Abstract
The association between proton pump inhibitors (PPI) use and risk of acute interstitial nephritis has been described. However, whether exposure to PPI associates with incident CKD, CKD progression, or ESRD is not known. We used Department of Veterans Affairs national databases to build a primary cohort of new users of PPI (n=173,321) and new users of histamine H2-receptor antagonists (H2 blockers; n=20,270) and followed these patients over 5 years to ascertain renal outcomes. In adjusted Cox survival models, the PPI group, compared with the H2 blockers group, had an increased risk of incident eGFR<60 ml/min per 1.73 m2 and of incident CKD (hazard ratio [HR], 1.22; 95% confidence interval [95% CI], 1.18 to 1.26; and HR, 1.28; 95% CI, 1.23 to 1.34, respectively). Patients treated with PPI also had a significantly elevated risk of doubling of serum creatinine level (HR, 1.53; 95% CI, 1.42 to 1.65), of eGFR decline >30% (HR, 1.32; 95% CI, 1.28 to 1.37), and of ESRD (HR, 1.96; 95% CI, 1.21 to 3.18). Furthermore, we detected a graded association between duration of PPI exposure and risk of renal outcomes among those exposed to PPI for 31–90, 91–180, 181–360, and 361–720 days compared with those exposed for ≤30 days. Examination of risk of renal outcomes in 1:1 propensity score-matched cohorts of patients taking H2 blockers versus patients taking PPI and patients taking PPI versus controls yielded consistent results. Our results suggest that PPI exposure associates with increased risk of incident CKD, CKD progression, and ESRD.
Keywords: ESRD, chronic kidney disease, renal progression, progression of chronic renal failure, Epidemiology and outcomes, clinical epidemiology
Numerous prior observations have suggested a relationship between exposure to proton pump inhibitors (PPI) and acute kidney injury and acute interstitial nephritis. Antoniou et al. conducted a population-based study involving Ontario residents aged 66 years and older who initiated PPI therapy and found an increased risk of both acute kidney injury and acute interstitial nephritis.1 Klepser et al. built a nested case-control study using claims data from a private insurer in a single Midwestern state and also found a significant association between PPI use and acute kidney injury.2 Blank et al. conducted a nested case-control study using routinely collected national health and drug dispensing data in New Zealand and found that current use of PPI was associated with increased risk of acute interstitial nephritis relative to past use.3 Data from adverse event reporting systems suggest that PPI is a common cause of drug-induced acute interstitial nephritis.4 While most patients recover kidney function, some may not fully recover and might develop CKD and progress to ESRD.5,6
While the association between PPI exposure and acute kidney disease has been well documented, it is unclear whether exposure to PPI is associated with an increased risk of incident CKD and progression to ESRD.4,7 In this report, we used national United States Department of Veterans Affairs (VA) databases to build a primary cohort of new users of PPI and new users of Histamine H2-receptor antagonists (H2 blockers), and additional cohorts for sensitivity analyses, including a 1:1 propensity score-matched cohort of PPI and H2 blockers, a 1:1 propensity score-matched cohort of PPI, and a control group, and examined the association between PPI exposure and risk of incident CKD, CKD progression, and ESRD among United States veterans without kidney disease at baseline (baseline eGFR>60 ml/min per 1.73 m2).
Results
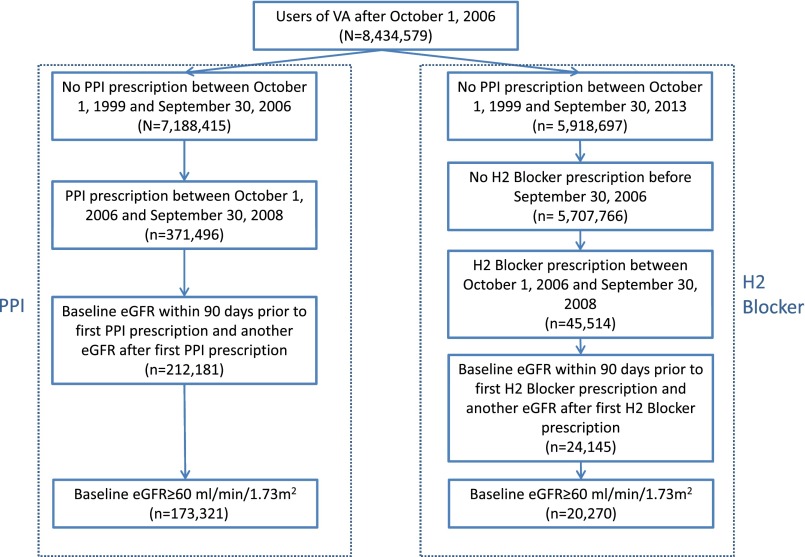
There were 20,270 and 173,321 participants in the H2 blockers, and PPI groups, respectively (Figure 1). The demographic and health characteristics of the two groups are described in Table 1.
Figure 1.
Flow diagram of cohort assembly of primary cohort of new users of PPI (n=173,321) and new users of H2 blockers (20,270).
Table 1.
Baseline characteristics of a cohort of new users of H2 blockers, and new users of PPI
| Baseline Characteristics | H2 Blockers (n=20,270) | PPI (n=173,321) | P Value | |
|---|---|---|---|---|
| Age (SD) | 55.40 (12.81) | 56.85 (11.85) | P<0.001 | |
| Baseline eGFR in ml/min per 1.73 m2 (SD) | 86.98 (15.88) | 86.56 (15.67) | P<0.001 | |
| Race | White (%) | 15,937 (78.62) | 137,174 (79.14) | P=0.01 |
| Black (%) | 3,784 (18.67) | 32,018 (18.47) | ||
| Other (%) | 549 (2.71) | 4,129 (2.38) | ||
| Sex | Male (%) | 18,929 (93.38) | 161,259 (93.04) | P=0.07 |
| Female (%) | 1,341 (6.62) | 12,062 (6.96) | ||
| Diabetes mellitus (%) | 8,923 (44.02) | 72,309 (41.72) | P<0.001 | |
| Hypertension (%) | 15,814 (78.02) | 136,782 (78.92) | P<0.01 | |
| Chronic lung disease (%) | 7,951 (39.23) | 66,955 (38.63) | P=0.10 | |
| Peripheral artery disease (%) | 5,009 (24.71) | 31,311 (18.07) | P<0.001 | |
| Cardiovascular disease (%) | 8,459 (41.73) | 71,807 (41.43) | P=0.41 | |
| Cerebrovascular disease (%) | 4,596 (22.67) | 26,457 (15.26) | P<0.001 | |
| Dementia (%) | 5,058 (24.95) | 32,380 (18.68) | P<0.001 | |
| Hyperlipidemia (%) | 14,785 (72.94) | 127,463 (73.54) | P=0.07 | |
| Hepatitis C (%) | 1,198 (5.91) | 14,892 (8.59) | P<0.001 | |
| HIV (%) | 55 (0.27) | 678 (0.39) | P<0.01 | |
| Gastroesophageal reflux disease (%) | 3,767 (18.58) | 86,804 (50.08) | P<0.001 | |
| Upper gastrointestinal tract bleeding (%) | 246 (1.21) | 7,898 (4.56) | P<0.001 | |
| Ulcer disease (%) | 666 (3.29) | 26,228 (15.13) | P<0.001 | |
| H. pylori infection (%) | 22 (0.11) | 4,052 (2.34) | P<0.001 | |
| Barrett esophagus (%) | 15 (0.07) | 3,207 (1.85) | P<0.001 | |
| Achalasia (%) | 1 (0.00) | 214 (0.12) | P<0.001 | |
| Stricture (%) | 33 (0.16) | 2,299 (1.33) | P<0.001 | |
| Esophageal adenocarcinoma (%) | 3 (0.01) | 291 (0.17) | P<0.001 | |
| Years of follow-up (IQR) | 5.00 (5.00, 5.00) | 5.00 (5.00, 5.00) | P<0.001 | |
| Days of having related prescription during follow-up (IQR) | 90 (30, 270) | 450 (90, 1260) | P<0.001 | |
IQR, interquartile range.
Association between PPI and Risk of eGFR<60 ml/min per 1.73 m2, and Risk of CKD
The incident rate for eGFR<60 ml/min per 1.73 m2 was 5408.24 (95% confidence interval [95% CI], 5248.96 to 5567.52) and 7241.27 (95% CI, 7176.61 to 7305.93) per 100,000 person-years for H2 blockers and PPI groups, respectively (Table 2). Unadjusted Cox survival model results are provided in Supplemental Table 1. In Cox survival models adjusted for demographic, eGFR, clinical comorbid conditions, and other health characteristics, we evaluated the risk of incident eGFR<60 ml/min per 1.73 m2; compared with users of H2 blockers, the PPI group showed an increased risk (hazard ratio [HR], 1.22; 95% CI, 1.18 to 1.26) (Table 2).
Table 2.
Association between PPI and risk of eGFR<60 ml/min per 1.73 m2, and risk of CKD
| Outcome | H2 Blockers (n=20,270) | PPI (n=173,321) | |
|---|---|---|---|
| Incident eGFR<60 ml/min per 1.73 m2 | Number of events (%) | 4,429 (21.85) | 48,171 (27.79) |
| Incident rate (95% CI) | 5408.24 (5248.96 to 5567.52) | 7241.27 (7176.61 to 7305.94) | |
| HR (95% CI) | 1.0 | 1.22 (1.18 to 1.26) | |
| Incident chronic kidney disease | Number of events (%) | 2,234 (11.02) | 26,193 (15.11) |
| Incident rate (95% CI) | 2569.86 (2463.30 to 2676.43) | 3683.12 (3638.52 to 3727.72) | |
| HR (95% CI) | 1.0 | 1.28 (1.23 to 1.34) |
Incident rate as incident per 100,000 person-years.
HRs were obtained from Cox models adjusted for baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, H. pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma.
The incident rate for CKD (defined as two measurements of eGFR<60 ml/min per 1.73 m2 at least 90 days apart) was 2569.86 (2463.30, 2676.43) and 3683.12 (95% CI, 3638.52 to 3727.72) per 100,000 person-years for H2 blockers and PPI groups, respectively (Table 2). Adjusted survival models showed that the risk of CKD was increased in those exposed to PPI (HR, 1.28; 95% CI, 1.23 to 1.34). The attributable risk for incident eGFR<60 ml/min per 1.73 m2 and incident CKD was 1.83% and 1.11%, respectively, and number needed to harm was 55 and 90, respectively.
Association between PPI and Risk of Kidney Disease Progression and ESRD
The incident rate of doubling of serum creatinine was 816.98 (758.86, 875.10) and 1387.02 (95% CI, 1360.81 to 1413.22) per 100,000 person-years for H2 blockers and PPI groups, respectively. The incident rate for >30% decline in eGFR was 4533.25 (4391.86, 4674.64) and 6170.27 (95% CI, 6112.51 to 6228.03) per 100,000 person-years, respectively (Table 3). In adjusted survival models, risk of doubling of serum creatinine and eGFR decline >30% was significantly elevated in those treated with PPI (HR, 1.53; 95% CI, 1.42 to 1.65; and HR, 1.32; 95% CI, 1.28 to 1.37, respectively) (Table 3). The attributable risk for doubling of serum creatinine and >30% decline in eGFR was 0.57% and 1.63%, respectively, and number needed to harm was 175 and 61, respectively.
Table 3.
Association between PPI and risk of kidney disease progression and risk of ESRD
| Outcome | H2 Blockers (n=20,270) | PPI (n=173,321) | |
|---|---|---|---|
| Doubling of serum creatinine | Number of events (%) | 759 (3.74) | 10,766 (6.21) |
| Incident rate (95% CI) | 816.98 (758.86 to 875.10) | 1387.02 (1360.81 to 1413.22) | |
| HR (95% CI) | 1.0 | 1.53 (1.42 to 1.65) | |
| >30% decline in eGFR | Number of events (%) | 3,949 (19.48) | 43,842 (25.30) |
| Incident rate (95% CI) | 4533.25 (4391.86 to 4674.64) | 6170.27 (6112.51 to 6228.03) | |
| HR (95% CI) | 1.0 | 1.32 (1.28 to 1.37) | |
| ESRD | Number of events (%) | 25 (0.12) | 329 (0.19) |
| Incident rate (95% CI) | 26.50 (16.11 to 36.88) | 41.25 (36.79 to 45.70) | |
| HR (95% CI) | 1.0 | 1.96 (1.21 to 3.18) | |
| ESRD or >50% decline in eGFR | Number of events (%) | 947 (4.67) | 12,952 (7.47) |
| Incident rate (95% CI) | 1024.27 (959.03 to 1089.51) | 1679.40 (1650.48 to 1708.32) | |
| HR (95% CI) | 1.0 | 1.47 (1.38 to 1.57) | |
Incident rate as incident per 100,000 person-years.
HRs were obtained from Cox models adjusted for baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, H. pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma.
Incident rate for the outcome of ESRD was significantly higher among those treated with PPI compared with H2 blockers (41.25 [95% CI, 36.79 to 45.70] and 26.50 [95% CI, 16.11 to 36.88] per 100,000 person-years, respectively). In adjusted survival models, the risk of ESRD was significantly increased in the PPI group (HR, 1.96; 95% CI, 1.21 to 3.18) (Table 3). Risk of ESRD, or >50% decline in eGFR was elevated in patients treated with PPI (HR, 1.47; 95% CI, 1.38 to 1.57) (Table 3). The attributable risk for ESRD and composite outcome of ESRD or >50% decline in eGFR was 0.01% and 0.66%, respectively, and number needed to harm was 6780 and 153, respectively.
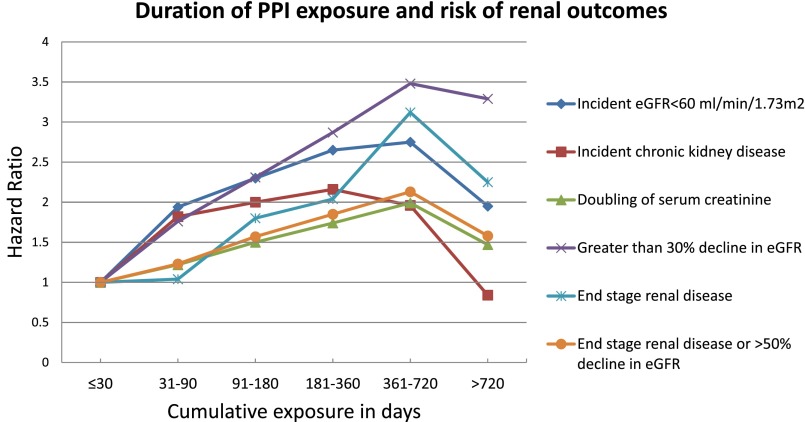
Duration of PPI Use and Risk of Renal Outcomes
We evaluated the association between duration of exposure and risk of renal outcomes among new users of PPI (n=173,321). Compared with those exposed for ≤30 days, there was a graded association between duration of exposure and risk of renal outcomes among those exposed for 31–90, 91–180, 181–360, and 361–720 days (Figure 2, Table 4). The association seems to diminish with exposure exceeding 720 days.
Figure 2.
Duration of PPI exposure and risk of renal outcomes among PPI users (n=173,321).
Table 4.
Duration of exposure to PPI and risk of renal outcomes among new users of PPI (n=173,321)
| Duration | Less or Equal to 30 Days | 31–90 Days | 91–180 Days | 181–360 Days | 361–720 Days | >720 days | |
|---|---|---|---|---|---|---|---|
| Incident eGFR<60 ml/min per 1.73 m2 | n (%) | 25,912 (14.95) | 31,192 (18.00) | 18,889 (10.90) | 20,770 (11.98) | 23,446 (13.53) | 53,112 (30.64) |
| HR (95% CI) | 1 | 1.94 (1.88 to 2.00) | 2.30 (2.22 to 2.39) | 2.65 (2.56 to 2.74) | 2.75 (2.66 to 2.85) | 1.95 (1.87 to 2.02) | |
| Incident CKD | n (%) | 23,621 (13.63) | 29,886 (17.24) | 18,338 (10.58) | 20,148 (11.62) | 23,293 (13.44) | 58,035 (33.48) |
| HR (95% CI) | 1 | 1.82 (1.74 to 1.89) | 2.00 (1.91 to 2.10) | 2.16 (2.06 to 2.26) | 1.96 (1.87 to 2.06) | 0.84 (0.79 to 0.89) | |
| Doubling of serum creatinine | n (%) | 19,602 (11.31) | 27,234 (15.71) | 16,989 (9.80) | 19,116 (11.03) | 23,603 (13.62) | 66,777 (38.53) |
| HR (95% CI) | 1 | 1.22 (1.13 to 1.30) | 1.50 (1.39 to 1.62) | 1.74 (1.61 to 1.87) | 1.99 (1.85 to 2.14) | 1.47 (1.37 to 1.59) | |
| >30% decline in eGFR | n (%) | 22,751 (13.13) | 29,291 (16.90) | 18,209 (10.51) | 20,444 (11.80) | 24,371 (14.06) | 58,255 (33.61) |
| HR (95% CI) | 1 | 1.76 (1.70 to 1.83) | 2.31 (2.22 to 2.40) | 2.87 (2.76 to 2.99) | 3.48 (3.34 to 3.61) | 3.29 (3.17 to 3.42) | |
| ESRD | n (%) | 18,529 (10.69) | 26,469 (15.27) | 16,649 (9.61) | 18,792 (10.84) | 23,500 (13.56) | 69,382 (40.03) |
| HR (95% CI) | 1 | 1.04 (0.70 to 1.56) | 1.80 (1.18 to 2.75) | 2.04 (1.33 to 3.12) | 3.12 (2.07 to 4.71) | 2.25 (1.46 to 3.47) | |
| ESRD or >50% decline in eGFR | n (%) | 19,799 (11.42) | 27,349 (15.78) | 17,105 (9.87) | 19,248 (11.11) | 23,695 (13.67) | 66,125 (38.15) |
| HR (95% CI) | 1 | 1.23 (1.16 to 1.31) | 1.57 (1.47 to 1.69) | 1.85 (1.72 to 1.98) | 2.13 (1.99 to 2.28) | 1.58(1.47 to 1.69) | |
HRs were obtained from Cox models adjusted for PPI duration, baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, H. pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma.
Beginning of follow up (T0) was defined as the date of last use of PPI before event occurrence.
PPI duration was computed between first PPI prescription date and T0.
Sensitivity Analyses
We examined the risk of renal outcomes in a 1:1 propensity score-matched cohort of new users of PPI (n=20,270) and new users of H2 blockers (n=20,270). The flowchart for the cohort design is provided in Supplemental Figure 1; Supplemental Table 2 provides description of demographics and health characteristics. The standardized difference for age, race, sex distribution, clinical comorbid conditions, and heath characteristics was <0.1, indicating balance between the two groups (Supplemental Table 2). Examination of the association of PPI use and renal outcomes yielded results consistent with those shown in the primary analysis (Table 5).
Table 5.
Risk of renal events in a 1:1 propensity score-matched cohort of new users of PPI (n=20,270) and new users of H2 blockers (n=20,270)
| Outcome | H2 Blockers (n=20,270) | PPI (n=20,270) | |
|---|---|---|---|
| Incident eGFR<60 ml/min per 1.73 m2 | Number of events (%) | 4,429 (21.85) | 5,204 (25.67) |
| Incident rate (95% CI) | 5408.24 (5249.96 to 5567.52) | 6563.33 (6385.01 to 6741.65) | |
| HR (95% CI) | 1.0 | 1.23 (1.17 to 1.30) | |
| Incident CKD | Number of events (%) | 2,234 (11.02) | 2,776 (13.70) |
| Incident rate (95% CI) | 2569.86 (2463.30 to 2676.43) | 3294.88 (3172.31 to 3417.44) | |
| HR (95% CI) | 1.0 | 1.28 (1.18 to 1.38) | |
| Doubling of serum creatinine | Number of events (%) | 759 (3.74) | 1,185 (5.85) |
| Incident rate (95% CI) | 816.98 (758.86 to 875.10) | 1300.96 (1226.89 to 1375.03) | |
| HR (95% CI) | 1.0 | 1.63 (1.47 to 1.81) | |
| >30% decline in eGFR | Number of events (%) | 3,949 (19.48) | 4,762 (23.49) |
| Incident rate (95% CI) | 4533.25 (4391.86 to 4674.64) | 5669.45 (5508.42 to 5830.47) | |
| HR (95% CI) | 1.0 | 1.32 (1.25 to 1.39) | |
| ESRD | Number of events (%) | 25 (0.12) | 38 (0.19) |
| Incident rate (95% CI) | 26.50 (16.11 to 36.88) | 40.69 (27.75 to 53.63) | |
| HR (95% CI) | 1.0 | 1.48 (0.49 to 4.50) | |
| ESRD or >50% decline in eGFR | Number of events (%) | 947 (4.67) | 1433 (7.07) |
| Incident rate (95% CI) | 1024.27 (959.03 to 1089.51) | 1582.80 (1500.85 to 1664.75) | |
| HR (95% CI) | 1.0 | 1.59 (1.45 to 1.74) | |
Incident rate as incident per 100,000 person-years.
HRs were obtained from Cox models adjusted for baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, gastroesophageal reflux disease, upper GI tract bleeding, ulcer disease, H. pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma.
We also examined the risk of renal outcomes in a 1:1 propensity matched cohort of new PPI users (n=173,321) and a control group (n=173,321) (see Concise Methods) (Supplemental Figure 2, Supplemental Table 3); the cohort was well balanced. Compared with the control group, patients treated with PPI exhibited an increased risk of renal outcomes, and results were consistent with those shown in the primary analyses (Table 6).
Table 6.
Risk of renal events in a 1:1 propensity matched cohort of new PPI users (173,321) and a control group (n=173,321)
| Outcome | Control (n=173,321) | PPI (n=173,321) | |
|---|---|---|---|
| Incident eGFR<60 ml/min per 1.73 m2 | Number (%) | 35,759 (20.63) | 48,171 (27.79) |
| Incident rate (95% CI) | 5105.97 (5053.05 to 5158.90) | 7241.27 (7176.61 to 7305.93) | |
| HR (95% CI) | 1.0 | 1.57 (1.54 to 1.60) | |
| Incident CKD | Number (%) | 17,426 (10.05) | 26,193 (15.11) |
| Incident rate (95% CI) | 2359.99 (2323.96 to 2394.01) | 3683.12 (3638.52 to 3727.72) | |
| HR (95% CI) | 1.0 | 1.81 (1.76 to 1.86) | |
| Doubling of serum creatinine | Number (%) | 6,039 (3.48) | 10,766 (6.21) |
| Incident rate (95% CI) | 770.38 (750.95 to 789.81) | 1387.02 (1360.81 to 1413.22) | |
| HR (95% CI) | 1.0 | 1.86 (1.80 to 1.93) | |
| >30% decline in eGFR | Number (%) | 31,781 (18.34) | 43,842 (25.30) |
| Incident rate (95% CI) | 4255.67 (4208.88 to 4302.46) | 6170.27 (6112.51 to 6228.03) | |
| HR (95% CI) | 1.0 | 1.67 (1.64 to 1.70) | |
| ESRD | Number (%) | 219 (0.13) | 329 (0.19) |
| Incident rate (95% CI) | 27.60 (23.94 to 31.25) | 41.25 (36.79 to 45.70) | |
| HR (95% CI) | 1.0 | 1.61 (1.26 to 2.04) | |
| ESRD or >50% decline in eGFR | Number (%) | 7,410 (4.28) | 12,952 (7.47) |
| Incident rate (95% CI) | 949.13 (927.52 to 970.74) | 1679.40 (1650.48 to 1708.32) | |
| HR (95% CI) | 1.0 | 1.83 (1.77 to 1.89) | |
Incident rate as incident per 100,000 person-years.
HRs were obtained from Cox models adjusted for baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, H. pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma.
As a test of calibration, we evaluated the association between PPI exposure and the outcome of AKI. The intent of this analysis was to examine the presence of an association where a priori observations suggest that an association is expected.1–3 The results suggest that patients in the PPI group have an increased risk of AKI (HR, 2.15; 95% CI, 2.00 to 2.32). To examine whether the association of PPI exposure and risk of chronic renal outcomes is mediated by occurrence of AKI, we controlled for AKI occurrence during exposure to acid-suppression therapy. The results suggest that associations remain significant (Table 7).
Table 7.
Risk of renal events in models additionally adjusted for AKI during exposure to acid-suppression therapy
| Outcome | H2 Blockers (n=20,270) | PPI (n=173,321) | |
|---|---|---|---|
| Incident eGFR<60 ml/min per 1.73 m2 | Number of patients with AKI during exposure to acid-suppression therapy (%) | 690 (3.40) | 10,903 (6.29) |
| HR (95% CI) | 1 | 1.20 (1.16 to 1.24) | |
| Incident CKD | Number of patients with AKI during exposure to acid-suppression therapy (%) | 710 (3.50) | 12,170 (7.02) |
| HR (95% CI) | 1 | 1.28 (1.22 to 1.34) | |
| Doubling of serum creatinine | Number of patients with AKI during exposure to acid-suppression therapy (%) | 749 (3.70) | 14,620 (8.44) |
| HR (95% CI) | 1 | 1.42 (1.32 to 1.54) | |
| >30% decline in eGFR | Number of patients with AKI during exposure to acid-suppression therapy (%) | 720 (3.55) | 11,797 (6.81) |
| H R (95% CI) | 1 | 1.28 (1.24 to 1.33) | |
| ESRD | Number of patients with AKI during exposure to acid-suppression therapy (%) | 760 (3.75) | 16,063 (9.27) |
| HR (95% CI) | 1 | 1.79 (1.10 to 2.89) | |
| ESRD or >50% decline in eGFR | Number of patients with AKI during exposure to acid-suppression therapy (%) | 748 (3.69) | 14,293 (8.25) |
| HR (95% CI) | 1 | 1.38 (1.29 to 1.47) | |
HRs were obtained from Cox models adjusted for AKI during exposure to acid-suppression therapy, baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, H. pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma.
We evaluated the association of PPI exposure and risk of renal events in a number of additional sensitivity analyses where we: (1) included the number of eGFR measurements for each patient as a covariate (Supplemental Table 4), (2) included use of nonsteroidal anti-inflammatory drugs (NSAIDs), defined as exposure to NSAIDs for 30 days or more before (Supplemental Table 5A) and during time in cohort (Supplemental Table 5B) as a covariate, (3) included baseline microalbumin-to-creatinine ratio as a covariate in a subcohort of patients where data were available (n=29,059) (Supplemental Table 6), (4) included serum bicarbonate as a covariate (n=174,322) (Supplemental Table 7) and (5) included the angiotensin-converting enzyme inhibitors (ACEI) and angiotensin-receptor blockers (ARB), defined as exposure to ACEI or ARB for 30 days or more before (Supplemental Table 8A) and during (Supplemental Table 8B) time in cohort as a covariate in the models. The results remained consistent in all sensitivity analyses.
Discussion
This study leverages the availability of a national comprehensive database in an integrated network of health systems to examine the association between PPI exposure and long-term renal outcomes. Among users of acid-suppression therapy, H2 blockers and PPI—2 classes of drugs generally prescribed for similar indications—we have shown that exposure to PPI is associated with increased risk of development of CKD, progression of kidney disease, and risk of ESRD. The results also suggest a graded relationship between duration of exposure and risk of renal outcomes. The results were consistent in multiple sensitivity analyses, including an assessment of risk in a 1:1 propensity score-matched and balanced cohort of H2 blocker and PPI users where risk of renal outcomes was significantly elevated in patients treated with PPI compared with those treated with H2 blockers, and a 1:1 propensity score-matched and balanced cohort of PPI users and controls where risk of renal outcomes was significantly increased in PPI users.
The results of our study further expand on the findings of a recently reported observational cohort study by Lazarus et al.8 The investigators followed 10,482 participants in the Atherosclerosis Risk in Communities Study and assessed the association between self-reported PPI use and the risk of incident CKD defined by diagnostic codes that indicated CKD at hospital discharge or death or by incident ESRD as determined through linkage with United States Renal Database System. In adjusted analyses, they found that participants who used PPIs at baseline had a significantly increased risk of incident CKD compared with nonusers. Similar associations were seen in the Geisinger Health System replication cohort of 248,751 participants, where incident CKD was defined as sustained eGFR<60 ml/min per 1.73 m2 or development of ESRD. In addition, twice-daily PPI dosing was shown to be associated with a higher risk of CKD than once-daily dosing. The study by Lazarus et al., and this study, reached remarkably similar conclusions using comparable study designs but in unrelated, population-based cohorts. Our study adopted a new user design, on the basis of pharmacy records, where the primary outcomes for incident CKD and CKD progression were defined using actual laboratory parameters (not ICD-9 codes). In addition to reporting an association between PPI and the risk of incident CKD, our results demonstrate that PPI use is also associated with an increased risk of CKD progression (doubling of serum creatinine, eGFR decline >30%) and ESRD; furthermore, we show a graded association between duration of exposure and risk of renal outcomes. The constellation of findings in our study lends further validity to the observations reported by Lazarus et al., further elucidates our understanding of the expanding spectrum of renal adverse events associated with PPI use, and suggests the need to exercise judicious use of PPI, limit exposure to the minimum dose necessary, and for close monitoring of renal function during PPI use.9
PPI are widely used and generally perceived as safe; they are often overprescribed, started inappropriately during a hospital stay, and their use extended for long-term duration without appropriate medical indication.10–12 Strid et al. examined the use of acid-suppressant drugs in patients with CKD and concluded that acid-suppression therapy is often prescribed without adequate indication, where PPI were the most common drug class used for acid suppression.13 Because of the wide use of PPI, the findings in this study may have public health relevance, in that, while seemingly benign, PPI use may be significantly associated with an increased risk of serious renal outcomes. We also note that, while the associations are significant, the incident rate of CKD, doubling of serum creatinine, eGFR decline >30%, and ESRD is relatively infrequent; therefore, while pharmacovigilance about safety of any approved therapeutic is a meritorious approach, the findings should not deter from prescription and use of PPI where medically indicated.
Recent examples that are relevant to PPI exposure and adverse outcomes include reports on risk of hypomagnesemia associated with PPI use among those admitted to intensive care units and in a population-based cohort study.14,15 It is notable that the risk of hypomagnesemia in PPI users was not observed in clinical trials and postmarketing studies. Randomized controlled trials are often undertaken in an idealized setting, are generally underpowered, and do not cover a sufficiently prolonged span of time to detect untoward events that may be rare and/or require a long time course for disease progression to manifest. The Food and Drug Administration postmarketing safety surveillance systems for drug and therapeutic biologic products are passive and rely on data obtained from manufacturers or through voluntary physician and consumer reporting.16 The systems may not capture long-term untoward outcomes.16 The newly established Sentinel Initiative aims to leverage the increasing availability of ‘Big Data’ and significant advances in analytics to proactively and systematically detect adverse signals associated with prescription medications and to uncover latent adverse events that are relatively rare and would not otherwise be observed in randomized clinical trials, postmarketing studies, or be captured through the passive surveillance mechanisms.16–18 The Sentinel Initiative, however, is informed (and often prompted) by observations (or signals) from clinical literature. Our results may help facilitate further discussion on PPI exposure and the risk of renal outcomes and, more broadly, on the role the scientific community could play in comprehensively fulfilling the promise of the Sentinel Initiative to protect and promote public health.17,19,20
The mechanism(s) underpinning the observed associations are not clear; several studies have suggested an association between PPI exposure and acute interstitial nephritis.1,3,4,6 PPI-induced acute interstitial nephritis is thought to be a cell-mediated immune response that maybe idiosyncratic, and likely represents a class effect and does not seem dose-dependent.4,21 It has been reported that 30–70% of patients with acute interstitial nephritis did not fully recover renal function, likely due to rapid development of interstitial fibrosis shortly after onset of the acute inflammatory process, especially in the setting of delayed diagnosis or treatment.4,5 This incomplete recovery of renal function, possibly along with chronic interstitial nephritis, leads to CKD and potentially CKD progression and ESRD.6,22 The relationship between AKI and subsequent development of CKD is supported by multiple observations, suggesting an important and growing role of AKI in the global epidemiology of CKD and ESRD and a bidirectional nexus between AKI and CKD and progression to ESRD.23–25 In our analyses, we observed that the association of PPI and renal outcomes remained significant even after controlling for AKI, suggesting that the described associations may be independent of clinically detectable AKI episodes and may be either the result of subclinical or unrecognized AKI or chronic indolent, but progressive, renal injury. PPI use may also cause severe hypomagnesemia,14,15 which is associated with faster eGFR decline in CKD patients and in patients with type 2 diabetes mellitus,26–28 progression to ESRD in diabetic nephropathy,29 decreased renal allograft survival,30 and, more recently, incident CKD.31 While our study did not examine this mechanistic link, it is hypothetically plausible that hypomagnesemia may mediate or partially explain the observed associations in this report.31
The results show a graded association between duration of exposure and risk of renal outcomes; however, the association seems to weaken in those exposed for more than 720 days, which is most likely a reflection of a survivorship bias—a phenomenon commonly referred to in pharmacoepidemiology as “depletion of susceptibles,” i.e., those remaining in the cohort are likely resistant to the effect of PPI on renal outcomes.32–34 In this study, we examined the risk of renal outcomes in a cohort design of new users of PPI and H2 blockers, a category of therapeutics (acid-suppression therapy) generally prescribed for similar medical indications which may reduce confounding by indication bias; we built multivariate Cox survival models adjusting for known confounders. While our study is sufficiently large, and the outcome is not particularly rare, we further tested the sensitivity of the results to changes in cohort design (and specification of statistical models) where associations were examined in two propensity score-matched and balanced cohorts (H2 blockers versus PPI, and PPI versus control).35 The results obtained using propensity score analyses were similar to those obtained using multivariate Cox regression analyses (i.e., were robust to changes in epidemiologic design), consistent with observations by Strümer et al. that, in most large studies, propensity score analyses do not yield substantially different risk estimates from conventional multivariate methods.36 Winklemeyer and Kurth note a limitation of both approaches, in that they cannot account for unmeasured and unknown confounders and suggest that traditional multivariate regression adjustment is preferable in pharmacoepidemiology studies when the sample size is sufficiently large and the outcome is not rare.37
Our study has a number of limitations. The cohort included mostly older white male United States’ veterans, thus the results may not be generalizable to less narrowly defined populations. The imperfect nature of administrative data and the retrospective design of the study may also lead to sampling bias and inaccurate measurements of the predictor variables. In order to minimize such measurement bias, we used definitions of comorbid illnesses that are validated for use in VA administrative data.38 In our analyses, we considered drug exposure as PPI prescription; since PPI is available over the counter in the United States, it is possible that some patients in this cohort may have obtained and used PPI without prescription. However, owing to financial considerations, this is not highly likely, and if it occurred in some patients, it will have biased the results against the primary hypothesis and resulted in underestimation of risk. While we report attributable risk to PPI use and number needed to harm, these numbers should not be extrapolated or otherwise generalized to other cohorts or the general population. The study has a number of strengths, including the use of national large-scale data from a network of integrated health systems which was captured during routine medical care which minimizes selection bias. We evaluated multiple outcomes in the continuum of CKD evolution, including development of CKD, progression of CKD, and the definite and terminal renal outcome of ESRD. We have taken considerable care to test the robustness of the associations in different cohort designs and numerous models in sensitivity analyses.
Concise Methods
Patients
Cohort for primary analyses
Using administrative data from the United States Department of Veterans Affairs, we identified users of the VA healthcare system who had no PPI prescription between October 1, 1999 and September 30, 2006. Patients were then further selected into PPI-treatment and H2-blockers groups. The PPI-treatment group selected patients who had at least one PPI prescription between October 1, 2006 and September 30, 2008. Patients in the PPI-treatment group were further restricted to those with a baseline eGFR>60 ml/min per 1.73 m2 within 90 days before their first PPI prescription and at least one other eGFR measurement after their first PPI prescription (n=173,321). The H2-blockers group included those who did not have a PPI prescription from October 1, 1999 until the end of follow-up on September 30, 2013, and had no H2 blocker prescription between October 1, 1999 and September 30, 2006. The H2-blocker group was restricted to those with a new prescription of H2 blockers between October 1, 2006 and September 30, 2008. They were also restricted to those with a baseline eGFR>60 ml/min per 1.73 m2 within 90 days before the first H2 blocker prescription and at least one other eGFR measurement after their first H2 blocker prescription (n=20,270) (Figure 1). Patients in cohort were followed for 5 years from their baseline eGFR measurement or until death. The study was approved by the Institutional Review Board of the VA Saint Louis Health Care System, Saint Louis, MO.
Data Sources
We used Department of Veterans Affairs databases including inpatient and outpatient medical SAS datasets (that include utilization data related to all inpatient and outpatient encounters within the VA system) to ascertain detailed patient demographic characteristics and comorbidity information based on Current Procedural Terminology codes, and ICD-9-CM diagnostic and procedure codes associated with inpatient and outpatient encounters.39–42 The VA Managerial Cost Accounting System Laboratory Results (a comprehensive database that includes VA-wide results for selected laboratory tests obtained in the clinical setting) provided information on outpatient and inpatient laboratory results. The VA Corporate Data Warehouse Production Outpatient Pharmacy domain provided information on prescriptions. The VA Vital Status and Beneficiary Identification Records Locator Subsystem files provided demographic characteristics and death follow-up through September 30, 2013.39,40 United States Renal Database System data provided information about occurrence of ESRD and date of first ESRD services.
Primary Predictor Variable
The primary predictor variable is outpatient use of PPI. Medications that contain esomeprazole, lansoprazole, omeprazole, pantoprazole, or rabeprazole were counted as PPI. Medications including ranitidine, cimetidine, and famotidine were counted as H2 blockers. Distribution of PPI and H2 blocker use is provided in Supplemental Table 9.
Outcomes
The primary outcomes in survival analyses were eGFR<60 ml/min per 1.73 m2, with CKD defined as two eGFRs<60 ml/min per 1.73 m2 at least 90 days apart, where the first eGFR measurement date was considered the date of CKD occurrence. Outcomes to capture kidney disease progression included >30% decline in eGFR, doubling of serum creatinine, ESRD,43–45 and ESRD or >50% decline in eGFR.45,46 All outcomes except ESRD were based on outpatient serum creatinine. Outcomes were ascertained for 5-year duration from time of cohort entry (where baseline eGFR was captured).
Covariates
Baseline covariates were ascertained from October 1, 1999 until baseline eGFR measure (T0), where baseline eGFR was defined as the eGFR within 90 days before first PPI or H2 blocker prescription between October 1, 2006, and September 30, 2008. Covariates included baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, and diseases associated with PPI use. eGFR was calculated using the abbreviated four-variable Chronic Kidney Disease Epidemiology Collaboration equation on the basis of age, sex, race, and outpatient serum creatinine.47 Race/ethnicity was categorized as white, black, or other (Latino, Asian, Native American, or other racial/ethnic minority groups). Diseases associated with PPI use included gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma.48 Comorbidities, except for hepatitis C and HIV, were assigned on the basis of relevant ICD-9-CM diagnostic and procedures codes and Current Procedural Terminology codes in the VA Medical SAS datasets.38,45,49,50 Hepatitis C and HIV were assigned based on laboratory results.
Statistical Analysis
t-test was used to detect mean difference for parametric continuous variables; Kruskal–Wallis test was used to detect difference for nonparametric continuous variables and chi-squared test was used to detect proportions difference between H2 blockers and PPI treatment. Incident rates per 100,000 person-years were computed for outcomes and confidence intervals were estimated based on normal distribution. Attributable risk and number needed to harm were calculated from incident rates. Cox proportional hazard regression models were used in the assessment of the association between PPI exposure and risk of renal outcomes. Multiple models were built to assess the relationship while controlling for different covariates.
We evaluated the association between duration of exposure and risk of renal outcomes among new users of PPI. Duration was defined in cumulative days of use and categorized as ≤30, 31–90, 91–180, 181–360, 361–720, or ≥721 days, where ≤30 days was used as the referent category. Time of cohort entry was defined as the date of last use of PPI before occurrence of renal event.32,51 Duration of PPI use was computed from the date of first PPI use until beginning of follow up.32,51 In regression analyses, a 95% CI of an HR that does not include unity was considered statistically significant. In all analyses a P value of 0.05 or less was considered statistically significant. All analyses were performed using SAS Enterprise Guide version 6.1 and 7.1.
Sensitivity Analyses
To further explore the possibility of hidden bias we undertook additional analyses examine the risk of renal outcomes in a 1:1 propensity score-matched cohorts of new users of H2 blockers who initiated a first prescription of H2 blockers between October 1, 2006, and September 30, 2008 (n=20,270) and new users of PPI who initiated a first prescription of PPI between October 1, 2006, and September 30, 2008 (n=20,270) (Supplemental Figure 1), and also between new users of PPI (n=173,321) who initiated a first prescription of PPI between October 1, 2006, and September 30, 2008, and a control group without PPI prescription between October 1, 2006, and September 30, 2008 (n=173,321) (Supplemental Figure 2). Propensity scores were calculated using a nonparsimonious logistic regression model with PPI exposure as the dependent variable, with predictor variables of baseline eGFR, age, race, sex, diabetes mellitus, hypertension, cardiovascular disease, peripheral artery disease, cerebrovascular disease, chronic lung disease, hepatitis C, HIV, dementia, gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, H. pylori infection, Barrett esophagus, achalasia, stricture, and esophageal adenocarcinoma. Nearest-neighbor matching without replacement was used, with a caliper distance set as 0.1, after the order of the treatment and control group was randomized.52,53
After 1:1 propensity score-matched cohorts of new users of PPI (n=20,270) and H2 blockers (n=20,270) (Supplemental Figure 1, Supplemental Table 2), and 1:1 propensity matched cohort of new PPI users (n=173,321) and a control group (n=173,321) (Supplemental Figure 2, Supplemental Table 3) were obtained, standardized differences were used to evaluate balance in distribution of baseline variables between PPI and control groups in matched cohorts, where a difference <0.1 was taken to indicate sufficient balance. Multivariate conditional Cox proportional hazards regression that stratified by matched pairs were conducted to examine the association between PPI and outcomes.
As a test of calibration, we evaluated the association between PPI exposure and the outcome of AKI during exposure to acid-suppression therapy and where AKI was defined as 0.3 mg/dl or 50% increase in serum creatinine within 30 days.45,49,54 To examine whether the association of PPI exposure and risk of chronic renal outcomes is mediated by occurrence of AKI, we controlled for AKI occurrence during exposure to acid-suppression therapy.
In order to further evaluate the consistency and robustness of the findings of our study, we examined the observed associations in a number of additional sensitivity analyses where we: (1) included the number of eGFR measurements from October 1, 1999 until time of cohort entry (T0) for each patient as a covariate, (2) included use of NSAIDs, defined in separate models as exposure to NSAIDs for 30 days or more before and during time in cohort, as a covariate, (3) included baseline microalbumin-to-creatinine ratio as a covariate in a subcohort of patients where data were available (n=29,059), (4) included serum bicarbonate as a covariate where it was treated as a continuous variable, and (5) included the ACEI and ARB, defined as exposure to ACEI or ARB for 30 days or more before and during time in cohort as a covariate in separate models.
Disclosures
None.
Supplementary Material
Acknowledgments
Kevin Martin from the Office of Information and Technology at the US Department of Veterans Affairs (VA) contributed to the development of SAS code for propensity score matching in a large cohort.
Support for VA/Centers for Medicare and Medicaid Services (CMS) data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Number/Data Use Agreement ID Al-Aly-01-A-1). This work was funded by a grant from the US Department of Veterans Affairs (for Z.A.A.).
The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Proton Pump Inhibitors and CKD,” on pages 2926–2928.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015121377/-/DCSupplemental.
References
- 1.Antoniou T, Macdonald EM, Hollands S, Gomes T, Mamdani MM, Garg AX, Paterson JM, Juurlink DN: Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open 3: E166–E171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klepser DG, Collier DS, Cochran GL: Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol 14: 150, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank ML, Parkin L, Paul C, Herbison P: A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 86: 837–844, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Praga M, González E: Acute interstitial nephritis. Kidney Int 77: 956–961, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Brewster UC, Perazella MA: Proton pump inhibitors and the kidney: critical review. Clin Nephrol 68: 65–72, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Perazella MA, Markowitz GS: Drug-induced acute interstitial nephritis. Nat Rev Nephrol 6: 461–470, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Rossert J: Drug-induced acute interstitial nephritis. Kidney Int 60: 804–817, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME: Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med 176: 238–246, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenfeld AJ, Grady D: Adverse Effects Associated With Proton Pump Inhibitors. JAMA Intern Med 176: 172–174, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Forgacs I, Loganayagam A: Overprescribing proton pump inhibitors. BMJ 336: 2–3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhry, MN, Soran, H, Ziglam, HM: Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. QJM 101: 445–448, 2008 [DOI] [PubMed]
- 12.Zink DA, Pohlman M, Barnes M, Cannon ME: Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 21: 1203–1209, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Strid H, Simrén M, Björnsson ES: Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol Dial Transplant 18: 570–575, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, Howell MD, Celi LA, Mukamal KJ: Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int 83: 692–699, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, Franco OH, Kuipers EJ, Hofman A, Zietse R, Stricker BH, Hoorn EJ: Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis 66: 775–782, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R: Developing the Sentinel System--a national resource for evidence development. N Engl J Med 364: 498–499, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Robb MA, Racoosin JA, Sherman RE, Gross TP, Ball R, Reichman ME, Midthun K, Woodcock J: The US Food and Drug Administration’s Sentinel Initiative: expanding the horizons of medical product safety. Pharmacoepidemiol Drug Saf 21[Suppl 1]: 9–11, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Psaty BM, Breckenridge AM: Mini-Sentinel and regulatory science--big data rendered fit and functional. N Engl J Med 370: 2165–2167, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Hackam DG, Mamdani M, Li P, Redelmeier DA: Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet 367: 413–418, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Marincola FM: In support of descriptive studies; relevance to translational research. J Transl Med 5: 21, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Härmark L, van der Wiel HE, de Groot MC, van Grootheest AC: Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol 64: 819–823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perazella MA, Luciano RL: Review of select causes of drug-induced AKI. Expert Rev Clin Pharmacol 8: 367–371, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Okusa MD, Chertow GM, Portilla D Acute Kidney Injury Advisory Group of the American Society of Nephrology : The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4: 520–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Laecke S, Nagler EV, Verbeke F, Van Biesen W, Vanholder R: Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med 126: 825–831, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Pham PC, Pham PM, Pham PA, Pham SV, Pham HV, Miller JM, Yanagawa N, Pham PT: Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 63: 429–436, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Pham PC, Pham PM, Pham PT, Pham SV, Pham PA, Pham PT: The link between lower serum magnesium and kidney function in patients with diabetes mellitus Type 2 deserves a closer look. Clin Nephrol 71: 375–379, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi Y, Shoji T, Hayashi T, Suzuki A, Shimizu M, Mitsumoto K, Kawabata H, Niihata K, Okada N, Isaka Y, Rakugi H, Tsubakihara Y: Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes Care 35: 1591–1597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzmacher R, Kendziorski C, Michael Hofman R, Jaffery J, Becker B, Djamali A: Low serum magnesium is associated with decreased graft survival in patients with chronic cyclosporin nephrotoxicity. Nephrol Dial Transplant 20: 1456–1462, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, Selvin E, Coresh J, Kao WH: Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int 87: 820–827, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams AL, Black MH, Zhang JL, Shi JM, Jacobsen SJ: Proton-pump inhibitor use and hip fractures in men: a population-based case-control study. Ann Epidemiol 24: 286–290, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Strom, BL: Pharmacoepidemiology, 2005.
- 34.Moride Y, Abenhaim L: Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol 47: 731–737, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Brookhart MA, Wyss R, Layton JB, Stürmer T: Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 6: 604–611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S: A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 59: 437–447, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkelmayer WC, Kurth T: Propensity scores: help or hype? Nephrol Dial Transplant 19: 1671–1673, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z: Renal Function Trajectories in Patients with Prior Improved eGFR Slopes and Risk of Death. PLoS One 11: e0149283, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy PA, Cowper DC, Seppala G, Stroupe KT, Hynes DM: Veterans Health Administration inpatient and outpatient care data: an overview. Eff Clin Pract 5[Suppl]: E4, 2002 [PubMed] [Google Scholar]
- 40.Oddone EZ, Eisen S: Veterans Affairs Research and Development: using science to improve health care for veterans. N C Med J 69: 35–37, 2008 [PubMed] [Google Scholar]
- 41.VIReC Research User Guide: VHA Medical SAS Outpatient Datasets FY2006. U.S. Department of Veterans Affairs. VA Information Resource Center: Hines, I, September 2007
- 42.VIReC Research User Guide: VHA Medical SAS Inpatient Datasets FY2006. U.S. Department of Veterans Affairs. VA Information Resource Center: Hines, I, September 2007
- 43.Al-Aly Z: Prediction of renal end points in chronic kidney disease. Kidney Int 83: 189–191, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS, Consortium CKDP CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowe M, Xie Y, Xian H, Balasubramanian S, Al-Aly Z: Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression [published online ahead of print January 29, 2016]. Kidney international doi: 10.1016/j.kint.2015.12.034 [DOI] [PubMed]
- 46.Rahman M, Yang W, Akkina S, Alper A, Anderson AH, Appel LJ, He J, Raj DS, Schelling J, Strauss L, Teal V, Rader DJ, Investigators CS CRIC Study Investigators : Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin J Am Soc Nephrol 9: 1190–1198, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL: Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med 28: 930–937, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z: Rate of Kidney Function Decline and Risk of Hospitalizations in Stage 3A CKD. Clin J Am Soc Nephrol 10: 1946–1955, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z: Estimated GFR Trajectories of People Entering Stage 4 CKD and Subsequent Kidney Disease Outcomes and Mortality [publsihed online ahead of print March 4, 2016]. Am J Kidney Dis doi: 10.1053/j.ajkd.2016.02.039 [DOI] [PubMed] [Google Scholar]
- 51.Johnson ES, Bartman BA, Briesacher BA, Fleming NS, Gerhard T, Kornegay CJ, Nourjah P, Sauer B, Schumock GT, Sedrakyan A, Stürmer T, West SL, Schneeweiss S: The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf 22: 1–6, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Austin PC: An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 46: 399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Austin PC, Grootendorst P, Anderson GM: A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 26: 734–753, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, Hung AM, Fly J, Speroff T, Ikizler TA, Matheny ME: Predictors of Recurrent AKI [published online ahead of print August 11, 2015]. J Am Soc Nephrol doi:ASN.2014121218 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.