Abstract
Globally, hepatitis C virus (HCV) infection affects approximately 130 million people and 3 million new infections occur annually. HCV is also recognized as an important cause of chronic liver disease in children. The absence of proofreading properties of the HCV RNA polymerase leads to a highly error prone replication process, allowing HCV to escape host immune response. The adaptive nature of HCV evolution dictates the outcome of the disease in many ways. Here, we investigated the molecular evolution of HCV in three unrelated children who acquired chronic HCV infection as a result of mother-to-child transmission, two of whom were also coinfected with HIV-1. The persistence of discrete HCV variants and their population structure were assessed using median joining network and Bayesian approaches. While patterns of viral evolution clearly differed between subjects, immune system dysfunction related to HIV coinfection or persistent HCV seronegativity stand as potential mechanisms to explain the lack of molecular evolution observed in these three cases. In contrast, treatment of HCV infection with PegIFN, which did not lead to sustained virologic responses in all 3 cases, was not associated with commensurate variations in the complexity of the variant spectrum. Finally, the differences in the degree of divergence suggest that the mode of transmission of the virus was not the main factor driving viral evolution.
Keywords: Hepatitis C virus, Vertical transmission, Molecular evolution
1. Introduction
Globally, hepatitis C virus (HCV) infection affects approximately 130 million people, in addition to 3 million new infections occurring annually (Alter, 2007). The HCV RNA-dependent RNA polymerase lacks proofreading and error correction mechanisms, and, as a consequence, the viral replication process is highly error prone (Moradpour et al., 2007). The high mutation rate of HCV favors a high degree of intrahost genetic diversity, allowing rapid adaptation that ultimately facilitates evasion from host immune responses (Cruz-Rivera et al., 2013; Escobar-Gutierrez et al., 2012; Fonseca-Coronado et al., 2012; Forns et al., 1999). HCV evolution is determined by a combination of evolutionary processes, including mutation and replication rates, natural selection, and genetic drift (Gray et al., 2012). This plasticity is a key biological property of HCV that enables it to reshape the structure of the intra-host viral population.
In several developed countries, HCV has become an important cause of chronic liver disease in children (Bortolotti et al., 2007; Slowik and Jhaveri, 2005). Viral transmission commonly occurs from mother to child in utero or during the peripartum period, but not as result of breastfeeding (Conte et al., 2000; Jhaveri et al., 2006). Reported rates of spontaneous HCV clearance in children vary greatly between studies (Ceci et al., 2001; Palomba et al., 1996; Tovo et al., 2000). Though pediatric HCV infection associated with mother-to-child transmission is thought to be generally asymptomatic (Farmand et al., 2011), the natural history of chronic hepatitis C in children is not completely understood (Le Campion et al., 2012). Molecular evolution of HCV in chronically-infected infants is characterized by a high degree of divergence that correlates with the development of immunity against the virus (Farci et al., 2006). In turn, high diversity has been associated with progression to chronic infection and poor response to treatment (Farci et al., 2000; Morishima et al., 2006). Thus, viral evolution is a critical factor that in many ways influences the outcome of HCV infection. Here, we compare the molecular evolution of HCV among three unrelated children who were infected via mother-to-child transmission, including two subjects who were co-infected with human immunodeficiency virus type 1(HIV-1).
2. Materials and methods
Patients’ characteristics are summarized in Table 1. Detailed case reports were described elsewhere (Canobio et al., 2004; Larouche et al., 2012). None of the children responded satisfactorily to treatment with pegylated interferon alfa-2b (PegIFN) and ribavirin (RBV) (Larouche et al., 2012; Quesnel-Vallieres et al., 2008) (Table 1). Serum was separated from venous blood samples by centrifugation. Viral RNA was isolated using the QIAamp Viral RNA Mini Kit (Qiagen, Mississauga, Canada). Hypervariable region 1 (HVR1) of the HCV E2 protein was amplified using previously-described primers and PCR conditions (Farci et al., 2000, 2002). Amplicons were sized on agarose gels, extracted, and subcloned into pCR2.1 using the Topo TA Cloning method (Invitrogen, Mississauga, Canada). Approximately 20 independent recombinants were randomly selected and subjected to unidirectional Sanger sequencing using an ABI 3730xl automated DNA sequencer (Applied Biosystems, Foster City, CA). Sequences were visualized and manually edited using Chromas v. 1.45 (Technelysium, Southport, Australia). The intra-host viral population structure, complexity and variant persistence were assessed using median joining network (MJN) analysis as implemented in Network v. 4.611, using an epsilon value of zero an calculating a full MJN (Bandelt et al., 1999; Fonseca-Coronado et al., 2012), assigning weights to individual nucleotide positions as described by Henikoff and Henikoff (1994), and map color analysis, respectively. Multiple nucleotide alignments were generated using MAFFT v. 7, using a subgenomic region of 264 nucleotides in lenght (Katoh and Standley, 2013). The structure of the viral population for each patient was assessed using a Bayesian approach as described elsewhere (Cheng et al., 2013). The clustering was performed with increasing levels in the hierarchy (2–20; Δ = 2) and 20 as the upper bound for number of clusters. Communities were defined as the number of genetically diverged groups in population as random variables.
Table 1.
Clinical characteristics of study subjects.
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Gestational age (weeks) | 35.7 | 39.0 | 39.0 |
| Birth weight (g) | 2560 | 3270 | 3160 |
| Mode of delivery | Caesarean sectiona | Vaginal | Vaginal |
| HCV genotype | 1a | 1b | 1a |
| Co-infection with HIV-1 | No | Yes (clade B) | Yes (clade B)b |
| PegIFN alfa-2b and ribavirin treatment | PegIFN: 50 μg/week ribavirin: 600 mg/day | PegIFN: 3 × 106 IU/m2 three times/week (BSA = 0.95m2) ribavirin: 200 mg BID (15 mg/[kg day]) | PegIFN: 80 mg once/week ribavirin: 400 mg BID (14 mg/[kg day]) |
| Duration of PegIFN alfa-2b and ribavirin treatment (weeks) | 30 (9.42–10.00 years of age) | 42 (6.4–7.2 years of age) | 12 (16.6–16.8 years of age) |
| SVR | No | No | No |
Membrane rupture for 24 h; cephalo-pelvic disproportion.
HIV-1 strain that carried mutations associated with major resistance to multiple antiretroviral agents (zidovudine, lamivudine, abacavir and atazanavir). PegIFN, pegylated interferon; BSA, body surface area; SVR, sustained virologic response.
3. Results
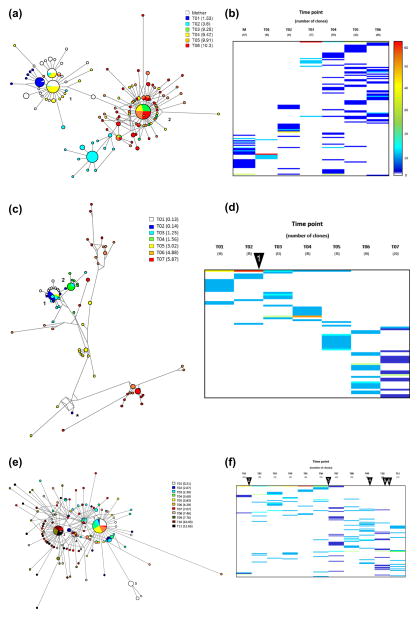
Patient 1, a female born to a mother infected with HCV genotype 1a, showed long-term HCV seronegativity in absence of evidence of HIV-1 infection (Larouche et al., 2012). MJN analysis showed that the viral population was composed of two distinct (1–2) but closely related subpopulations (Fig. 1A). Interestingly, and despite the extended duration of the follow-up, the viral variants obtained from the mother when the child was 9.9 years of age were closely related to these two subpopulations, indicating a very limited molecular evolution in the mother. One of the viral variants from the mother was the center of the network and directly connected both subpopulations. In addition, all viral variants from the mother were grouped within subpopulation 1, with no viral variants found in subpopulation 2. All viral variants from the first time point (T01) in the child were also grouped within subpopulation 1. In contrast, variants identified at later time points were generally grouped within subpopulation 2. Viral variants from T02, T04 and T05 were found intermixed in both sub-populations, whereas viral variants from time points T03 and T06 were found exclusively in subpopulation 2. The complexity of the variant spectrum and the persistence of individual variants were assessed using a color map approach. As shown in Fig. 1B, the viral populations from the mother and the patient’s earlier time points (T01–T03) showed considerably less complexity in comparison to the later time points (Fig. 1B). In general, the nucleotide distances were rather small (mean = 1.47, range = 0–7).
Fig. 1.
HCV intrahost molecular evolution. Median-joining network (A, C and E) and color map (B, D and F) approaches were used to assess the architecture of the viral population at each time point in all patients. In the network, each node represents a unique haplotype within the viral population. The size of the node reflects the frequency, in absolute numbers, of each viral variant. The length of the link represents the nucleotide differences between the two different haplotypes. Numbers depict the major variants in each network. Viral persistence of individual viral variants was assessed using color maps. Each line represents a unique variant during the follow up, and the percentages are color coded. Number of clones analyzed at each time point is shown between brackets. Warmer colors represent higher frequencies, while cooler colors depict lower frequencies. Arrowheads represent introduction of or modifications to regimens of antiretroviral therapy administered to coinfected patients. Arrowhead 1 (D): introduction of combination treatment with zidovudine, lamivudine and ritonavir; Arrowhead 2 (F): introduction of zidovudine monotherapy; Arrowhead 3 (F): combination treatment with zidovudine, lamivudine and saquinavir; Arrowhead 4 (F): combination treatment with amprenavir, didanosine and stavudine; Arrowhead 5 (F): combination treatment with lamivudine, stavudine, ritonavir and nelfinavir; Arrowhead 6 (F): combination treatment with didanosine, stavudine, lopinavir and ritonavir (Canobio et al., 2004).
Patient 2, a male who was infected with HCV genotype 1b and co-infected with HIV-1, was treated with zidovudine from birth, with zidovudine-lamivudine combination starting at 6 weeks of age, and with combination antiretroviral therapy (cART; lamivudine, stavudine, and ritonavir) starting at 1.16 years of age (Canobio et al., 2004). The structure of the viral population was considerably more complex than the one observed in Patient 1 (Fig. 1C). Two major viral variants were identified and grouped viral sequences from the first four time points. Interestingly, one variant identified at the second time point was considerably distant from the two major variants and to some degree closer to sequences identified at time point T05 (Fig. 1C; indicated by “*”). Later time points were highly divergent and occupied larger extensions of the sequence space. The viral variants identified through time points T01–T04 showed important similarities in terms of nucleotide sequence (Fig. 1D). Likewise, the profiles of the viral populations observed in the later time points (T05–T07) also exhibited considerable similarities.
Patient 3, a male who was infected with HCV genotype 1a and also co-infected HIV-1, showed moderate immunosuppression and was repeatedly found to be HCV-seronegative. The subject was treated with zidovudine monotherapy until age 6 and various cART regimens were subsequently introduced (Canobio et al., 2004). At least five different major variants were identified among samples obtained from this patient (Fig. 1E). The major variants were genetically similar and included viral sequences that were identified throughout the follow-up period. Major sequence 1 and 3 comprised variants isolated from ten different time points, including early and late time points. Conversely, major variants 2 and 4 primarily comprised sequences identified in the later time points. The remaining major sequence (5) included viral variants from the first time point. Several viral variants persisted throughout the follow-up period (Fig. 1F). Later time points (T07–T11) showed a higher degree of complexity in comparison to the early time points.
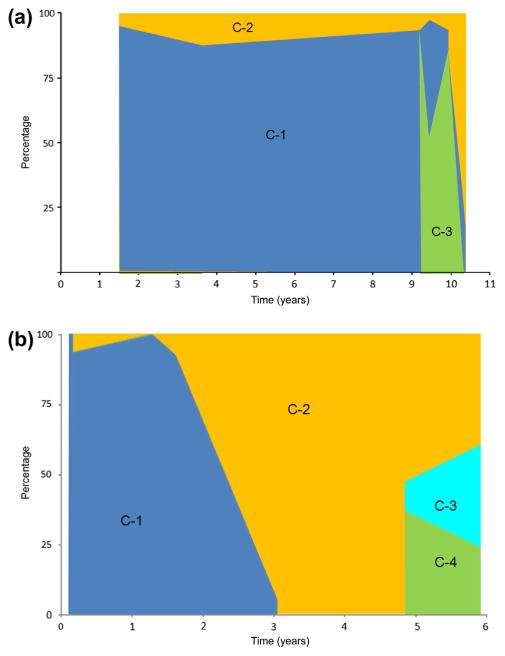
HCV circulates within the infected host as co-evolving viral communities or subpopulations (Ramachandran et al., 2011). Therefore, the structure of the viral population for each patient was assessed using a Bayesian approach (Cheng et al., 2013). Patient 1 exhibited three different viral communities (Fig. 2A). Communities 1 and 2 were present throughout the follow-up period. However, a third viral community arose at 9.25 years of age and became the predominant population at the late time points. Community 1 was the predominant subpopulation throughout the observation period. In the case of Patient 2, 4 HCV communities were identified. Communities 1 and 2 represented the major subpopulations at early time points. However, community 1 was no longer present at late time points. Two extra communities (3 and 4) arose at the late time points accounting for more than 50% of the entire viral population. Viral variants found in Patient 3 formed one unique community (data not shown).
Fig. 2.
Population structure analysis. All viral variants from Patient 1 (A) and Patient 2 (B) were analyzed used using a Bayesian approach to identify the communities within the population. The frequency of any given community at different time points is color coded for each community.
4. Discussion
In this study, we have shown that HCV evolution can exhibit profound differences among children who were infected as a result of mother-to-child transmission. Importantly, these 3 patients exhibited some degree of immunologic dysfunction: Patient 1 was persistently seronegative, while Patient 2 and Patient 3 were co-infected with HIV-1 and were born at a time when highly active antiretroviral therapy was not readily available. Our study does not include cases with no preexisting conditions, and therefore, the long term molecular evolution of HCV under normal immunological pressure could not be assessed. Importantly, limited genetic diversity has been previously reported among pediatric cases associated with vertical transmission (Gismondi et al., 2009). In general, molecular evolution of HCV is heterogeneous (Cruz-Rivera et al., 2013; Ray et al., 2005). However, under specific circumstances, HCV molecular evolution can be significantly slow. Patient 1 showed no anti-HCV antibody response and few IFN-γ producing cells in the peripheral blood (Larouche et al., 2012). The weak immune response exhibited by the subject presumably exerted a limited pressure over the prevailing viral population, consequently allowing limited divergence for an extended period of time. This situation is reminiscent of what was reported in HCV-infected children suffering from X-linked agammaglobulinemia, a hereditary deficit that interferes with the development of humoral immune responses (Gaud et al., 2003). Interestingly, the viral population from the mother, which was sampled 9.91 years after the birth of the child, also remained in close proximity to the viral variants from the subject. While the limited divergence could be explained at least partially by development of tolerance in the child, the slow molecular evolution of HCV in the mother is a remarkable finding (Larouche et al., 2012).
HIV co-infection has been reported to be associated with limited genetic complexity of HCV variant spectra (Lopez-Labrador et al., 2007). Consistent with this, the diversity of HCV quasispecies is influenced by seroconversion to HIV-1 and varies as a function of CD4 cell counts in co-infected subjects (Mao et al., 2001; Roque-Afonso et al., 2002). Conversely, patient 2 exhibited a high degree of HCV intra-host genetic variation. In general, the composition of the viral population changed over time, occupying different regions of the sequence space (Fig. 1D). Patient 3 also exhibited a rather high degree of HCV complexity that was associated with initiation of antiretroviral therapy (Canobio et al., 2004), a phenomenon that was previously reported in co-infected adults and which is thought to relate to the rapid restoration of immune pressure as a result of effective control of HIV-1 replication and associated immune suppression (Blackard et al., 2004; Shuhart et al., 2006).
In contrast, treatment of HCV infection with PegIFN, which did not lead to sustained virologic responses in all 3 cases, was not associated with commensurate variations in the complexity of the variant spectrum (Table 1). This lack of variation in the quasi-species following introduction of IFN treatment was previously shown to predict non-response to IFN treatment in adults chronically infected with HCV (Farci et al., 2002). However, the restricted sample size and the heterogeneity of the cases described in the present study limit the formulation of any strong conclusions, and further studies with larger number of patients will be required to confirm these observations.
The extended timeframe in this follow-up study (i.e. 6–11 years) allowed for a comprehensive depiction of the landscape of the sequence space in which these HCV strains evolved. It is important to mention that in the two patients displaying high degree of HCV genetic diversity, it was possible to identify viral variants from the early points in the late time points. This indicates that certain variants can persist for long periods of times. We hypothesize that the entirety of the variant spectra was not sampled in all time points because of the limited sensitivity of the methodology. Recent studies have shown the advantages of using next generation sequencing platforms for the analysis of the HCV intra-host variability (Fonseca-Coronado et al., 2012).
Examination of HCV molecular evolution has shown the coexistence of genetically distinct viral lineages that are not detected at all time points in infected patients (Alfonso et al., 2005; Ramachandran et al., 2011; Smith et al., 2010). Here, the Bayesian analysis showed a relative small number of communities circulating among these cases. Patients 1 and 2 showed a predominant community at the early time points that was significantly reduced in the later time points. In both patients, the rise of new communities at the late time points was the characteristic feature. Interestingly, Patient 3 only exhibited one community throughout the follow-up. Adaptation to late stages of infection has been previously reported, although, the participating mechanisms responsible for this adaptation have not been elucidated (Ramachandran et al., 2011). However, temporal cooperation between viral subpopulations at different stages of chronic infection has been proposed as a plausible scenario. Thus, a particular viral population at a given stage elicits a specific effect on the host and reduces the host selection pressure for the next viral population until the final population achieves a stable state (Ramachandran et al., 2011).
In summary, HCV exhibits a varied array of genetic variability and heterogeneity among children who acquired HCV infection via mother-to-child transmission. The characteristic plasticity and dynamic processes responsible for the genetic variation displayed by HCV appears to allow for a rapid adaptation to changes in the microenvironment where the viral population resides. This genetic variation represents an important challenge for the development of prophylactic measures.
Acknowledgments
This research protocol was approved by the Ethics Review Board of CHU Sainte-Justine, where patients were enrolled. Supported in part by the Canadian Institutes for Health Research (CIHR)/Health Canada Research Initiative on Hepatitis C (grant No. EOP-41537), by CANFAR, the Canadian Foundation for AIDS Research (grant No. 013515), and by the Réseau SIDA-maladies infectieuses of the Fonds de la recherche du Québec-santé (FRQS). A.L. is the recipient of graduate scholarships from Fondation CHU Sainte-Justine and FRQS. This work was partially supported by Proyecto PAPIIT TA200112, Dirección General de Asuntos del Personal Academico, Universidad Nacional Autónoma de México y Fondo Sectorial de Investigación en Salud y Seguridad Social 2012 C01-181585, Consejo Nacional de Ciencia y Tecnología.
References
- Alfonso V, Mbayed VA, Sookoian S, Campos RH. Intra-host evolutionary dynamics of hepatitis C virus E2 in treated patients. J Gen Virol. 2005;86:2781–2786. doi: 10.1099/vir.0.81084-0. [DOI] [PubMed] [Google Scholar]
- Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Blackard JT, Yang Y, Bordoni P, Sherman KE, Chung RT AIDS Clinical Trials Group 383 Study Team. Hepatitis C virus (HCV) diversity in HIV–HCV-coinfected subjects initiating highly active antiretroviral therapy. J Infect Dis. 2004;189:1472–1481. doi: 10.1086/382959. [DOI] [PubMed] [Google Scholar]
- Bortolotti F, Iorio R, Resti M, Camma C, Marcellini M, Giacchino R, Marazzi MG, Verucchi G, Zancan L, Barbera C, Maggiore G, Vajro P, Giannattasio A, Bartolacci S. Epidemiological profile of 806 Italian children with hepatitis C virus infection over a 15-year period. J Hepatol. 2007;46:783–790. doi: 10.1016/j.jhep.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Canobio S, Guilbert CM, Troesch M, Samson J, Lemay M, Pelletier VA, Bernard-Bonnin AC, Kozielski R, Lapointe N, Martin SR, Soudeyns H. Differing patterns of liver disease progression and hepatitis C virus (HCV) quasispecies evolution in children vertically coinfected with HCV and human immunodeficiency virus type 1. J Clin Microbiol. 2004;42:4365–4369. doi: 10.1128/JCM.42.9.4365-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, Mautone A, Impedovo L, Ierardi E, Mastroianni M, Bettocchi S, Selvaggi L. Vertical transmission of hepatitis C virus in a cohort of 2447 HIV-seronegative pregnant women: a 24-month prospective study. J Pediatr Gastroenterol Nutr. 2001;33:570–575. doi: 10.1097/00005176-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Cheng L, Connor TR, Siren J, Aanensen DM, Corander J. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol Biol Evol. 2013;30:1224–1228. doi: 10.1093/molbev/mst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751–755. doi: 10.1002/hep.510310328. [DOI] [PubMed] [Google Scholar]
- Cruz-Rivera M, Carpio-Pedroza JC, Escobar-Gutierrez A, Lozano D, Vergara-Castaneda A, Rivera-Osorio P, Martinez-Guarneros A, Chacon CA, Fonseca-Coronado S, Vaughan G. Rapid hepatitis C virus divergence among chronically infected individuals. J Clin Microbiol. 2013;51:629–632. doi: 10.1128/JCM.03042-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Gutierrez A, Vazquez-Pichardo M, Cruz-Rivera M, Rivera-Osorio P, Carpio-Pedroza JC, Ruiz-Pacheco JA, Ruiz-Tovar K, Vaughan G. Identification of hepatitis C virus transmission using a next-generation sequencing approach. J Clin Microbiol. 2012;50:1461–1463. doi: 10.1128/JCM.00005-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci P, Quinti I, Farci S, Alter HJ, Strazzera R, Palomba E, Coiana A, Cao D, Casadei AM, Ledda R, Iorio R, Vegnente A, Diaz G, Tovo PA. Evolution of hepatitis C viral quasispecies and hepatic injury in perinatally infected children followed prospectively. Proc Natl Acad Sci USA. 2006;103:8475–8480. doi: 10.1073/pnas.0602546103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH, Alter HJ. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- Farci P, Strazzera R, Alter HJ, Farci S, Degioannis D, Coiana A, Peddis G, Usai F, Serra G, Chessa L, Diaz G, Balestrieri A, Purcell RH. Early changes in hepatitis C viral quasispecies during interferon therapy predict the therapeutic outcome. Proc Natl Acad Sci USA. 2002;99:3081–3086. doi: 10.1073/pnas.052712599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmand S, Wirth S, Henneke P. Reply to the correspondence letter by Dr. Giuseppe Indolfi “Spontaneous clearance of hepatitis C virus in vertically infected children. Any clue for treatment?”. Eur J Pediatr. 2011 doi: 10.1007/s00431-011-1582-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fonseca-Coronado S, Escobar-Gutierrez A, Ruiz-Tovar K, Cruz-Rivera MY, Rivera-Osorio P, Vazquez-Pichardo M, Carpio-Pedroza JC, Ruiz-Pacheco JA, Cazares F, Vaughan G. Specific detection of naturally occurring hepatitis C virus mutants with resistance to telaprevir and boceprevir (protease inhibitors) among treatment-naive infected individuals. J Clin Microbiol. 2012;50:281–287. doi: 10.1128/JCM.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns X, Purcell RH, Bukh J. Quasispecies in viral persistence and pathogenesis of hepatitis C virus. Trends Microbiol. 1999;7:402–410. doi: 10.1016/s0966-842x(99)01590-5. [DOI] [PubMed] [Google Scholar]
- Gaud U, Langer B, Petropoulou T, Thomas HC, Karayiannis P. Changes in hypervariable region 1 of the envelope 2 glycoprotein of hepatitis C virus in children and adults with humoral immune defects. J Med Virol. 2003;69:350–356. doi: 10.1002/jmv.10296. [DOI] [PubMed] [Google Scholar]
- Gismondi MI, Becker PD, Diaz Carrasco JM, Guzman CA, Campos RH, Preciado MV. Evolution of hepatitis C virus hypervariable region 1 in immunocompetent children born to HCV-infected mothers. J Viral Hepat. 2009;16:332–339. doi: 10.1111/j.1365-2893.2009.01071.x. [DOI] [PubMed] [Google Scholar]
- Gray RR, Salemi M, Klenerman P, Pybus OG. A new evolutionary model for hepatitis C virus chronic infection. PLoS Pathog. 2012;8:e1002656. doi: 10.1371/journal.ppat.1002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG. Position-based sequence weights. J Mol Biol. 1994;243:574–578. doi: 10.1016/0022-2836(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Jhaveri R, Grant W, Kauf TL, McHutchison J. The burden of hepatitis C virus infection in children: estimated direct medical costs over a 10-year period. J Pediatr. 2006;148:353–358. doi: 10.1016/j.jpeds.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larouche A, Gaetan G, El-Bilali N, Quesnel-Vallieres M, Martin SR, Alvarez F, Shoukry NH, Soudeyns H. Seronegative hepatitis C virus infection in a child infected via mother-to-child transmission. J Clin Microbiol. 2012;50:2515–2519. doi: 10.1128/JCM.00622-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Campion A, Larouche A, Fauteux-Daniel S, Soudeyns H. Pathogenesis of hepatitis C during pregnancy and childhood. Viruses. 2012;4:3531–3550. doi: 10.3390/v4123531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Labrador FX, Dove L, Hui CK, Phung Y, Kim M, Berenguer M, Wright TL. Trends for genetic variation of hepatitis C virus quasispecies in human immunodeficiency virus-1 coinfected patients. Virus Res. 2007;130:285–291. doi: 10.1016/j.virusres.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q, Ray SC, Laeyendecker O, Ticehurst JR, Strathdee SA, Vlahov D, Thomas DL. Human immunodeficiency virus seroconversion and evolution of hepatitis C virus quasispecies. J Virol. 2001;75:3259–3267. doi: 10.1128/JVI.75.7.3259-3267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Morishima C, Polyak SJ, Ray R, Doherty MC, Di Bisceglie AM, Malet PF, Bonkovsky HL, Sullivan DG, Gretch DR, Rothman AL, Koziel MJ, Lindsay KL. Hepatitis C virus-specific immune responses and quasi-species variability at baseline are associated with nonresponse to antiviral therapy during advanced hepatitis C. J Infect Dis. 2006;193:931–940. doi: 10.1086/500952. [DOI] [PubMed] [Google Scholar]
- Palomba E, Manzini P, Fiammengo P, Maderni P, Saracco G, Tovo PA. Natural history of perinatal hepatitis C virus infection. Clin Infect Dis. 1996;23:47–50. doi: 10.1093/clinids/23.1.47. [DOI] [PubMed] [Google Scholar]
- Quesnel-Vallieres M, Lemay M, Lapointe N, Martin SR, Soudeyns H. HCV quasispecies evolution during treatment with interferon alfa-2b and ribavirin in two children coinfected with HCV and HIV-1. J Clin Virol. 2008;43:236–240. doi: 10.1016/j.jcv.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Campo DS, Dimitrova ZE, Xia GL, Purdy MA, Khudyakov YE. Temporal variations in the hepatitis C virus intrahost population during chronic infection. J Virol. 2011;85:6369–6380. doi: 10.1128/JVI.02204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med. 2005;201:1753–1759. doi: 10.1084/jem.20050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque-Afonso AM, Robain M, Simoneau D, Rodriguez-Mathieu P, Gigou M, Meyer L, Dussaix E. Influence of CD4 cell counts on the genetic heterogeneity of hepatitis C virus in patients coinfected with human immunodeficiency virus. J Infect Dis. 2002;185:728–733. doi: 10.1086/339297. [DOI] [PubMed] [Google Scholar]
- Shuhart MC, Sullivan DG, Bekele K, Harrington RD, Kitahata MM, Mathisen TL, Thomassen LV, Emerson SS, Gretch DR. HIV infection and antiretroviral therapy: effect on hepatitis C virus quasispecies variability. J Infect Dis. 2006;193:1211–1218. doi: 10.1086/502974. [DOI] [PubMed] [Google Scholar]
- Slowik MK, Jhaveri R. Hepatitis B and C viruses in infants and young children. Semin Pediatr Infect Dis. 2005;16:296–305. doi: 10.1053/j.spid.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Smith JA, Aberle JH, Fleming VM, Ferenci P, Thomson EC, Karayiannis P, McLean AR, Holzmann H, Klenerman P. Dynamic coinfection with multiple viral subtypes in acute hepatitis C. J Infect Dis. 2010;202:1770–1779. doi: 10.1086/657317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovo PA, Pembrey LJ, Newell ML. Persistence rate and progression of vertically acquired hepatitis C infection. European paediatric hepatitis C virus infection. J Infect Dis. 2000;181:419–424. doi: 10.1086/315264. [DOI] [PubMed] [Google Scholar]