Abstract
Erythropoiesis-stimulating agents (ESAs) are commonly used to treat anemia in patients with CKD, including those receiving dialysis, although clinical trials have identified risks associated with ESA use. We evaluated the effects of changes in dialysis payment policies and product labeling instituted in 2011 on mortality and major cardiovascular events across the United States dialysis population in an open cohort study of patients on dialysis from January 1, 2005, through December 31, 2012, with Medicare as primary payer. We compared observed rates of death and major cardiovascular events in 2011 and 2012 with expected rates calculated on the basis of rates in 2005–2010, accounting for differences in patient characteristics and influenza virulence. An abrupt decline in erythropoietin dosing and hemoglobin concentration began in late 2010. Observed rates of all-cause mortality, cardiovascular mortality, and myocardial infarction in 2011 and 2012 were consistent with expected rates. During 2012, observed rates of stroke, venous thromboembolic disease (VTE), and heart failure were lower than expected (absolute deviation from trend per 100 patient-years [95% confidence interval]: −0.24 [−0.08 to −0.37] for stroke, −2.43 [−1.35 to −3.70] for VTE, and −0.77 [−0.28 to −1.27] for heart failure), although non–ESA-related changes in practice and Medicare payment penalties for rehospitalization may have confounded the results. This initial evidence suggests that action taken to mitigate risks associated with ESA use and changes in payment policy did not result in a relative increase in death or major cardiovascular events and may reflect improvements in stroke, VTE, and heart failure.
Keywords: dialysis, health policy, erythropoietin, cardiovascular events, mortality
Anemia is a common complication of advanced CKD and ESRD. In the 1970s and 1980s, ESRD was frequently accompanied by severe anemia, with hemoglobin concentrations typically <7–8 g/dl.1 With the introduction of recombinant erythropoietin (epoetin alfa) in 1989, and its rapid adoption thereafter, patients receiving dialysis were able to maintain higher hemoglobin concentrations and receive fewer blood transfusions.2
During the next two decades, with increasing use of erythropoiesis-stimulating agents (ESAs) in the dialysis population and other clinical settings, investigators attempted to determine the optimal approach to anemia correction in patients with ESRD and nondialysis-requiring CKD. While ESAs were effective in raising hemoglobin concentrations, a large randomized trial comparing hematocrit targets of 30% (partial correction) and 42% (full correction) in patients with cardiovascular disease receiving hemodialysis reported an increase in the risk of vascular access thrombosis and a trend toward higher mortality and risk of acute myocardial infarction (MI) in patients randomly assigned to the higher hematocrit target.3 Two randomized trials compared higher hemoglobin targets with lower targets in patients with nondialysis-requiring CKD treated with epoetin; one trial showed an increased risk of cardiovascular events with higher hemoglobin targets.4 Finally, a placebo-controlled trial of darbepoetin in patients with nondialysis-requiring CKD and type 2 diabetes mellitus similarly showed no effect on a composite cardiovascular endpoint but identified a nearly two-fold increase in the risks of stroke and venous thromboembolic disease (VTE) in patients randomly assigned to darbepoetin.5
In 2011 federal agencies instituted two major changes relevant to ESAs. On January 1, 2011, the Centers for Medicare & Medicaid Services (CMS) enacted an expanded capitation payment policy for dialysis and related care (the ESRD Prospective Payment System, often referred to as “bundling”). On June 24, 2011, the US Food and Drug Administration (FDA) approved modified product labels for epoetin and darbepoetin, advising against the use of ESAs in patients with ESRD and hemoglobin concentrations of 11.0 g/dl or higher.6,7 Concurrently, quality improvement programs were introduced for hospitals in the United States that were intended to reduce readmissions rates for MI, heart failure, and pneumonia and improve patient satisfaction.8–10
We undertook the following study to evaluate whether changes in epoetin alfa prescribing practices that evolved after major regulatory and payment policy changes enacted in 2011 were associated with changes in mortality and major cardiovascular events in patients receiving dialysis.
Results
Selected baseline characteristics of patients entering each annual cohort are shown in Table 1; additional baseline characteristics are outlined in Supplemental Table 3. Demographic factors were similar across all years. More recent years were characterized by longer dialysis vintage, a higher proportion of patients with diabetes mellitus, and a lower proportion of patients with GN as the primary cause of ESRD.
Table 1.
Trends in selected baseline characteristics among patients on dialysis, 2005–2012
| Characteristic | Year | |||||||
|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | |
| Patients, n | 235,883 | 238,052 | 241,437 | 248,198 | 251,805 | 263,500 | 275,527 | 285,433 |
| Dialysis modality, % | ||||||||
| In-center hemodialysis | 92.2 | 92.3 | 92.7 | 92.8 | 92.9 | 92.8 | 92.5 | 92.0 |
| Peritoneal dialysis | 7.8 | 7.7 | 7.3 | 7.2 | 7.1 | 7.2 | 7.5 | 8.0 |
| Mean age, yr | 62.2 | 62.2 | 62.2 | 62.2 | 62.1 | 62.2 | 62.3 | 62.3 |
| Mean dialysis duration, yr | 4.3 | 4.3 | 4.4 | 4.5 | 4.6 | 4.7 | 4.8 | 4.9 |
| Male sex, % | 53.3 | 53.6 | 53.8 | 54.1 | 54.2 | 54.2 | 54.3 | 54.3 |
| Race, % | ||||||||
| White | 43.2 | 42.4 | 42.1 | 41.7 | 41.2 | 40.8 | 40.6 | 40.2 |
| Black | 38.2 | 38.7 | 38.7 | 38.6 | 38.6 | 38.8 | 38.8 | 38.6 |
| Other | 18.6 | 18.9 | 19.2 | 19.7 | 20.2 | 20.4 | 20.7 | 21.2 |
| ESRD cause, % | ||||||||
| Diabetes | 43.1 | 43.4 | 43.7 | 43.9 | 44.1 | 44.3 | 44.3 | 44.4 |
| Hypertension | 29.2 | 29.2 | 29.1 | 29.1 | 29.0 | 29.2 | 29.4 | 29.5 |
| GN | 11.7 | 11.3 | 11.0 | 10.7 | 10.4 | 10.1 | 9.8 | 9.7 |
| Other | 16.0 | 16.1 | 16.3 | 16.4 | 16.4 | 16.4 | 16.5 | 16.4 |
| Mean BMI, kg/m2 | 28.0 | 28.3 | 28.6 | 28.9 | 29.1 | 29.4 | 29.6 | 29.7 |
| Atrial fibrillation, % | 7.4 | 7.8 | 7.9 | 7.4 | 6.9 | 7.1 | 7.4 | 7.5 |
| CAD/atherosclerosis, % | 21.6 | 20.9 | 20.0 | 20.0 | 19.5 | 19.0 | 18.3 | 17.4 |
| Cancer, % | 2.3 | 2.2 | 2.2 | 2.2 | 2.3 | 2.2 | 2.2 | 2.2 |
| CHF, % | 21.1 | 21.2 | 21.0 | 20.1 | 19.2 | 19.1 | 19.1 | 18.9 |
| COPD, % | 9.4 | 9.7 | 9.8 | 9.0 | 8.1 | 8.2 | 8.1 | 7.9 |
| Cardiovascular disease, % | 45.6 | 45.5 | 45.0 | 44.5 | 43.7 | 43.5 | 43.0 | 42.1 |
| Diabetes, % | 25.3 | 24.9 | 24.2 | 24.3 | 23.8 | 23.9 | 23.6 | 23.1 |
| GI bleeding, % | 5.2 | 5.0 | 4.8 | 4.7 | 4.5 | 4.5 | 4.6 | 4.6 |
| HTN, % | 42.4 | 42.5 | 42.2 | 42.0 | 41.3 | 41.2 | 40.8 | 40.0 |
| LV hypertrophy, % | 5.7 | 5.4 | 5.4 | 5.0 | 4.8 | 4.6 | 4.2 | 4.0 |
| MI/acute coronary syndrome, % | 5.7 | 5.4 | 5.2 | 5.2 | 5.0 | 5.0 | 4.8 | 4.8 |
| Other cardiac disease, % | 16.1 | 15.7 | 15.4 | 14.6 | 13.9 | 13.4 | 12.9 | 12.5 |
| Pulmonary HTN, % | 1.4 | 1.4 | 1.4 | 1.8 | 2.0 | 2.2 | 2.4 | 2.7 |
| PVD, % | 14.1 | 14.0 | 13.5 | 13.2 | 13.0 | 12.7 | 12.1 | 11.2 |
| Stroke, % | 6.8 | 6.7 | 6.5 | 6.8 | 6.8 | 6.7 | 6.4 | 6.2 |
| TIA, % | 1.6 | 1.6 | 1.5 | 1.5 | 1.5 | 1.5 | 1.4 | 1.4 |
| Valvular disease, % | 9.6 | 9.7 | 9.8 | 9.0 | 8.2 | 7.8 | 7.7 | 7.5 |
Clinical characteristics were defined based on claims obtained from the inpatient setting during the six months preceding the index date. BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; HTN, hypertension; LV, left ventricular; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Changes in Hemoglobin Concentration and ESA Dosing
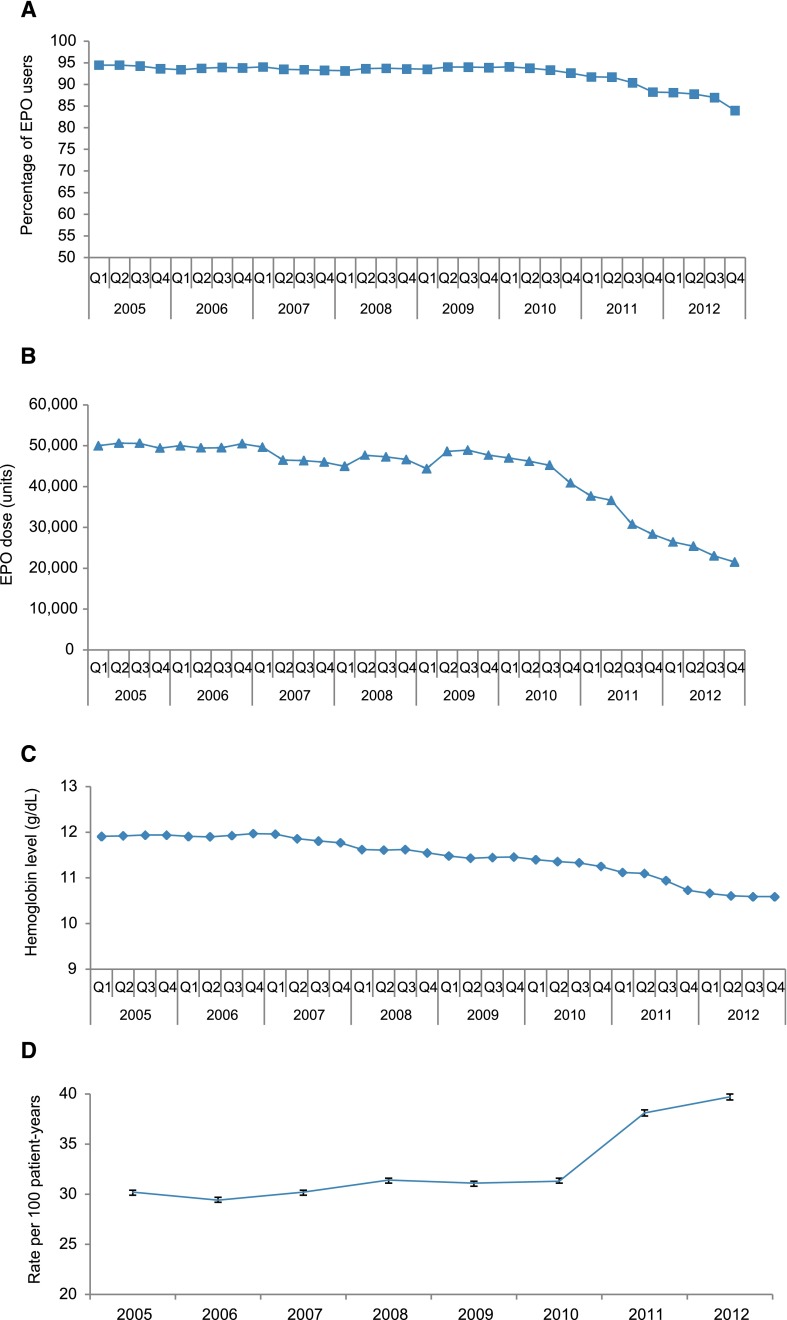
Figure 1, A–D, shows trends in the proportion of patients receiving epoetin alfa, median epoetin alfa dose, mean hemoglobin concentration, and rates of blood transfusion.
Figure 1.
Reduced proportion of patients receiving epoetin alfa (EPO), reduced EPO doses, reduced hemoglobin concentrations just preceding and after regulatory changes, and subsequent increase in use of transfusion are shown. Trends in percentage of epoetin alfa (EPO) users (A), median EPO dose per 30 outpatient days (B), median hemoglobin levels (C), and transfusion rates (95% CI) (D) among patients on dialysis, 2005–2012.
All-Cause and Cardiovascular Mortality
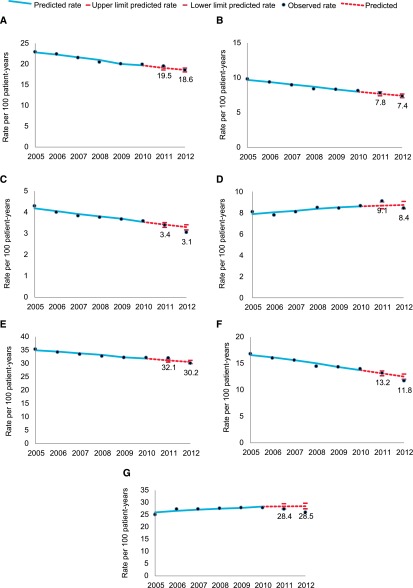
Figure 2, A and B, shows projected and observed all-cause and cardiovascular mortality. Observed all-cause and cardiovascular mortality were within the predicted range during 2011 and 2012 (Supplemental Table 4).
Figure 2.
No change in expected rates of all-cause or cardiovascular death, myocardial infarction or a composite endpoint (death or MI or stroke), and lower than expected rates of stroke, VTE and heart failure are shown. Trends in the observed and predicted rates of all-cause death (A), cardiovascular death (B), stroke (C), MI (D), the composite endpoint (E), heart failure (F), and VTE (G) among patients on dialysis, 2005–2012. Composite endpoint describes rates of all-cause death, stroke, and MI.
Major Cardiovascular Events
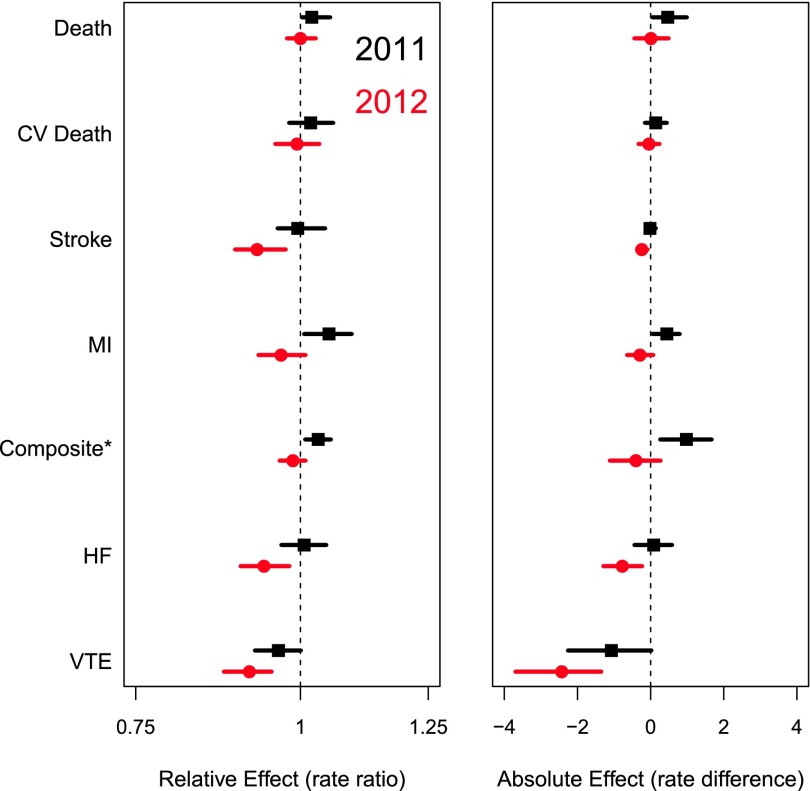
Figure 2, C–G, shows projected and observed rates of stroke, MI, the composite endpoint, heart failure, and VTE, respectively. Observed rates were within the predicted range during 2011 for all outcomes. During 2012, observed rates of stroke (absolute deviation from trend, −0.24 per 100 patient-years; 95% CI, −0.08 to −0.37), VTE (−2.43; 95% CI, −1.35 to −3.70), and heart failure (−0.77; 95% CI, −0.28 to −1.27) were lower than expected. Figure 3 shows a Forest plot of absolute and relative differences in observed versus expected event rates in 2011 and 2012.
Figure 3.
Rate ratio (RR) and absolute rate differences and their associated 95% confidence intervals for all outcomes for the individual years 2011 and 2012, comparing observed versus expected are shown. Relative effect (rate ratio) and absolute effect (rate difference) of observed to expected rates of all-cause death, cardiovascular (CV) death, stroke, MI, the composite endpoint, heart failure (HF), and VTE in 2011 and 2012. Expected rates are based on 2005–2010 data. *Composite endpoint describes rates of all-cause death, stroke, and MI.
Changes in Event Rates in the General Medicare Population
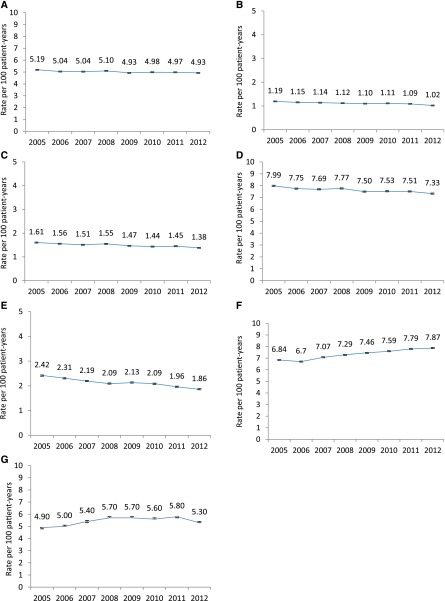
Baseline characteristics of patients entering each annual non-ESRD Medicare cohort are shown in Supplemental Table 5. To examine secular trends in the non-ESRD Medicare population, Figure 4, A–G, shows corresponding rates of all-cause mortality, stroke, MI, the composite endpoint, heart failure, VTE, and transfusion in a 5% sample of Medicare beneficiaries. A direct comparison of the rates of stroke and MI over time in the dialysis and non-ESRD Medicare populations is presented in Supplemental Figure 1, which shows similar trends as noted in the dialysis population.
Figure 4.
Similar trends in each of the outcomes in the general population with the exception of VTE, raising the possibility that trends observed in the dialysis population were related to secular trends across the entire population rather than changes related to epoetin alfa use are shown. Trends in rates (95% CI) of all-cause death (A), stroke (B), MI (C), the composite endpoint (D), heart failure (E), VTE (F), and transfusion (G) in the non-ESRD Medicare population, 2005–2012. Composite endpoint describes rates of all-cause death, stroke, and MI.
Discussion
The current analysis of approximately 250,000 United States patients receiving maintenance dialysis in each calendar year between 2005 and 2012 highlights the secular trends in declining hemoglobin concentration in this population and the accelerated decline in epoetin alfa dosing that began in the latter part of 2010 and continued after expanded capitation of ESRD services was introduced in January 2011. The new bundled payment included provision of ESAs, intravenous (IV) or oral iron formulations, and IV or oral calcitriol and active vitamin D analogues. Capitated payment changed the incentives for ESRD-related medication use and was likely to have contributed to the decline in ESA dosing and hemoglobin concentration and the associated increase in blood transfusions. The modified ESA product labels, released in June 2011, replaced the conventional hemoglobin target of 10–12 g/dl with recommendations to use ESAs when the hemoglobin concentration falls <10 g/dl and to reduce or interrupt dosing as the hemoglobin approaches or exceeds 11 g/dl. These revisions were implemented to reduce the potential risk of adverse cardiovascular events, variably identified in randomized controlled trials that targeted hemoglobin concentrations of ≥13 g/dl. After accounting for declining secular trends in mortality and hospitalization events since 2005,15 we have shown that rates of all-cause and cause-specific mortality declined as expected on the basis of secular trends, although rates of stroke, VTE, and heart failure were lower than expected in 2012.
To accurately predict the risks of these events in calendar years 2011 and 2012, we needed to account for differences in the clinical profile of the dialysis population as well as the effect of seasonal diseases, particularly influenza virulence. Our findings indicate that changes in CMS payment policy and ESA labeling contributed to clinically meaningful declines in the proportion of patients receiving epoetin alfa (approximately 10%), epoetin alfa dose (approximately 40%), and hemoglobin concentration (approximately 1 g/dl), and a corresponding increase in blood transfusion (approximately 30%) during 2011 and 2012. These changes in practice were not associated with changes in all-cause or cardiovascular mortality beyond what was expected due to secular trends, consistent with results of previous studies on the correction of anemia with ESAs and mortality.16–22
Our findings are consistent with selected results from randomized clinical trials. An increased risk of VTE was seen in patients randomly assigned to the higher hematocrit target in the Normal Hematocrit Trial, and an increased risk of stroke (and VTE) was seen in patients randomly assigned to darbepoetin (versus placebo) in the Trial to Reduce Cardiovascular Events With Aranesp Therapy. Beginning in 2011, a larger proportion of patients receiving dialysis were untreated with ESAs than before 2011, potentially reducing risk. While a trend toward more heart failure events was observed in patients randomly assigned to the higher hemoglobin target in the Correction of Hemoglobin and Outcomes in Renal Insufficiency trial, diagnostic criteria for heart failure in ESRD are inconsistently applied; hence, we have less confidence in results herein pertaining to heart failure than to those pertaining to stroke or VTE.
There are several strengths to our approach. Given the near-complete capture in Medicare data of patients receiving dialysis in the United States, our results should be generalizable to a large proportion of the dialysis population. We included patients on peritoneal dialysis as well as hemodialysis. We used methods to ensure completeness of all Medicare services, particularly at the end of each calendar year, by appropriately attributing all services that were received in one calendar year but billed in the following calendar year. This additional step ensured that our results are not subject to data reporting delays that would effectively reduce event rates at the end of each calendar year.
This study also had several limitations. Our analytic approach relies on the assumption that the extrapolated secular trend provides an accurate representation of what would have happened in the absence of policy change. This assumption would be violated if there were material changes between the pre- and postpolicy periods in the following: (1) the clinical profile of patients, (2) medical coding, (3) the capture of outcome events, and (4) treatment practices or hospitalization polices. On the basis of available data, population characteristics were similar throughout the study period, thereby reducing the potential for meaningful bias by changes in underlying disease severity. In 2010, the number of diagnosis fields on Medicare claims increased from ten to 25, which could have affected our findings, although sensitivity analyses varying the sensitivity and specificity of our outcome definitions did not materially change results. To minimize differences in hospital admission and disposition practices over time, we developed outcome definitions that specifically incorporated visits to the emergency department or short-term observation stays for patients with heart failure to minimize potential under-reporting of events.
We were unable to evaluate changes in the use of oral medications because these are captured only in Medicare Part D claims. Restricting the study sample to patients with Part D coverage would have sharply reduced the size and generalizability of the study sample. Moreover, investigators from the Dialysis Outcomes and Practice Patterns Study have suggested that medication treatment patterns for cardiovascular disease in ESRD have not changed materially during this period.23 Medicare implemented hospital payment policy changes in 2010, 2011, and 2012 that were part of the Affordable Care Act quality improvement initiative, including financial penalties for hospitals with high rehospitalization rates; these changes could have altered hospitalization (and rehospitalization) practices for selected cardiovascular events, including heart failure.8,10 Changes in guidelines for the prevention and treatment of stroke and VTE published in 20118,10 could have affected our results. Indeed, rates of stroke and heart failure were also lower in 2012 than in previous years in the general Medicare population, lessening our confidence that changes in ESA prescribing practices were causally related to the observed results.
Finally, during the same time frame, providers undertook extensive efforts to reduce the use of catheters for hemodialysis access. It is possible that lower rates of catheter use (and thereby higher rates of use of arteriovenous fistulas and grafts) may have contributed to lower rates of some adverse events among patients undergoing hemodialysis. We elected not to adjust for the use of IV iron or active vitamin D analogues, other medications included in the bundled payment. After payment reform, there was a shift away from IV and toward oral vitamin D use,23,24 and US Renal Data System Standard Analysis Files contained no data fields for oral drugs, precluding adequate adjustment. Appropriately characterizing and controlling for IV iron administration would have been problematic given the difficulty of identifying in administrative data different patterns of use (e.g., bolus versus maintenance dosing)25 and the relatively low use in patients receiving peritoneal dialysis.26
The natural experiment design provides a unique opportunity to evaluate the effect of policy changes on relatively low-frequency events (such as stroke) accounting for secular changes. However, it is impossible to rule out the potential for residual error resulting from other secular (non–ESA-related) changes in clinical practice not captured through the existing data. In the case of stroke, these could include increased use of percutaneous cerebrovascular intervention, anticoagulants, or antiplatelet agents. Because we restricted our population to patients receiving maintenance dialysis for at least 9 months, we underestimate rates of major cardiovascular events during the first year of dialysis, a period of exceptionally high risk.27–31 Finally, because of the lag in processing of Medicare claims, no more than 1.5 years of data following the policy changes were available for this study.
In conclusion, we evaluated changes in capitated payment for dialysis and related services and ESA labeling instituted in the United States during 2011 to assess the extent to which these changes were associated with changes in mortality and cardiovascular events beyond expected secular trends. Observed rates of all-cause and cardiovascular mortality were not different from those expected. Observed rates of stroke, VTE, and heart failure were lower than expected, although non–ESA-related changes in practice and Medicare payment penalties for rehospitalization may have confounded the results. This study provides initial evidence suggesting that the FDA action aimed at mitigating risks associated with ESA use and changes in payment policy instituted by CMS did not result in an unintended relative increase in death or any major cardiovascular events and may reflect improvements in stroke, VTE, and heart failure.
CONCISE METHODS
Data Sources
We used data from Medicare final action claims for patients receiving dialysis during 2001–2012 and general Medicare 5% sample data covering Part A institutional claims (inpatient, outpatient, skilled nursing facility, hospice, or home health agency) and noninstitutional Part B physician/supplier claims. We obtained patient demographic characteristics and cause of ESRD from the ESRD Medical Evidence Report (form CMS 2728) and mortality data (all-cause and cause-specific) from the ESRD Death Notification form (CMS 2746).
Study Population and Design
We conducted an open cohort study of patients on dialysis with Medicare as primary payer during the study period January 1, 2005, through December 31, 2012. We included patients on a rolling basis, with their start of follow-up being January 1, 2005, or the first day of the calendar month subsequent to meeting the inclusion criteria (the “index date”). Patients in each calendar year between 2005 and 2012 contributed person-time to the calendar year(s) during which they met eligibility criteria. General inclusion criteria were age ≥18 years, receipt of in-center hemodialysis or peritoneal dialysis for ≥9 months, and Medicare as primary payer for ≥6 months.
Baseline and Follow-up
We defined the baseline period as the time from the index date back to the earliest time Medicare claims data were available or January 1, 2001. Thus, each patient had a minimum baseline period of 6 months, and the period length varied among patients. This design allowed utilization of all available covariate information when defining comorbidity from claims.11 To account for the time-varying effects of comorbidities, we included a variable to indicate the presence or absence of a comorbid condition, as well as a variable denoting the time between the latest ascertainment of that condition and the beginning of follow-up (<6 months versus ≥6 months).12 We also included a variable to indicate the source of the comorbidity claim (inpatient versus outpatient). We assessed covariates at the index date and updated covariates on January 1 of each calendar year.
Follow-up began on the index date and continued until the first occurrence of the following censoring events: (1) death; (2) kidney transplantation; (3) modality (hemodialysis or peritoneal dialysis) switch, if the switch persisted for ≥60 days; (4) loss of Medicare as primary payer; or (5) darbepoetin or peginesatide administration. We censored all remaining eligible patients on December 31, 2012.
Exposure, Covariates, and Outcomes
The primary exposure of interest was calendar year. At baseline, we assessed demographic characteristics (age, sex, race, ethnicity), primary cause of ESRD, vintage (time since initiation of dialysis), body mass index, and comorbid conditions (listed in Supplemental Table 1). For each calendar year from 2005 through 2012, we determined quarterly epoetin alfa use (percentage) and dose, as well as monthly hemoglobin concentrations. We defined influenza virulence by tracking outpatient visits for influenza-like illness in the general population using data from the US Centers for Disease Control and Prevention. We considered the following outcomes: all-cause mortality, cardiovascular mortality, stroke, MI, a composite endpoint (death or stroke or MI), heart failure, VTE, and red blood cell transfusion; we used validated algorithms for defining these events in claims data (Supplemental Table 2).13,14
Statistical Analyses
We estimated rates of all-cause mortality, cardiovascular mortality, stroke, MI, and the composite endpoint during each calendar year (2005–2012) in the dialysis and non-ESRD Medicare populations. We also estimated rates of heart failure and VTE in the dialysis population. To account for secular trends in the outcomes of interest, we used mixed-effects Poisson regression models with year as a random effect to fit a log linear trend line between 2005 and 2010. We adjusted models for a wide array of demographic and clinical factors, including multiple comorbid conditions and outpatient visits for influenza-like illness. We applied the resulting models to patients in 2011 and 2012 separately to calculate patient-level predicted event rates. We used bootstrapping with 600 iterations to calculate 95% confidence intervals (95% CIs) around predicted rates, as well as the absolute and relative differences between observed and predicted rates for 2011 and 2012. We applied this general approach for each outcome. Additional details of the analytical methods are presented in the Supplemental Material. We used SAS 9.2 or above (Cary, NC) for all analyses.
Disclosures
Several of the authors report receiving research grants from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Chertow serves on the Board of Directors of Satellite Healthcare, has served as a scientific advisor to Akebia, Amgen, Inc., Keryx, and Vifor, and has received research support from Amgen, Inc. Drs. Monda, Beaubrun, Pollock, and Bradbury work at Amgen, Inc. and hold Amgen, Inc. stock. Dr. Ashfaq worked at Amgen, Inc. at the time of manuscript submission and is currently employed at AstraZeneca. Dr. Gilbertson has provided consultation to Amgen, Inc., DaVita Clinical Research, and Affymax. Drs. Gilbertson, Collins, Herzog, and Liu work at the Chronic Disease Research Group, which has received research support from Amgen, Inc. Dr. Collins has provided consultation to NxStage, AstraZeneca, Relypsa, and Amgen. Dr. Herzog has served as a scientific consultant for Abbvie, Affymax, Amgen, Inc., BMS, Fibrogen, GSK, Keryx, Matinas Bio Pharma, Medtronic, Relypsa, ZS Pharma, and ClearView Healthcare Partners, and owns stock in Boston Scientific, Johnson & Johnson, General Electric, and Merck. Dr. Brookhart has received research support from Amgen, Inc. and AstraZeneca, has served as a scientific advisor for Amgen, Inc., GlaxoSmithKline (GSK), and Merck (honoraria/payment received by the institution), and has received consulting fees from RxAnte, Inc. and World Health Information Consultants. Dr. Stürmer receives salary support as Director of the Comparative Effectiveness and Research (CER) Strategic Initiative, North Carolina Translational and Clinical Sciences (NC TraCS) Institute, and as Director of the Center for Pharmacoepidemiology (current members: GSK, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, Inc., AstraZeneca) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer owns stock in Novartis, Roche, BASF, AstraZeneca, Johnson & Johnson, and Novo Nordisk. Dr. Winkelmayer reports having received honoraria for having served on scientific advisory, event adjudication, or data safety monitoring boards for Akebia, Amgen, Inc., AstraZeneca, Bayer, Medtronic, Relypsa, and Zoll. Dr. Rothman is a full time employee of the Research Triangle Institute, an independent non-profit research organization that does work for government agencies and pharmaceutical companies.
Supplementary Material
Acknowledgments
We applied to and received approval from the Human Subjects Research Committee of the Hennepin County Medical Center/Hennepin Healthcare System, Inc. Financial support was provided by Amgen, Inc.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “ESRD Payment Reform: First Do No Harm,” on pages 2924–2926.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015111232/-/DCSupplemental.
References
- 1.Churchill DN, Taylor DW, Cook RJ, LaPlante P, Barre P, Cartier P, Fay WP, Goldstein MB, Jindal K, Mandin H, McKenzie JK, Muirhead N, Parfrey PS, Posen GA, Slaughter D, Ulan RA, Werb R: Canadian Hemodialysis Morbidity Study. Am J Kidney Dis 19: 214–234, 1992 [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 3.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Amgen Inc.: Prescribing information for Epogen (epoetin alfa). Approved May 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103234s5281lbl.pdf. Accessed September 22, 2015
- 7.Amgen Inc.: Prescribing information for Aranesp (darbepoetin alfa). Revised March 2015. Available at http://pi.amgen.com/united_states/aranesp/ckd/aranesp_pi_hcp_english.pdf. Accessed September 22, 2015
- 8.Joynt KE, Jha AK: A path forward on Medicare readmissions. N Engl J Med 368: 1175–1177, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Medicare Program; Proposed Changes to the Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Proposed Fiscal Year 2011 Rates; Effective Date of Provider Agreements and Supplier Approvals; and Hospital Conditions of Participation for Rehabilitation and Respiratory Care Services Medicaid Program: Accreditation Requirements for Providers of Inpatient Psychiatric Services for Individuals Under Age 21. Federal Register. 2010. Available at https://www.federalregister.gov/articles/2010/05/04/2010-9163/medicare-program-proposed-changes-to-the-hospital-inpatient-prospective-payment-systems-for-acute. Accessed September 22, 2015
- 10.Medicare Program; Hospital Inpatient Value-Based Purchasing Program. Federal Register. 2011. Available at https://www.federalregister.gov/articles/2011/05/06/2011-10568/medicare-program-hospital-inpatient-value-based-purchasing-program. Accessed September 22, 2015 [PubMed]
- 11.Brunelli SM, Gagne JJ, Huybrechts KF, Wang SV, Patrick AR, Rothman KJ, Seeger JD: Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidemiol Drug Saf 22: 542–550, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbertson DT, Bradbury BD, Wetmore JB, Weinhandl ED, Monda KL, Liu J, Brookhart MA, Gustafson SK, Roberts T, Collins AJ, Rothman KJ: Controlling confounding of treatment effects in administrative data in the presence of time-varying baseline confounders [published online ahead of print November 26, 2015]. Pharmacoepidemiol Drug Saf doi: 10.1002/pds.3922 [DOI] [PubMed] [Google Scholar]
- 13.Tirschwell DL, Longstreth WT Jr: Validating administrative data in stroke research. Stroke 33: 2465–2470, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH: Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: Estimating positive predictive value on the basis of review of hospital records. Am Heart J 148: 99–104, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Saran R, Li Y, Robinson B: USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 16.Bradbury BD, Wang O, Critchlow CW, Rothman KJ, Heagerty P, Keen M, Acquavella JF: Exploring relative mortality and epoetin alfa dose among hemodialysis patients. Am J Kidney Dis 51: 62–70, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bradbury BD, Danese MD, Gleeson M, Critchlow CW: Effect of Epoetin alfa dose changes on hemoglobin and mortality in hemodialysis patients with hemoglobin levels persistently below 11 g/dL. Clin J Am Soc Nephrol 4: 630–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Thamer M, Cotter D, Kaufman J, Hernán MA: Estimated effect of epoetin dosage on survival among elderly hemodialysis patients in the United States. Clin J Am Soc Nephrol 4: 638–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradbury BD, Do TP, Winkelmayer WC, Critchlow CW, Brookhart MA: Greater Epoetin alfa (EPO) doses and short-term mortality risk among hemodialysis patients with hemoglobin levels less than 11 g/dL. Pharmacoepidemiol Drug Saf 18: 932–940, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Wang O, Kilpatrick RD, Critchlow CW, Ling X, Bradbury BD, Gilbertson DT, Collins AJ, Rothman KJ, Acquavella JF: Relationship between epoetin alfa dose and mortality: findings from a marginal structural model. Clin J Am Soc Nephrol 5: 182–188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brookhart MA, Schneeweiss S, Avorn J, Bradbury BD, Liu J, Winkelmayer WC: Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA 303: 857–864, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Weinhandl ED, Gilbertson DT, Collins AJ: Association of mean weekly epoetin alfa dose with mortality risk in a retrospective cohort study of Medicare hemodialysis patients. Am J Nephrol 34: 298–308, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Dialysis Outcomes and Practice Patterns Study Program: DOPPS Practice Monitor. August 2015. Available at http://www.dopps.org/DPM. Accessed September 22, 2015
- 24.Brunelli SM, Monda KL, Burkart JM, Gitlin M, Neumann PJ, Park GS, Symonian-Silver M, Yue S, Bradbury BD, Rubin RJ: Early trends from the Study to Evaluate the Prospective Payment System Impact on Small Dialysis Organizations (STEPPS). Am J Kidney Dis 61: 947–956, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV: Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 24: 1151–1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Renal Data System : USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 27.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, Kerr PG, Morgenstern H, Li Y, Pisoni RL, Saran R, Tentori F, Akizawa T, Fukuhara S, Port FK: Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 82: 570–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soucie JM, McClellan WM: Early death in dialysis patients: Risk factors and impact on incidence and mortality rates. J Am Soc Nephrol 7: 2169–2175, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Foley RN, Collins AJ: End-stage renal disease in the United States: An update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.