Abstract
Common variants in the UMOD gene encoding uromodulin, associated with risk of hypertension and CKD in the general population, increase UMOD expression and urinary excretion of uromodulin, causing salt-sensitive hypertension and renal lesions. To determine the effect of selective pressure on variant frequency, we investigated the allelic frequency of the lead UMOD variant rs4293393 in 156 human populations, in eight ancient human genomes, and in primate genomes. The T allele of rs4293393, associated with CKD risk, has high frequency in most modern populations and was the one detected in primate genomes. In contrast, we identified only the derived, C allele in Denisovan and Neanderthal genomes. The distribution of the UMOD ancestral allele did not follow the ancestral susceptibility model observed for variants associated with salt-sensitive hypertension. Instead, the global frequencies of the UMOD alleles significantly correlated with pathogen diversity (bacteria, helminths) and prevalence of antibiotic-resistant urinary tract infections (UTIs). The inverse correlation found between urinary levels of uromodulin and markers of UTIs in the general population substantiates the link between UMOD variants and protection against UTIs. These data strongly suggest that the UMOD ancestral allele, driving higher urinary excretion of uromodulin, has been kept at a high frequency because of its protective effect against UTIs.
Keywords: genetic renal disease, chronic kidney disease, kidney tubule, tubular epithelium, Urinary tract infection
Uromodulin (Tamm–Horsfall protein) is the most abundant protein excreted in the urine.1,2 Uromodulin is exclusively produced in the kidney by epithelial cells lining the thick ascending limb (TAL) of the loop of Henle, where it is sorted to the apical plasma membrane and released into the tubular lumen by proteolytic cleavage. Although the biologic role of uromodulin remains mysterious, studies in knockout mice have shown that uromodulin regulates NaCl transport processes operating in the TAL,3,4 protects against urinary tract infection (UTI),5 and regulates innate immunity.6
The interest in uromodulin was boosted when genetic studies revealed that mutations of the UMOD gene, which encodes uromodulin, can cause rare forms of autosomal dominant tubulointerstitial kidney diseases.7,8 These disorders may include impaired urinary concentrating ability, hyperuricemia, and/or gout, and they often lead to CKD and ESRD between the second and fourth decade of life. Analyses in renal biopsies and in mouse models of autosomal dominant tubulointerstitial kidney disease revealed intracellular aggregates of mutant uromodulin in the TAL cells, coupled with a systematic decrease of uromodulin excretion in the urine.9–11
In parallel, multiple genome-wide association studies (GWAS) and sequencing efforts have identified common single nucleotide polymorphisms (SNPs) in UMOD that are strongly associated with eGFR and the risk for developing CKD in the general population.12–16 The SNPs in UMOD were also associated with the risk of developing hypertension and kidney stones.17,18 The top variants from these GWAS studies consistently map in the same linkage disequilibrium block encompassing the UMOD promoter. Recently, we demonstrated that these UMOD risk variants directly increase the expression of uromodulin in vitro and in vivo, while increased uromodulin expression causes salt-sensitive hypertension and kidney lesions in mice and humans (Table 1).19 Meta-analyses have shown that the UMOD variant rs4293393, strongly associated with eGFR and BP, is also the top variant associated with uromodulin level in urine, confirming its biologic effect on promoter activity.16
Table 1.
The rs4293393 promoter variant of the UMOD gene
| rs4293393 | Documented in Archaic Humansa | Frequencyb | UMOD Expression | Urinary Uromodulin Levels | GWAS CKD, Hypertension | GWAS Kidney Stones | Potential Role for UTI | Potential Selective Pressure |
|---|---|---|---|---|---|---|---|---|
| Ancestral T allele | No | 0.80 | High | High | Risk (higher >65 yr)c | Protective | Protective | Positive or Neutral |
| Derived C allele | Yes | 0.20 | Low | Low | Protective (higher >65 yr)c | Risk | Deleterious | Negative or Neutral |
| References | 19 | 16,19 | 12–14,17,18,80,81 | 18 |
The unusually high frequency of the UMOD risk variants (70%–80% in Africans and Europeans and 92%–95% in East Asians), combined with strong biologic activity, suggests the action of some sort of selective pressure.20 Based on known properties of uromodulin, such pressures could be linked to salt avidity or, alternatively, to antimicrobial activity. The aims of the present study were to characterize the ancestry of the top risk UMOD promoter variant rs4293393, to explore whether the high prevalence of this variant is due to selective pressure, and to investigate the nature of such a potential selection.
UMOD Variant in Nonhuman Primates and Ancient Hominids
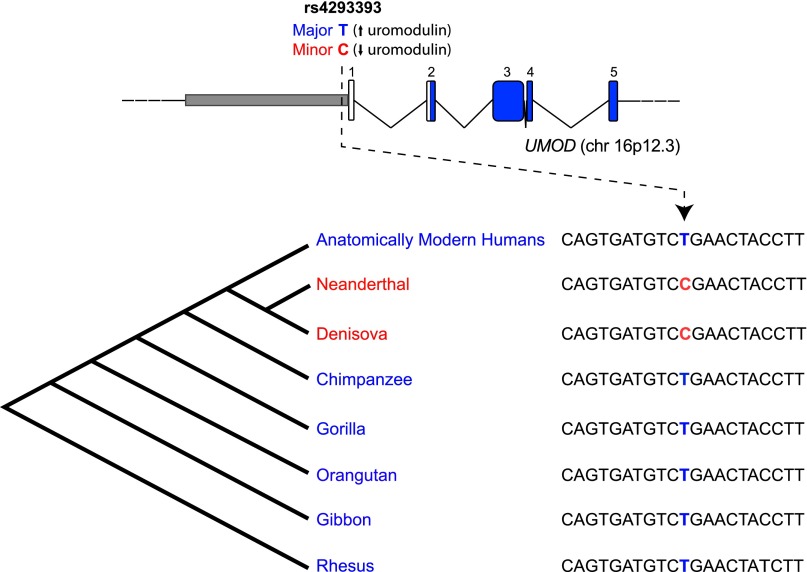
The top rs4293393 UMOD variant identified in previous GWAS has two alleles: T, the major allele associated with risk of hypertension and CKD, and C, the minor allele. To place the observed modern human variation in its evolutionary context, we evaluated the allelic status of rs4293393 in 25 individuals from the four chimpanzee subspecies,21 and in available genomes of other nonhuman primates. All the individuals analyzed were homozygous for the T allele, identifying it as the ancestral one (Figure 1).
Figure 1.
Genomic sequence variation at UMOD variant rs4293393. Schematic representation of the UMOD locus showing the localization of the top variant rs4293393 in the gene promoter. The alignment shows the genetic variation in the genomic region surrounding the variant. Phylogenetic relationship among the considered species is also shown. Only the major, T allele is detected in all ancient genomes that are collectively indicated as “Anatomically Modern Humans”. By contrast, only the minor, C allele is detected in the archaic genomes of Denisova and Neanderthal. The characteristics of the analyzed genomes are listed in Supplemental Table 1.
We next evaluated the UMOD allelic status in the two ancient hominid genomes at high coverage currently available, namely the Denisova and the Neanderthal,22,23 from which present-day humans diverged some 550,000–750,000 years ago, respectively,23,24 and in anatomically modern human samples spanning a period 3900–45,000 years ago (Supplemental Table 1). This investigation showed that only the ancestral, T allele is present in the genomes of anatomically modern humans, whereas the archaic hominids were homozygous for the derived, C allele (Figure 1).
Since the genomic region around rs4293393 is not among those identified in the modern human genome as of putative Neanderthal origin,23 an explanation for the observed pattern could be that the derived, C allele arose in the human lineage after separation from the one leading to the chimpanzees (6 million years ago), but before Neanderthals and Denisovans separated from modern human lineage as distinct groups (Figure 1).23 The probability of being homozygous for the derived allele of two individuals randomly chosen from a population with the allele frequencies of current European populations (about 0.20) is 0.0016. Although a meaningful statistical comparison cannot be based on two individuals only, this low probability suggests that archaic hominids did have a higher frequency of the derived rs4293393 allele than modern Europeans. In turn, this supports the possibility that the recent evolution of modern humans did not allow the derived allele to rise to high frequencies, as it probably did in archaic humans, or that the evolutionary forces acting on archaic and modern humans were somehow different.
Does the Ancestral Susceptibility Model Apply to UMOD Variants?
Since the T allele of rs4293393, associated with risk for CKD and hypertension, is the ancestral one and is associated with salt retention, we hypothesized that the ancestral susceptibility model could be applied to the UMOD locus.25 This model pertains to alleles that underwent ancient adaptations. With a shift in environment and lifestyle, the ancestral alleles no longer confer a selective advantage but rather increase the risk of common diseases, while the derived, protective alleles become neutral or moderately advantageous and increase in frequency. This model potentially applies to variants influencing the risk of hypertension, since ancient populations adapted to hot, humid areas by retaining salt. In turn, these ancestral alleles favoring salt retention increase the risk of hypertension in modern populations living in cooler, temperate climates. The ancestral susceptibility model predicts a relatively recent change in the selective pressure, approximately at the time of modern human dispersal from Africa (about 50,000–70,000 years ago).26 It is supported by a change of allelic frequencies along the latitude, reflecting environmental adaptations, with African populations showing higher frequencies of the ancestral (risk) allele, and non-African populations showing higher frequencies of the derived (protective) allele.27
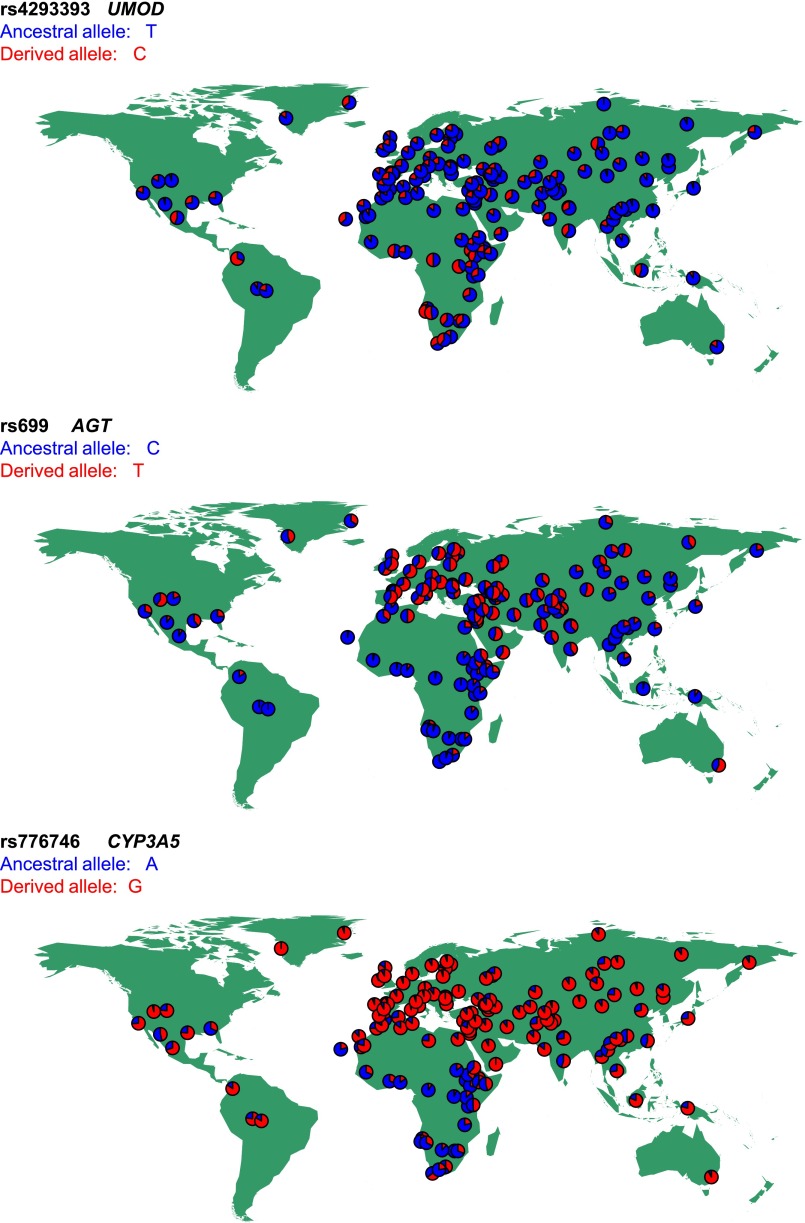
To test this hypothesis, we used worldwide databases of genomic variation and compared the distribution of rs4293393 with classic variants associated with salt conservation and ancestral susceptibility. The ancestral A-6G and T235M variants of the AGT gene coding for angiotensinogen are increasing the risk of hypertension, whereas the derived allele is protective and carries the signature of positive natural selection.28,29 Similarly, the ancestral allele of CYP3A5, coding for a member of the cytochrome P450 superfamily of enzymes involved in steroid synthesis, is associated with increased BP in blacks whereas the derived, protective variant CYP3A5*3 results in a nonfunctional protein.30,31 The populations for which we have genetic information for rs699 (AGT), rs776746 (CYP3A5), and rs4293393 (UMOD), along with allele frequencies and references, are listed in Supplemental Table 2. The observed geographic pattern for the rs4293393 UMOD variant reveals that the most frequent allele outside Africa is the ancestral one, whereas the derived allele reaches its highest frequencies in populations from Equatorial Africa and South America. This distribution does not fit the ancestral susceptibility model, being in fact somewhat opposite to that observed for AGT and CYP3A5 (Figure 2).
Figure 2.
The allelic frequency distribution of the rs4293393 UMOD variant does not fit the ancestral susceptibility model. Distribution of ancestral (blue) and derived (red) allele frequencies in 156 worldwide populations at the following variants: rs4293393 of the UMOD gene (top), rs699 of the AGT gene (central), and rs776746 of the CYP3A5 gene (bottom).
We also tested the distribution of UMOD rs4293393 against that of two closely linked variants (T555I, rs75770792; Q568P, rs111253292) in the CORIN gene, as described by Dries et al.32 The derived allele (I555/P568) of CORIN is associated with increased risk of hypertension and cardiac hypertrophy due to decreased conversion of proatrial natriuretic peptide into active proatrial natriuretic peptide, a cardiac hormone that regulates BP and salt–water balance.33 The distribution of UMOD was fundamentally different from that of CORIN which is characterized by the exclusive presence of the ancestral, protective allele in most of the populations analyzed, with the exception of few groups from Africa or of African origin (e.g., Yoruba in Ibadan Nigeria, African Caribbeans in Barbados) showing a very minor frequency (<10%) of the derived, risk allele (Supplemental Figure 1, Supplemental Table 3).
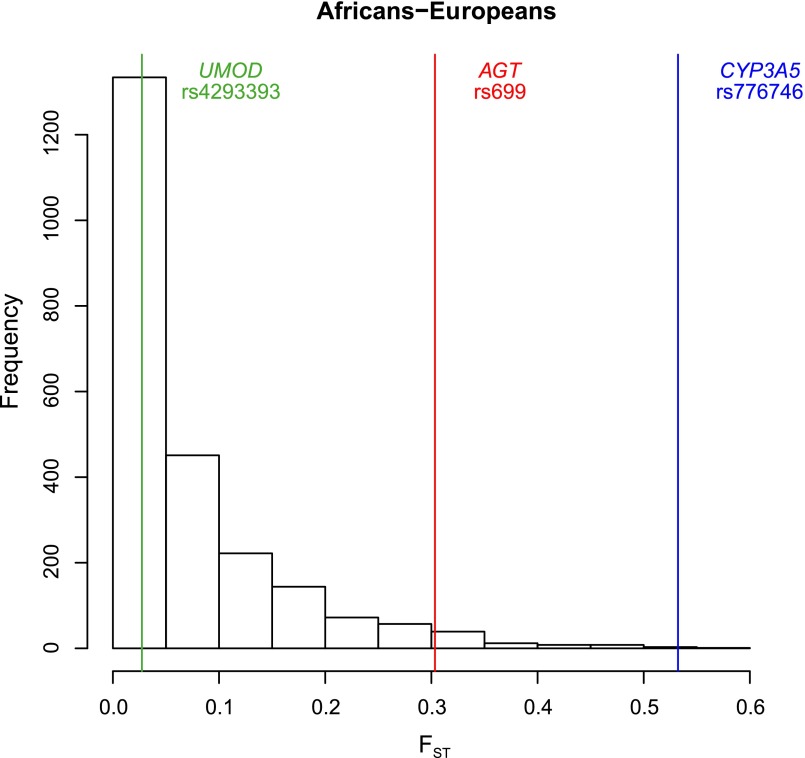
Analysis of molecular variance showed that differences in allele frequencies between African versus European populations were much lower for the UMOD variant (4.8% between groups) than at AGT and CYP3A5 loci (45.2% and 64.9%, respectively) (Table 2). Although significant, the amount of genetic variation attributable to differences among groups for rs4293393 is comparable with that known for whole genome neutral variants.34 Furthermore, the African versus European fixation index (FST) estimated for the UMOD variant does not depart from the distribution of the FST values observed at random intergenic (and hence presumably neutral) regions (Figure 3). These data suggest that the derived allele of UMOD has not been under strong selective pressure. A possible explanation for these findings is that the UMOD derived allele did not confer the selective advantage expected under the ancestral susceptibility model, hence it did not rapidly increase in frequency. Other selective regimes, or more sophisticated models incorporating demography and environmental factors, are needed to account for the current frequency and distribution of the UMOD variant.
Table 2.
Analysis of molecular variance of selected variants in African versus European populations
| Marker | Variations between Groups (%) | Variations between Populations within Groups (%) | Variations within Populations (%) |
|---|---|---|---|
| UMOD (rs4293393) | 4.82 | 3.93 | 91.25 |
| AGT (rs699) | 45.20 | 3.20 | 51.60 |
| CYP3A5 (rs776746) | 64.93 | 10.83 | 24.24 |
Figure 3.
Allelic frequencies of the rs4293393 UMOD variant do not show significant variation between African and European populations. Comparison between the FST values estimated between African and European populations for 2000 random noncoding regions (histogram), rs4293393 of the UMOD gene, rs699 of the AGT gene, and rs776746 of the CYP3A5 gene.
Dating and Testing Positive Selection of the UMOD Derived Variant
To evaluate in greater detail the possibility of positive selection on the UMOD derived allele, we incorporated past demography in the model. For that purpose, we reconstructed the haplotypes of the carriers of the derived allele for surrounding neutral markers. This way, we could infer recombination rates, from which we obtained estimates of the age of the variant in the population and of its intrinsic rate of growth.35 Assuming a generation time of 25 years,36 this analysis allowed us to estimate that the UMOD derived allele started increasing in frequency approximately 15,000 years ago, with limited differences among African and non-African populations (Table 3). The population sizes we chose were based on census data. An alternative, somewhat arbitrary choice of population size (i.e., 10 million) did not substantially affect the results. The estimated growth rates associated with the derived UMOD allele are low (and hence compatible with neutral allele-frequency changes), but still comparable with those found in loci affected by recent positive selection, e.g., protective variants for type 2 diabetes, i.e., between 1.021 and 1.027.37 Based on the same haplotype data, we could then explicitly test for positive selection by computing the integrated haplotype scores, so as to compare the decay of homozygosity around the UMOD rs4293393 variant for the ancestral and the derived alleles, in each population. The results obtained were not significant, providing further evidence that the derived allele of rs4293393 has not been under selective pressure (Supplemental Figure 2).
Table 3.
Joint estimate of growth rate (r) and age (g) of the derived allele of UMOD rs4293393
| Population | Census Size | Growth Rate (r) | Age of Mutation (g) |
|---|---|---|---|
| CEU | 5,000,000 | 1.02 (1.01 to 1.02) | 13,938 (11,905 to 17,133) |
| GIH | 5,000,000 | 1.01 (1.01 to 1.02) | 17,755 (15,223 to 21,780) |
| LWK | 5,300,000 | 1.01 (1.01 to 1.02) | 18,173 (15,485 to 22,570) |
| MEX | 5,000,000 | 1.02 (1.02 to 1.03) | 12,868 (10,920 to 16,265) |
| MKK | 850,000 | 1.01 (1.01 to 1.02) | 19,180 (16,205 to 23,358) |
| YRI | 36,000,000 | 1.02 (1.01 to 1.02) | 19,340 (16,918 to 23,400) |
| Population | Arbitrary Size | Growth Rate (r) | Age of Mutation (g) |
|---|---|---|---|
| CEU | 10,000,000 | 1.02 (1.02 to 1.03) | 13,838 (11,943 to 16,878) |
| GIH | 10,000,000 | 1.02 (1.01 to 1.02) | 17,655 (15,288 to 21,485) |
| LWK | 10,000,000 | 1.01 (1.01 to 1.02) | 18,080 (15,563 to 22,265) |
| MEX | 10,000,000 | 1.02 (1.02 to 1.03) | 12,775 (10,973 to 15,960) |
| MKK | 10,000,000 | 1.01 (1.01 to 1.02) | 18,773 (16,375 to 22,383) |
| YRI | 10,000,000 | 1.02 (1.02 to 1.03) | 19,510 (16,780 to 23,963) |
CEU, European ancestry; GIH, Gujarati Indians in Houston; LWK, Luhya in Webuye, Kenya; MEX, Mexican ancestry in Los Angeles; MKK, Maasai in Kinyawa, Kenya; YRI, Yoruba in Ibadan, Nigeria. The 95% confidence interval for growth rate and age estimates is shown in brackets.
Global Correlations of UMOD Alleles with Environmental Variables and Prevalence of Uropathogens
The discrepancies between the distribution of allelic frequencies of UMOD rs4293393 and that of other variants involved in salt retention and BP control led us to envisage alternative mechanisms of selection, related to environmental factors. Genome-wide signatures of natural selection can be driven by adaptation to local environments or pathogen diversity.38,39 Furthermore, the number of different pathogen species in a certain geographical region can be a good estimator of the selective pressure for the population.40–42 Based on multilevel biologic evidence, we hypothesized that the functional UMOD variant may play a role in protection against UTIs. Indeed, thanks to its biochemical properties, i.e., high-mannose glycans and Sda carbohydrate antigen, uromodulin acts as an incidental receptor that can effectively bind pathogens of the urinary tract, typically type 1 fimbriated Escherichia coli, competing with their binding to urothelial cells.43 Accordingly, uromodulin knockout mice show decreased bacterial clearance and increased susceptibility to UTIs by E. coli,5,44 and patients harboring heterozygous UMOD mutations, associated with lower levels of uromodulin in the urine, may present with repeated UTIs.45,46
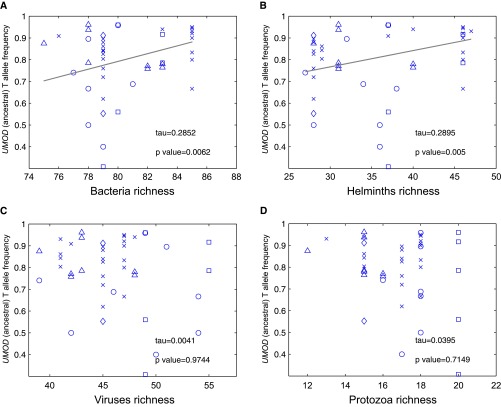
We thus assessed whether the rs4293393 UMOD variant displays signals of genetic adaptation to local environmental variables including climate, diet, and pathogens. We exploited the Human Genome Diversity Project (HGDP-CEPH),47 containing genotypes of more than 650,000 SNPs for about 1,000 individuals sampled in 52 human populations worldwide, in order to correlate the frequency of the ancestral, risk allele in these populations with the diversity of environmental variables. This analysis revealed that the frequencies of the rs4293393 ancestral allele are significantly correlated with pathogen diversity, bacteria and helminths in particular, but not with other pathogens (viruses and protozoa) or other environmental variables, including climate and diet regimen (Figure 4). When considering all SNPs in the HGDP database having a similar minor allele frequency (±0.01), the UMOD variant is above the 85th percentile (0.15) of the empirical P value distribution, which is consistent with previous estimates of the proportion of the human genome that underwent pathogen-driven selection.39 Of note, the two SNPs in AGT and CYP3A5 involved in salt retention were not correlated with diversity of bacteria and helminths (data not shown).
Figure 4.
The frequencies of the UMOD ancestral allele correlate with bacteria and helminth diversity. Correlation between the frequency of the UMOD ancestral T allele for rs4293393 in different populations (data from the Human Genome Diversity Panel, HGDP-CEPH) and diversity of (A) bacteria, (B) helminths, (C) viruses, and (D) protozoa. The trend was estimated using the Theil–Sen method. Populations from different continents are represented by different symbols: Africa (○), Asia (x), America (□), Oceania (◊), Europe (△).
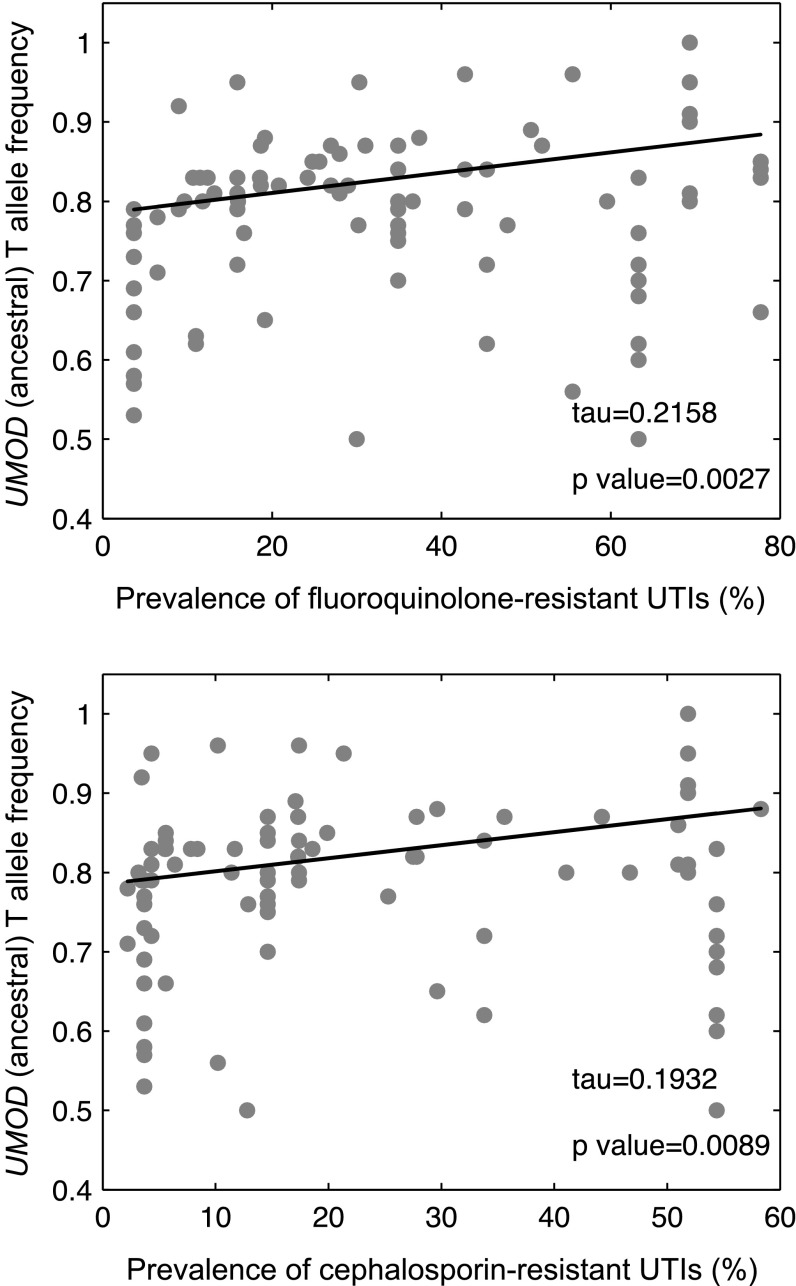
We next analyzed data about the global prevalence of multidrug-resistant Gram-negative bacteria, including uropathogenic E. coli, which reflects geographical variations in the prevalence of UTIs.48,49 There was a significant correlation between the UMOD ancestral allele frequencies and the prevalence of antibiotic (fluoroquinolone and cephalosporin) resistance in Gram-negative uropathogens (Figure 5, Supplemental Table 4). The empirical P values for these correlations were 0.17 and 0.14, respectively, similar to the correlation with pathogen diversity. These data suggest a higher frequency of the UMOD ancestral allele in areas where the prevalence of UTIs is higher.
Figure 5.
The frequencies of the UMOD ancestral allele correlate with the prevalence of antibiotic resistance in Gram-negative pathogens. Correlation between the frequency of the ancestral T allele for UMOD variant rs4293393 in different populations and prevalence (%) of antibiotic-resistant Gram-negative pathogens of the urinary tract, as available in Zowawi et al.48 The trend was estimated using the Theil–Sen method. The complete list of population and prevalence data are reported in Supplemental Table 4.
Uromodulin and Markers of UTIs in the General Population
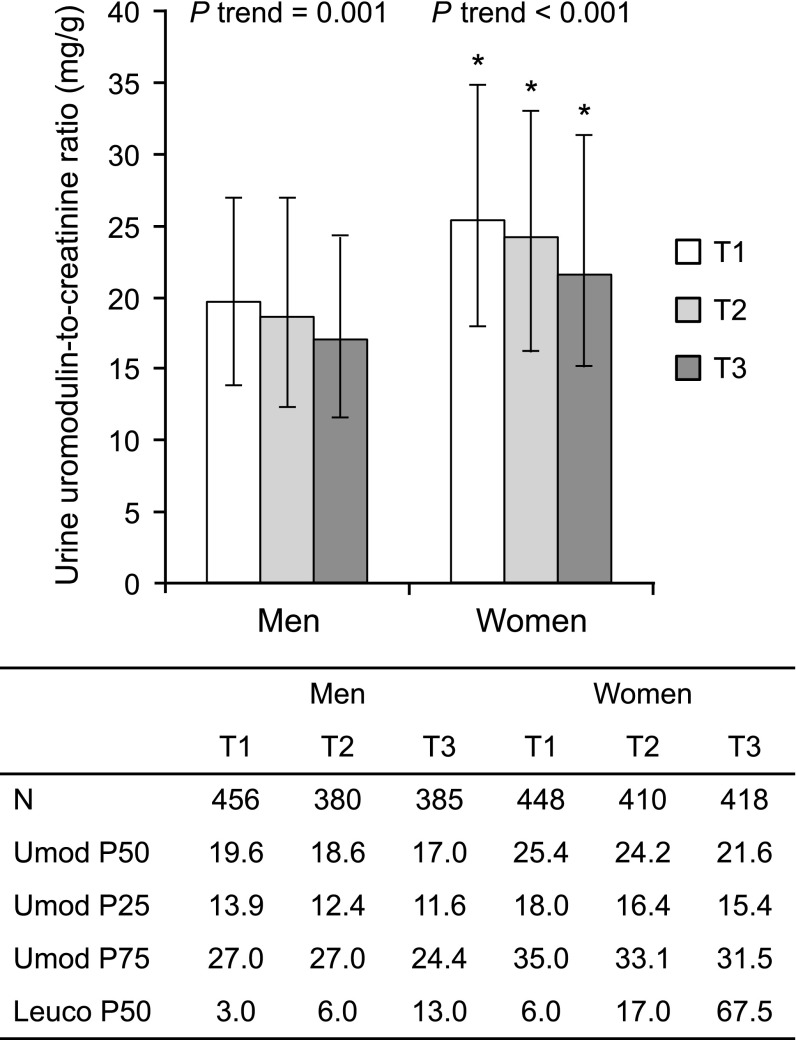
Meta-GWAS have shown that the UMOD ancestral variant is strongly associated with higher levels of uromodulin in the urine in large cohorts.16 Furthermore, the UMOD genotype is an independent predictor of urinary uromodulin in the general population.50 In order to substantiate a protective effect of uromodulin, we analyzed its urinary levels against markers of UTIs in the population-based Cohorte Lausannoise (CoLaus) cohort (n=5447). The relationship between the urinary uromodulin/creatinine ratio in spot urine and the amount of urinary leukocytes grouped in tertiles is shown in Figure 6. In both men and women, urinary leukocytes were higher when uromodulin levels were lower. In each tertile, men had a significantly lower median uromodulin/creatinine ratio than women (P<0.001). Men also had lower median leukocyte counts than women (P<0.001). Overall, 54 participants among the 2497 CoLaus participants with available information had positive nitrites in spot urine. When accounting for urinary creatinine, age, and sex, urinary uromodulin was negatively associated with the presence of urinary nitrites (Table 4). In the entire CoLaus cohort, the urinary uromodulin/creatinine ratio in spot urine, which is significantly dependent of the UMOD genotype, was negatively associated with circulating ultrasensitive C-reactive protein (CRP) in premenopausal women but not in postmenopausal women nor in men (Supplemental Table 5). Furthermore, we could detect an association between rs12917707 (which is in almost complete linkage disequilibrium [R2 = 0.95, D’ = 1] with rs4293393) and ultrasensitive CRP in the overall CoLaus cohort (n=4803; Tobit regression, P<0.04). When stratified by sex and menopausal status, this association was present only in premenopausal women (n=1081; Tobit regression, P<0.004), who are at much greater risk of UTIs.
Figure 6.
Inverse relationship between urinary levels of uromodulin and urinary leukocyte counts in CoLaus. N represents sample sizes in each tertile (total n=2497). Bars represent median uromodulin/creatinine ratio in spot urine in each sex-specific tertile of urinary leukocytes. Data are uromodulin-to-creatinine medians (Umod P50) and whiskers interquartile range (Umod P25 and Umod P75). P values for trend are from a nonparametric test for trend across sex-specific leukocyte tertiles. *P<0.001 for median test comparing men and women. Leuco P50: median leukocyte counts in each tertile.
Table 4.
Multiple logistic regression for factors associated with the presence of urinary nitrites in the CoLaus study
| Parameter (N=2497) | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age (yr) | 1.04 | 1.02 to 1.08 | 0.001 |
| Sex (1= women, 0= men) | 4.01 | 2.02 to 7.98 | <0.001 |
| Square-root urinary creatinine (mg/dl) | 1.19 | 1.08 to 1.30 | <0.001 |
| Square-root urinary uromodulin (μg/ml) | 0.74 | 0.60 to 0.90 | 0.002 |
Positive nitrites were detected in 54 of 2497 successive CoLaus participants.
Taken together, these data suggest that the high prevalence of the UMOD ancestral allele, which is associated with risk of salt-sensitive hypertension and CKD, is not explained by the ancestral susceptibility model. The global distribution of the UMOD variant is clearly different from the AGT and CYP3A5 variants, classically associated with that model. It is also different from other variants (e.g., CORIN) influencing salt retention. Instead, the global correlations between the ancestral allele of UMOD and pathogen diversity and uropathogenic E. coli prevalence, as well as its influence on the level of uromodulin in urine, suggest that this allele has been kept at high frequency due to its protective effect against UTIs and their consequences in terms of fitness and reproduction. This hypothesis is supported by the inverse correlation between urinary levels of uromodulin and markers of UTIs in the general population, and by the fact that the frequency of the derived allele of UMOD has not been under selective pressure and remains low (Table 1). In the case of UMOD, there is no balancing selection: the fact that the association of UMOD variants with CKD is clearly age-dependent, only being evident after the age of 65 years,14,18 implies that the derived allele should have no direct impact on reproductive fitness.
Among the various environmental factors, pathogens have posed the main selective pressure, shaping the human genome and leading to local genetic adaptations.39 The gene targets of such pathogen-driven selection are mostly related to immune response and include MHC class I, blood group antigens, and interleukins and their receptors. However, genes not directly participating in immune response may also be involved: for instance, genes encoding complex sialoglycoproteins which can function as incidental pathogen receptors are known to be targeted by pathogen-driven selection in humans.51 This is particularly relevant when considering that uromodulin contains complex sialylated and fucosylated oligosaccharides which are key to its binding to type 1 fimbriated E. coli and to its capability to modulate the susceptibility and virulence of UTIs.52,53 UTIs are among the most common bacterial infections, with particularly high incidence and risk of recurrence in young women and potential complications affecting pregnancy outcome and even leading to septic shock and death.54,55 Increasing evidence shows that host defense mechanisms against uropathogenic E. coli, which causes >80% of UTIs, influence the recurrence and severity of UTIs.56 At physiologic concentrations, uromodulin abolishes the binding of E. coli to uroplakin receptors and, through its interactions with host factors like IgG heavy and light chains, facilitates neutrophil migration.57,58 Conversely, uropathogenic E. coli were more abundant, caused more severe renal infections and acute mortality, and persisted longer in uromodulin knockout mice.5 Our data, showing for the first time an inverse correlation of urinary uromodulin levels and local and systemic markers of UTIs, support the view that higher levels of uromodulin in the urine, determined by the presence of the UMOD ancestral allele,50 exert a protective effect against UTIs in the general population.
Besides direct binding to uropathogens, the protective effect of the UMOD ancestral allele may also depend on the ability of uromodulin to regulate innate immunity by activating dendritic cells via Toll-like receptor 4 (TLR4).6 Interestingly, the geographical distribution of the rs4293393 alleles is comparable (52% correlation between worldwide frequencies of the two ancestral alleles) with that of the nonsynonymous rs4986790 variant (Asp299Gly) in TLR4, associated with increased risk of infections mediated by Gram-negative bacteria, including E. coli,59 and risk of UTIs in children.60 Like UMOD, this TLR4 variant showed a significant correlation with the diversity of bacteria and helminths (data not shown). Other studies, mostly on polymorphisms in TLR genes, established the importance of the early response against invading pathogens that is essentially provided by innate immunity.52
Of note, the UMOD ancestral allele may also have an additional, protective effect against infections by impacting on tubular salt handling. Uromodulin is known to regulate apical transport systems in the TAL and in downstream nephron segments.19,61 An increased ability to retain salt would confer an advantage in the case of other types of infections associated with salt-losing, particularly at a young age.
The global correlations between the UMOD ancestral allele frequencies and pathogen diversity or prevalence of UTIs support the hypothesis that this allele has been kept at higher frequencies in areas with stronger selective pressure for its protective effect. Selection would hence have acted on an already present allele, following the model of selection on standing variation.62 In this case, correlations between genetic variants and environmental variables may indeed be the most appropriate to assess evidence of selection,63 as observed for the ancestral -3826A allele in UCP1, that has been kept at higher frequencies in populations at high latitude exposed to low solar radiation.64 The UMOD situation is therefore different from the paradigm of selection at the APOL1 locus, where it is the derived allele that confers selective advantage against pathogens.65,66
It is interesting to note that among the populations that drive the correlation between UMOD variant and pathogens, the three African populations that show the lowest ancestral allele frequency (Biaka Pigmies, Mbuti Pigmies, San) (Supplemental Table 2), associated with low levels of pathogen diversity, are characterized by the highest values of gathering activity.39 This is consistent with gathering activity being associated to mobile populations that are less prone to infectious disease transmission compared with sedentary cultures with increasing population density.67,68 This concept may also be extended to the intriguing observation that both anatomically archaic humans were homozygous for the UMOD derived allele, whereas all anatomically modern humans showed homozygosity for the ancestral allele. While little is known about Denisova demography, archaeological data suggest that Neanderthal population sizes were around one tenth of those of their contemporary anatomically modern counterparts.69 Therefore, we speculate that differences in population densities between Neanderthals and pre-Neolithic Homo sapiens could justify different selective regimes driven by pathogens, hence different frequencies of the UMOD derived allele.70
There is no published GWAS for susceptibility to UTIs, and thus no direct evidence for an UMOD locus influencing the trait. This absence is explained at least in part by the difficulty to obtain reliable and standardized urinary samples, and to ascertain UTIs in a population setting. Additional genetic analyses directly evaluating the influence of the UMOD genotype on the UTI phenotype will thus be important.
We acknowledge that our study provides circumstantial evidence rather than direct proof or a clear signature of adaptation. Formal tests for the effect of positive selection assume that the selective process affects the derived allele. When, as is the case for UMOD, the candidate allele is ancestral, there is no way to generate formal expectations of its frequency and distribution. This is because we do not know the set of neutral evolutionary changes leading to the distribution of allele frequencies at the moment when selection begins to operate. Therefore, a neutral model accounting for the high frequency of the UMOD ancestral (CKD risk) variants cannot be rejected.
However, several lines of evidence concur to strongly suggest an evolutionary advantage for carriers of the ancestral UMOD allele (summarized in Table 1), including: (1) robust biochemical evidence showing that uromodulin binds type 1 fimbriated E. coli, (2) increased susceptibility to UTIs in uromodulin knockout mice, (3) inverse correlation between uromodulin urinary levels and markers of UTIs in the general population, (4) global spatial correlations of UMOD allelic frequencies with pathogen diversity and prevalence of resistant uropathogenic E. coli strains, and (5) correlation between the distribution patterns of allelic frequencies of UMOD and of a well established TLR4 variant associated with UTI susceptibility. Further work should provide opportunities to substantiate the link between the UMOD gene, uromodulin, and a protective effect against damaging consequences of UTIs in the general population.
Concise Methods
Phylogenetic Analysis of UMOD Variant rs4293393
To study the phylogenetic context of the UMOD rs4293393 we investigated the region surrounding the variant in four nonhuman primates (Gorilla, Orangutan, Gibbon, and Rhesus) from the University of California at Santa Cruz genome browser (http://genome.ucsc.edu/), in 25 chimpanzee genome sequences from Prado-Martinez et al.,21 in two ancient hominid genomes (i.e., Neanderthal and Denisova), and in anatomically modern human samples spanning a period 3900–45,000 years ago. Information on the characteristics of the ancient samples and references are provided in Supplemental Table 1. We extracted the sequence information from complete genomes using VCFtools.71 Sequences spanning rs4293393 were aligned to the modern human genome sequence (assembly GRCh37/hg19).
Analysis of Allelic Frequency Distribution of UMOD, AGT, CYP3A5, and CORIN Variants
To compare the allele frequency distribution of the UMOD (rs4293393), AGT (rs699), and CYP3A5 (rs776746) variants, we collected genomic data from worldwide datasets of genomic variation (see Supplemental Table 2 for details and references). From these datasets we selected the populations reporting the allele frequencies information for at least one of the three variants, while having a sample size of at least ten individuals; the final dataset resulted in 156 populations from all over the world. Since most of the datasets used above do not include information about the CORIN variant (rs75770792), we checked its presence in all other available datasets. We identified 44 worldwide populations with a sufficient number (>8) of individuals typed for both CORIN- and UMOD-relevant SNPs (rs75770792 and rs4293393, respectively) (see Supplemental Table 3 for details and references), thus allowing a direct comparison between the distributions of allele frequencies.
We combined the data and estimated the allele frequencies using PLINK72; we then plotted the allele frequencies on a geographic map with the mapplots R package. The analysis of molecular variance for UMOD (rs4293393), AGT (rs699), and CYP3A5 (rs776746) was conducted with the pegas R package.73 From 8473 variants that are shared among the 156 populations, we selected 2351 intergenic (i.e., neutral) loci using the Variant Effect Predictor tool.74 We extracted the allele frequencies information at these loci in the 156 populations with PLINK, and then we calculated the FST values with 4P software.75
Dating of the UMOD rs4293393 Variant
To date the UMOD derived variant we used the Austerlitz likelihood-based method.35 This maximum likelihood algorithm requires a preliminary reconstruction of the haplotypes of the carriers of the derived allele for surrounding neutral markers. Then the number of generations elapsed since the variant entered the population and the variant’s intrinsic growth rate are estimated from the percentage of recombinant haplotypes and from the carrier frequency of the derived allele in the population. As this method requires reconstructing the haplotypes carrying the derived variant, we exploited the International HapMap Project populations, dating the derived variant in each population separately. To reconstruct the haplotypes we chose nine SNPs on each side of the target variant, at about 20, 35, 50, 75, 100, 125, 150, 200, and 250 kb distance from the target variant, respectively. We used the International HapMap Project data from populations for which phase information were available (i.e., CEU, GIH, LWK, MKK, and YRI) to compute the integrated haplotype scores with the rehh R package. The integrated haplotype score estimates were standardized per allelic frequency bins using genome-wide data.76 The same populations were then used to estimate the time elapsed since the appearance of the derived allele and its intrinsic growth rate with the Austerlitz et al. method as implemented in their Mathematica notebook.35 The MEX population was only used for dating the derived allele, as no ancestry information was available.
Correlation of Allelic Frequencies of rs4293393 with Environmental Parameters
Genotypes of rs4293393 in individuals from the 52 populations of the HGDP-CEPH panel were retrieved from the correspondent website (Human Genome Diversity Panel Database Version 3.0, http://www.cephb.fr/hgdp/index.php). We considered the data of pathogen richness provided in Fumagalli et al.,39 which represent the quantification of the number of different pathogen species, detailed for bacteria, protozoa, helminths, and viruses, for the 21 countries where HGDP-CEPH populations are distributed, as retrievable from the Global Infectious Disease and Epidemiology Network database (2002) (http://gideononline.com).
We derived data concerning prevalence of resistance to fluoroquinolones and third-generation cephalosporins in Gram-negative urinary pathogens from Zowawi et al.48 Where more than one study for a given country was available, we calculated and used the average prevalence. We could associate prevalence indexes to 96 (fluoroquinolone resistance) or 92 (cephalosporin resistance) populations of the 156 for which we extracted allelic frequencies of the rs4293393 UMOD variant (Supplemental Table 2). The complete list of population and antibiotic-resistant UTI prevalence is reported in Supplemental Table 4.
Correlations between allelic frequencies of the candidate SNP and pathogen richness or UTI prevalence were computed by the Kendall rank correlation coefficient, a nonparametric statistic, implemented in Matlab R2014a. Since both pathogen diversity and UTI prevalence data were available as country (political unit)-based values, we did not apply a correction for spatial autocorrelation and we relied on the simplest index of association available, as previously done in similar studies.41,77
Urinary Uromodulin Levels and Markers of UTIs in the General Population
Recruitment
The CoLaus study aims to identify new molecular determinants of cardiovascular risk in the white population from the city of Lausanne (Switzerland). The study was approved by the Institutional Ethics Committee of the University of Lausanne and all participants provided written informed consent. The sampling procedure has been previously described.78 Briefly, a simple, nonstratified random sample of the overall population of Lausanne, aged 35–75 years, was drawn. Recruitment for baseline examination began in June 2003 and ended in May 2006. The participation rate was 41%. All participants (N=5447) were seen in the morning after a minimum fasting time of 8 hours.
Data Collection
A standardized questionnaire was used to assess lifestyle, including alcohol and smoking habits. Participants were classified as either current smokers or nonsmokers. For the purpose of the present analysis, regular alcohol consumption was considered as present whenever participants reported to drink at least one unit of alcohol per day. Body mass was estimated as weight divided by height in meters squared, as previously described.78 Venous blood samples (50 ml) were drawn in the fasting state. Ultrasensitive CRP was assessed by immunoassay and latex HS (IMMULITE 1000–High, Diagnostic Products Corporation, Los Angeles, CA) with maximum intra- and interbatch coefficients of variation of 1.3% and 4.6%, respectively. Uromodulin was measured in morning spot urine using a validated ELISA,79 with a sensitivity of 2.8 ng ml−1, a linearity of 1.0, an interassay variability of 3.3% and an intra-assay variability of 5.5%. Urinary creatinine levels (normalization) were measured using the Synchron System Creatinine Assay (Unicell DxC Synchron, Beckman Coulter, Inc., Brea, CA,), following the manufacturer's instructions. In a subset of 2497 consecutive participants, a urinary test strip was used to determine the presence (versus absence) of nitrite in spot urine using a Clinitek Atlas analyzer (SIEMENS, Tarrytown, NY) and the determination of leukocyte counts by automatic counting.
Statistical Analyses
We conducted multiple logistic regression to explore the association between presence of urinary nitrites and square-root-transformed urinary uromodulin concentration, while including age, sex, and square-root-transformed urinary creatinine concentration as covariates in the model. We used a median test to compare urinary leukocytes, or uromodulin-to-creatinine levels, between men and women and a nonparametric test for trend to compare uromodulin-to-creatinine levels across sex-specific tertiles of urine leukocytes. We explored the association of log-transformed ultrasensitive CRP with quintiles of urinary uromodulin-to-creatinine ratio or with the rs12917707 using multiple Tobit regression to account for the left-censored dependent variable. We generated quintiles of urinary uromodulin-to-creatinine ratio separately in men and premenopausal and postmenopausal women. Fully adjusted models included age, body mass index, smoking, alcohol consumption, eGFR Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), HDL-cholesterol, log-triglyceride, lipid-lowering drug, type 2 diabetes, and hypertension as covariates. We used a likelihood ratio test, including four dummy variables, to test the effect of all quintiles. Using separate models, we included a categorical variable coded from 1 to 5 to generate a single P value for linear trend across quintiles.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors acknowledge Denise Darman (University of Ferrara, Italy), Nadine Nägele, and Sonia Youhanna (University of Zurich, Switzerland) for technical help, and Lorena Madrigal (University of South Florida), Ozren Polasek (University of Split, Croatia), Catharina Svanborg (University of Lund, Sweden), and Gérard Waeber (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland) for helpful discussions. They also wish to acknowledge the constructive remarks of the reviewers that contributed to extend and strengthen the original findings.
This work was supported by the Fonds National de la Recherche Scientifique and the Fonds de la Recherche Scientifique Médicale (Brussels, Belgium), the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 305608 (EURenOmics), the Gebert-Rüef Foundation, the Swiss National Science Foundation project grant 310030_146490 and NCCR Kidney.CH Program (to O.D.), Telethon-Italy (TCR08006, GGP14263) and the Italian Ministry of Health (grant RF-2010-2319394) (to L.R.), the European Research Council (ERC-2011-AdvG_295733 grant, LanGeLin) (to G.B. and S.G.), the Italian Ministry for Research and Universities (grant no. PRIN 2010-2011) (to G.B.), and The European Union Project ShockOmics (grant agreement no. 602706, to L.P.). The CoLaus study was supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine, University Hospital Center of Lausanne, Switzerland and the Swiss National Science Foundation (grants 33CSCO-122661, 33CS30-139468, and 33CS30-148401).
Part of the results detailed in this manuscript were presented as an oral communication at the 2014 Annual Meeting of the American Society of Nephrology, November 11–16, 2014, in Philadelphia, PA.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015070830/-/DCSupplemental.
References
- 1.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O: The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Tamm I, Horsfall FL Jr: Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med 74: 106–108, 1950 [PubMed] [Google Scholar]
- 3.Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, Raffi H, Rampoldi L, Uchida S, Hille C, Dosche C, Kumar S, Castañeda-Bueno M, Gamba G, Bachmann S: Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem 286: 30200–30210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renigunta A, Renigunta V, Saritas T, Decher N, Mutig K, Waldegger S: Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem 286: 2224–2235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S: Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65: 791–797, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Anders H-J, Schaefer L: Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ: Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 39: 882–892, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckardt K-U, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O: Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int 88: 676–683, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Dahan K, Devuyst O, Smaers M, Vertommen D, Loute G, Poux J-M, Viron B, Jacquot C, Gagnadoux M-F, Chauveau D, Büchler M, Cochat P, Cosyns J-P, Mougenot B, Rider MH, Antignac C, Verellen-Dumoulin C, Pirson Y: A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol 14: 2883–2893, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Rampoldi L, Caridi G, Santon D, Boaretto F, Bernascone I, Lamorte G, Tardanico R, Dagnino M, Colussi G, Scolari F, Ghiggeri GM, Amoroso A, Casari G: Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet 12: 3369–3384, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Bernascone I, Janas S, Ikehata M, Trudu M, Corbelli A, Schaeffer C, Rastaldi MP, Devuyst O, Rampoldi L: A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum Mol Genet 19: 2998–3010, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Köttgen A, Glazer NL, Dehghan A, Hwang S-J, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen Y-DI, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WHL, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köttgen A, Pattaro C, Böger CA, Fuchsberger C, Olden M, Glazer NL, Parsa A, Gao X, Yang Q, Smith AV, O’Connell JR, Li M, Schmidt H, Tanaka T, Isaacs A, Ketkar S, Hwang S-J, Johnson AD, Dehghan A, Teumer A, Paré G, Atkinson EJ, Zeller T, Lohman K, Cornelis MC, Probst-Hensch NM, Kronenberg F, Tönjes A, Hayward C, Aspelund T, Eiriksdottir G, Launer LJ, Harris TB, Rampersaud E, Mitchell BD, Arking DE, Boerwinkle E, Struchalin M, Cavalieri M, Singleton A, Giallauria F, Metter J, de Boer IH, Haritunians T, Lumley T, Siscovick D, Psaty BM, Zillikens MC, Oostra BA, Feitosa M, Province M, de Andrade M, Turner ST, Schillert A, Ziegler A, Wild PS, Schnabel RB, Wilde S, Munzel TF, Leak TS, Illig T, Klopp N, Meisinger C, Wichmann H-E, Koenig W, Zgaga L, Zemunik T, Kolcic I, Minelli C, Hu FB, Johansson A, Igl W, Zaboli G, Wild SH, Wright AF, Campbell H, Ellinghaus D, Schreiber S, Aulchenko YS, Felix JF, Rivadeneira F, Uitterlinden AG, Hofman A, Imboden M, Nitsch D, Brandstätter A, Kollerits B, Kedenko L, Mägi R, Stumvoll M, Kovacs P, Boban M, Campbell S, Endlich K, Völzke H, Kroemer HK, Nauck M, Völker U, Polasek O, Vitart V, Badola S, Parker AN, Ridker PM, Kardia SLR, Blankenberg S, Liu Y, Curhan GC, Franke A, Rochat T, Paulweber B, Prokopenko I, Wang W, Gudnason V, Shuldiner AR, Coresh J, Schmidt R, Ferrucci L, Shlipak MG, van Duijn CM, Borecki I, Krämer BK, Rudan I, Gyllensten U, Wilson JF, Witteman JC, Pramstaller PP, Rettig R, Hastie N, Chasman DI, Kao WH, Heid IM, Fox CS: New loci associated with kidney function and chronic kidney disease. Nat Genet 42: 376–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pattaro C, Köttgen A, Teumer A, Garnaas M, Böger CA, Fuchsberger C, Olden M, Chen M-H, Tin A, Taliun D, Li M, Gao X, Gorski M, Yang Q, Hundertmark C, Foster MC, O’Seaghdha CM, Glazer N, Isaacs A, Liu C-T, Smith AV, O’Connell JR, Struchalin M, Tanaka T, Li G, Johnson AD, Gierman HJ, Feitosa M, Hwang S-J, Atkinson EJ, Lohman K, Cornelis MC, Johansson Å, Tönjes A, Dehghan A, Chouraki V, Holliday EG, Sorice R, Kutalik Z, Lehtimäki T, Esko T, Deshmukh H, Ulivi S, Chu AY, Murgia F, Trompet S, Imboden M, Kollerits B, Pistis G, Harris TB, Launer LJ, Aspelund T, Eiriksdottir G, Mitchell BD, Boerwinkle E, Schmidt H, Cavalieri M, Rao M, Hu FB, Demirkan A, Oostra BA, de Andrade M, Turner ST, Ding J, Andrews JS, Freedman BI, Koenig W, Illig T, Döring A, Wichmann HE, Kolcic I, Zemunik T, Boban M, Minelli C, Wheeler HE, Igl W, Zaboli G, Wild SH, Wright AF, Campbell H, Ellinghaus D, Nöthlings U, Jacobs G, Biffar R, Endlich K, Ernst F, Homuth G, Kroemer HK, Nauck M, Stracke S, Völker U, Völzke H, Kovacs P, Stumvoll M, Mägi R, Hofman A, Uitterlinden AG, Rivadeneira F, Aulchenko YS, Polasek O, Hastie N, Vitart V, Helmer C, Wang JJ, Ruggiero D, Bergmann S, Kähönen M, Viikari J, Nikopensius T, Province M, Ketkar S, Colhoun H, Doney A, Robino A, Giulianini F, Krämer BK, Portas L, Ford I, Buckley BM, Adam M, Thun GA, Paulweber B, Haun M, Sala C, Metzger M, Mitchell P, Ciullo M, Kim SK, Vollenweider P, Raitakari O, Metspalu A, Palmer C, Gasparini P, Pirastu M, Jukema JW, Probst-Hensch NM, Kronenberg F, Toniolo D, Gudnason V, Shuldiner AR, Coresh J, Schmidt R, Ferrucci L, Siscovick DS, van Duijn CM, Borecki I, Kardia SL, Liu Y, Curhan GC, Rudan I, Gyllensten U, Wilson JF, Franke A, Pramstaller PP, Rettig R, Prokopenko I, Witteman JC, Hayward C, Ridker P, Parsa A, Bochud M, Heid IM, Goessling W, Chasman DI, Kao WH, Fox CS CARDIoGRAM Consortium ICBP Consortium CARe Consortium Wellcome Trust Case Control Consortium 2 (WTCCC2) : Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet 8: e1002584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köttgen A, Yang Q, Shimmin LC, Tin A, Schaeffer C, Coresh J, Liu X, Rampoldi L, Hwang S-J, Boerwinkle E, Hixson JE, Kao WHL, Fox CS: Association of estimated glomerular filtration rate and urinary uromodulin concentrations with rare variants identified by UMOD gene region sequencing. PLoS One 7: e38311, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olden M, Corre T, Hayward C, Toniolo D, Ulivi S, Gasparini P, Pistis G, Hwang S-J, Bergmann S, Campbell H, Cocca M, Gandin I, Girotto G, Glaudemans B, Hastie ND, Loffing J, Polasek O, Rampoldi L, Rudan I, Sala C, Traglia M, Vollenweider P, Vuckovic D, Youhanna S, Weber J, Wright AF, Kutalik Z, Bochud M, Fox CS, Devuyst O: Common variants in UMOD associate with urinary uromodulin levels: a meta-analysis. J Am Soc Nephrol 25: 1869–1882, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, Hastie CE, Menni C, Monti MC, Delles C, Laing S, Corso B, Navis G, Kwakernaak AJ, van der Harst P, Bochud M, Maillard M, Burnier M, Hedner T, Kjeldsen S, Wahlstrand B, Sjögren M, Fava C, Montagnana M, Danese E, Torffvit O, Hedblad B, Snieder H, Connell JMC, Brown M, Samani NJ, Farrall M, Cesana G, Mancia G, Signorini S, Grassi G, Eyheramendy S, Wichmann HE, Laan M, Strachan DP, Sever P, Shields DC, Stanton A, Vollenweider P, Teumer A, Völzke H, Rettig R, Newton-Cheh C, Arora P, Zhang F, Soranzo N, Spector TD, Lucas G, Kathiresan S, Siscovick DS, Luan J, Loos RJF, Wareham NJ, Penninx BW, Nolte IM, McBride M, Miller WH, Nicklin SA, Baker AH, Graham D, McDonald RA, Pell JP, Sattar N, Welsh P, Munroe P, Caulfield MJ, Zanchetti A, Dominiczak AF Global BPgen Consortium : Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet 6: e1001177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, Edvardsson V, Sulem P, de Vegt F, d’Ancona FCH, den Heijer M, Wetzels JFM, Franzson L, Rafnar T, Kristjansson K, Bjornsdottir US, Eyjolfsson GI, Kiemeney LA, Kong A, Palsson R, Thorsteinsdottir U, Stefansson K: Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet 6: e1001039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, Citterio L, Demaretz S, Trevisani F, Ristagno G, Glaudemans B, Laghmani K, Dell’Antonio G, Loffing J, Rastaldi MP, Manunta P, Devuyst O, Rampoldi L Swiss Kidney Project on Genes in Hypertension (SKIPOGH) team : Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med 19: 1655–1660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TFC, McCarroll SA, Visscher PM: Finding the missing heritability of complex diseases. Nature 461: 747–753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prado-Martinez J, Sudmant PH, Kidd JM, Li H, Kelley JL, Lorente-Galdos B, Veeramah KR, Woerner AE, O’Connor TD, Santpere G, Cagan A, Theunert C, Casals F, Laayouni H, Munch K, Hobolth A, Halager AE, Malig M, Hernandez-Rodriguez J, Hernando-Herraez I, Prüfer K, Pybus M, Johnstone L, Lachmann M, Alkan C, Twigg D, Petit N, Baker C, Hormozdiari F, Fernandez-Callejo M, Dabad M, Wilson ML, Stevison L, Camprubí C, Carvalho T, Ruiz-Herrera A, Vives L, Mele M, Abello T, Kondova I, Bontrop RE, Pusey A, Lankester F, Kiyang JA, Bergl RA, Lonsdorf E, Myers S, Ventura M, Gagneux P, Comas D, Siegismund H, Blanc J, Agueda-Calpena L, Gut M, Fulton L, Tishkoff SA, Mullikin JC, Wilson RK, Gut IG, Gonder MK, Ryder OA, Hahn BH, Navarro A, Akey JM, Bertranpetit J, Reich D, Mailund T, Schierup MH, Hvilsom C, Andrés AM, Wall JD, Bustamante CD, Hammer MF, Eichler EE, Marques-Bonet T: Great ape genetic diversity and population history. Nature 499: 471–475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer M, Kircher M, Gansauge M-T, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prüfer K, de Filippo C, Sudmant PH, Alkan C, Fu Q, Do R, Rohland N, Tandon A, Siebauer M, Green RE, Bryc K, Briggs AW, Stenzel U, Dabney J, Shendure J, Kitzman J, Hammer MF, Shunkov MV, Derevianko AP, Patterson N, Andrés AM, Eichler EE, Slatkin M, Reich D, Kelso J, Pääbo S: A high-coverage genome sequence from an archaic Denisovan individual. Science 338: 222–226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prüfer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, Heinze A, Renaud G, Sudmant PH, de Filippo C, Li H, Mallick S, Dannemann M, Fu Q, Kircher M, Kuhlwilm M, Lachmann M, Meyer M, Ongyerth M, Siebauer M, Theunert C, Tandon A, Moorjani P, Pickrell J, Mullikin JC, Vohr SH, Green RE, Hellmann I, Johnson PLF, Blanche H, Cann H, Kitzman JO, Shendure J, Eichler EE, Lein ES, Bakken TE, Golovanova LV, Doronichev VB, Shunkov MV, Derevianko AP, Viola B, Slatkin M, Reich D, Kelso J, Pääbo S: The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505: 43–49, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reich D, Green RE, Kircher M, Krause J, Patterson N, Durand EY, Viola B, Briggs AW, Stenzel U, Johnson PLF, Maricic T, Good JM, Marques-Bonet T, Alkan C, Fu Q, Mallick S, Li H, Meyer M, Eichler EE, Stoneking M, Richards M, Talamo S, Shunkov MV, Derevianko AP, Hublin J-J, Kelso J, Slatkin M, Pääbo S: Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468: 1053–1060, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Rienzo A, Hudson RR: An evolutionary framework for common diseases: the ancestral-susceptibility model. Trends Genet 21: 596–601, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Tassi F, Ghirotto S, Mezzavilla M, Vilaça ST, De Santi L, Barbujani G: Early modern human dispersal from Africa: genomic evidence for multiple waves of migration. Investig Genet 6: 13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson EE, Kuttab-Boulos H, Witonsky D, Yang L, Roe BA, Di Rienzo A: CYP3A variation and the evolution of salt-sensitivity variants. Am J Hum Genet 75: 1059–1069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S-J, Chiang F-T, Chen WJ, Liu P-H, Hsu K-L, Hwang J-J, Lai L-P, Lin J-L, Tseng C-D, Tseng Y-Z: Three single-nucleotide polymorphisms of the angiotensinogen gene and susceptibility to hypertension: single locus genotype vs. haplotype analysis. Physiol Genomics 17: 79–86, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Nakajima T, Wooding S, Sakagami T, Emi M, Tokunaga K, Tamiya G, Ishigami T, Umemura S, Munkhbat B, Jin F, Guan-Jun J, Hayasaka I, Ishida T, Saitou N, Pavelka K, Lalouel J-M, Jorde LB, Inoue I: Natural selection and population history in the human angiotensinogen gene (AGT): 736 complete AGT sequences in chromosomes from around the world. Am J Hum Genet 74: 898–916, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Givens RC, Lin YS, Dowling ALS, Thummel KE, Lamba JK, Schuetz EG, Stewart PW, Watkins PB: CYP3A5 genotype predicts renal CYP3A activity and blood pressure in healthy adults. J Appl Physiol (1985) 95: 1297–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E: Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27: 383–391, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho S-I, Wu Q, Post W, Drazner MH: Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 112: 2403–2410, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q: Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res 103: 502–508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbujani G, Ghirotto S, Tassi F: Nine things to remember about human genome diversity. Tissue Antigens 82: 155–164, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Austerlitz F, Kalaydjieva L, Heyer E: Detecting population growth, selection and inherited fertility from haplotypic data in humans. Genetics 165: 1579–1586, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenner JN: Cross-cultural estimation of the human generation interval for use in genetics-based population divergence studies. Am J Phys Anthropol 128: 415–423, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Ségurel L, Austerlitz F, Toupance B, Gautier M, Kelley JL, Pasquet P, Lonjou C, Georges M, Voisin S, Cruaud C, Couloux A, Hegay T, Aldashev A, Vitalis R, Heyer E: Positive selection of protective variants for type 2 diabetes from the Neolithic onward: a case study in Central Asia. Eur J Hum Genet 21: 1146–1151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coop G, Witonsky D, Di Rienzo A, Pritchard JK: Using environmental correlations to identify loci underlying local adaptation. Genetics 185: 1411–1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admetlla A, Pattini L, Nielsen R: Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet 7: e1002355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prugnolle F, Manica A, Charpentier M, Guégan JF, Guernier V, Balloux F: Pathogen-driven selection and worldwide HLA class I diversity. Curr Biol 15: 1022–1027, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Seixas S, Ivanova N, Ferreira Z, Rocha J, Victor BL: Loss and gain of function in SERPINB11: an example of a gene under selection on standing variation, with implications for host-pathogen interactions. PLoS One 7: e32518, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forni D, Pozzoli U, Cagliani R, Tresoldi C, Menozzi G, Riva S, Guerini FR, Comi GP, Bolognesi E, Bresolin N, Clerici M, Sironi M: Genetic adaptation of the human circadian clock to day-length latitudinal variations and relevance for affective disorders. Genome Biol 15: 499, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serafini-Cessi F, Monti A, Cavallone D: N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J 22: 383–394, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Mo L, Zhu X-H, Huang H-Y, Shapiro E, Hasty DL, Wu X-R: Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 286: F795–F802, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Bleyer AJ, Trachtman H, Sandhu J, Gorry MC, Hart TC: Renal manifestations of a mutation in the uromodulin (Tamm Horsfall protein) gene. Am J Kidney Dis 42: E20–E26, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Wolf MTF, Beck BB, Zaucke F, Kunze A, Misselwitz J, Ruley J, Ronda T, Fischer A, Eifinger F, Licht C, Otto E, Hoppe B, Hildebrandt F: The Uromodulin C744G mutation causes MCKD2 and FJHN in children and adults and may be due to a possible founder effect. Kidney Int 71: 574–581, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, Cann HM, Barsh GS, Feldman M, Cavalli-Sforza LL, Myers RM: Worldwide human relationships inferred from genome-wide patterns of variation. Science 319: 1100–1104, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Zowawi HM, Harris PNA, Roberts MJ, Tambyah PA, Schembri MA, Pezzani MD, Williamson DA, Paterson DL: The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol 12: 570–584, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Hilbert DW: Antibiotic resistance in urinary tract infections: current issues and future solutions. In: Urinary Tract Infections, edited by Tenke P, 2011 Available from: http://www.intechopen.com/books/urinary-tract-infections/antibiotic-resistance-in-urinary-tract-infections-current-issues-and-future-solutions. Accessed: December 10, 2015 [Google Scholar]
- 50.Troyanov S, Delmas-Frenette C, Bollée G, Youhanna S, Bruat V, Awadalla P, Devuyst O, Madore F: Clinical, Genetic, and Urinary Factors Associated with Uromodulin Excretion. Clin J Am Soc Nephrol 11: 62–69, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cagliani R, Sironi M: Pathogen-driven selection in the human genome. Int J Evol Biol 2013: 204240–204246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ragnarsdóttir B, Lutay N, Grönberg-Hernandez J, Köves B, Svanborg C: Genetics of innate immunity and UTI susceptibility. Nat Rev Urol 8: 449–468, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Ragnarsdóttir B, Svanborg C: Susceptibility to acute pyelonephritis or asymptomatic bacteriuria: host-pathogen interaction in urinary tract infections. Pediatr Nephrol 27: 2017–2029, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Stamm WE: An epidemic of urinary tract infections? N Engl J Med 345: 1055–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Dielubanza EJ, Mazur DJ, Schaeffer AJ: Management of non-catheter-associated complicated urinary tract infection. Infect Dis Clin North Am 28: 121–134, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Ulett GC, Totsika M, Schaale K, Carey AJ, Sweet MJ, Schembri MA: Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr Opin Microbiol 16: 100–107, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR: Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 276: 9924–9930, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Schmid M, Prajczer S, Gruber LN, Bertocchi C, Gandini R, Pfaller W, Jennings P, Joannidis M: Uromodulin facilitates neutrophil migration across renal epithelial monolayers. Cell Physiol Biochem 26: 311–318, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Agnese DM, Calvano JE, Hahm SJ, Coyle SM, Corbett SA, Calvano SE, Lowry SF: Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis 186: 1522–1525, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Karoly E, Fekete A, Banki NF, Szebeni B, Vannay A, Szabo AJ, Tulassay T, Reusz GS: Heat shock protein 72 (HSPA1B) gene polymorphism and Toll-like receptor (TLR) 4 mutation are associated with increased risk of urinary tract infection in children. Pediatr Res 61: 371–374, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Wolf MTF, Wu X-R, Huang C-L: Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int 84: 130–137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Przeworski M, Coop G, Wall JD: The signature of positive selection on standing genetic variation. Evolution 59: 2312–2323, 2005 [PubMed] [Google Scholar]
- 63.Novembre J, Di Rienzo A: Spatial patterns of variation due to natural selection in humans. Nat Rev Genet 10: 745–755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hancock AM, Clark VJ, Qian Y, Di Rienzo A: Population genetic analysis of the uncoupling proteins supports a role for UCP3 in human cold resistance. Mol Biol Evol 28: 601–614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M, Winkler CA, Kopp J, Rotimi C, Adeyemo A, Doumatey A, Ayodo G, Alper SL, Pollak MR, Friedman DJ, Raper J: Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci U S A 111: E2130–E2139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolfe ND, Dunavan CP, Diamond J: Origins of major human infectious diseases. Nature 447: 279–283, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu H, Nigmatulina K, Eckhoff P: The scaling of contact rates with population density for the infectious disease models. Math Biosci 244: 125–134, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Mellars P, French JC: Tenfold population increase in Western Europe at the Neandertal-to-modern human transition. Science 333: 623–627, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Karlsson EK, Kwiatkowski DP, Sabeti PC: Natural selection and infectious disease in human populations. Nat Rev Genet 15: 379–393, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R 1000 Genomes Project Analysis Group : The variant call format and VCFtools. Bioinformatics 27: 2156–2158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC: PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Excoffier L, Smouse PE, Quattro JM: Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F: Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 26: 2069–2070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benazzo A, Panziera A, Bertorelle G: 4P: fast computing of population genetics statistics from large DNA polymorphism panels. Ecol Evol 5: 172–175, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voight BF, Kudaravalli S, Wen X, Pritchard JK: A map of recent positive selection in the human genome. PLoS Biol 4: e72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Bresolin N, Clerici M, Sironi M: The landscape of human genes involved in the immune response to parasitic worms. BMC Evol Biol 10: 264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Firmann M, Mayor V, Vidal PM, Bochud M, Pécoud A, Hayoz D, Paccaud F, Preisig M, Song KS, Yuan X, Danoff TM, Stirnadel HA, Waterworth D, Mooser V, Waeber G, Vollenweider P: The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord 8: 6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O: Determination of uromodulin in human urine: influence of storage and processing. Nephrol Dial Transplant 29: 136–145, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Pattaro C, De Grandi A, Vitart V, Hayward C, Franke A, Aulchenko YS, Johansson A, Wild SH, Melville SA, Isaacs A, Polasek O, Ellinghaus D, Kolcic I, Nöthlings U, Zgaga L, Zemunik T, Gnewuch C, Schreiber S, Campbell S, Hastie N, Boban M, Meitinger T, Oostra BA, Riegler P, Minelli C, Wright AF, Campbell H, van Duijn CM, Gyllensten U, Wilson JF, Krawczak M, Rudan I, Pramstaller PP EUROSPAN consortium : A meta-analysis of genome-wide data from five European isolates reveals an association of COL22A1, SYT1, and GABRR2 with serum creatinine level. BMC Med Genet 11: 41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Böger CA, Gorski M, Li M, Hoffmann MM, Huang C, Yang Q, Teumer A, Krane V, O’Seaghdha CM, Kutalik Z, Wichmann H-E, Haak T, Boes E, Coassin S, Coresh J, Kollerits B, Haun M, Paulweber B, Köttgen A, Li G, Shlipak MG, Powe N, Hwang S-J, Dehghan A, Rivadeneira F, Uitterlinden A, Hofman A, Beckmann JS, Krämer BK, Witteman J, Bochud M, Siscovick D, Rettig R, Kronenberg F, Wanner C, Thadhani RI, Heid IM, Fox CS, Kao WH CKDGen Consortium : Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD. PLoS Genet 7: e1002292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR 1000 Genomes Project Consortium : A global reference for human genetic variation. Nature 526: 68–74, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.