smad2/3 primary antibodies or mouse anti–human YAP/TAZ and rabbit anti–TEAD1 primary antibodies. Proximity of YAP/TAZ to Smad2/3 or TEAD1 was detected by the addition of anti–mouse plus and anti–rabbit minus proximity ligation assay probes followed by ligation, rolling circle amplification, and hybridization with fluorescently labeled oligonucleotides. Fluorescent spots were imaged and quantified as nuclear proximity ligation assay signal intensity divided by nuclear volume.
Animals and UUO Surgery
Study 1
To assess YAP/TAZ localization in α-SMA+ renal fibroblasts, male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and underwent either left-sided UUO or sham surgery. Seven days after surgery, mice were euthanized, and the left kidneys were harvested, immersion fixed in 10% neutral buffered formalin, and embedded in paraffin.
Study 2 (UUO: Early Verteporfin Treatment)
To examine whether early verteporfin treatment could prevent renal fibrosis, male C57BL/6 mice (The Jackson Laboratory) underwent either left-sided UUO or sham surgery. Mice undergoing UUO surgery were randomized to receive every other day intraperitoneal injections of either verteporfin (100 mg/kg) dissolved in 10% DMSO or DMSO vehicle control as per a previously published protocol.19 Treatment began immediately after surgery (day 0) and was continued until study end, which was 7 days after surgery.
Study 3 (UUO: Late Verteporfin Treatment)
To examine whether late verteporfin treatment could prevent the progression of established fibrosis, male C57BL/6 mice (The Jackson Laboratory) underwent left-sided UUO or sham surgery. Mice undergoing UUO surgery were randomized to receive intraperitoneal injections of either verteporfin (100 mg/kg) or DMSO vehicle as described for study 2. Treatment in this study, however, began on day 7 post–UUO, a time point when renal fibrosis is already established in the obstructed left kidney, and it was continued for an additional 7 days. All mice were euthanized 14 days after UUO or sham surgery.
Tissue Collection, Preparation, Histology, and In Situ Hybridization
At study end, the left kidneys of all mice were harvested. Frozen kidney sections were stained with antibodies against Smad2/3 and α-SMA. Formalin-fixed tissues were embedded in paraffin and sectioned before staining with picrosirius red (Sigma-Aldrich) or antibodies against α-SMA, YAP/TAZ, and type 4 collagen.51,52 Stained sections were digitally analyzed using Aperio Imagescope software (Leica Biosystems, Concord, ON, Canada) as previously described.51–53 In situ hybridization was performed on formalin–fixed, paraffin–embedded mouse kidney sections as previously described,54 and the hybridized antisense probe was visualized with alkaline phosphatase–conjugated antidigoxigenin (Roche, Basel, Switzerland) and BM purple AP substrate (Roche).
Statistical Analyses
A minimum of three independent experiments was performed for all in vitro studies. Data presented are means±SEMs. Between-group differences were measured using one-way ANOVA with Fisher least significant difference post hoc analysis where appropriate. Statistical analysis was performed using GraphPad Prism for Mac 6.0 (GraphPad Software Inc., San Diego, CA).
Study Approval
All animal studies were approved by the St. Michael’s Hospital Animal Ethics Committee and conformed to the Canadian Council on Animal Care guidelines.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Philip Marsden, Dr. Lisa Robinson, Dr. Jim Scholey, Dr. Craig Simmons, and Dr. Helen McNeill for helpful discussions and Dr. Amy Won and Dr. Christopher Yip for technical assistance with initial gel optimization.
S.G.S. was supported by a Natural Sciences and Engineering Research Council Canada Graduate Scholarship (Master’s). A.M.S. was supported by a Queen Elizabeth II Graduate Scholarship in Science and Technology and a Li Ka Shing Knowledge Institute Scholarship. M.F.T. is supported by a Queen Elizabeth II Graduate Scholarship in Science and Technology. S.M. is supported by a postdoctoral fellowship from the Canadian Diabetes Association. J.L.W. is the Mary Janigan Research Chair in Molecular Cancer Therapeutics. This work was supported, in part, by the following funding sources awarded to D.A.Y.: a Kidney Research Scientist Core Education and National Training Program (KRESCENT) Infrastructure grant, a Kidney Foundation of Canada Biomedical grant KFOC130031, a J.P. Bickell Foundation Medical Research grant, a Canadian Institutes of Health Research operating grant 201503MOP-341962, and the St. Michael’s Hospital Foundation. D.A.Y. is supported by a KRESCENT New Investigator and Canadian Diabetes Association Clinician Scientist award.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050499/-/DCSupplemental.
References
- 1.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attisano L, Wrana JL: Signal transduction by the TGF-beta superfamily. Science 296: 1646–1647, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Massagué J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Leask A, Abraham DJ: TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Olsen AL, Bloomer SA, Chan EP, Gaça MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG: Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 301: G110–G118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells RG: Tissue mechanics and fibrosis. Biochim Biophys Acta 1832: 884–890, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V: Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16: 1009–1017, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG: Increased stiffness of the rat liver precedes matrix deposition: Implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 293: G1147–G1154, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Salameh N, Larrat B, Abarca-Quinones J, Pallu S, Dorvillius M, Leclercq I, Fink M, Sinkus R, Van Beers BE: Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology 253: 90–97, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Perepelyuk M, Terajima M, Wang AY, Georges PC, Janmey PA, Yamauchi M, Wells RG: Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol 304: G605–G614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y: Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ: Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123: 1096–1108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ: Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyer TA, Weiss A, Khomchuk Y, Huang K, Ogunjimi AA, Varelas X, Wrana JL: Switch enhancers interpret TGF-β and Hippo signaling to control cell fate in human embryonic stem cells. Cell Reports 5: 1611–1624, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL: TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10: 837–848, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL: The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell 19: 831–844, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S: Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S: A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154: 1047–1059, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D: Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26: 1300–1305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA: Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G: Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159: 1009–1020, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan L, Sebe A, Péterfi Z, Masszi A, Thirone AC, Rotstein OD, Nakano H, McCulloch CA, Szászi K, Mucsi I, Kapus A: Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell 18: 1083–1097, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szászi K, Kapus A: Fate-determining mechanisms in epithelial-myofibroblast transition: Major inhibitory role for Smad3. J Cell Biol 188: 383–399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speight P, Nakano H, Kelley TJ, Hinz B, Kapus A: Differential topical susceptibility to TGFβ in intact and injured regions of the epithelium: Key role in myofibroblast transition. Mol Biol Cell 24: 3326–3336, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL: Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE: Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341: 1240104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umale S, Deck C, Bourdet N, Dhumane P, Soler L, Marescaux J, Willinger R: Experimental mechanical characterization of abdominal organs: Liver, kidney & spleen. J Mech Behav Biomed Mater 17: 22–33, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Nicolle S, Vezin P, Palierne JF: A strain-hardening bi-power law for the nonlinear behaviour of biological soft tissues. J Biomech 43: 927–932, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Arndt R, Schmidt S, Loddenkemper C, Grünbaum M, Zidek W, van der Giet M, Westhoff TH: Noninvasive evaluation of renal allograft fibrosis by transient elastography--a pilot study. Transpl Int 23: 871–877, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Michels S, Schmidt-Erfurth U: Photodynamic therapy with verteporfin: A new treatment in ophthalmology. Semin Ophthalmol 16: 201–206, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL: A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 24: 72–85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL: The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFbeta-TrCP E3 ligase. J Biol Chem 285: 37159–37169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R: Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci U S A 98: 974–979, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao S, Alarcón C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, Massagué J: Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell 36: 457–468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ: Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piersma B, de Rond S, Werker PM, Boo S, Hinz B, van Beuge MM, Bank RA: YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am J Pathol 185: 3326–3337, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, Hoorens A, Reynaert H, Halder G, van Grunsven LA: The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol 63: 679–688, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Ramakrishnan SK, Triner D, Centofanti B, Maitra D, Győrffy B, Sebolt-Leopold JS, Dame MK, Varani J, Brenner DE, Fearon ER, Omary MB, Shah YM: Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci Signal 8: ra98, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donohue E, Balgi AD, Komatsu M, Roberge M: Induction of covalently crosslinked p62 oligomers with reduced binding to polyubiquitinated proteins by the autophagy inhibitor verteporfin. PLoS One 9: e114964, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alarcón C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, Sapkota G, Pan D, Massagué J: Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 139: 757–769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aragón E, Goerner N, Zaromytidou AI, Xi Q, Escobedo A, Massagué J, Macias MJ: A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev 25: 1275–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luchsinger LL, Patenaude CA, Smith BD, Layne MD: Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J Biol Chem 286: 44116–44125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM: LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J 27: 1830–1846, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD: Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell 158: 143–156, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Codelia VA, Sun G, Irvine KD: Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol 24: 2012–2017, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fine LG, Norman JT: Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Tse JR, Engler AJ: Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol 10: 10.16, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Kandow CE, Georges PC, Janmey PA, Beningo KA: Polyacrylamide hydrogels for cell mechanics: Steps toward optimization and alternative uses. Methods Cell Biol 83: 29–46, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Godbout C, Follonier Castella L, Smith EA, Talele N, Chow ML, Garonna A, Hinz B: The mechanical environment modulates intracellular calcium oscillation activities of myofibroblasts. PLoS One 8: e64560, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsson I, Bahram F, Li X, Gualandi L, Koch S, Jarvius M, Söderberg O, Anisimov A, Kholová I, Pytowski B, Baldwin M, Ylä-Herttuala S, Alitalo K, Kreuger J, Claesson-Welsh L: VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J 29: 1377–1388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuen DA, Connelly KA, Advani A, Liao C, Kuliszewski MA, Trogadis J, Thai K, Advani SL, Zhang Y, Kelly DJ, Leong-Poi H, Keating A, Marsden PA, Stewart DJ, Gilbert RE: Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. PLoS One 5: e9543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuen DA, Connelly KA, Zhang Y, Advani SL, Thai K, Kabir G, Kepecs D, Spring C, Smith C, Batruch I, Kosanam H, Advani A, Diamandis E, Marsden PA, Gilbert RE: Early outgrowth cells release soluble endocrine antifibrotic factors that reduce progressive organ fibrosis. Stem Cells 31: 2408–2419, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuen DA, Kepecs DM, Zhang Y, Advani S, Thai K, Connelly KA, Gilbert RE: Repeated treatment with bone marrow cell secretory products maintains long-term renoprotection in experimental chronic kidney disease: A placebo-controlled trial. Can J Kidney Health Dis 2: 44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkinson DG: RNA detection using non-radioactive in situ hybridization. Curr Opin Biotechnol 6: 20–23, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.