Abstract
Background
Red blood cell transfusions (RBCT) carry risk of transfusion-related immunodulation that may impact postoperative recovery. This study examined the association between perioperative RBCT and short-term postoperative outcomes following gastrectomy for gastric cancer.
Methods
Using the American College of Surgeons National Surgical Quality Improvement Program database, we compared outcomes of patients (transfused v. nontransfused) undergoing elective gastrectomy for gastric cancer (2007–2012). Outcomes were 30-day major morbidity, mortality and length of stay. The association between perioperative RBCT and outcomes was estimated using modified Poisson, logistic, or negative binomial regression.
Results
Of the 3243 patients in the entire cohort, we included 2884 patients with nonmissing data, of whom 535 (18.6%) received RBCT. Overall 30-day major morbidity and mortality were 20% and 3.5%, respectively. After adjustment for baseline and clinical characteristics, RBCT was independently associated with increased 30-day mortality (relative risk [RR] 3.1, 95% confidence interval [CI] 1.9–5.0), major morbidity (RR 1.4, 95% CI 1.2–1.8), length of stay (RR 1.2, 95% CI 1.1–1.2), infections (RR 1.4, 95% CI 1.1–1.6), cardiac complications (RR 1.8, 95% CI 1.0–3.2) and respiratory failure (RR 2.3, 95% CI 1.6–3.3).
Conclusion
Red blood cell transfusions are associated with worse postoperative short-term outcomes in patients with gastric cancer. Blood management strategies are needed to reduce the use of RBCT after gastrectomy for gastric cancer.
Abstract
Contexte
Les transfusion de globules rouges (TGR) entrainent une immunosuppression qui peut entraver la récupération post-opératoire. Cette étude évalue l’association entre les TGR péri-opératoires et l’issue post-opératoire après gastrectomie pour cancer gastrique (CG).
Méthodes
Le registre de l’ACS-NSQIP fut utilisé pour comparer l’issue des patients subissant une gastrectomie élective pour CG de 2007 à 2012, selon la TGR. La morbidité majeure et mortalité à 30 jours, et la durée d’hospitalisation furent analysées. L’association entre la TGR et les résultats post-opératoires fut estimée par régressions de Poisson modifiée, logistique, et binomiale.
Résultats
Parmi 3243 gastrectomies, 2884 patients avec des données complètes furent inclus, dont 535 (18,6 %) furent transfusés. La morbidité globale à 30 jours était 20 % et la mortalité 3,5 %. Après avoir contrôlé pour les caractéristiques démographiques et cliniques pertinentes, les TGR démontraient une association indépendante avec une morbidité majeure (risque relatif [RR] 3,1; intervalle de confiance [IC] à 95 % 1,9–5,0), une mortalité (RR 1,4; IC à 95 % 1,2–1,8), et une durée d’hospitalisation (RR 1,2; IC à 95 % 1,1–1,2) accrues. Les TGR étaient aussi associées aux complications infectieuses (RR 1,4; IC à 95 % 1,1–1,6), cardiaques (RR 1,8; IC à 95 % 1,0–3,2), et respiratoires (RR 1,4; IC à 95 % 1,6–3,3).
Conclusion
Les TGR sont associées à une détérioration de l’issue post-opératoire après gastrectomie pour CG, dont la morbidité majeure, la mortalité, et la durée d’hospitalisation. Des stratégies multidisciplinaires de gestion du risque transfusionnel sont nécessaires afin de limiter l’utilisation des TGRs après gastrectomie pour CG.
As the second leading cause of cancer-related death worldwide, gastric cancer poses a substantial health care and societal burden.1 While advances in perioperative chemotherapy and targeted therapy contribute to improving long-term outcomes for gastric cancer, 2,3 surgery remains the cornerstone of multimodal gastric cancer treatment, with survival contingent on complete resection.4 Advances in surgical technique and perioperative care have led to 5% perioperative mortality in high-volume centres.5 Further reduction of surgical mortality and morbidity is still needed to limit their impact on quality of life and to enable patients to take advantage of systemic therapies when available.
Red blood cell transfusions (RBCT) have been associated with an increased risk of postoperative morbidity, perhaps owing to transfusion-related immune modulation. 6,7 With up to 60% presenting with perioperative anemia, patients undergoing surgery for gastric malignancy are at particular risk for needing RBCT.8,9 The current literature looking at the impact of RBCT on gastric cancer surgery is restricted to studies with small sample sizes from individual hospitals.10–13
The purpose of the present study was to estimate the effect of RBCT on 30-day postoperative outcomes following elective gastrectomy for gastric cancer using the large multi-institutional American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) data set.
Methods
Study design and population
We conducted a retrospective study using a cohort of patients entered in the ACS-NSQIP registry between Jan. 1, 2007, and Dec. 31, 2012, who underwent a gastrectomy, defined by Current Procedural Terminology (CPT) codes, and had a postoperative diagnosis of gastric cancer (ICD-9 code 151.x). We excluded patients who were undergoing an emergent operation, who were younger than 18 years, or for whom we were missing data on the following key variables: sex, American Society of Anesthesiologists (ASA) class, preoperative hematocrit level, and baseline cardiovascular comorbidities (cardiac heart failure, myocardial infarction, angina, hypertension). Albeit infrequent, there were sometimes missing values for the following variables: ASA class (< 1%), sex (< 1%), hematocrit (< 7%) and cardiac history (10%). The majority of the missing data pertained to patients treated in more recent years (2011–2012). Very few patients treated between 2007 and 2010 were missing data. To understand how this increasing level of missing data over time impacted the results, we completed a sensitivity analysis restricted to patients who were operated during the timeframe with complete data (2007–2010).
Data sources
The ACS-NSQIP database is a multicentre prospective registry designed to evaluate risk-adjusted outcomes of surgical patients. A total of 525 institutions, including both teaching and nonteaching hospitals, participate and are representative of various regions in North America. Variables collected include demographic characteristics, preoperative risk factors, procedural indication and details, and 30-day detailed postoperative morbidity and mortality. Data are collected by trained abstractors and validated for accuracy.14 The ACS-NSQIP data collection and auditing methods are presented elsewhere.15–18
Patients’ demographic and clinical characteristics and their treatment details were collected from the gastrectomy admission records in the ACS-NSQIP database. The extent of surgery was defined according to CPT codes, and procedures were subdivided into total gastrectomy (CPT 43620, 43621 and 43622), subtotal gastrectomy (CPT 43631, 43632, 43633 and 43634) and multivisceral resection (CPT 38120, 38102 and 38129 for splenectomy; 44140, 44141 and 44160 for colectomy; 48146, 48145 and 48140 for pancreatectomy; 44121, 44120 and 44125 for enterectomy; 47100, 47120 and 47122 for hepatectomy; and 43124, 43117, 43118, 43101, 43121, 43122 and 43123 for esophagectomy). Cardiac comorbidities were defined as a history of congestive heart failure (in the 30 d before surgery), myocardial infarction (in the 6 mo before surgery), angina (in the 30 d before surgery), or hypertension requiring antihypertensive medication (in the 30 d before surgery). We used the World Health Organization definition of anemia: hematocrit less than 40%.19 Perioperative RBCT was defined as receiving packed red blood cells (PRBC) intraoperatively or within 72 h postoperatively.14 A dichotomous transfusion variable was created. None of the patients were missing data on this variable.
Outcomes
Primary outcomes were 30-day mortality and major morbidity. Major morbidity was defined as the occurrence of deep or organ-space surgical site infection (SSI), wound dehiscence, pneumonia, pulmonary embolism, prolonged mechanical ventilation beyond 48 h, unplanned re-intubation, renal failure, sepsis, myocardial infarction, cardiac arrest, or stroke. Postoperative mortality was defined as death within 30 days of the operation.
Secondary outcomes included system-specific 30-day morbidity grouped into infectious events (SSI, pneumonia, urinary tract infection, sepsis, septic shock), cardiac events (myocardial infarction, cardiac arrest), respiratory failure (prolonged mechanical ventilation > 48 h, unplanned reintubation), venous thromboembolic events (pulmonary embolism, deep vein thrombosis), unplanned reoperation as well as hospital length of stay.14
Statistical analysis
Comparative statistics between the transfused and nontransfused patients were assessed with independent samples t tests (normally distributed) and Wilcoxon rank sum tests (skewed) for continuous data, and χ2 tests for categorical data. Categorical data are reported as absolute numbers (n) and proportions (%), and continuous data are reported as means with interquartile ranges (IQR). “Unknown” categories were created for missing data.
We used a modified Poisson regression analysis to examine the association between RBCT and common dichotomous outcomes (> 10%), while logistic regression was used for uncommon dichotomous outcomes (≤ 10%). Length of stay values were treated as count data, but since the data were skewed and violated the assumptions of Poisson regression, negative binomial regression was used to measure the association between transfusion and length of stay. We selected covariates a priori based on timing (known preoperatively), clinical relevance (considered when assessing a patient for risk of adverse perioperative events) and existing literature (established relationship with worse surgical outcomes). Variables on the causal pathway were excluded from the multivariate regression in order to estimate the total effect of blood transfusions on worse surgical outcomes. The most parsimonious model was selected to maintain adequate study power. Multivariate analyses were adjusted for the following variables: age, body mass index (BMI; < 20, 20–29, 30–39, ≥ 40, unknown), race, ASA class, preoperative hematocrit values, cardiac comorbidities (a composite variable of hypertension, history of congestive heart failure, angina or myocardial infarction), bleeding disorder, preoperative international normalized ratio (INR; normal, abnormal, unknown), preoperative bilirubin (normal, abnormal, unknown), extent of gastrectomy and surgical procedure (total gastrectomy v. subtotal gastrectomy, and whether or not a multivisceral resection was performed), the year of the operation and operative duration. Results are reported as relative risks (RR) with 95% confidence intervals (CI). All statistical analyses were done using SAS version 9.3 for Windows (SAS Institute Inc.). We considered results to be significant at p < 0.05.
Results
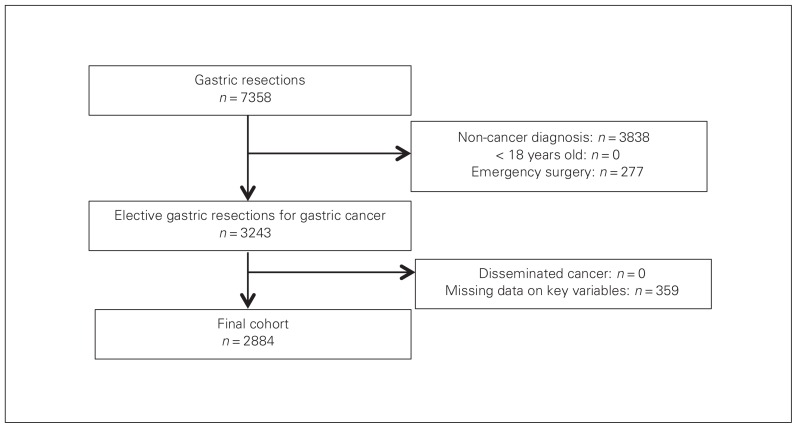
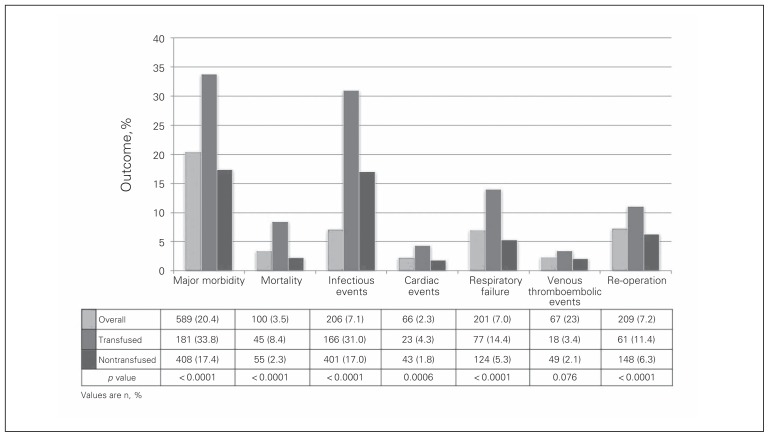
We identified 3243 patients in the cohort, 2884 of whom satisfied inclusion criteria (Fig. 1). Of those, 535 (18.6%) received RBCT. Demographic and clinical characteristics are presented in Table 1. Transfused patients were older (p < 0.001), had a higher ASA class (p < 0.001) and were more likely to have diabetes (p = 0.003), chronic obstructive pulmonary disease (p < 0.001), or a bleeding disorder (p < 0.001) than the nontransfused patients. Transfused patients were also more likely to have a total gastrectomy that included a multivisceral resection (Table 2). The mean operative duration was 30 minutes longer for transfused patients (p < 0.001). The 30-day postoperative outcomes are detailed in Figure 2. Patients receiving RBCT were more likely to experience 30-day major morbidity (33.8% v. 17.4%, p < 0.001) and had higher mortality (8.4% v. 2.3%, p < 0.001) than patients who did not receive RBCT.
Fig. 1.
Flow of patients through the study.
Table 1.
Demographic and clinical characteristics of the included patients based on transfusion status
| Characteristic | Group; no. (%) or mean (IQR) | p value | |
|---|---|---|---|
| Transfused (n = 535) | Nontransfused (n = 2349) | ||
| Age, yr | < 0.001 | ||
| < 40 | 10 (1.9) | 77 (3.3) | |
| 40–64 | 142 (26.5) | 862 (36.7) | |
| 65–74 | 168 (31.4) | 683 (29.0) | |
| ≥ 75 | 215 (40.2) | 727 (31.0) | |
| Male sex | 320 (59.8) | 1366 (58.1) | 0.48 |
| Race | 0.05 | ||
| White | 255 (47.7) | 1132 (48.2) | |
| Black | 83 (15.5) | 323 (13.8) | |
| Other | 46 (8.6) | 293 (12.5) | |
| Unknown | 151 (28.2) | 601 (25.6) | |
| ASA Score | < 0.001 | ||
| I | 3 (0.56) | 30 (1.3) | |
| II | 99 (18.5) | 761 (32.4) | |
| III | 363 (67.9) | 1434 (61.1) | |
| IV/V | 70 (13.1) | 124 (5.3) | |
| BMI | 0.13 | ||
| < 20 | 56 (10.5) | 203 (8.6) | |
| 20–29 | 352 (65.8) | 1576 (67.0) | |
| 30–40 | 99 (18.5) | 476 (20.3) | |
| ≥ 40 | 23 (4.3) | 62 (2.6) | |
| Unknown | 5 (0.93) | 32 (1.4) | |
| Diabetes | 134 (22.8) | 453 (19.3) | 0.003 |
| Active smoker | 80 (15.7) | 431 (18.4) | 0.06 |
| COPD | 47 (8.8) | 113 (4.8) | < 0.001 |
| Dyspnea | < 0.001 | ||
| At rest | 13 (2.4) | 12 (0.51) | |
| With moderate exertion | 91 (17.0) | 235 (10.0) | |
| Hypertension | 353 (66.0) | 1417 (60.3) | 0.015 |
| Cardiac comorbidity | 535 (67.1) | 1422 (60.6) | 0.005 |
| Bleeding disorder | 38 (7.1) | 74 (3.2) | < 0.001 |
| Corticosteroid use | 11 (2.1) | 40 (1.7) | 0.58 |
| Weight loss > 10% | 112 (20.9) | 326 (13.9) | < 0.001 |
| Preoperative albumin < 3g/dL | 358 (66.9) | 1710 (72.8) | < 0.001 |
| Preoperative serum albumin (g/dL) | 3.4 (3.0–3.9) | 3.8 (3.5–4.2) | < 0.001 |
| Preoperative hematocrit (%) | 31.7 (28.4–37.7) | 36.9 (33.7–40.4) | < 0.001 |
| Preoperative INR | 1.1 (1.0–4.4) | 1.0 (1.0–3.6) | < 0.001 |
| Preoperative chemotherapy (within 30 d of surgery) | 29 (5.4) | 159 (6.8) | 0.47 |
| Preoperative radiation therapy (within 30 d of surgery) | 10 (1.9) | 54 (2.3) | 0.79 |
ASA = American Society of Anesthesiologists; BMI = body mass index; COPD = chronic pulmonary obstructive disease; INR = international normalized ratio; IQR = interquartile range.
Table 2.
Operative characteristics for the included patients, based on transfusion status
| Variable | Group; no. (%) or mean (IQR) | p value | |
|---|---|---|---|
| Transfused | Nontransfused | ||
| Operative duration, min | 253 (180–305) | 223 (150–274) | < 0.001 |
| Operative year | 0.09 | ||
| 2007 | 89 (16.6) | 369 (15.7) | |
| 2008 | 90 (16.8) | 415 (17.7) | |
| 2009 | 109 (20.4) | 446 (19.0) | |
| 2010 | 81 (15.4) | 470 (20.0) | |
| 2011 | 92 (17.2) | 328 (14.0) | |
| 2012 | 74 (13.8) | 321 (13.7) | |
| Procedure | < 0.001 | ||
| Total gastrectomy | 243 (45.4) | 841 (35.8) | |
| Subtotal gastrectomy | 292 (54.6) | 1508 (64.2) | |
| Multivisceral resection | 84 (15.7) | 205 (8.7) | < 0.001 |
| Colectomy | 25 (4.7) | 37 (1.6) | |
| Pancreatectomy | 28 (5.2) | 32 (1.4) | |
| Enterectomy | 14 (2.6) | 41 (1.8) | |
| Hepatectomy | 28 (5.2) | 96 (4.1) | |
| Esophagectomy | 7 (1.3) | 16 (0.7) | |
| Splenectomy | 29 (5.4) | 40 (1.7) | |
IQR = interquartile range.
Fig. 2.
Postoperative outcomes for patients undergoing gastrectomy for gastric cancer based on transfusion status.
Results of the multivariate analysis are presented in Table 3. Transfusion was independently associated with increased 30-day mortality (RR 3.1, 95% CI 1.9–5.0) and major morbidity (RR 1.4, 95% CI 1.2–1.8), after adjusting for baseline and clinical characteristics. Postoperative infections (RR 1.4, 95% CI 1.1–1.6), cardiac events (RR 1.8, 95% CI 1.0–3.2) and respiratory failure (RR 2.3, 95% CI 1.6–3.3) were increased with RBCT, whereas no association was observed for venous thromboembolic events (RR 1.1, 95% CI 0.6–2.1). Patients receiving RBCT were significantly more likely to undergo an unplanned reoperation (RR 1.6, 95% CI 1.1–2.3). After adjustment, patients receiving RBCT had a longer hospital length of stay (RR 1.2, 95%CI 1.1–1.2).
Table 3.
Association of RBCT and postoperative outcomes following gastrectomy for gastric cancer
| Outcome | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
|
|
|
|||
| RR (95% CI) | p value | RR (95%CI) | p value | |
| Major morbidity | 1.95 (1.68–2.26) | < 0.001 | 1.44 (1.18–1.76) | < 0.001 |
|
| ||||
| Mortality | 3.83 (2.55–5.75) | < 0.001 | 3.06 (1.89–4.95) | < 0.001 |
|
| ||||
| Postoperative infections | 1.81 (1.55–2.12) | < 0.001 | 1.35 (1.14–1.61) | < 0.001 |
|
| ||||
| Cardiac events | 2.41 (1.44–4.03) | < 0.001 | 1.77 (0.97–3.23) | 0.06 |
|
| ||||
| Respiratory failure | 3.02 (2.23–4.08) | < 0.001 | 2.34 (1.64–3.34) | < 0.001 |
|
| ||||
| Venous thromboembolic events | 1.63 (0.94–2.83) | 0.08 | 1.14 (0.61–2.12) | 0.69 |
|
| ||||
| Reoperation | 1.91 (1.40–2.62) | < 0.001 | 1.59 (1.11–2.28) | 0.011 |
|
| ||||
| Length of stay | 1.36 (1.28–1.44) | < 0.001 | 1.15 (1.08–1.22) | < 0.001 |
CI = confidence interval; RBCT = red blood cell transfusion; RR = relative risk.
Adjusted for: age, sex, race, body mass index, American Society of Anesthesiologists class, hematocrit, bilirubin, international normalized ratio, cardiac comorbidities, bleeding disorder, primary operation, multivisceral resection and operative duration.
The conclusions of the analyses did not change when the cohort was restricted to the calendar years with the most complete data (2007–2010).
Discussion
Using the ACS-NSQIP data, we present the impact of RBCT on short-term outcomes following resections for gastric cancer. After adjusting for potential confounders, transfusion was associated with a 3-fold increase in the risk of death, and a 1.5-fold increase in the risk of major morbidity. Transfused patients also had a prolonged hospital stay compared with their nontransfused counterparts.
A large proportion of patients undergoing gastrectomy for gastric cancer are anemic from either intraoperative blood loss or low-rate tumour bleeding.8 Thus, transfusions are common in this patient group, and the effect of transfusion on surgical outcomes is an important issue. Studies of other gastrointestinal surgeries have highlighted the increased morbidity and delayed recovery associated with RBCT, some using the ACS-NSQIP.20 However, these analyses included a variety of surgical procedures with equally variable transfusion risk and morbidity profiles. Owing to the nature of the disease, high frequency of preoperative anemia and requirement for extensive lymph node dissection, patients with gastric cancer present a specific risk for perioperative transfusion requirement.8 The evidence regarding RBCT and its impact on gastric cancer surgery specifically remains limited. Most studies to date have focused on the effects of RBCT on long-term oncologic outcomes, reporting worse survival and recurrence patterns for transfused patients.10,11 Only 1 study investigated short-term outcomes, reporting an increased overall 30-day morbidity with RBCT (60% v. 14.2%, p = 0.024) for a sample of 588 patients at a single institution.13 No morbidity details were presented, such that one cannot decipher the type of complications associated with RBCT.13 Our large multi-institutional sample size provides strong evidence of the detrimental effects of RBCT on postoperative outcomes for gastric cancer, for both local and systemic complications. Unplanned reoperations were also associated with RBCT, but this could have been due to postoperative bleeding requiring both RBCT and re-interventions.
It is believed that RBCT-induced healing impairment results from transfusion-related immune modulation, which can exacerbate the stress-induced postoperative immunosuppressive state.10,21–23 While the exact underlying mechanism of transfusion-related immune modulation is unclear, several hypotheses have been proposed, such as leukocyte-mediated immmunosuppression in allogeneic blood,24,25 a transfusion-induced reduction in natural killer cells and interleukin-2, and the infusion of incompatible major histocompatibility complex antigens between donor and recipient. In the early postoperative course following gastric cancer resection, the increased infectious and cardiopulmonary complications observed in transfused patients may not be solely related to transfusion-related immune modulation; transfusion-associated circulatory overload, allergic reactions, transfusion-related acute lung injury, hemolytic reactions and graft-versus-host disease also have to be considered.14,26 However, the occurrence of these adverse events is well documented and rare enough that they cannot account for all the morbidity excess associated with RBCT that we observed.7 Therefore, there is a potential for impact of RBCT on postoperative morbidity beyond that of traditional direct transfusion-related adverse reactions.
Postoperative morbidity carries repercussions beyond the immediate surgical recovery period. In addition to prolonging it, the increased complication rate seen following transfusions may hinder the delivery of adjuvant cancer therapy. Evidence from breast, colorectal and pancreatic cancer has shown that delays in receipt of adjuvant therapy negatively impacts disease-free survival, disease-specific survival and overall survival.27–30 Given the importance of systemic therapy in gastric cancer survival, minimizing postoperative morbidity in order to get patients, in a timely manner, to their intended oncologic therapy is paramount.2,31 We identified RBCT as being associated with increased postoperative morbidity and thus as a potentially modifiable factor that can be addressed to improve gastric cancer outcomes.
Despite evidence and clinical guidelines supporting restrictive strategies for blood transfusions, practices still vary significantly,32–35 such that RBCT is still considered an overused treatment.36,37 When such gaps between practice and guidelines exist, the most effective strategy to ensure successful changes requires practice-specific data on which to base tailored approaches to knowledge translation.38 This study provides procedure-specific evidence to do so. Comprehensive transfusion reduction initiatives involving blood conservation consultants, institutional guidelines and the use of alternative transfusion strategies have successfully been implemented.39,40 Similar strategies for patients with gastric cancer could be adopted and could also include medical interventions like the use of iron supplementation (alone or in combination with erythropoietin for anemic patients) and antifibrinolytic agents to reduce the need for RBCT.41,42 Further research is required to determine whether blood-conserving tactics (with potential ensuing reduction in transfusion rates) would actually translate into improved outcomes in gastric cancer surgery.
While some large retrospective databases risk containing information bias, the ACS-NSQIP registry has repeatedly been shown to provide accurate and valid data.15–18 The ACS-NSQIP database lacks cancer-specific variables, which may result in uncontrolled confounding, and this could in turn result in an over- or underestimate of the effect of blood transfusions on perioperative morbidity. For example, staging data are not collected. Operations in patients with more advanced disease can be technically challenging, potentially increasing the risk for transfusion and postoperative morbidity. While we used and corrected for extent of gastric resection (total v. subtotal gastrectomy, and multivisceral resection) and operative duration as surrogate markers for technical complexity, those variables do not fully account for the potential influence of cancer stage on receipt of RBCT. Refinement of the timing of RBCT into intra- or postoperative could not be obtained with the data available within ACS-NSQIP. While the impact of RBCT on outcomes is unlikely to differ whether it was administered intra- or postoperatively, such information could be useful in tailoring knowledge-translation efforts to improve adherence to restrictive transfusion guidelines to operating room or postoperative care teams. Finally, potential institutional-level variation in practice and outcomes could not be accounted for in this analysis, since institution and physician information is not available in ACS-NSQIP owing to privacy policies.
Moreover, we acknowledge the challenge of establishing causality in retrospective studies. Ascertaining whether transfusions are the cause of major morbidity directly or whether they are a response to surgical complications is difficult. However, the ACS-NSQIP defines the receipt of perioperative RBCT as occurring within the first 72 h following gastric resection or intraoperatively, thus RBCT administration is likely to have preceded complications. Clinical variables, including hemoglobin level before transfusion, dictating the indication for RBCT were not available. Many factors play a role in the decision to transfuse a patient, and some may contribute to increased morbidity independently of RBCT. Therefore, this analysis could not decipher the indication for individual patients’ transfusions and identify the subgroup of patients in which RBCT could have been avoided. Transfused and nontransfused patients differed significantly at baseline, as evidenced in Table 2, with transfused patients appearing less healthy and therefore at higher risk of postoperative morbidity. To mitigate selection and confounding biases inherent to retrospective studies, we corrected for all known variables associated with RBCT in our robust regression models. In particular, age was included in the multivariate model as a continuous variable in an attempt to account for the increased frequency of transfusion with older age. However, we acknowledge that this does not account for unknown confounders or for residual confounding that may result from potential imperfect measure of comorbidities.
Nevertheless, this study provides results with high external validity, given it is, to our knowledge, the largest and first multi-institutional appraisal of its kind. It provides procedure-specific evidence to raise awareness about the need to minimize use of RBCT when it can be safely avoided for elective gastric cancer resection. Stronger procedure-specific evidence to support change in practice could be obtained with a randomized study design aimed at comparing the impact of a comprehensive blood management strategy focused on restrictive transfusion guidelines compared with traditional practice on postpancreatectomy outcomes.
Conclusion
Perioperative RBCT was associated with increased 30-day mortality, major morbidity and hospital length of stay following elective gastrectomies for gastric cancer. This information furthers the rationale to minimize the use of RBCT in surgical patients when it can be safely avoided. Blood conservation strategies to reduce unnecessary transfusions and their detrimental effects on gastric cancer postoperative outcomes are needed and could be examined in future studies.
Footnotes
Part of this work was presented as on oral presentation at the 2015 International Gastric Cancer Association Annual Meeting, June 4–6, 2015, Sao Paulo, Brazil.
Disclaimer: The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Competing interests: None declared.
Contributors: M. Elmi, N. Coburn and J. Hallet designed the study. All authors analyzed the data. M. Elmi, A. Mahar, D. Kagedan and J. Hallet wrote the article, which all authors reviewed and approved for publication.
References
- 1.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann oncol. 2003;14(Suppl 2):ii31–6. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Karpeh MS, Klimstra DS, et al. Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg. 1999;3:24–33. doi: 10.1016/s1091-255x(99)80004-3. [DOI] [PubMed] [Google Scholar]
- 5.Coupland VH, Lagergren J, Luchtenborg M, et al. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004–2008. Gut. 2013;62:961–6. doi: 10.1136/gutjnl-2012-303008. [DOI] [PubMed] [Google Scholar]
- 6.Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a metaanalysis. J Trauma. 2003;54:908–14. doi: 10.1097/01.TA.0000022460.21283.53. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien SF, Yi QL, Fan W, et al. Current incidence and residual risk of HIV, HBV and HCV at Canadian Blood Services. Vox Sang. 2012;103:83–6. doi: 10.1111/j.1423-0410.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 8.Grotto HZ. Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol. 2008;25:12–21. doi: 10.1007/s12032-007-9000-8. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Ojima T, Iwahashi M, Nakamori M, et al. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J Gastrointest Surg. 2009;13:1821–30. doi: 10.1007/s11605-009-0973-9. [DOI] [PubMed] [Google Scholar]
- 11.Maeta M, Shimizu N, Oka A, et al. Perioperative allogeneic blood transfusion exacerbates surgical stress-induced postoperative immunosuppression and has a negative effect on prognosis in patients with gastric cancer. J Surg Oncol. 1994;55:149–53. doi: 10.1002/jso.2930550304. [DOI] [PubMed] [Google Scholar]
- 12.Zhou HY, Yi W, Wang J, et al. Association of perioperative allogeneic blood transfusions and prognosis of patients with gastric cancer after curative gastrectomy. Am J Surg. 2014;208:80–7. doi: 10.1016/j.amjsurg.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Jung DH, Lee HJ, Han DS, et al. Impact of perioperative hemoglobin levels on postoperative outcomes in gastric cancer surgery. Gastric cancer. 2013;16:377–82. doi: 10.1007/s10120-012-0196-8. [DOI] [PubMed] [Google Scholar]
- 14.Alam A, Lin Y, Lima A, et al. The prevention of transfusion-associated circulatory overload. Transfus Med Rev. 2013;27:105–12. doi: 10.1016/j.tmrv.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: Why is it what it is? Am J Surg. 2009;198(Suppl):S19–27. doi: 10.1016/j.amjsurg.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217:336–46.e1. doi: 10.1016/j.jamcollsurg.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Shiloach M, Frencher SK, Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Davenport DL, Holsapple CW, Conigliaro J. Assessing surgical quality using administrative and clinical data sets: a direct comparison of the University Health System Consortium Clinical Database and the National Surgical Quality Improvement Program data set. Am J Med Qual. 2009;24:395–402. doi: 10.1177/1062860609339936. [DOI] [PubMed] [Google Scholar]
- 19.Iron deficiency anemia. World Health Organ Tech Rep Ser. 1959 [PubMed] [Google Scholar]
- 20.Bernard AC, Davenport DL, Chang PK, et al. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931–7. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med. 1994;118:371–9. [PubMed] [Google Scholar]
- 22.Opelz G. Improved kidney graft survival in nontransfused recipients. Transplant Proc. 1987;19:149–52. [PubMed] [Google Scholar]
- 23.Peters WR, Fry RD, Fleshman JW, et al. Multiple blood transfusions reduce the recurrence rate of Crohn’s disease. Dis Colon Rectum. 1989;32:749–53. doi: 10.1007/BF02562122. [DOI] [PubMed] [Google Scholar]
- 24.Kirkley SA. Proposed mechanisms of transfusion-induced immunomodulation. Clin Diagn Lab Immunol. 1999;6:652–7. doi: 10.1128/cdli.6.5.652-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16:144–60. doi: 10.1053/tmrv.2002.31463. [DOI] [PubMed] [Google Scholar]
- 27.Aloia TA, Zimmitti G, Conrad C, et al. Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol. 2014;110:107–14. doi: 10.1002/jso.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correa-Gallego C, Gonen M, Fischer M, et al. Perioperative complications influence recurrence and survival after resection of hepatic colorectal metastases. Ann Surg Oncol. 2013;20:2477–84. doi: 10.1245/s10434-013-2975-9. [DOI] [PubMed] [Google Scholar]
- 29.Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–94. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 30.Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–42. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 31.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 32.Hutton B, Fergusson D, Tinmouth A, et al. Transfusion rates vary significantly amongst Canadian medical centres. Can J Anaesth. 2005;52:581–90. doi: 10.1007/BF03015766. [DOI] [PubMed] [Google Scholar]
- 33.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 34.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 35.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proceedings from the National Summit on Overuse. The Joint Commission; [accessed 2014 Nov. 1]. Available: www.jointcommission.org/overuse_summit/ [Google Scholar]
- 37.American Society of Hematology releases list of commonly used tests and treatments to question as part of Choosing Wisely campaign. [accessed 2014 Nov. 1]. Available: www.choosingwisely.org/american-society-of-hematology-releases-list-of-commonly-used-tests-choosing-wiselys/
- 38.Davis DA, Taylor-Vaisey A. Translating guidelines into practice: a systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ. 1997;157:408–16. [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman J, Luke K, Escobar M, et al. Experience of a network of transfusion coordinators for blood conservation (Ontario Transfusion Coordinators [ONTraC]) Transfusion. 2008;48:237–50. doi: 10.1111/j.1537-2995.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 40.Froman JP, Mathiason MA, Kallies KJ, et al. The impact of an integrated transfusion reduction initiative in patients undergoing resection for colorectal cancer. Am J Surg. 2012;204:944–50. doi: 10.1016/j.amjsurg.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;(3):CD001886. doi: 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallet J, Hanif A, Callum J, et al. The impact of perioperative iron on the use of red blood cell transfusions in gastrointestinal surgery: a systematic review and meta-analysis. Transfus Med Rev. 2014;28:205–11. doi: 10.1016/j.tmrv.2014.05.004. [DOI] [PubMed] [Google Scholar]