Abstract
The introduction of corticosteroids and later, cyclophosphamide dramatically improved survival in patients with proliferative lupus nephritis, and combined administration of these agents became the standard-of-care treatment for this disease. However, treatment failures were still common and the rate of progression to ESRD remained unacceptably high. Additionally, treatment was associated with significant morbidity. Therefore, as patient survival improved, the goals for advancing lupus nephritis treatment shifted to identifying therapies that could improve long-term renal outcomes and minimize treatment-related toxicity. Unfortunately, progress has been slow and the current approaches to the management of lupus nephritis continue to rely on high-dose corticosteroids plus a broad-spectrum immunosuppressive agent. Over the past decade, an improved understanding of lupus nephritis pathogenesis fueled several clinical trials of novel drugs, but none have been found to be superior to the combination of a cytotoxic agent and corticosteroids. Despite these trial failures, efforts to translate mechanistic advances into new treatment approaches continue. In this review, we discuss current therapeutic strategies for lupus nephritis, briefly review recent advances in understanding the pathogenesis of this disease, and describe emerging approaches developed on the basis of these advances that promise to improve upon the standard-of-care lupus nephritis treatments.
Keywords: lupus nephritis, systemic lupus erythematosus, clinical trial, glomerular disease, immunosuppression
The introduction of corticosteroids in the 1950s profoundly changed the management of systemic lupus erythematosus (SLE) and consequently lupus nephritis (LN).1 Prior to the routine use of corticosteroids, LN carried a dismal patient survival rate of 17% at 5 years.2 A landmark study by Pollak et al. demonstrated that proliferative LN improved after treatment with high-dose corticosteroids,3 and as corticosteroids became standard-of-care (SOC) for LN, patient survival rates for proliferative disease improved to 55% at 5 years.2,4 Despite these benefits, long-term high-dose corticosteroids had significant treatment-associated toxicity, patients still developed progressive renal failure, and mortality remained high. Then in the 1970s it was shown that the addition of cytotoxic agents to corticosteroids could prevent progressive kidney failure,5 and with the addition of cyclophosphamide patient survival improved to 80% at 5 years.6
However, over the past 30 years the treatment of LN has stagnated. All mainstream approaches to management continue to incorporate high-dose corticosteroids plus an immunosuppressive agent, although high-dose cyclophosphamide is no longer the immunosuppressive of choice. Short-term complete renal response rates are only 10%–40% at 12 months, long-term outcomes have not improved further, and as many as 30% of LN patients will still progress to ESRD.4,7–10 Novel alternative therapies have been tested but to date no new approaches to the treatment of LN have been shown to be superior to cyclophosphamide plus corticosteroids.11,12
Despite these sobering facts, advances in our understanding of the pathogenesis of LN13 are fueling efforts to translate this knowledge into new management approaches. These efforts will be described here.
The Baseline: SOC Approaches to the Treatment of LN
Initial Treatment
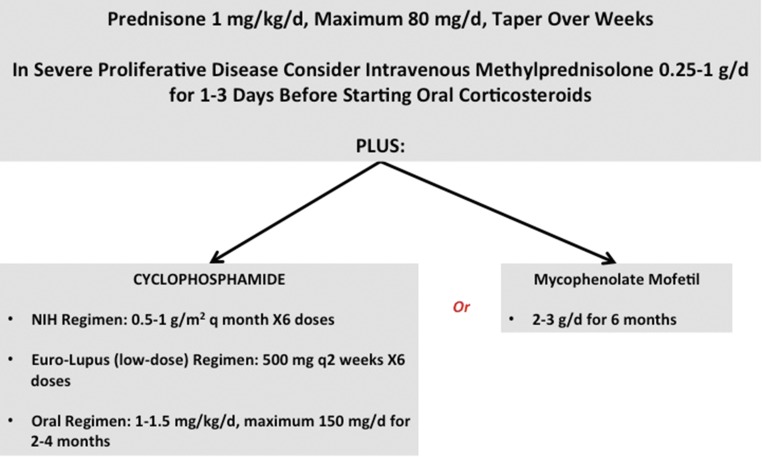
The initial treatment of active proliferative LN is termed induction and the mainstream approaches to induction are shown in Figure 1. The principle goal of induction is to rapidly attenuate immune complex–mediated renal inflammation to permit the injured renal parenchyma to heal. However, the landmark National Institutes of Health (NIH) study that led to the routine use of cyclophosphamide plus prednisone for induction revealed another, equally important goal of induction therapy in LN. Patients treated with cyclophosphamide and corticosteroids had fewer renal relapses and a lower incidence of future kidney failure than patients treated with corticosteroids alone, but this benefit was not realized for 3–5 years.5 The addition of cyclophosphamide did not improve short-term renal outcomes compared with corticosteroids, but did preserve kidney function in the long-term. These findings raised the possibility that in addition to controlling inflammation and its deleterious effects, the right type of induction therapy could also alter the immune system sufficiently to preserve kidney function long-term. New therapies for LN induction should therefore be measured against the combination of cyclophosphamide and corticosteroids in terms of both immediate and long-term renal outcomes. Unfortunately, in clinical trials of induction therapies the long-term assessment of kidney function is often neglected.
Figure 1.
Current standard of care treatment protocols for LN induction therapy. Patients with proliferative forms of LN are treated with oral corticosteroids, typically prednisone starting at 1 mg/kg per day and tapered over weeks to months. In severe disease with rapid deterioration of kidney function, high-dose intravenous methylprednisolone (0.25–1 g/d) is often given for 1–3 days preceding oral corticosteroids. In addition to corticosteroids one of four immunosuppressive regimens using cyclophosphamide or MMF is generally used. The NIH high-dose regimen consists of monthly intravenous pulses of cyclophosphamide dosed at 0.5–1 g/m2 for 6 months. Oral cyclophosphamide dosed at 1–1.5 mg/kg per day for 2–4 months provides a cumulative cyclophosphamide burden similar to the NIH regimen. In both cases cyclophosphamide is dosed based on nonobese body weight. The Euro-Lupus (low-dose) intravenous cyclophosphamide regimen is dosed at 500 mg every 2 weeks for six total doses. Cumulative cyclophosphamide for the Euro-Lupus regimen is 3 g, which is at least 50% lower than the NIH regimen. MMF is given for 6 months and dosed at 2–3 g/d.
An example of an LN induction trial that considered short- and long-term outcomes is the Euro-Lupus Nephritis Trial (ELNT) which compared the NIH cyclophosphamide regimen to a low-dose cyclophosphamide regimen (Figure 1). The ELNT and NIH regimens were equally effective for short-term remission induction9 and long-term (5 and 10 year) renal preservation,14 but there were fewer adverse events and a lower cumulative cyclophosphamide burden with ELNT.9,14 The generalizability of ELNT was questioned because it had predominantly white patients. However, the short-term efficacy of low-dose cyclophosphamide was recently verified in a racially diverse North American population15 and a southeast Asian cohort.16
Like the ELNT investigators, others sought to eliminate the cyclophosphamide-specific risks of premature ovarian failure and future malignancies by substituting mycophenolate mofetil (MMF) for cyclophosphamide in LN induction.17 Several trials suggested equivalence or superiority of MMF in achieving short-term renal responses,18,19 however in the largest trial, the Aspreva Lupus Management Study (ALMS), MMF and cyclophosphamide were found to be equivalent at the 24-week end point. Adverse event rates were similar for each drug, but as expected, the types of adverse events were different. Importantly, a 3-year follow-up study of ALMS (ALMS Maintenance) showed a trend for patients who had received induction with cyclophosphamide to have better long-term kidney outcomes than those induced with MMF regardless of choice of maintenance immunosuppression.20 Other studies have also questioned whether long-term kidney outcomes with MMF are equivalent to those with cyclophosphamide.21 A recent meta-analysis of 187 studies done between 1970 and 2015 evaluated the change in ESRD risk over time in LN.22 This study found that the 10- and 15-year risk of developing ESRD due to LN decreased by 10% from 1970 to the mid-1990s which coincided with the introduction of cyclophosphamide use for induction therapy. No further risk reduction was seen after the mid-1990s and instead a slight increase in risk was seen in the late 2000s, coinciding with an increase in MMF use for LN induction therapy.22
Maintenance Therapy
The duration of induction therapy is generally 3–6 months (Figure 1). Attaining a complete clinical or histologic renal remission by the end of induction occurs in only a modest number of patients.7–10,23 Furthermore, LN often relapses and ongoing treatment is needed to limit disease flares over time. Therefore, the principal goal of maintenance therapy is to consolidate the response that was achieved by induction therapy into a complete clinical remission and to keep patients relapse-free while minimizing treatment toxicity. Intravenous cyclophosphamide given quarterly was the first maintenance regimen evaluated for LN, but was associated with significant toxicity.24 Subsequent studies found that maintenance with azathioprine or MMF was more effective and less toxic than cyclophosphamide in preventing ESRD or death after 1–3 years of follow-up.24 Since then two large prospective, randomized controlled trials (RCTs) directly compared MMF to azathioprine for maintenance.14,20 The MAINTAIN nephritis trial randomized 105 white patients with LN to MMF or azathioprine and found no difference between these treatments in time to first renal flare.14 In contrast, 227 responders in the multiracial ALMS trial were rerandomized to MMF or azathioprine for maintenance, and MMF was found to be superior to azathioprine in preventing the composite end point of death, ESRD, doubling of serum creatinine, LN flare, or need for rescue therapy.20 Additionally, the patients maintained on azathioprine tended to do worse if induced with MMF. Although MMF is the therapy of choice for LN maintenance for most patients, azathioprine is effective and using these drugs can be individualized to specific patient circumstances. For example, azathioprine can be safely used in pregnancy whereas MMF is contraindicated.
MMF is an enzyme inhibitor and therapeutic drug monitoring of mycophenolic acid levels may be important in individualizing MMF dosing and checking compliance. Azathioprine is a prodrug of 6-mercaptopurine, which integrates into DNA. Monitoring drug levels may not be helpful, however pharmacogenomic testing of thiopurine methyl transferase, the enzyme that inactivates 6-mercaptopurine, may help avoid toxicity in patients with thiopurine methyl transferase deficiency.25
One of the most important questions concerning maintenance therapy is its duration. There are few convincing data to answer this question. The optimal length of maintenance therapy is unclear, with durations of 12–36 months studied in clinical trials.20,24,26 Indefinite treatment may be considered in individual cases based on disease severity and relapse risk.27 A current NIH-sponsored trial (identifier: NCT01946880) is evaluating disease flare in patients weaned off MMF maintenance therapy.
Pathogenesis Lite: A Brief Consideration of Mechanisms and Targets in LN
A detailed discussion of LN pathogenesis is beyond the scope of this review. However, it is relevant to briefly summarize current thoughts on pathogenesis to understand the rationale behind emerging therapies.
LN first comes to clinical attention after the kidney exceeds a threshold of inflammatory injury mediated by intrarenal immune complexes that have activated proinflammatory pathways. IFN-α released from plasmacytoid dendritic cells stimulates the production of antigen presenting cells, promotes autoreactive B cell differentiation to plasma cells, and increases the production of CD4 helper T (TH) cells and CD8 memory T cells, thus driving autoantibody expression and eventually immune complex formation.28–31 This can occur in the kidney interstitium as well as systemically. Plasmacytoid dendritic cells enter the kidney in LN.32 LN biopsies often have T and B cell aggregates and occasionally germinal centers in the tubulointerstitial compartment, and clonally-restricted antibody production from interstitial plasma cells has been demonstrated.33,34 Intrarenal immune complexes activate the C pathway, augmenting tissue injury and inflammation.35–38 Additionally, B cells present autoantigens to T cells and activate proinflammatory cytokine expression. T helper cell (TH1) cytokines are overexpressed in LN kidneys and are associated with inflammation through macrophage, C, and Fc receptor pathway activation.39,40 TH1 cells also promote B cell differentiation, proliferation, and aid class switching of autoantibodies.41,42 TH17 and CD4-CD8 T cells promote intrarenal IL-17 expression in LN.43 IL-17 is a proinflammatory cytokine that may also drive T cells away from maturing into a regulatory T cell phenotype that can suppress autoantibody production and attenuate the immune response.44–46
It is therefore reasonable to think of active LN as an inflammatory process occurring in parallel to a background, tonic level of systemic and intrarenal autoimmunity that can continually replenish the proinflammatory mediators needed to injure the kidney. The approach to LN management thus needs to be two-pronged: attenuation of inflammation to curtail further renal damage and suppression of autoimmunity to prevent exacerbations of disease activity (induction and maintenance therapy). Anti-inflammatory treatments should improve kidney function acutely (e.g., corticosteroids) but may not be sufficient to prevent long-term renal damage. Alternatively, therapies that target autoimmunity would not be expected to acutely resolve inflammation, but should prevent further disease flares and preserve the kidneys. It is likely that several recent therapeutic failures of novel LN drugs may have been due to trial end points focused on short-term improvements using drugs better suited for suppressing autoimmunity and achieving long-term benefits.
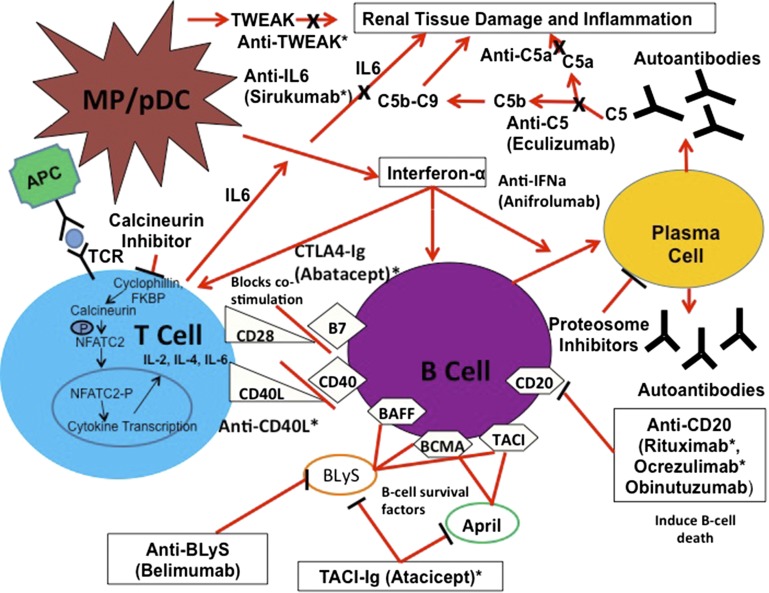
This high-level overview suggests B cells, T cells, C, and specific cytokines are potential therapeutic targets in LN. Therapeutic vulnerabilities in these pathways can be discerned by examining the effectors at a more granular level (Figure 2). For example, B cell activating factors like BAFF (BLyS) are needed for proliferation and survival of B cells.47 Serum BAFF is increased in LN,48,49 and BAFF mRNA has been found in glomeruli from patients with proliferative LN.50
Figure 2.
Novel therapies target the principal components of the immune system that contribute to LN pathogenesis. This schema illustrates current thoughts on the cells, cytokines, and growth factors and their interactions that amplify kidney injury and facilitate ongoing autoimmunity in LN. During LN circulating plasmacytoid dendritic cells enter the kidney and release IFN-α, and IFN-α then stimulates antigen presenting cells, promotes B cell differentiation into plasma cells, and facilitates production of TH1 and TH2 cells. B cells also present autoantigens to T cells which leads to T cell activation and release of proinflammatory cytokines such as IL-6. B cell and T cell proliferation is dependent upon costimulation which occurs independently from antigen presentation through interactions between CD28:B7 and CD40L:CD40 located on T and B cells respectively. Additionally, the B cell stimulators Blys and APRIL function to activate B cells and prolong survival. Autoreactive plasma cells produce autoantibodies that bind autoantigens and form immune complexes. These immune complexes deposit in the renal parenchyma, activate the alternative complement pathway, and recruit proinflammatory cells to the kidney leading to tissue damage and inflammation. The putative points of interaction of novel therapeutics and pathogenic mechanisms are indicated. Therapies with an asterisk have already been studied in clinical trials. Other therapies that are currently being studied or that we would like to see studied are also shown.
Lupus T cells have a lower activation threshold than normal T cells and signaling through the T cell receptor is associated with more calcium influx leading to calcineurin activation and eventually enhanced T cell expression of CD40 ligand (CD40L).51 CD40L is a costimulatory molecule that binds CD40 and activates B cells.52 Additionally, CD40L upregulates CD80 on B cells and CD80 interacts with CD28 on T cells.52 CD28/CD80 is a major costimulatory circuit for lymphocytes. This interaction can be blocked by CTLA4,53 and in murine models of LN the CTLA4-Ig fusion protein abatacept attenuates disease.54,55
IFN-α seems to be a master regulatory cytokine in approximately 50% of patients with SLE who display upregulation of IFN-α–inducible genes in peripheral blood leukocytes (the IFN signature).56,57 IFN-α–inducible genes are also upregulated in the kidney at LN flare.58
In lupus the C system plays dichotomous roles.59 The classical C pathway provides protection against lupus onset, as is evident by the association of deficiencies in classic C pathway genes with lupus or lupus-like disease.60,61 C can also protect against LN by promoting the clearance of circulating immune complexes and apoptotic debris, by preventing the formation of large immune complexes through the classical pathway, and by solubilizing immune complex precipitates through the alternative pathway.62 In contrast, C activation by both classical and alternative pathways contributes to LN disease activity, although the alternative pathway may be more critical in driving kidney tissue damage.63 Experimental models of LN support the importance of the alternative pathway.35–38 Blocking the alternative pathway at several levels in these models ameliorated disease.64,65
As suggested above and illustrated in Figure 2, all of these components of the immune system interact with each other to amplify inflammation and facilitate autoimmunity. Therapeutics designed to target these pathways have been developed and their interactions with LN pathways are shown in Figure 2 and discussed in detail below.
The Future: Emerging Therapies for LN
Table 1 presents an overview of the therapeutic landscape of LN. Included in the table are several recently completed trials which failed to meet their end points but provided important insights to inform future trials. Additionally, all current trials of novel LN therapeutics are listed.
Table 1.
Recent and Current Clinical Trials for Lupus Nphritis
| Drug | Trial Stage | Target | Phase/Status or Mechanism | Reference/Clinical Trial Number |
|---|---|---|---|---|
| Completed Clinical Trials | ||||
| Abatacept – BMS | Completed clinical trials | CTLA4-B7 interaction | Phase 3 – Failed to meet end point | 8 |
| Abatacept – ACCESS | Completed clinical trials | CTLA4-B7 interaction | Phase 2 – Failed to meet end point | 15 |
| Anti-CD40L | Completed clinical trials | CD40-ligand | Phase 2 – Terminated | 89 |
| Anti-TWEAK | Completed clinical trials | TWEAK | Phase 2 – Terminated | NCT01499355 |
| Bortezomib | Completed clinical trials | Plasma cells | Phase 4 – Terminated | 85 |
| Laquinamod | Completed clinical trials | Inflammation | Phase 2 – Encouraging | 101 |
| Rituximab | Completed clinical trials | CD20 | Phase 3 – Failed to meet end point | 10 |
| Ocrelizumab | Completed clinical trials | CD20 | Phase 3 – Failed to meet end point | 88 |
| Sirukumab | Completed clinical trials | IL-6 | Phase 2 – Failed to meet end point | 102 |
| Tabalumab | Completed clinical trials | B lymphocyte stimulator | Phase 3 – Failed to meet end point | 68 |
| Active Clinical Trials | ||||
| Anifrolumab | Active clinical trials | IFN-α | Phase 2 – Recruiting | NCT02547922 |
| Belimumab | Active clinical trials | B lymphocyte stimulator | Phase 3 – Recruitment closed | NCT01639339 |
| Ixazomib | Active clinical trials | Plasma cells | Phase 1 – Recruiting | NCT02176486 |
| Obinutuzumab | Active clinical trials | CD20 | Phase 2 – Recruiting | NCT02550652 |
| Rituximab | Active clinical trials | CD20/steroid reduced | Phase 3 – Recruiting | NCT01773616 |
| Rituximab/Belimumab | Active clinical trials | CD20/B lymphocyte stimulator | Phase 2 – Recruiting | NCT02260934 |
| Voclosporin | Active clinical trials | Calcineurin | Phase 2 – Recruitment closed | NCT02141672 |
| Therapies for Consideration | ||||
| Eculizumab | Therapy for consideration | C5 | Anti-inflammatory | 98 |
| Anti-C5aR (CCX168) | Therapy for consideration | C5a | Anti-inflammatory | 103 |
| Anti–IL-17 | Therapy for consideration | IL-17 | Anti-inflammatory | 104 |
BMS, Bristol-myers squibb; ACCESS, Abatacept and Cyclophosphamide Combination Therapy for Lupus Nephritis.
B Cell Depletion
B cells have a central role in LN pathogenesis (Figure 2) and are therefore attractive therapeutic targets. Several uncontrolled studies reported a benefit of the B cell depleting agent rituximab, an anti-CD20 monoclonal antibody, however early enthusiasm was tempered by the failure of the Lupus Nephritis Assessment with Rituximab Study (LUNAR), a large RCT that compared SOC plus rituximab to SOC plus placebo.10 Despite these mixed results there is continued interest in studying rituximab and other B cell depleting agents for treatment of LN. Additionally, trial design issues have been raised for LUNAR.
One important consideration is that in LUNAR rituximab was evaluated for short-term (52 weeks) effects as an induction drug even though it does not directly attenuate inflammation. Although rituximab does attenuate disease early in some autoimmune processes, it may not be anticipated to do so in LN, as it does not directly or rapidly affect inflammation. Instead, B cell depletion may have more effect if examined over a longer time, and at the 76-week LUNAR follow-up some separation between the rituximab and placebo arms became apparent.10 There is evidence that B cell depletion does affect autoimmunity. Belimumab, a humanized anti-BAFF monoclonal antibody, was found to be effective in nonrenal SLE.66 Although patients with severe LN were excluded from the belimumab RCTs, a post hoc analysis of the cohorts demonstrated a decreased rate of renal flares in patients treated with belimumab compared with placebo.67 However, in two equally large studies of tabalumab, another anti-BAFF antibody, in nonrenal SLE, there was no difference in renal flare rate between placebo and tabalumab.68 A phase 3 RCT is currently underway to evaluate belimumab directly in LN, and importantly, it will continue for at least 2 years (identifier: NCT01639339).
Another possible reason for the failure of LUNAR is that rituximab did not adequately deplete B cells. Whereas routine flow cytometry usually demonstrates B cell depletion after rituximab, high-sensitivity flow cytometry has shown that over half the patients still had circulating B cells.69 Furthermore, B cells are present in the tubulointerstitial compartment of the kidney in LN and it is intriguing to speculate that these interstitial B cells could contribute to organ-specific autoimmunity. The limited available evidence from animal models and data from cancer literature suggests that B cell depletion in solid tissues and secondary lymphoid organs is generally incomplete and variable between individuals. One recent study did demonstrate intrarenal B cell depletion after rituximab treatment for allograft rejection.70
To address this concern, a trial of B cell depletion in LN is being repeated with obinutuzumab, a type 2 chimeric, anti-CD20 monoclonal antibody that causes more complete peripheral and lymphoid tissue B cell depletion than rituximab.71,72 It is possible that obinutuzumab will perform better than rituximab by more effectively depleting peripheral and renal interstitial B cells. Tissue depletion will be assessed by an optional repeat kidney biopsy at 52 weeks (identifier: NCT02550652).
Finally, BAFF levels increase with B cell depletion73 and it is suspected that B cell repopulation in a BAFF-rich environment promotes the production of autoreactive B cells by bypassing tolerance checkpoints. Murine studies have shown that autoreactive B cells are preferentially repopulated after initial depletion.74–76 High circulating BAFF levels have been associated with SLE flare.48,77 Taken together, these data suggest that B cell repopulation in a low BAFF environment after rituximab is given might lead to a more tolerant B cell repertoire, less autoreactivity, and a sustained clinical response. The Immune Tolerance Network CALIBRATE study will test this hypothesis by sequential administration of cyclophosphamide plus rituximab followed by belimumab in a multicenter RCT (identifier: NCT02260934).
Plasma Cell Inhibition
Although B cell depletion for LN has been emphasized, it is important to note that B cell depleting drugs do not directly eliminate plasma cells, but do so indirectly by eliminating their precursors, and this takes time. Long-lived plasma cells produce and maintain autoantibodies in SLE and have been detected during flare.78–80 In lupus prone mice, autoreactive plasma cells have been found to localize in the tubulointerstitium during nephritic flares.79 Current SOC therapies do not adequately suppress long-lived plasma cells and therefore a key component driving disease in SLE remains unchecked.81
Proteasome inhibitors target plasma cells. The boronic acid derivatives bortezomib, carfilzomib, delanzomib, and ixazomib are proteasome inhibitors used to treat multiple myeloma by inducing apoptosis in plasma cells. Murine models of SLE and LN have shown that proteasome inhibitors are effective in treating lupus and can ameliorate and prevent nephritis.82,83 These drugs attenuate IFN-α production by blocking toll-like receptor–mediated plasmacytoid dendritic cell activity, inhibit induction of several NF-κB–dependent proinflammatory cytokines, and appear to increase T regulatory cells (Tregs) in experimental lupus models.83,84 Thus proteasome inhibitors may have a dual effect in the induction phase of LN treatment, acting as anti-inflammatory and antiautoimmunity agents. Proteasome inhibitors have received little attention in human lupus. Bortezomib was given to 12 patients with refractory SLE, half with LN.85 After 3 months, double-stranded DNA levels, circulating plasma cells, and proteinuria fell. This study was terminated due to neurotoxicity, a known side effect of bortezomib.85 Newer generation proteasome inhibitors, including carfilzomib and ixazomib citrate, may be associated with less neurotoxicity.86 Ixazomib citrate, an oral proteasome inhibitor, is currently being evaluated for treatment of proliferative LN in a phase 1 study of patients who have had incomplete responses to SOC therapy (identifier: NCT02176486).
Minimizing Corticosteroids in LN Therapy
Corticosteroid use seems almost mandatory for LN treatment, but this notion is being challenged by the Rituximab and Mycophenolate Mofetil without Oral Steroids for Lupus Nephritis (RITUXILUP) trial that combines B cell depletion with MMF and limited intravenous methylprednisolone pulses while eliminating oral corticosteroids entirely. The RITUXILUP protocol was evaluated in a single-center study of 50 patients with class 3, 4, or 5 LN.87 All patients received two 1 g doses of Rituximab plus 500 mg of methylprednisolone on days 1 and 15 followed by maintenance with MMF. At 52 weeks half the patients achieved a complete clinical response and 34% had a partial response. These response rates are comparable to those reported for several recent large RCTs.7–10 Adverse events were infrequent and less than with current SOC. To validate these findings, a noninferiority prospective, multicenter RCT evaluating the efficacy of the RITUXILUP regimen is underway (identifier: NCT01773616). Of note, high-dose methylprednisolone is an effective anti-inflammatory treatment. In an RCT comparing SOC to B cell depletion with ocrelizumab, a humanized anti-CD20 antibody, >70% of patients who received 1000 mg of methylprednisolone at the beginning of induction achieved a complete response regardless of intervention group.88 These data suggest that the 1000 mg of methylprednisolone given in the RITUXILUP trial may be sufficient to control inflammation while providing rituximab and MMF the time needed to suppress autoimmunity.
Blocking Costimulation in LN
The CD40/CD40L costimulatory pathway was targeted with an anti-CD40L antibody in human lupus. The trial was halted prematurely because several thromboembolic events occurred with anti-CD40L.89 However, in the 18 patients who received at least three doses of anti-CD40L there were some response signals including reductions in antidouble-stranded DNA levels, increases in C, and resolution of nephritic sediment, but only two patients met the primary end point of a 50% reduction in proteinuria with stable renal function.89 A re-engineered anti-CD40L Fab′ fragment that is not thrombogenic is currently in early stage development and may be tested in LN.90
The CD28/CD80 costimulatory pathway in LN has been targeted with abatacept in three RCTs. Two of these trials failed to demonstrate any benefit of adding abatacept to SOC LN induction treatment.8,15 Data from the third trial are pending. Although abatacept does not have any direct anti-inflammatory activity, these trials evaluated it as an induction therapy with short-term (6–12 months) response end points. Because abatacept blocks one of the pathways important in autoimmunity it may have performed better if it had been evaluated as an antiflare therapy. Evidence from the Abatacept and Cyclophosphamide Combination Therapy for Lupus Nephritis (ACCESS) trial, which compared abatacept plus low-dose cyclophosphamide to placebo plus low-dose cyclophosphamide, supports this idea. Patients in the abatacept arm who achieved a complete renal remission at 24 weeks were followed for an additional 6 months without maintenance therapy. At 12 months only one of 22 abatacept patients had a new flare, compared with three of 21 placebo patients who received azathioprine maintenance. Blocking costimulation may be most effective in LN as a treatment for maintenance of remission, but this will need to be formally tested.
Calcineurin Inhibitors and a Multitarget Approach to LN Therapy
Because many components of the immune system are simultaneously engaged in the generation of systemic and renal autoimmunity (Figure 2), it may be too optimistic to assume that intervening in a single pathway or depleting a single cell type will be sufficient to treat LN. In an effort to interfere with several aspects of the immune system at once in LN, a multitarget approach has been tested in a large Asian cohort.91 The multitarget regimen of corticosteroids, tacrolimus, and MMF was compared with a SOC regimen of corticosteroids plus intravenous cyclophosphamide. The calcineurin inhibitor (CNI) tacrolimus was used to better target T cells. CNIs block T cell activation (Figure 2) and prevent the release of several proinflammatory cytokines.92 This trial demonstrated a significantly higher rate of complete renal remission in the multitarget group compared with SOC at 6 months. However, this result should be interpreted with caution because reduction in proteinuria is the major clinical determinant of renal remission in LN and CNIs can attenuate proteinuria through immune and nonimmune mechanisms.93 We suggest that the usual clinical means of assessing renal remission may not be applicable in LN patients treated with a CNI, and it may be necessary to consider post-treatment kidney biopsies to ensure resolution of immune renal injury. Repeat biopsies were done in a small number of patients in this trial and the kidneys of the multitarget and cyclophosphamide showed similar improvement. Other caveats in the interpretation of this trial are that follow-up was very short, and the results may not be applicable to non-Asian populations. Long-term data on kidney survival after a CNI-based induction regimen are needed. Some of these issues will be addressed in a current 24-week trial comparing a multitarget regimen using the new generation CNI voclosporin to SOC in a racially diverse population (identifier: NCT02141672).
CNIs (plus corticosteroids) have also been used as the only immunosuppressive agents in the treatment of proliferative LN. The Cyclofa-Lune study compared cyclosporine to intravenous cyclophosphamide in white patients, and renal outcomes were similar between the groups at 18 months and 7.7 years (median follow-up).94 In another study, Chinese patients with LN were treated with tacrolimus or MMF for 6 months, and azathioprine maintenance in responders, and followed for 5 years.95 The primary outcome of a stable serum creatinine and a 24-hour urine protein-to-creatinine ratio of <1 at 6 months was achieved in 59% of the MMF group and 62% of the tacrolimus group. However, reversible increases of ≥30% in serum creatinine were only seen with tacrolimus, and compared with MMF over 5 years, the tacrolimus patients tended to have more renal flares. These data support the need for careful long-term studies in CNI-treated LN patients to ensure that favorable kidney outcomes are due to controlling immune injury and not masking proteinuria through nonimmune mechanisms.
Type 1 IFN inhibition
IFN-α appears to have a central regulatory role in SLE and LN, and therefore potential as a treatment target. Anifrolumab, a monoclonal antibody against IFN-α receptor 1, has recently been evaluated in nonrenal SLE and was shown to be significantly more effective than placebo, primarily in patients who had a high type 1 IFN gene signature.96 The TULIP-LN1 study will evaluate the safety and efficacy of anifrolumab plus SOC for the treatment of proliferative LN (identifier: NCT02547922). Of note, during screening for TULIP-LN1 the type 1 IFN signature will be measured in potential participants and patients will be stratified into high- or low-IFN groups. This is particularly important, as few LN clinical trials have measured the biologic target of a novel therapy prior to treatment. Treating patients not expressing a drug’s biologic target may have contributed to past trial failures.
C Inhibition
As discussed above, although both pathways likely contribute to LN pathogenesis, the alternative pathway appears to be critical for actual kidney tissue damage, mediated through C activation products that drive inflammation (C3bi, C5a), chemotaxis (C5a), and direct cellular injury (the membrane attack complex, C5b-9).97 Inhibition of the alternative pathway may be a useful adjunct during LN induction by attenuating inflammation, and may be corticosteroid sparing. Despite considerable experimental and clinical evidence demonstrating a role for C in LN, C inhibition has not been actively pursed to treat LN except for one phase 1 trial that demonstrated the safety of terminal C inhibition in SLE.98 There are several anti-C therapies in development or available.99 Candidates for testing in LN include eculizumab, a monoclonal antibody directed at C component C5, and a small molecule inhibitor of the C5a receptor that can be given orally and is being trialed in ANCA-associated vasculitis (identifiers: NCT02222155 and NCT01363388).100
Summary and Future Considerations
The goal of LN treatment is to preserve long-term kidney health, and current practice suggests this is best done by inducing an early renal remission and preventing further episodes of active disease. Commonly used SOC treatments are suboptimal and toxic. Several novel approaches to treatment that have more specific effects on the immune system have been studied with disappointing results. Lessons learned from these trials have, to some extent, informed the next wave of investigation into novel treatment solutions for LN, and will hopefully result in one or more new therapies than can be matched specifically to an individual’s disease.
Disclosures
B.H.R. is a consultant for Genetech (San Francisco, CA), Pfizer (New York, NY), Glaxo-Smith Kline (Brentford, UK), Mallinckrodt (Saint Louis, MO), Biogen Idec (Cambridge, MA), AstraZeneca (London, UK), and Pharmalink AB (Stockholm, Sweden). S.V.P. received a Fellowship Grant from Mallinckrodt Pharmaceuticals and has participated on an advisory board for Alexion Pharmaceuticals (Cheshire, CT).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Heller BI, Jacobson WE, Hammarsten JF. Effects of Cortisone in Glomerulonephritis and the Nephropathy of Disseminated Lupus Erythematosus. Am J Med 10: 520, 1951 [DOI] [PubMed]
- 2.Cameron JS: Lupus nephritis. J Am Soc Nephrol 10: 413–424, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Pollak VE, Pirani CL, Schwartz FD: The natural history of the renal manifestations of systemic lupus erythematosus. 1964. J Am Soc Nephrol 8: 1189–1198; discussion 1189–1195, 1997 [DOI] [PubMed]
- 4.Costenbader KH, Desai A, Alarcón GS, Hiraki LT, Shaykevich T, Brookhart MA, Massarotti E, Lu B, Solomon DH, Winkelmayer WC: Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 63: 1681–1688, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin HA 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL: Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 314: 614–619, 1986 [DOI] [PubMed] [Google Scholar]
- 6.Ginzler EM, Bollet AJ, Friedman EA: The natural history and response to therapy of lupus nephritis. Annu Rev Med 31: 463–487, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sánchez-Guerrero J, Solomons N, Wofsy D; Aspreva Lupus Management Study Group : Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 20: 1103–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furie R, Nicholls K, Cheng TT, Houssiau F, Burgos-Vargas R, Chen SL, Hillson JL, Meadows-Shropshire S, Kinaszczuk M, Merrill JT: Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol 66: 379–389, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed ER, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 46: 2121–2131, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G, Group LI; LUNAR Investigator Group : Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 64: 1215–1226, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejía JC, Aydintug AO, Chwalinska-Sadowska H, de Ramón E, Fernández-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR; European Working Party on Systemic Lupus Erythematosus : Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 82: 299–308, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Danila MI, Pons-Estel GJ, Zhang J, Vilá LM, Reveille JD, Alarcón GS: Renal damage is the most important predictor of mortality within the damage index: data from LUMINA LXIV, a multiethnic US cohort. Rheumatology (Oxford) 48: 542–545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lech M, Anders HJ: The pathogenesis of lupus nephritis. J Am Soc Nephrol 24: 1357–1366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Cauli A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 69: 61–64, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Group AT; ACCESS Trial Group : Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol 66: 3096–3104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathi M, Goyal A, Jaryal A, Sharma A, Gupta PK, Ramachandran R, Kumar V, Kohli HS, Sakhuja V, Jha V, Gupta KL: Comparison of low-dose intravenous cyclophosphamide with oral mycophenolate mofetil in the treatment of lupus nephritis. Kidney Int 89: 235–242, 2015 [DOI] [PubMed]
- 17.Faurschou M, Sorensen IJ, Mellemkjaer L, Loft AG, Thomsen BS, Tvede N, Baslund B: Malignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol 35: 100–105, 2008 [PubMed] [Google Scholar]
- 18.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN; Hong Kong-Guangzhou Nephrology Study Group : Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. N Engl J Med 343: 1156–1162, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, Appel GB: Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 353: 2219–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, Eitner F, Appel GB, Contreras G, Lisk L, Solomons N, Group A; ALMS Group : Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 365: 1886–1895, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Rovin BH, Parikh SV, Hebert LA, Chan TM, Mok CC, Ginzler EM, Hooi LS, Brunetta P, Maciuca R, Solomons N: Lupus nephritis: induction therapy in severe lupus nephritis--should MMF be considered the drug of choice? Clin J Am Soc Nephrol 8: 147–153, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Tektonidou MG, Dasgupta A, Ward MM: Risk of End-stage Renal Disease in Patients with Lupus Nephritis, 1970 to 2015 A systematic review and Bayesian meta-analysis. Arthritis Rheum 2016. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26815601. Accessed May 6, 2016 [DOI] [PMC free article] [PubMed]
- 23.Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, Nagaraja H, Indrakanti D, Rovin BH: Histologic versus clinical remission in proliferative lupus nephritis [published online ahead of print August 6, 2015]. Nephrology Dial Transplant doi:10.1093/ndt/gfv296 [DOI] [PMC free article] [PubMed]
- 24.Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O’Nan P, Roth D: Sequential therapies for proliferative lupus nephritis. N Engl J Med 350: 971–980, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Maltzman JS, Koretzky GA: Azathioprine: old drug, new actions. J Clin Invest 111: 1122–1124, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houssiau FA, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, Fiehn C, de Ramon Garrido E, Gilboe IM, Tektonidou M, Blockmans D, Ravelingien I, le Guern V, Depresseux G, Guillevin L, Cervera R, Group MNT; MAINTAIN Nephritis Trial Group : Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis 69: 2083–2089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dörner T, Doria A, Ferrario F, Floege J, Houssiau FA, Ioannidis JP, Isenberg DA, Kallenberg CG, Lightstone L, Marks SD, Martini A, Moroni G, Neumann I, Praga M, Schneider M, Starra A, Tesar V, Vasconcelos C, van Vollenhoven RF, Zakharova H, Haubitz M, Gordon C, Jayne D, Boumpas DT; European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association : Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 71: 1771–1782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallagher KM, Lauder S, Rees IW, Gallimore AM, Godkin AJ: Type I interferon (IFN alpha) acts directly on human memory CD4+ T cells altering their response to antigen. J Immunol 183: 2915–2920, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J: Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19: 225–234, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Ramos HJ, Davis AM, Cole AG, Schatzle JD, Forman J, Farrar JD: Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates. Blood 113: 5516–5525, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rönnblom L, Alm GV, Eloranta ML: The type I interferon system in the development of lupus. Semin Immunol 23: 113–121, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Tucci M, Quatraro C, Lombardi L, Pellegrino C, Dammacco F, Silvestris F: Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum 58: 251–262, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR: In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186: 1849–1860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR: Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 63: 865–874, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao L, Haas M, Quigg RJ: Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol 22: 285–295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekine H, Ruiz P, Gilkeson GS, Tomlinson S: The dual role of complement in the progression of renal disease in NZB/W F(1) mice and alternative pathway inhibition. Mol Immunol 49: 317–323, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, Boackle SA, Colten HR, Gilkeson GS: Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol 164: 786–794, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC: C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol 16: 3572–3582, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T, Fukuda K, Kanai H, Nakashima H, Otsuka T, Hirakata H: Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum 44: 2097–2106, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F: Overexpression of interleukin-12 and T helper 1 predominance in lupus nephritis. Clin Exp Immunol 154: 247–254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster MH: T cells and B cells in lupus nephritis. Semin Nephrol 27: 47–58, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kyttaris VC, Tsokos GC: T lymphocytes in systemic lupus erythematosus: an update. Curr Opin Rheumatol 16: 548–552, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC: Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol 181: 8761–8766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK: Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Iikuni N, Lourenço EV, Hahn BH, La Cava A: Cutting edge: Regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol 183: 1518–1522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhn A, Beissert S, Krammer PH: CD4(+)CD25 (+) regulatory T cells in human lupus erythematosus. Arch Dermatol Res 301: 71–81, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Davidson A: Targeting BAFF in autoimmunity. Curr Opin Immunol 22: 732–739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, Recta V, Zhong J, Freimuth W: Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 58: 2453–2459, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF: Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus 22: 873–884, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Neusser MA, Lindenmeyer MT, Edenhofer I, Gaiser S, Kretzler M, Regele H, Segerer S, Cohen CD: Intrarenal production of B-cell survival factors in human lupus nephritis. Mod Pathol 24: 98–107, 2011 [DOI] [PubMed]
- 51.Kyttaris VC, Wang Y, Juang YT, Weinstein A, Tsokos GC: Increased levels of NF-ATc2 differentially regulate CD154 and IL-2 genes in T cells from patients with systemic lupus erythematosus. J Immunol 178: 1960–1966, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ: Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 229: 152–172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lenschow DJ, Walunas TL, Bluestone JA: CD28/B7 system of T cell costimulation. Annu Rev Immunol 14: 233–258, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Daikh DI, Wofsy D: Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J Immunol 166: 2913–2916, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A: Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol 171: 489–497, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW: Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100: 2610–2615, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, Furst DE, Gorn A, McMahon M, Taylor M, Brahn E, Hahn BH, Tsao BP: Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum 54: 2951–2962, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Parikh SV, Malvar A, Song H, Alberton V, Lococo B, Vance J, Zhang J, Yu L, Rovin BH: Characterising the immune profile of the kidney biopsy at lupus nephritis flare differentiates early treatment responders from non-responders. Lupus Sci Med 2: e000112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Birmingham DJ, Hebert LA: The Complement System in Lupus Nephritis. Semin Nephrol 35: 444–454, 2015 [DOI] [PubMed] [Google Scholar]
- 60.Navratil JS, Korb LC, Ahearn JM: Systemic lupus erythematosus and complement deficiency: clues to a novel role for the classical complement pathway in the maintenance of immune tolerance. Immunopharmacology 42: 47–52, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Walport MJ: Complement and systemic lupus erythematosus. Arthritis Res 4[Suppl 3]: S279–S293, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schifferli JA, Steiger G, Hauptmann G, Spaeth PJ, Sjöholm AG: Formation of soluble immune complexes by complement in sera of patients with various hypocomplementemic states. Difference between inhibition of immune precipitation and solubilization. J Clin Invest 76: 2127–2133, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birmingham DJ, Irshaid F, Nagaraja HN, Zou X, Tsao BP, Wu H, Yu CY, Hebert LA, Rovin BH: The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus 19: 1272–1280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S: Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J Immunol 180: 1231–1238, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Sekine H, Kinser TT, Qiao F, Martinez E, Paulling E, Ruiz P, Gilkeson GS, Tomlinson S: The benefit of targeted and selective inhibition of the alternative complement pathway for modulating autoimmunity and renal disease in MRL/lpr mice. Arthritis Rheum 63: 1076–1085, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF; BLISS-76 Study Group : A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 63: 3918–3930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dooley MA, Houssiau F, Aranow C, D’Cruz DP, Askanase A, Roth DA, Zhong ZJ, Cooper S, Freimuth WW, Ginzler EM; BLISS-52 and -76 Study Groups : Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 22: 63–72, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Rovin BH, Dooley MA, Radhakrishnan J, Ginzler EM, Forrester TD, Anderson PW: The impact of tabalumab on the kidney in systemic lupus erythematosus: results from 2 phase 3 randomized, clinical trials [published online ahead of print May 24, 2016]. Lupus doi: 10.1177/0961203316650734 [DOI] [PubMed] [Google Scholar]
- 69.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, Ponchel F, Rawstron AC, Emery P: B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 63: 3038–3047, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Steinmetz OM, Lange-Hüsken F, Turner JE, Vernauer A, Helmchen U, Stahl RA, Thaiss F, Panzer U: Rituximab removes intrarenal B cell clusters in patients with renal vascular allograft rejection. Transplantation 84: 842–850, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Döhner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M: Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370: 1101–1110, 2014 [DOI] [PubMed] [Google Scholar]
- 72.Mössner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, Ferrara C, Sondermann P, Jäger C, Strein P, Fertig G, Friess T, Schüll C, Bauer S, Dal Porto J, Del Nagro C, Dabbagh K, Dyer MJ, Poppema S, Klein C, Umaña P: Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollard RP, Abdulahad WH, Vissink A, Hamza N, Burgerhof JG, Meijer JM, Visser A, Huitema MG, Spijkervet FK, Kallenberg CG, Bootsma H, Kroese FG: Serum levels of BAFF, but not APRIL, are increased after rituximab treatment in patients with primary Sjogren’s syndrome: data from a placebo-controlled clinical trial. Ann Rheum Dis 72: 146–148, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Miller JP, Stadanlick JE, Cancro MP: Space, selection, and surveillance: setting boundaries with BLyS. J Immunol 176: 6405–6410, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R: Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20: 785–798, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H: A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med 203: 393–400, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cambridge G, Isenberg DA, Edwards JC, Leandro MJ, Migone TS, Teodorescu M, Stohl W: B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann Rheum Dis 67: 1011–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Cheng Q, Mumtaz IM, Khodadadi L, Radbruch A, Hoyer BF, Hiepe F: Autoantibodies from long-lived ‘memory’ plasma cells of NZB/W mice drive immune complex nephritis. Ann Rheum Dis 72: 2011–2017, 2013 [DOI] [PubMed] [Google Scholar]
- 79.Espeli M, Bökers S, Giannico G, Dickinson HA, Bardsley V, Fogo AB, Smith KG: Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol 22: 296–305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiepe F, Dörner T, Hauser AE, Hoyer BF, Mei H, Radbruch A: Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol 7: 170–178, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Grammer AC, Lipsky PE: B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther 5[Suppl 4]: S22–S27, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hainz N, Thomas S, Neubert K, Meister S, Benz K, Rauh M, Daniel C, Wiesener M, Voll RE, Amann K: The proteasome inhibitor bortezomib prevents lupus nephritis in the NZB/W F1 mouse model by preservation of glomerular and tubulointerstitial architecture. Nephron, Exp Nephrol 120: e47–e58, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Seavey MM, Lu LD, Stump KL, Wallace NH, Ruggeri BA: Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. Int Immunopharmacol 12: 257–270, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Weng J, Lai P, Lv M, Lin S, Ling W, Geng S, Luo C, Liu X, Du X: Bortezomib modulates regulatory T cell subpopulations in the process of acute graft-versus-host disease. Clin Lab 59: 51–58, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Alexander T, Sarfert R, Klotsche J, Kühl AA, Rubbert-Roth A, Lorenz HM, Rech J, Hoyer BF, Cheng Q, Waka A, Taddeo A, Wiesener M, Schett G, Burmester GR, Radbruch A, Hiepe F, Voll RE: The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 74: 1474–1478, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, Jackson SR, Stoppa AM, Simpson DR, Gimsing P, Palumbo A, Garderet L, Cavo M, Kumar S, Touzeau C, Buadi FK, Laubach JP, Berg DT, Lin J, Di Bacco A, Hui AM, van de Velde H, Richardson PG; TOURMALINE-MM1 Study Group : Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 374: 1621–1634, 2016 [DOI] [PubMed] [Google Scholar]
- 87.Pepper R, Griffith M, Kirwan C, Levy J, Taube D, Pusey C, Lightstone L, Cairns T: Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Nephrol Dial Transplant 24: 3717–3723, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Mysler EF, Spindler AJ, Guzman R, Bijl M, Jayne D, Furie RA, Houssiau FA, Drappa J, Close D, Maciuca R, Rao K, Shahdad S, Brunetta P: Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 65: 2368–2379, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, Vaishnaw A, Group BGLNT; BG9588 Lupus Nephritis Trial Group : A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum 48: 719–727, 2003 [DOI] [PubMed] [Google Scholar]
- 90.Shock A, Burkly L, Wakefield I, Peters C, Garber E, Ferrant J, Taylor FR, Su L, Hsu YM, Hutto D, Amirkhosravi A, Meyer T, Francis J, Malcolm S, Robinson M, Brown D, Shaw S, Foulkes R, Lawson A, Harari O, Bourne T, Maloney A, Weir N: CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther 17: 234, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, Chen J, Lin H, Liu F, He Y, He Y, Miao L, Chen N, Li Y, Gu Y, Shi W, Hu W, Liu Z, Bao H, Zeng C, Zhou M: Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med 162: 18–26, 2015 [DOI] [PubMed] [Google Scholar]
- 92.Tsuda K, Yamanaka K, Kitagawa H, Akeda T, Naka M, Niwa K, Nakanishi T, Kakeda M, Gabazza EC, Mizutani H: Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naïve T cells into cytokine-producing mature T cells. PLoS One 7: e31465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathieson PW: Proteinuria and immunity--an overstated relationship? N Engl J Med 359: 2492–2494, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Zavada J, Pesickova S, Rysava R, Olejarova M, Horák P, Hrncír Z, Rychlík I, Havrda M, Vítova J, Lukác J, Rovensky J, Tegzova D, Böhmova J, Zadrazil J, Hána J, Dostál C, Tesar V: Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: the Cyclofa-Lune study. Lupus 19: 1281–1289, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Mok CC, Ying KY, Yim CW, Siu YP, Tong KH, To CH, Ng WL: Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: a randomised controlled trial and long-term follow-up. Ann Rheum Dis 75: 30–36, 2016 [DOI] [PubMed] [Google Scholar]
- 96.Furie R, Merrill JT, Werth VT, Khamashta M, Kalunian K, Brohawn P, Illei GG, Drappa J, Wang L, Yoo S: Anifrolumab, an anti-interferon alpha receptor monoclonal antibody, in moderate to severe systemic lupus erythematosus. Am Coll Rheumatol 2015; Abstract #3223: San Francisco, CA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Biesecker G, Koffler D: Immunopathology of the membrane attack complex in systemic lupus erythematosus nephritis. Arthritis Rheum 25: 876–879, 1982 [DOI] [PubMed] [Google Scholar]
- 98.Barilla-Labarca ML, Toder K, Furie R: Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol 148: 313–321, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Gilkeson GS. Complement-Targeted Therapies in Lupus. Current Treat Options Rheum 1: 10–18, 2015
- 100.Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, Seitz LC, Penfold ME, Gan L, Hu P, Lu B, Gerard NP, Gerard C, Schall TJ, Jaen JC, Falk RJ, Jennette JC: C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol 25: 225–231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jayne DAG, Mao D: The Pharmacokinetics of Laquinimod and Mycophenolate Mofetil during Treatment of Active Lupus Nephritis. In: Form A ed., American Society of Nephrology Kidney Week: 2013, SA-PO1094 [Google Scholar]
- 102.Aranow C, Vollenhoven R, Rovin BH: A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept Study to Evaluate the Efficacy and Safety of Sirukumab in Patients with Active Lupus Nephritis. Abstract form, American College of Rheumatology National Meeting: 2015 [Google Scholar]
- 103.Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ: C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol 35: 2496–2506, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Zickert A, Amoudruz P, Sundström Y, Rönnelid J, Malmström V, Gunnarsson I: IL-17 and IL-23 in lupus nephritis - association to histopathology and response to treatment. BMC Immunol 16: 7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]