Abstract
An intronic variant at the complement factor H (CFH) gene on chromosome 1q32 (rs6677604) associates with risk of IgA nephropathy (IgAN), but the association signal has not been uniformly replicated in Han Chinese populations. We investigated whether the causal sequence variant resides in the CFH gene or the neighboring complement factor H–related 1 (CFHR1) gene and CFHR3, which harbor an 84-kb combined deletion (CFHR3,1Δ) in linkage disequilibrium with rs6677604. Imputation of 1000 Genomes Project data did not suggest new causal single–nucleotide variants within the CFH cluster. We next performed copy number analysis across the CFH locus in two independent Han Chinese case-control cohorts (combined n=3581). The CFHR3,1Δ and rs6677604-A alleles were rare (4.4% in patients and 7.1% in controls) and in strong linkage disequilibrium with each other (r2=0.95); of these alleles, CFHR3,1Δ associated more significantly with decreased risk of IgAN (odds ratio [OR], 0.56; 95% confidence interval [95% CI], 0.46 to 0.70; P=8.5 × 10−8 versus OR, 0.61; 95% CI, 0.50 to 0.75; P=1.6 × 10−6 for rs6677604-A). Moreover, CFHR3,1Δ explained all of the association signal at rs6677604 and remained significant after conditioning on rs6677604 genotype (P=0.01). Exploratory analyses of clinical and histopathologic parameters using the Oxford classification criteria revealed a suggestive association of CFHR3,1Δ with reduced tubulointerstitial injury (OR, 0.46; 95% CI, 0.25 to 0.79). These data indicate that dysregulated activity of the alternative complement pathway contributes to IgAN pathogenesis in both Asians and Europeans and implicate CFHR3,1Δ as the functional allele at this locus.
Keywords: genetic renal disease, IgA nephropathy, complement, human genetics
IgA nephropathy (IgAN), a common form of GN, is characterized by mesangial proliferation and mesangial IgA deposits. In two recent genome–wide association studies (GWASs),1,2 we detected a major susceptibility locus for IgAN within the complement factor H (CFH) gene cluster on chromosome 1q32. The top single–nucleotide polymorphism (SNP) in this region, rs6677604, is located in intron 12 of CFH and protective in IgAN. However, the causal allele driving the association signal at the CFH locus has not been identified. A common 84-kb deletion of the complement factor H–related 3 (CFHR3) and CFHR1 genes (CFHR3,1Δ), which is in linkage disequilibrium (LD) with rs6677604, has been considered as the likely causal allele,1–4 but this has not been formally tested by direct genotyping of CFHR3,1Δ in large case-control cohorts. Moreover, several rare copy number variants (CNVs), such as solitary deletions of CFHR1 and CFHR3 (CFHR1Δ and CFHR3Δ, respectively), have been detected in this region, but their functional role remains uncertain.5–7
The detection of CFHR3,1Δ and other CNVs is complicated by the extensive sequence homology across the CFH gene cluster on chr1q32 (called the Regulator of Complement Activation [RCA] locus), and these CNVs are, therefore, not discernible with standard DNA microarrays.8,9 Consequently, we performed multiplex ligation–dependent probe amplification (MLPA) analysis and quantitative PCR genotyping specifically targeting the CFH gene cluster to comprehensively evaluate CNVs in two large independent Han Chinese case-control cohorts (combined n=3581). The goals of this study were threefold: (1) to directly genotype CFHR3,1Δ in Han Chinese patients with IgAN and healthy controls and formally test its association with IgAN, (2) to determine whether CFHR3,1Δ and/or other rare CNVs explain the association between rs6677604 and IgAN at the CFH locus, and (3) to determine if CFHR3,1Δ is associated with clinical disease parameters and Oxford biopsy scores.
Results
To find causal variants driving the GWAS association signal at the CFH locus (rs6677604), we first searched for novel coding single–nucleotide variants (SNVs) in LD with rs6677604 in the 1000 Genomes Project data but did not identify any SNVs that could potentially account for the association. To detect potentially causal noncoding variants, we next imputed the CFH gene cluster of the Beijing cohort (which was genotyped genome wide1) using the 1000 Genomes Project reference data and performed systematic conditional analyses to detect novel, previously untyped SNVs that may explain the rs6677604 association. We detected multiple loci with significant nominal association, but conditional analysis confirmed that rs6677604 is the top SNV signal within this interval (P=1.4 × 10−5). Consultation of expression quantitative trait loci in the Genotype-Tissue Expression (GTEx) Project10 confirmed that rs6677604-A is associated with significantly lower expression of CFHR1 and CFHR3 but not CFH or any other neighboring genes (Supplemental Figure 1, Supplemental Table 1).
The above data motivated a systematic search for CNVs that are in LD with rs6677604 as potentially causal variants within the RCA locus. Therefore, we performed MLPA–based CNV analysis across the RCA interval in a total of 1929 patients with biopsy-proven IgAN and 1652 healthy controls across two independent Han Chinese cohorts (Supplemental Table 2). We detected multiple rare CNVs across this region. The most frequent CNV was the known 84-kb deletion of both CFHR3 and CFHR1 (CFHR3,1Δ), with a frequency of 7.0% in the Han Chinese controls, which is considerably lower compared with allelic frequencies observed in Europeans (22.5%–27.5%).7,11 Consistent with prior reports,5,6 we also detected additional rare CNVs involving single-gene deletion of CFHR1 or CFHR3 (frequencies of 1.9% and 0.7%, respectively) (Supplemental Figure 2, Table 1). In accordance with prior studies in Europeans,5 the rs6677604-A allele nearly perfectly tagged CFHR3,1Δ in our Asian cohorts (r2=0.95; D′=0.98) (Supplemental Table 3). Only a few individuals with the rs6677604-G allele harbored CFHR3,1Δ, and none carried single-gene deletions.
Table 1.
Allelic associations for rs6677604 and different CNVs within CFHR3 and CFHR1
| Variants | Beijing Cohort, n=2067, 1185 Patients/882 Controls | Shanghai Cohort, n=1472, 722 Patients/750 Controls | Cohorts Combined, n=3539, 1907 Patients/1632 Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MAF Patients/Controls | OR (95% CI) | P Value | MAF Patients/Controls | OR (95% CI) | P Value | MAF Patients/Controls | OR (95% CI) | P Value | |
| rs6677604 (A) | 0.040/0.072 | 0.55 (0.42 to 0.72) | 1.4 × 10−5 | 0.051/0.070 | 0.71 (0.52 to 0.96) | 0.02 | 0.044/0.071 | 0.61 (0.50 to 0.75) | 1.6 × 10−6 |
| CFHR3-CFHR1 | |||||||||
| Del-Del | 0.037/0.071 | 0.51 (0.38 to 0.67) | 2.3 × 10−6 | 0.046/0.069 | 0.65 (0.48 to 0.90) | 8.3 x 10−3 | 0.040/0.070 | 0.56 (0.46 to 0.70) | 8.5 × 10−8 |
| Wt-Del | 0.0076/0.0085 | 0.89 (0.45 to 1.78) | 0.74 (NS) | 0.012/0.011 | 1.10 (0.56 to 2.16) | 0.78 (NS) | 0.0094/0.0098 | 0.96 (0.59 to 1.56) | 0.87 (NS) |
| Del-Wt | 0.0025/0.0034 | 0.74 (0.24 to 2.31) | 0.61 (NS) | 0.006/0.004 | 1.57 (0.55 to 4.42) | 0.40 (NS) | 0.0039/0.0037 | 1.07 (0.50 to 2.29) | 0.86 (NS) |
| Wt-Dup | 0.0042/0.0034 | 1.24 (0.45 to 3.43) | 0.68 (NS) | 0.003/0.005 | 0.59 (0.17 to 2.03) | 0.40 (NS) | 0.0037/0.0040 | 0.92 (0.43 to 1.97) | 0.83 (NS) |
MAF, minor allele frequency; Del, deletion; Wt, wild type; Dup, duplication.
CFHR3,1Δ Is Strongly Protective against IgAN in Two Han Chinese Cohorts
In the Beijing cohort (1194 patients and 902 controls), CFHR3,1Δ was strongly protective against IgAN (odds ratio [OR], 0.51; 95% CI, 0.38 to 0.67; P=2.3 × 10−6), and this effect was stronger compared with its tag SNP, rs6677604 (OR, 0.55; 95%CI, 0.42 to 0.72; P=1.4 × 10−5) (Table 1). Because the Beijing cohort was previously genotyped at a genome-wide level, we phased multi-SNP haplotypes containing CFHR3Δ and CFHR1Δ and identified 10 distinct haplotypes across this interval. We next performed haplotype–based association tests (Supplemental Table 4A) using the H1 haplotype as reference, because it has equal frequency in the patients with IgAN and controls (7.4%). We identified two distinct haplotypes that carried CFHR3,1Δ: the common haplotype H2 (overall frequency =5.0%; OR, 0.54; 95% confidence interval [95% CI], 0.38 to 0.77) and the rare haplotype H3 (overall frequency =0.3%; OR, 0.14; 95% CI, 0.02 to 1.25). The combined protective OR for these two haplotypes was 0.52 (95% CI, 0.36 to 0.74). Choosing a different reference haplotype (H8) did not alter the results of this analysis (Supplemental Table 4B).
The analysis of the CNV types in the Shanghai cohort (735 patients and 750 controls) confirmed the protective effect of CFHR3,1Δ (OR, 0.65; 95%CI, 0.48 to 0.90; P=8.3 × 10−3), which was also stronger compared with rs6677604 (OR, 0.71; 95% CI, 0.52 to 0.96; P=0.02) (Table 1). In the combined Beijing and Shanghai cohorts, CFHR3,1Δ achieved near genome–wide significance (OR, 0.56; 95% CI, 0.46 to 0.70; P=8.5 × 10−8) (Table 1). This effect was significantly stronger compared with the pooled effect of the tag SNP, rs6677604 (OR, 0.61; 95% CI, 0.50 to 0.75; P=1.6 × 10−6). None of the other rare CNVs were associated with IgAN susceptibility, although our power was limited by the rarity of these alleles. Meta-analysis of the two cohorts also showed that the CFHR3,1Δ association is nearly an order of magnitude more significant than the rs6677604 association (Supplemental Table 5A).
CFHR3, 1Δ Accounts for the GWAS Association Signal at the RCA Locus
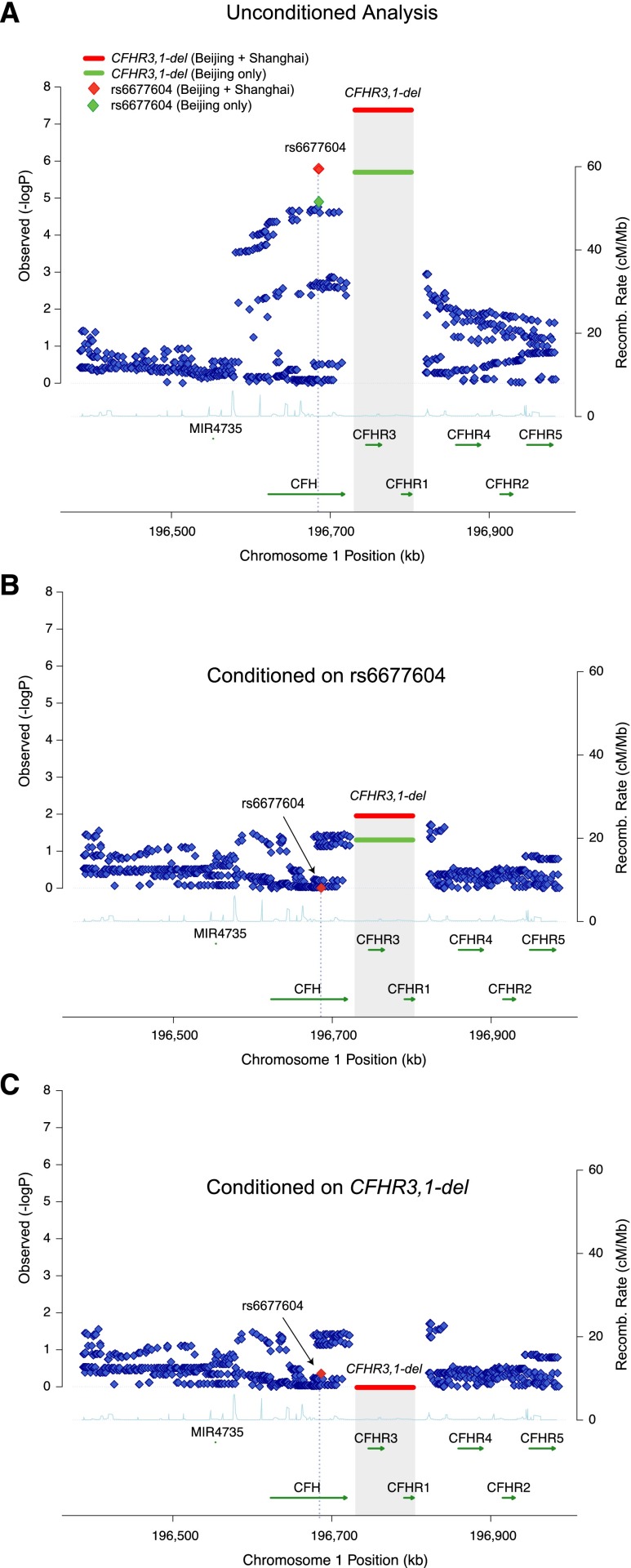
To determine if the GWAS signal at rs6677604 can be fully explained by CFHR3,1Δ, we performed conditional association analyses (Figure 1B, Table 2). When we conditioned the association of rs6677604 on CFHR3,1Δ, the protective effect of rs6677604 was no longer discernible (conditioned OR, 1.25; 95% CI, 0.69 to 2.27; P=0.46). Conversely, the estimated effect of CFHR3,1Δ remained statistically significant after controlling for rs6677604 (conditioned OR, 0.45; 95% CI, 0.25 to 0.84; P=0.01). Meta-analysis of the two cohorts also showed similar results (Supplemental Table 5B). These findings indicate that the protective effect of CFHR3,1Δ fully explains the observed effect of rs6677604 on IgAN.
Figure 1.
High–resolution regional plots for the CFH locus, including a complete set of the 1000 Genomes Project phase 3 imputed markers. (A) First–pass association analysis: CFHR3,1Δ polymorphism represents the top association in the region. (B) Conditional association results after adjusting for rs6677604 genotypes: the association of CFHR3,1Δ persists after accounting for the effect of rs6677604. (C) The reciprocal conditioning on CFHR3,1Δ completely abrogates any association of rs6677604. The y axis corresponds to the level of statistical significance (−log–transformed P values for the test of association); the shaded area represents the deletion of CFHR3 and CFHR1 genes, with the horizontal bar indicating the level of statistical significance for the MLPA-typed deletion. Green symbols represent the association results for the Beijing cohort (1185 patients and 882 controls); red symbols correspond to combined results for the Beijing and Shanghai cohorts (1907 patients and 1632 controls).
Table 2.
Conditional analysis rs6677604 and CFHR3,1Δ
| Tested Variant | Conditioning Variant | Beijing Cohort, n=2067, 1185 Patients/882 Controls | Shanghai Cohort, n=1472, 722 Patients/750 Controls | Cohorts Combined, n=3539, 1907 Patients/1632 Controls | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| rs6677604 | None | 0.55 (0.42 to 0.72) | 1.4 × 10−5 | 0.71 (0.52 to 0.96) | 0.02 | 0.61 (0.50 to 0.75) | 1.6 × 10−6 |
| rs6677604 | ΔCFHR3/CFHR1 | 1.20 (0.52 to 2.80) | 0.67 | 1.38 (0.59 to 3.21) | 0.46 | 1.25 (0.69 to 2.27) | 0.46 |
| ΔCFHR3/CFHR1 | None | 0.51 (0.38 to 0.67) | 2.3 × 10−6 | 0.65 (0.48 to 0.90) | 8.3 × 10−3 | 0.56 (0.46 to 0.70) | 8.5 × 10−8 |
| ΔCFHR3/CFHR1 | rs6677604 | 0.42 (0.18 to 1.02) | 0.05 | 0.48 (0.20 to 1.15) | 0.09 | 0.45 (0.25 to 0.84) | 0.01 |
Suggestive Association of CFHR3,1Δ with Reduced Glomerulosclerosis and Tubulointerstitial Injury by Oxford MEST Scoring
We next performed exploratory analyses of the effect of CFHR3,1Δ on clinical parameters and kidney histopathology as defined by the most recent Oxford classification system (Table 3). In total, 604 patients had biopsy slides available for Oxford scoring. In this dataset, carriers of CFHR3,1Δ had nominal associations for lower occurrence of glomerular segmental adhesions and sclerosis (S-score OR, 0.59; 95% CI, 0.36 to 0.98; P=0.04) as well as interstitial fibrosis and tubular atrophy (T-score OR, 0.46; 95% CI, 0.25 to 0.79; P=6.9 × 10−3). These associations were again more significant compared with rs6677604 (Supplemental Table 6). CFHR3,1Δ was not associated with any other clinical parameters, including eGFR, systolic BP, diastolic BP, hyperuricemia, gross hematuria, or degree of proteinuria (Supplemental Table 7). The association with the T score remains suggestive after correction for 12 independent tests (Bonferroni-corrected P=0.08).
Table 3.
Association analysis of Oxford pathology parameters with CFHR3,1Δ
| Oxford Classification, % | CFHR3,1Δ Status | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Wt/Wt, n=523 | Wt/Del or Del/Del, n=81 | |||
| M1 | 228 (43.6) | 23 (28.4) | 0.77 (0.44 to 1.31) | 0.34 |
| E1 | 265 (50.7) | 38 (46.9) | 1.23 (0.74 to 2.02) | 0.42 |
| S1 | 381 (72.8) | 50 (61.7) | 0.59 (0.36 to 0.98) | 0.04 |
| T1 and T2 | 169 (32.3) | 19 (23.5) | 0.46 (0.25 to 0.79) | 6.9 × 10−3 |
Effect size and P value was adjusted by the cohort membership. Wt, wild type; Del, deletion; M, mesangial hypercellularity; E, endocapillary hypercellularity; S, segmental glomerulosclerosis; T, tubular atrophy/interstitial fibrosis.
Discussion
The complement system plays a critical role at the interface of hemostasis and immunity. In this study, we performed fine mapping of the CFH gene cluster, which had been detected at genome-wide significance in three multiethnic genetic studies of IgAN.1–3 In those studies, the top signal rs6677604 was highly significant in European populations but was not uniformly significant in Asian cohorts.1–3 In addition, the evidence of association for rs6677604 was very modest in the most recent GWAS involving >8000 Chinese patients with IgAN (meta–analytic P value =0.001).12 Here, we show that the combined deletion of CFHR1 and CFHR3 explains the rs6677604 signal at the CFH and locus, indicating that this deletion is the likely functional allele in this interval. Furthermore, by directly genotyping this allele, we achieved near genome–wide significance in two modestly sized Han Chinese cohorts. These findings are further supported by the GTEx Project data showing that rs6677604-A is associated with significantly lower expression of CFHR1 and CFHR3 across multiple tissues, including the liver, which is a major source of complement production (Supplemental Figure 1, Supplemental Table 1). Altogether, these data indicate that the CFHR3,1Δ association was poorly detectable in East Asians–only GWASs, because this allele is rare in this population, and the functional allele was not directly genotyped.12 Our findings identify the functional variant at this interval, and by confirming the association in Han Chinese cohorts, we also show that dysregulated activity of the alternative complement pathway contributes to disease pathogenesis in both Asians and Europeans.
In IgAN, the mesangial deposits in glomeruli contain components of the alternative (C3 and Properdin) and terminal pathways (C5, C9, and membrane attack complex) in the absence of initiating elements of the classic pathway (C1q or C4), suggesting activation of the alternative pathway.13,14 Although IgA is not thought to activate complement and circulating C3 and C4 levels are within normal limits in patients with IgAN, several studies have described increased plasma levels of C3 proteolytic fragments (e.g., iC3b and C3d), which are produced through the alternative pathway.15–17 The polymeric form of IgA and the Fab fragment of immobilized human IgA may be able to activate C3 in alternative pathway–specific conditions,18–20 but the mechanisms of the alternative pathway activation remain unclear.
CFH is a major regulator of the alternative complement pathway, protecting against its excessive activation.8,21,22 The function of CFHR genes, which share significant sequence homology with CFH, is less well understood. Recent data indicate that CFHR1, CFHR2, and CFHR5 can form heterodimers via their N-terminal domains and essentially act as CFH antagonists by competing with CFH for C3b binding without exerting any intrinsic complement–regulatory activity.21,22 Hence, genetic rearrangements that generate hybrid CFHR genes result in competitive deregulation of the alternative complement pathway and inhibit CFH action, leading to GN.21–24 Conversely, in the setting of genetic inactivation of CFHR genes (e.g., CFHR3,1Δ), the unopposed action of CFH leads to more pronounced downregulation of the alternative complement pathway.21,22 Other studies have shown that variants within the CFH locus affect circulating CFH, C3, and C3a levels, again pointing to their functional relevance.4,25 Additional studies have shown that C3a and C5a, cleaved products of complement activation, induce tubulointerstitial injury via the C3a and C5a receptors.16,26 Consistent with these data, we observed a suggestive association of CFHR3,1Δ with reduced tubulointerstitial injury in IgAN—the Oxford parameter most consistently associated with progression of kidney disease in IgAN. In this study, this association does not seem to be driven by glomerular injury or proteinuria, because we did not detect any association with these parameters. Although these data are intriguing, these findings will require confirmation in larger multiethnic cohorts, with detailed analysis of parameters, such as proteinuria, glomerulosclerosis scores, and progression rates.
Genetic variation in the CFH gene clusters has been associated with susceptibility to multiple immune and infectious disorders, including age–related macular degeneration,5,27,28 atypical hemolytic uremic syndrome,29–31 C3 glomerulopathy,21–24 SLE,32 and susceptibility to meningococcal disease.33 The CFH locus is also associated with variation in C3 and C4 levels25 as well as myeloperoxidase levels.34 The interplay between CFHR3,1Δ and risk of immune-mediated diseases is complex: CFHR3,1Δ is protective against IgAN and age–related macular degeneration5 but increases susceptibility to atypical hemolytic uremic syndrome29 as well as SLE.32 Interestingly, patients with atypical hemolytic uremic syndrome homozygous for CFHR3,1Δ alleles may be susceptible to developing autoantibodies to CFH, suggesting that complete absence of CFHR1 and CFHR3 proteins may promote autoimmunity.35,36 CFHR3,1Δ frequency also varies significantly among world populations, reaching ≤40% in some African populations.2 Recently, we have shown that variation in risk allele frequencies for IgAN may represent adaptation to local pathogens.2 The variable frequency of CFHR3,1Δ together with its opposing effects on different complex diseases suggest a role for balancing selection in determining genetic predisposition to IgAN.
Future studies may clarify the exact mechanisms accounting for activation of the alternative pathway in IgAN and contrasting effects of CFHR3,1Δ on different complex traits. Careful genotype-phenotype correlation studies in larger cohorts might better define phenotypic associations with CFHR3,1Δ, and animal studies may provide novel insight into the functional effect of individual complement factors on kidney injury, ultimately leading to better therapeutic options.
Concise Methods
Study Cohorts
We studied a total of 1929 patients with biopsy–diagnosed unrelated primary IgAN and 1652 healthy unrelated controls of Han Chinese ancestry (Supplemental Table 2). Characteristics of these cohorts were previously described.1,2 Patients and controls were recruited at the Renal Division, Peking University First Hospital (Beijing cohort: 1194 patients and 902 controls) and the Department of Nephrology, Shanghai Jiaotong University Ruijin Hospital (Shanghai cohort: 735 patients and 750 controls). All patients had IgAN defined by classic light microscopy findings together with dominant and mesangial staining for IgA by immunofluorescence (at least 2+ on a semiquantitative scale from 0 to 3+). Patients with systemic diseases, such as SLE, Henoch–Schonlein purpura, and chronic liver disease, were excluded from the study. Baseline demographic and clinical data were collected from all patients at the time of renal biopsy. All biopsies were reviewed and scored by experienced pathologists and underwent standardized scoring using the recently developed Oxford MEST system.37,38 The controls were recruited among healthy blood donors from the same geographic region. In terms of demographics, 2128 (59.4%) were men, and 1453 (40.6%) were women; average age was 26 (range =11–80) years old. All study subjects provided informed consent to participate in our genetic studies, and the study was approved by the Columbia Institutional Review Board as well as local ethics committees. All subjects provided informed consent to participate in the study.
SNP Genotyping
The Beijing cohort was genotyped with the Illumina Human 610-Quad Bead Chip.1,2 The Shanghai cohort was genotyped for top loci that emerged in a more recent multiethnic GWAS of IgAN1,2 with the Sequenom MassARRAY (MALDI-TOF) and KASP Genotyping System. We implemented standard quality control measures as previously described.1,2
The 1000 Genomes Project Imputation
We performed imputation analysis on the Beijing cohort using Markov Chain Haplotyping (MACH) software, with phase 1 phased data from the 1000 Genomes Project panel for the reference panel (all Asian subpopulations) using MACH and Minimac2 softwares.39–41 We applied strict quality control filters pre- and postimputation as described previously,1,2 including r2>0.8 for all imputed markers included in the analysis. Primary association analyses of the imputed data were performed using dosage association in PLINK.
MLPA
We performed the MLPA assay by using the SALSA MLPA Kit P236-A2 ARMD (MRC-Holland, Amsterdam, The Netherlands) according to the manufacturer’s protocol. This kit contains 13 probes for the CFH gene, eight probes for CFHR3, five probes for CFHR1, and four probes for CFHR2 as well as five probes in the flanking genes KCNT2 and CFHR5. Briefly, we used 100 ng genomic DNA (20 ng/μl) per reaction. We used DNA pooled from 40 rs6677604-GG homozygotes (normal copy number of CFHR3 and CFHR1) as a negative control and DNA pooled from 40 rs6677604-AG heterozygotes (heterozygous deletion of CFHR3 and CFHR1) as a positive control. Blank, negative, and positive controls were included in each experiment. Fragment analyses were performed on an ABI Prism Model 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA). CNVs were determined according to the manufacturer’s instructions. The samples for MLPA were selected on the basis of the genotype at rs6677604. In total, using this approach, we confirmed the CFHR3,1Δ genotypes in all rs6677604-AA (n=16), all rs6677604-AG (n=376), and 207 (6.5%) of the randomly selected rs6677604-GG individuals. The CFHR3,1Δ statuses of the remaining rs6677604-GG homozygotes were determined by Taqman Quantitative Real–Time PCR (Applied Biosystems) as described below. All CNVs detected by quantitative PCR in rs6677604-GG homozygotes were confirmed by MLPA. The MLPA results underwent standard quality controls procedures according to the manufacturer’s instructions. Briefly, visual examination of the peak patterns using a raw data checklist and a peak pattern flowchart was performed to evaluate each sample. Only data that passed the peak pattern filter were used in downstream analyses. After intra- and intersample normalization, we regarded probe ratios <0.7 as a copy loss and probe ratios >1.3 as a copy gain. The CopyCaller software generated two quality metrics for each analyzed sample: a confidence estimate (confidence score) and a deviation estimate (Z score). We only accepted copy number calls with confidence scores ≥95% and |Z score| <1.75. The samples that failed these quality control requirements were repeated, at most, three times and discarded if conclusive genotypes could not be called (Supplemental Table 8).
Quantitative Real–Time PCR
Quantitative real–time PCR was carried out on genomic DNA using the Taqman Copy Number Real–Time Detection System (Applied Biosystems). Briefly, each sample of 20 ng DNA with a concentration of 5 ng/μl was plated along with a blank control (a mix of all PCR reagents without DNA), a negative control (40 pooled rs6677604-GG homozygotes), and a positive control (40 pooled rs6677604-AG samples with heterozygous deletion of both CFHR3 and CFHR1). Two sets of predesigned primers, including CFHR1 (Assay ID Hs04197581_cn; Applied Biosystems) and RNaseP (standard reference; Applied Biosystems), were used for quantitative real–time PCR. A duplex system was used, and quantitative real–time PCR was performed on the 7300 Real–Time PCR System (Applied Biosystems). CNV calls were determined using the Copy Caller Software (Applied Biosystems) with a known calibrator sample method. All CNVs detected using this method were also confirmed by MLPA as described above.
Primary Statistical Analyses
All allelic association tests for SNPs and CNVs were performed with PLINK v1.07.42 The case-control analyses as well as association tests of CFHR3,1-del with clinical and histopathologic variables were first performed individually for the Beijing and Shanghai cohorts; then, combined statistics were derived using a cohort-stratified approach. The conditional analyses of rs6677604 and CFHR3,1-del were implemented using logistic regression in PLINK v1.07. For multimarker analysis in the Beijing cohort, we first phased all haplotypes and estimated their frequencies. Because extremely rare haplotypes increase the degrees of freedom while providing little power for detecting associations, all haplotypes with frequencies <0.1% were collapsed into a single group. For the purpose of global haplotype tests, these rare haplotypes were constrained to have the same pooled OR, and this estimate was treated as a nuisance parameter in the likelihood ratio tests, so that rare haplotypes are not individually tested for association.43 For all other haplotypes, we estimated the ORs and the corresponding 95% CIs in reference to the most common haplotype that carried no putative functional alleles.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank all study participants for their contribution to this work.
This study was supported by grants R01DK082753 (to A.G.), R01DK095510 (to A.G.), and R01MD009223 (to A.G.). J.X. was supported by a fellowship from the International Society of Nephrology, Natural Science Foundation of China 81370015 and 81570598, and Shanghai Science and Technology Committee grant 14430721000. K.K. was supported by grants K23DK090207, R03DK099564, and R01DK105124 and a Carl W. Gottschalk Research Scholar grant from the American Society of Nephrology. N.C. was supported by National Basic Research Program of China 973 grant 2012CB517604.
We thank Dr. Pietro Canetta for his helpful comments.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015111210/-/DCSupplemental.
References
- 1.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG: Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46: 1187–1196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG: Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, Shi SF, Liu LJ, Yu F, Zhao MH, Novak J, Gharavi AG, Zhang H: Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol 26: 1195–1204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U: A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet 38: 1173–1177, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL: Deletion of CFHR3 and CFHR1 genes in age-related macular degeneration. Hum Mol Genet 17: 971–977, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Fritsche LG, Lauer N, Hartmann A, Stippa S, Keilhauer CN, Oppermann M, Pandey MK, Köhl J, Zipfel PF, Weber BH, Skerka C: An imbalance of human complement regulatory proteins CFHR1, CFHR3 and factor H influences risk for age-related macular degeneration (AMD). Hum Mol Genet 19: 4694–4704, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J: Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 26: 1503–1512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pouw RB, Vredevoogd DW, Kuijpers TW, Wouters D: Of mice and men: The factor H protein family and complement regulation. Mol Immunol 67: 12–20, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Consortium GT, GTEx Consortium : Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 348: 648–660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid-Kubista KE, Tosakulwong N, Wu Y, Ryu E, Hecker LA, Baratz KH, Brown WL, Edwards AO: Contribution of copy number variation in the regulation of complement activation locus to development of age-related macular degeneration. Invest Ophthalmol Vis Sci 50: 5070–5079, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Li M, Foo JN, Wang JQ, Low HQ, Tang XQ, Toh KY, Yin PR, Khor CC, Goh YF, Irwan ID, Xu RC, Andiappan AK, Bei JX, Rotzschke O, Chen MH, Cheng CY, Sun LD, Jiang GR, Wong TY, Lin HL, Aung T, Liao YH, Saw SM, Ye K, Ebstein RP, Chen QK, Shi W, Chew SH, Chen J, Zhang FR, Li SP, Xu G, Tai ES, Wang L, Chen N, Zhang XJ, Zeng YX, Zhang H, Liu ZH, Yu XQ, Liu JJ: Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun 6: 7270, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauterberg EW, Lieberknecht HM, Wingen AM, Ritz E: Complement membrane attack (MAC) in idiopathic IgA-glomerulonephritis. Kidney Int 31: 820–829, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Couser WG, Baker PJ, Adler S: Complement and the direct mediation of immune glomerular injury: A new perspective. Kidney Int 28: 879–890, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Wyatt RJ, Kanayama Y, Julian BA, Negoro N, Sugimoto S, Hudson EC, Curd JG: Complement activation in IgA nephropathy. Kidney Int 31: 1019–1023, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Zwirner J, Burg M, Schulze M, Brunkhorst R, Götze O, Koch KM, Floege J: Activated complement C3: A potentially novel predictor of progressive IgA nephropathy. Kidney Int 51: 1257–1264, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Janssen U, Bahlmann F, Köhl J, Zwirner J, Haubitz M, Floege J: Activation of the acute phase response and complement C3 in patients with IgA nephropathy. Am J Kidney Dis 35: 21–28, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hiemstra PS, Gorter A, Stuurman ME, Van Es LA, Daha MR: Activation of the alternative pathway of complement by human serum IgA. Eur J Immunol 17: 321–326, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Hiemstra PS, Biewenga J, Gorter A, Stuurman ME, Faber A, van Es LA, Daha MR: Activation of complement by human serum IgA, secretory IgA and IgA1 fragments. Mol Immunol 25: 527–533, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Janoff EN, Fasching C, Orenstein JM, Rubins JB, Opstad NL, Dalmasso AP: Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J Clin Invest 104: 1139–1147, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC, Lea SM: Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A 110: 4685–4690, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tortajada A, Yébenes H, Abarrategui-Garrido C, Anter J, García-Fernández JM, Martínez-Barricarte R, Alba-Domínguez M, Malik TH, Bedoya R, Cabrera Pérez R, López Trascasa M, Pickering MC, Harris CL, Sánchez-Corral P, Llorca O, Rodríguez de Córdoba S: C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest 123: 2434–2446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y, Voskarides K, Deltas C, Palmer A, Frémeaux-Bacchi V, de Cordoba SR, Maxwell PH, Pickering MC: Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valoti E, Alberti M, Tortajada A, Garcia-Fernandez J, Gastoldi S, Besso L, Bresin E, Remuzzi G, Rodriguez de Cordoba S, Noris M: A novel atypical hemolytic uremic syndrome-associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H-dependent complement regulation. J Am Soc Nephrol 26: 209–219, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Sun J, Gao Y, Tan A, Zhang H, Hu Y, Feng J, Qin X, Tao S, Chen Z, Kim ST, Peng T, Liao M, Lin X, Zhang Z, Tang M, Li L, Mo L, Liang Z, Shi D, Huang Z, Huang X, Liu M, Liu Q, Zhang S, Trent JM, Zheng SL, Xu J, Mo Z: Genome-wide association study for serum complement C3 and C4 levels in healthy Chinese subjects. PLoS Genet 8: e1002916, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao L, Wang Y, Haas M, Quigg RJ: Distinct roles for C3a and C5a in complement-induced tubulointerstitial injury. Kidney Int 80: 524–534, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J: Complement factor H polymorphism in age-related macular degeneration. Science 308: 385–389, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raychaudhuri S, Ripke S, Li M, Neale BM, Fagerness J, Reynolds R, Sobrin L, Swaroop A, Abecasis G, Seddon JM, Daly MJ: Associations of CFHR1-CFHR3 deletion and a CFH SNP to age-related macular degeneration are not independent. Nat Genet 42: 553–555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zipfel PF, Edey M, Heinen S, Józsi M, Richter H, Misselwitz J, Hoppe B, Routledge D, Strain L, Hughes AE, Goodship JA, Licht C, Goodship TH, Skerka C: Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet 3: e41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse HM, Schirmer S, Gropp K, Enghardt T, Wallich R, Hälbich S, Mihlan M, Schlötzer-Schrehardt U, Zipfel PF, Skerka C: Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 114: 2439–2447, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Wright AF: A rare variant in CFH directly links age-related macular degeneration with rare glomerular nephropathies. Nat Genet 43: 1176–1177, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, Kaufman KM, Langefeld CD, Williams AH, Comeau ME, Ziegler JT, Marion MC, Adler A, Glenn SB, Alarcón-Riquelme ME, Pons-Estel BA, Harley JB, Bae SC, Bang SY, Cho SK, Jacob CO, Vyse TJ, Niewold TB, Gaffney PM, Moser KL, Kimberly RP, Edberg JC, Brown EE, Alarcon GS, Petri MA, Ramsey-Goldman R, Vilá LM, Reveille JD, James JA, Gilkeson GS, Kamen DL, Freedman BI, Anaya JM, Merrill JT, Criswell LA, Scofield RH, Stevens AM, Guthridge JM, Chang DM, Song YW, Park JA, Lee EY, Boackle SA, Grossman JM, Hahn BH, Goodship TH, Cantor RM, Yu CY, Shen N, Tsao BP BIOLUPUS Network GENLES Network : Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet 7: e1002079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davila S, Wright VJ, Khor CC, Sim KS, Binder A, Breunis WB, Inwald D, Nadel S, Betts H, Carrol ED, de Groot R, Hermans PW, Hazelzet J, Emonts M, Lim CC, Kuijpers TW, Martinon-Torres F, Salas A, Zenz W, Levin M, Hibberd ML International Meningococcal Genetics Consortium : Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat Genet 42: 772–776, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Reiner AP, Hartiala J, Zeller T, Bis JC, Dupuis J, Fornage M, Baumert J, Kleber ME, Wild PS, Baldus S, Bielinski SJ, Fontes JD, Illig T, Keating BJ, Lange LA, Ojeda F, Müller-Nurasyid M, Munzel TF, Psaty BM, Rice K, Rotter JI, Schnabel RB, Tang WH, Thorand B, Erdmann J, Jacobs DR Jr., Wilson JG, Koenig W, Tracy RP, Blankenberg S, März W, Gross MD, Benjamin EJ, Hazen SL, Allayee H CARDIoGRAM Consortium : Genome-wide and gene-centric analyses of circulating myeloperoxidase levels in the charge and care consortia. Hum Mol Genet 22: 3381–3393, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Józsi M, Strobel S, Dahse HM, Liu WS, Hoyer PF, Oppermann M, Skerka C, Zipfel PF: Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood 110: 1516–1518, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Józsi M, Licht C, Strobel S, Zipfel SL, Richter H, Heinen S, Zipfel PF, Skerka C: Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 111: 1512–1514, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR: MaCH: Using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 34: 816–834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR: Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet 44: 955–959, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchsberger C, Abecasis GR, Hinds DA: minimac2: Faster genotype imputation. Bioinformatics 31: 782–784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC: PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudbridge F: Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered 66: 87–98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.