Abstract
Metastasis is the primary cause of mortality in osteosarcoma. Targeting metastasis is a major strategy in osteosarcoma treatment. As a traditional Chinese medicine, Trillium tschonoskii Maxim has been widely used in the therapy of various diseases, including cancer. However, currently there is no evidence regarding the anti-metastasic effect of Paris saponin VII (PS VII), which is extracted from Trillium tschonoskii Maxim, on osteosarcoma cells and its underling mechanisms. The present study aimed to examine the effect of PS VII on the migration and invasion of osteosarcoma cells. Viability and proliferation of osteosarcoma cells were examined by MTT assay. Migration and invasion of osteosarcoma cells was then detected using scratch wound healing assays and Transwell assays, respectively. Additionally, the expression of matrix metalloproteinase (MMP)-2 and -9 was determined at the mRNA and protein level following treatment with PS VII. Mitogen-activated protein kinase (MAPK) expression was also detected by western blot analysis. Finally, an inhibitor of p38 MAPK was used to verify the effect of PS VII on the expression of MMP-2 and -9, as well as the migration and invasion osteosarcoma cells. This demonstrated that the proliferation, migration and invasion of the osteosarcoma cells were suppressed following treatment with PS VII. PS VII downregulated the expression of MMP-2 and -9 in a dose- and time-dependent manner. PS VII also exerted its ability to downregulate the phosphorylation of p38 MAPKs. Furthermore, by using a p38 inhibitor, SB203580, the role of PS VII in MMP-2 and -9 expression and osteosarcoma cell invasion was revealed. Taken together, these results demonstrated that PS VII suppresses the migration and invasion of osteosarcoma cells via the p38 MAPK signaling pathway.
Keywords: Paris saponin VII, migration, invasion, osteosarcoma cells, p38
Introduction
Osteosarcoma is the most common malignant primary bone tumor in adolescents and children, comprising ~20% of all types of primary bone cancer (1–3). Complete radical surgical resection is the current treatment for local osteosarcoma, typically followed by chemotherapy to improve the prognosis and long-term survival rate. However, due to distal metastases, osteosarcoma has a poor prognosis. It has been previously reported that <30% of patients with osteosarcoma survive for 5 years following the detection of lung metastasis (4). Due to the poor prognosis and high mortality rate attributed to the metastatic ability of osteosarcoma, identification of novel agents to obtain improved treatment outcomes is a major clinical challenge in osteosarcoma therapy.
Osteosarcoma cells are highly invasive, so the suppression of their invasive ability maybe effective in the treatment of osteosarcoma. A series of complex mechanisms, including migration, invasion, adhesion to endothelial cells, and degradation of the extracellular matrix (ECM), are involved in the process of osteosarcoma metastasis (5,6). Evidence has demonstrated the crucial role of matrix metalloproteinases (MMPs) in the degradation of the ECM and cancer metastasis (7,8). MMP-2 and -9 can degrade the main component of the ECM, type IV collagen, so are considered to be involved in cancer metastasis (9,10). Previous studies have demonstrated that mitogen-activated protein kinase (MAPK) family members, including extracellular signal-regulated kinases (ERK) 1/2, p38 and c-Jun N-terminal kinase (JNK), are important for the regulation of MMP-2 and -9 production (11,12).
Due to the high motility rate and increasing drug resistance of osteosarcoma, increasing numbers of studies have focused on natural products as novel anti-tumor therapeutics for its treatment (13,14). Trillium tschonoskii Maxim has long been used in the treatment of neurasthenia, hypertension, cancer and as an analgesic (15). As a type of steroidal saponin, Paris saponin VII (PS VII; Fig. 1A) is extracted from Trillium tschonoskii Maxim. Previous reports have demonstrated that PS VII exhibits anti-tumor capacity in different types of tumor. Li et al (16,17) have demonstrated that PS VII suppresses the growth of colorectal cancer cells via the Ras pathway in vitro and in vivo. Fan et al (18) demonstrated that PS VII suppresses colorectal cancer cell metastasis by regulating MMP-2 and -9 production. PS VII has also been reported to suppress the proliferation and induce the apoptosis of cervical and breast cancer cells (19,20). Furthermore, a recent study reported that PS VII inhibits the migration and invasion of lung cancer cells (21). However, to the best of our knowledge, no evidence has reported whether PS VII exerts any direct effect on osteosarcoma migration and invasion, or the mechanisms that mediate this effect.
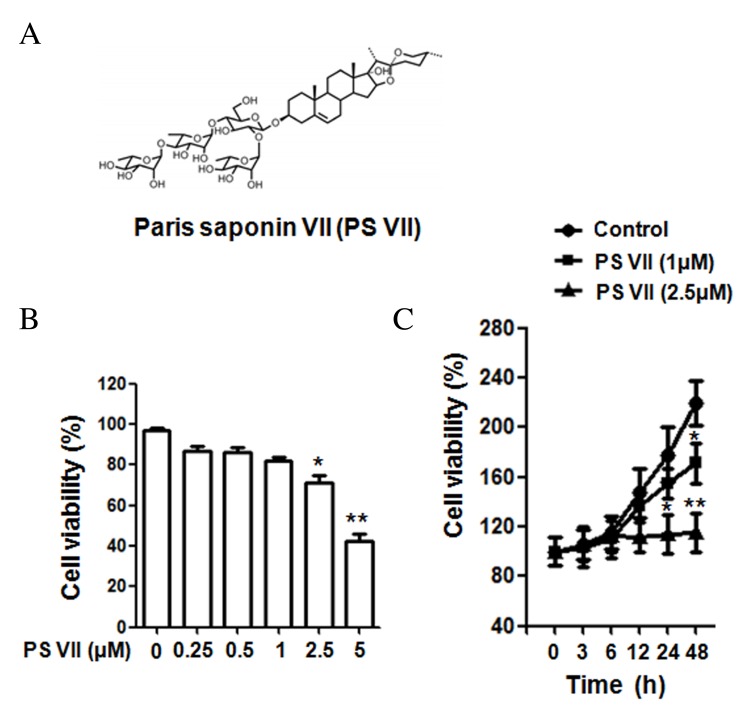
Figure 1.
Chemical structure of PS VII and the effect of PS VII on U2OS osteosarcoma cell proliferation. (A) Chemical structure of PS VII. (B) Viability of U2OS cells was examined by MTT assay following 24 h treatment with 0, 0.25, 0.5, 1, 2.5 and 5 μM PS VII. (C) Viability of U2OS cells was examined by MTT assay following 0, 3, 6, 12, 24 and 48 h treatment with 0 (control), 1 and 2.5 μM PS VII. Values are presented as the mean ± standard deviation from three independent experiments. *P<0.05, **P<0.01 vs. control. PS VII, Paris saponin VII; MTT, 3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide.
The current study evaluated the effects of PS VII on osteosarcoma cell migration and invasion, and the underlying mechanisms.
Materials and methods
Cell lines and reagents
The U2OS osteosarcoma cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and was routinely cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 37°C. Rabbit antibodies recognizing p38 (catalog no. 8690), phospho (p)-p38 (catalog no. 4631), ERK1/2 (catalog no. 4695), p-ERK1/2 (catalog no. 4370), JNK (catalog no. 9252), p-JNK (catalog no. 4668), MMP-2 (catalog no. 13132) and MMP-9 (catalog no. 13667) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit anti-GAPDH (catalog no. G9545) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (catalog no. ZDR-5306) was obtained from ZSBG-BIO (Beijing, China). PS VII with a purity of >98% was purchased from PureOne Biotechnology Co. (Shanghai, China). SB203580, a selective inhibitor of p38, was purchased from Sigma-Aldrich.
Cell viability and proliferation assays
Cell viability and proliferation were examined with 3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The U2OS osteosarcoma cells (5,000 cells/well) in 100 μl medium were seeded into 96-well plates. Following treatment with PS VII of various concentrations (0, 0.25, 0.5, 1, 2.5 and 5 μM), for various time points (0, 3, 6, 12, 24 and 48 h), 20 μl MTT (5 mg/ml) was added into each well. Following incubation for 4 h, 100 μl of dimethyl sulfoxide was added to each well for another 15 min. Finally, the absorbance values were determined by microplate luminometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from U2OS osteosarcoma cells using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and then reverse transcribed to cDNA with RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The qPCR analysis was performed using a LightCycler (Bio-Rad Laboratories, Inc.), with SYBR® Fast qPCR Mix (catalog no. RR430S; Takara Biotechnology Co., Ltd., Dalian, China). The primer sequences used for qPCR were as follows: MMP-9, forward 5′-GACGCAGACATCGTCATCCA-3′ and reverse 5′-CACAACTCGTCATCGTCGAAA-3′; MMP-2, forward 5′-TTGATGGCATCGCTCAGATC-3′ and reverse 5′-TTGTCACGTGGCGTCACAGT-3′; and GAPDH, forward 5′-TGTGGGCATCAATGGATTTGG-3′ and reverse 5′-ACACCATGTATTCCGGGTCAAT-3′. An initial denaturation step at 95°C for 10 sec was followed by 40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec, extension at 72°C for 30 sec, and a final extension step at 72°C for 3 min. Melting curves were assessed to confirm the specificity of the products generated for each set of primers. Subsequent to the amplification, ΔΔCq comparative method was used to normalize the relative levels of gene expression to GAPDH (22). Experiments were performed in triplicate.
Western blotting
Following incubation with PS VII, the U2OS osteosarcoma cells were collected and protein was extracted using protein lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). Lysed proteins were centrifuged at 10,000 × g, 4°C for 15 min, and protein concentrations were determined using the bicinchoninic acid assay (Beyotime Institute of Biotechnology). Equal quantities of protein (10 μg) from cell lysates were loaded onto 12% gels and separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Following separation, proteins were transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA) and then blocked with 5% fat-free milk in Tris-buffered saline with 0.1% Tween-20 (TBST) at room temperature for 1 h, and incubated with primary antibodies (diluted 1:1,000) overnight at 4°C. The membranes were then washed with TBST and incubated with an HRP-conjugated secondary antibody (diluted 1:10,000) for 1 h at room temperature. Immune complexes were detected with enhanced chemiluminescence reagents (EMD Millipore), and the blots were quantified by densitometric analysis using the Alpha Imager 2200 (ProteinSimple, San Jose, CA, USA).
Scratch wound healing assay
A scratch wound healing assay was applied to evaluate the migration of the U2OS osteosarcoma cells. In brief, the U2OS cells (1×106/well) were seeded in 6-well plates cultured with DMEM supplemented with 10% FBS. When reaching confluency, each well was straight scratched with a 200 μl pipette tip. To evaluate the effect of PS VII on the migration of U2OS cells, 1 μM PS VII was added to the plates. Following 24 h of incubation, the wound healing areas were imaged and the distance between two cell edges was analyzed by ImageJ software version 1.48 (National Institutes of Health, Bethesda, MD, USA). Control cells not stimulated with PS VII were analyzed at 0 and 24 h.
In vitro invasion assay
To assess the effect of PS VII on the invasive ability of U2OS osteosarcoma cells, the Transwell system was applied. The U2OS cells were cultured in Boyden chambers, with 8-μm pore filter inserts, in 24-well plates. The pore inserts were pre-coated with BD Matrigel™ Basement Membrane Matrix (BD Biosciences, San Jose, CA, USA) overnight. The U2OS cells (1×105 cells/well) were suspended in 100 μl DMEM supplemented with 1% FBS and were added to the upper chamber. DMEM with 10% FBS and 1 μM PS VII was added to the lower chamber. After 24 h of incubation, the cells attaching to the lower surface were fixed with 100% methanol for 10 min at room temperature and stained with 0.1% crystal violet. Images of 5 random high-power fields (magnification, ×200) were captured of each sample and counted to assess the average number of invasive cells.
Statistical analysis
The data are expressed as the mean ± standard deviation. All experiments were repeated at least three times. Comparisons among values for all groups were performed by one-way analysis of variance. Holm's test was applied for analysis of differences between two different groups. P<0.05 was considered to indicate a statistically significant difference.
Results
PS VII suppresses the cell viability of osteosarcoma cells
The structure of PS VII is presented in Fig. 1A. To determine the effect of PS VII on the cell viability of U2OS osteosarcoma cells, MTT assays were performed using doses of 0, 0.25, 0.5, 1, 2.5 and 5 μM PS VII. As presented in Fig. 1B, at doses 2.5 and 5 μM, PS VII significantly reduced the viability of U2OS cells after 24 h treatment, compared with the untreated control (P=0.042 and P=0.0072, respectively). However, at doses <2.5 μM (0.25, 0.5 and 1 μM), the suppression was not significantly different compared with the untreated control. PS VII at concentrations of 1 and 2.5 μM were subsequently used to treat the cells at various time points (0, 3, 6, 12, 24 and 48 h), as presented in Fig. 1C. After 24 h of treatment, PS VII significantly inhibited the cell proliferation at a dose of 2.5 μM compared with the untreated control (P=0.033), while proliferation was not inhibited with 1 μM PS VII at 24 h. However, after 48-h stimulation, 1 (P=0.026) and 2.5 (P=0.0045) μM PS VII significantly inhibited cell proliferation compared with the control group. As a result, PS VII at a dose of 1 μM for 24 h was used for subsequent migration and invasion experiments to exclude the effect on cell proliferation.
PS VII inhibits the migration and invasion of osteosarcoma cells
The wound healing assay was performed to evaluate the effect of PS VII on the migration of osteosarcoma cells. The U2OS osteosarcoma cells were treated with 1 μM PS VII for 24 h. As presented in Fig. 2A, migration of U2OS cells was suppressed by 1 μM PS VII compared with untreated control cells (P=0.0082). The results of the wound healing assay indicated that healing of the scratch was significantly reduced following treatment with PS VII. To further examine the effect of PS VII on cell invasion, a Transwell assay was used, in which U2OS cells were treated with 1 μM PS VII for 24 h. As demonstrated in Fig. 2B, the invasion of U2OS cells was suppressed by 1 μM PS VII compared with untreated control cells (P=0.0055), suggesting that PS VII inhibited the invasive ability of osteosarcoma cells.
Figure 2.
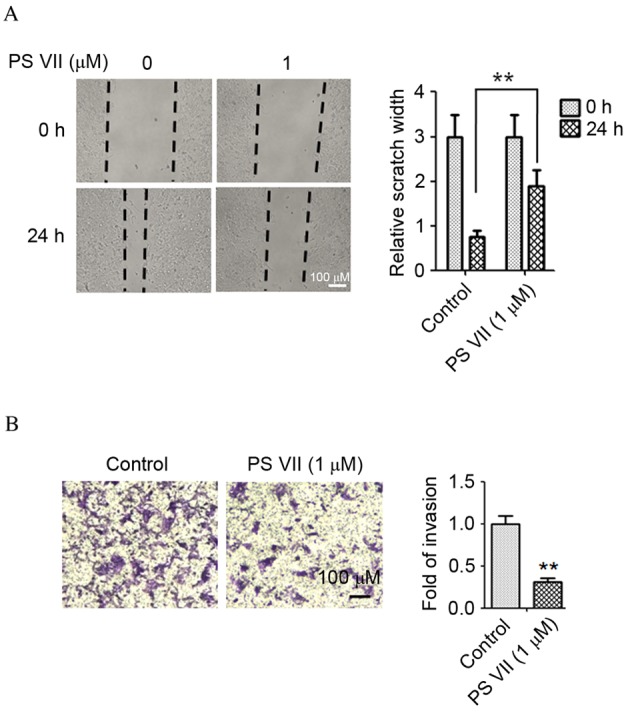
PS VII inhibits the migration and invasion of U2OS osteosarcoma cells. (A) U2OS cell migration was assessed by a wound healing assay following treatment with 0 or 1 μM PS VII 24 h. (B) U2OS cell invasion was assessed by Transwell assay following treatments with 1 μM PS VII for 24 h and staining with crystal violet. Values are presented as the mean ± standard deviation of three independent experiments. **P<0.01 vs. control. PS VII, Paris saponin VII.
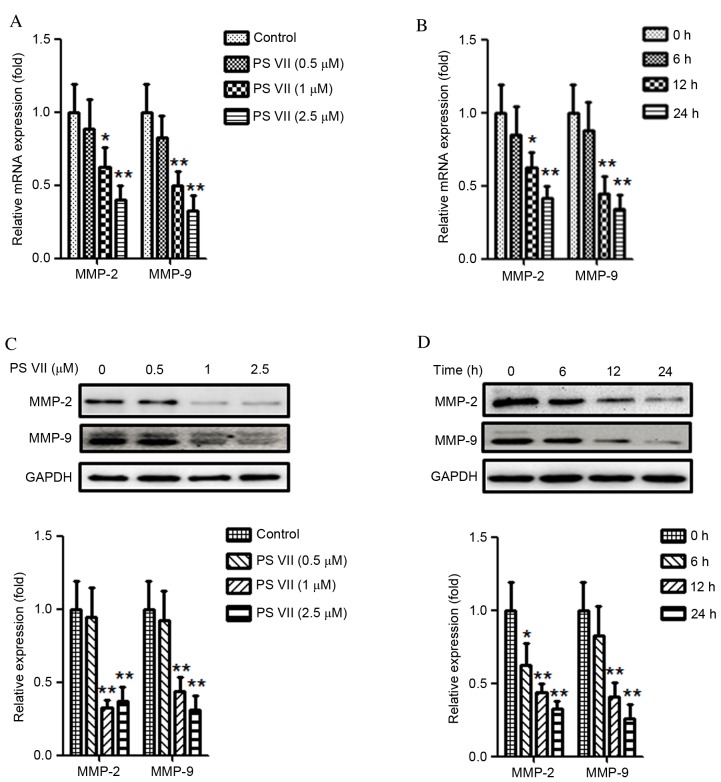
PS VII reduces the production of MMP-2 and -9 in osteosarcoma cells
Previous studies have demonstrated that MMPs, particularly MMP-2 and -9, are crucial for the migration and invasion of cancer cells (23,24). Thus the effect of PS VII on MMP-2 and -9 expression in U2OS osteosarcoma cells was examined. U2OS cells were stimulated with 0, 0.5, 1 or 2.5 μM PS VII for 24 h. As demonstrated in Fig. 3A, treatment with 1 and 2.5 μM PS VII significantly reduced the relative mRNA expression of MMP-2 (P=0.037 and P=0.0067, respectively) and MMP-9 (P=0.0082 and P=0.0071, respectively) in U2OS osteosarcoma cells compared with control cells. U2OS cells were subsequently treated with 1 μM PS VII for 0, 6, 12 and 24 h (Fig. 3B). Following treatment with 1 μM PS VII for 6 h, the relative mRNA expression of MMP-2 and -9 did not significantly change. However, when the treatments were extended to 12 and 24 h, the relative mRNA expression levels of MMP-2 (P=0.044 and P=0.0062, respectively) and MMP-9 (P=0.0064 and P=0.0055, respectively) were significantly decreased compared with the 0 h control. Quantitative western blots were subsequently used to examine the levels of MMP-2 and -9 protein expression following identical treatments. As demonstrated in Fig. 3C, following treatment with 1 and 2.5 μM PS VII, the protein expression of MMP-2 (P=0.0034 and P=0.0042, respectively) and MMP-9 (P=0.0049 and P=0.0036, respectively) was significantly reduced compared with control treatment. Similarly to the mRNA levels, the protein expression also decreased gradually following treatment with 1 μM PS VII for 6 (MMP-2, P=0.041), 12 (MMP-2, P=0.0062; MMP-9, P=0.0056) and 24 (MMP-2, P=0.0051; MMP-9, P=0.0046) h compared with at 0 h (Fig. 3D). These results, therefore, demonstrated that PS VII suppressed the production of MMP-2 and -9 in a dose- and time-dependent manner at the mRNA and protein levels. Furthermore, the results of the present study indicated that the inhibitory effect of PS VII on U2OS cell migration and invasion may be associated with MMP-2 and -9 production.
Figure 3.
PS VII reduces the expression of MMP-2 and -9 of U2OS osteosarcoma cells. (A) MMP-2 and -9 mRNA levels were assessed by qPCR following treatment of U2OS cells with 0 (control), 0.5, 1 and 2.5 μM PS VII for 24 h. (B) MMP-2 and -9 mRNA levels were assessed by qPCR following treatment of U2OS cells with 1 μM PS VII for 0 (control), 6, 12 and 24 h. (C) MMP-2 and -9 protein expression was assessed by quantitative western blot analysis following stimulation of U2OS cells with 0 (control), 0.5, 1 and 2.5 μM PS VII for 24 h. (D) MMP-2 and -9 protein expression was assessed by quantitative western blot analysis following stimulation of U2OS cells with 1 μM PS VII for 0 (control), 6, 12 and 24 h. Values are presented as the mean ± standard deviation from three independent experiments. *P<0.05, **P<0.01 vs. control. PS VII, Paris saponin VII; MMP, matrix metalloproteinase; qPCR, quantitative polymerase chain reaction; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
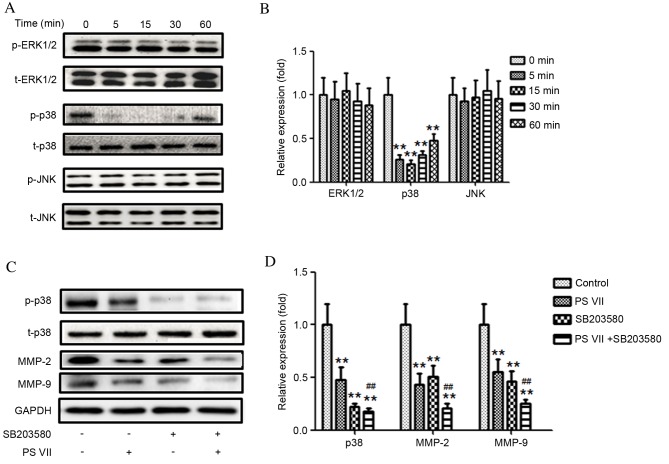
p38 MAPK signaling pathway modulates PS VII-regulated MMP-2/9 production
MAPK signaling pathways are important for tumor development. Inactivation of MAPK signaling has previously been demonstrated to inhibit tumor cell migration and invasion (25). To examine the association between PS VII and the production of MMP-2 and -9, phosphorylation levels of three members of the MAPK family, ERK1/2, p38 and JNK, was detected following treatment of U2OS cells with 1 μM PS VII for 0, 5, 15, 30 and 60 min (Fig. 4A and B). The relative level of p-p38 was significantly reduced compared with the 0 min control following 5 (P=0.0057), 15 (P=0.0044) and 30 (P=0.0061) min of treatment and the effect had begun to gradually increase by 60 min of treatment (P=0.0076; Fig. 4B). However, the expression of p-ERK1/2 and p-JNK remained unchanged compared with the 0 min control (Fig. 4B). These results indicated that the p38 MAPK signaling pathway may be involved in MMP-2 and -9 production in U2OS osteosarcoma cells.
Figure 4.
p38 mitogen-activated protein kinase, but not ERK1/2 or JNK, modulates PS VII-mediated production of MMP-2 and -9 in U2OS osteosarcoma cells. (A) p-ERK1/2, ERK1/2, p-p38, p38, p-JNK and JNK protein expression was assessed by western blot following stimulation of U2OS cells with 1 μM PS VII for 0 (control), 5, 15, 30 and 60 min, and (B) quantitatively analyzed (*P<0.05, **P<0.01 vs. 0 min control). (C) p-p38, p38, MMP-2, MMP-9 and GADPH (blot control) protein expression was assessed by western blot following stimulation of U2OS cells with 1 μM PS VII for 24 h, ± 50 μM SB203580 for 1 h prior to PS VII treatment and (D) quantitatively analyzed (**P<0.01 vs. no-treatment control; ##P<0.01, vs. PS VII-treated cells). Data are presented as the mean ± standard deviation from three independent experiments. PS VII, Paris saponin VII; p-, phosphorylated; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; t-, total; MMP, matrix metalloproteinase; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
To investigate whether the effect of PS VII is p38-dependent, a p38 inhibitor, SB203580, was used to block p38 activation. As demonstrated in Fig. 4C and D, inhibition of p38 activity with SB203580 further reduced the expression of MMP-2 (P=0.0055) and -9 (P=0.0056) compared with PS VII treatment alone. These results indicate that inhibition of MMP-2 and -9 production by PS VII may be p38-dependent.
Inhibition of the p38 MAPK signaling pathway enhances the anti-metastasic effect of PS VII in osteosarcoma cells
To further investigate whether the anti-metastasic effect of PS VII was attributable to p38 MAPK signaling inactivation, the effect of SB203580 on U2OS cell migration in the presence and absence of PS VII was examined. As demonstrated in Fig. 5A, the wound healing assay indicated that the PS VII-induced inhibition of cell migration was significantly enhanced by the p38 inhibitor compared with PS VII treatment only (P=0.032). Similarly, Transwell assays indicated that inhibition of p38 MAPK significantly enhanced the PS VII-induced inhibition of osteosarcoma cell invasion (P=0.044; Fig. 5B). Therefore, the present study supports the hypothesis that inhibition of metastasis in osteosarcoma cells by PS VII is modulated by suppression of MMP-2 and -9 production via the p38 MAPK signaling pathway.
Figure 5.
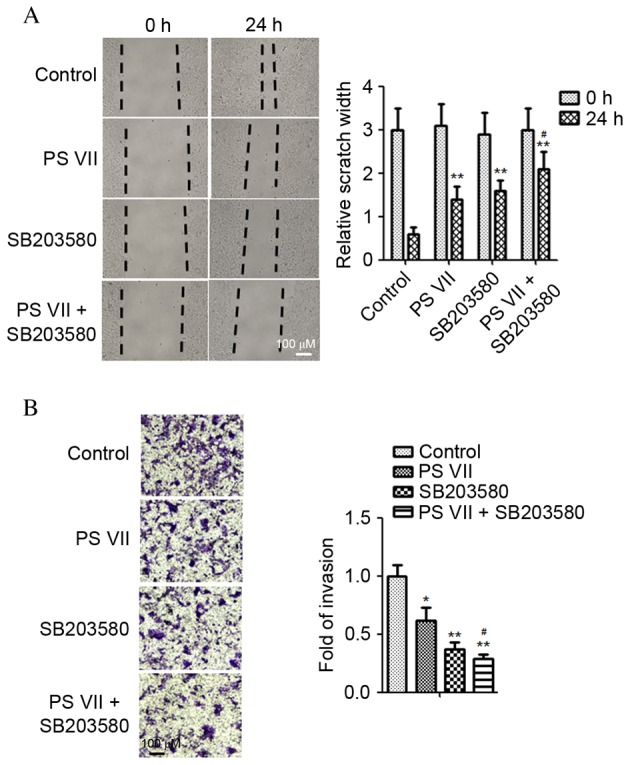
Inhibition of p38 mitogen-activated protein kinase signaling enhances the anti-metastasic effect of PS VII in U2OS osteosarcoma cells. (A) U2OS cell migration was assessed by a wound healing assay following treatment with 1 μM PS VII and/or 50 μM SB203580 for 24 h. (B) U2OS cell invasion was assessed by Transwell assay following treatment with 1 μM PS VII and/or 50 μM SB203580 for 24 h. Values are presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 vs. control; #P<0.05, ##P<0.01 vs. PS VII-treated cells. PS VII, Paris saponin VII.
Discussion
Investigation of metastasis and the underlying molecular mechanisms is important for aiding the development of effective agents to suppress cancer metastasis. Natural products are currently receiving increased attention in cancer therapy research for their anti-tumor properties and reduced side-effects compared with traditional chemotherapeutics (26,27). Previous studies have demonstrated that PS VII suppresses cell proliferation, migration and invasion in colorectal (16–18), cervical (19), breast (20) and lung (21) cancer cells. Although previous studies have demonstrated the anti-tumor activity of PS VII, the effect of PS VII on osteosarcoma cell migration and invasion, and the molecular mechanisms had not been investigated. The present study initially evaluated the effect of PS VII on the viability of osteosarcoma cells, and revealed that 1 μM PS VII significantly inhibited cell proliferation after 48 h of treatment, with no difference before 24 h. A stimulation time point of 24 h and stimulation dose of 1 μM was therefore used in subsequent experiments, to exclude the influence on proliferation. To the best of our knowledge, the present study is the first to have demonstrated that PS VII effectively inhibits osteosarcoma cell migration and invasion, and that PS VII suppresses osteosarcoma cell migration and invasion by inhibiting MMP-2 and -9 production via the p38 MAPK signaling pathway. This is, therefore, the first evidence of the anti-metastatic activity of PS VII on osteosarcoma cells and a molecular mechanism for such activity.
Metastasis of osteosarcoma is a complicated process and is often correlated with the hydrolysis of the ECM by a number of proteolytic enzymes, including MMPs, which are proteinases involved in the migration and invasion of malignant cells (5,6,23,24). Among them, MMP-2 and -9 are crucial for the initiation of metastasis and invasion (9,10). Previous studies have demonstrated the importance of MMP-2 and -9 in osteosarcoma progression, with high MMP-2 expression associated with poor prognosis (28–30). The current study examined the suppressive effect of PS VII on the migration and invasion of U2OS osteosarcoma cells by using scratch wound healing and Transwell assays, and established that reduced expression of MMP-2 and -9 following PS VII treatment was associated with the suppression of migration and invasion in U2OS osteosarcoma cells. This indicates that metastasis inhibition by PS VII may be associated with MMP-2 and -9 activity.
Previous studies have reported that MAPK family members, including ERK, p38 and JNK, may be involved in MMP-2 and -9 production, thus promoting tumor proliferation and metastasis (11,12,31). The p38 MAPK signaling pathway is involved in the modulation of proliferation, apoptosis, migration and invasion of cancer cells (32–34). In the present study, p38 expression in osteosarcoma cells was determined by western blot, and the phosphorylation of p38 was observed to be reduced when treated with PS VII. To further confirm that the p38 signaling pathway was involved in PS VII-induced osteosarcoma cells metastasis suppression, p38 activity was blocked using the p38 inhibitor, SB203580. Inhibition of p38 phosphorylation by SB203580 was demonstrated to enhance MMP-2 and -9 downregulation induced by PS VII. Furthermore, inhibition of p38 activity by SB203580 further suppressed osteosarcoma cell migration and invasion. PS VII was, therefore, demonstrated to inhibit osteosarcoma cell metastasis through suppression of p38 MAPK activity, resulting in downregulation of MMP-2 and -9, and further suggesting that p38 MAPK is upstream of MMP-2 and -9 in the signaling cascade.
Therefore, to the best of our knowledge, the current study presents the first evidence that PS VII suppresses the migration and invasion of osteosarcoma cells by reducing MMP-2 and MMP-9 production. Furthermore, the effect of PS VII is modulated by the p38 MAPK signaling pathway. Thus, PS VII is indicated to be a potential novel therapeutic agent in osteosarcoma therapy, and a candidate for further study in vivo.
References
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh WW, Holsinger FC, Levy A, Palla FS, Anderson PM. Osteosarcoma of the jaw in children and young adults. Head Neck. 2012;34:981–984. doi: 10.1002/hed.21850. [DOI] [PubMed] [Google Scholar]
- 4.Mohseny AB, Machado I, Cai Y, Schaefer KL, Serra M, Hogendoorn PC, Llombart-Bosch A, Cleton-Jansen AM. Functional characterization of osteosarcoma cell lines provides representative models to study the human disease. Lab Invest. 2011;91:1195–1205. doi: 10.1038/labinvest.2011.72. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977–987. doi: 10.1093/carcin/bgl221. [DOI] [PubMed] [Google Scholar]
- 6.Lu KH, Yang HW, Su CW, Lue KH, Yang SF, Hsieh YS. Phyllanthus urinaria suppresses human osteosarcoma cell invasion and migration by transcriptionally inhibiting u-PA via ERK and Akt signaling pathways. Food Chem Toxicol. 2013;52:193–199. doi: 10.1016/j.fct.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Stallings-Mann M, Radisky D. Matrix metalloproteinase-induced malignancy in mammary epithelial cells. Cells Tissues Organs. 2007;185:104–110. doi: 10.1159/000101310. [DOI] [PubMed] [Google Scholar]
- 8.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990;1:99–106. [PubMed] [Google Scholar]
- 10.Himelstein BP, Canete-Soler R, Bernhard EJ, Dilks DW, Muschel RJ. Metalloproteinases in tumor progression: The contribution of MMP-9. Invasion Metastasis. 1994;14:246–258. [PubMed] [Google Scholar]
- 11.Kim BS, Park JY, Kang HJ, Kim HJ, Lee J. Fucoidan/FGF-2 induces angiogenesis through JNK- and p38-mediated activation of AKT/MMP-2 signalling. Biochem Biophys Res Commun. 2014;450:1333–1338. doi: 10.1016/j.bbrc.2014.06.137. [DOI] [PubMed] [Google Scholar]
- 12.Jin YJ, Park I, Hong IK, Byun HJ, Choi J, Kim YM, Lee H. Fibronectin and vitronectin induce AP-1-mediated matrix metalloproteinase-9 expression through integrin α(5)β(1)/α(v) β(3)-dependent Akt, ERK and JNK signaling pathways in human umbilical vein endothelial cells. Cell Signal. 2011;23:125–134. doi: 10.1016/j.cellsig.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Yang HL, Yang YJ, Wang L, Lee SC. Overcome cancer cell drug resistance using natural products. Evid Based Complement Alternat Med. 2015;2015:767136. doi: 10.1155/2015/767136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Xiao M, Guo L, Wang L, Tang L, Xu Y, Yan F, Chen F. Genetic diversity and genetic structure of an endangered species, Trillium tschonoskii. Biochem Genet. 2005;43:445–458. doi: 10.1007/s10528-005-6782-2. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Sun Y, Fan L, Zhang F, Meng J, Han J, Guo X, Zhang D, Zhang R, Yue Z, et al. Paris saponin VII inhibits growth of colorectal cancer cells through Ras signaling pathway. Biochem Pharmacol. 2014;88:150–157. doi: 10.1016/j.bcp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Liu C, Xiao D, Han J, Yue Z, Sun Y, Fan L, Zhang F, Meng J, Zhang R, et al. Trillium tschonoskii steroidal saponins suppress the growth of colorectal Cancer cells in vitro and in vivo. J Ethnopharmacol. 2015;168:136–145. doi: 10.1016/j.jep.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 18.Fan L, Li Y, Sun Y, Yue Z, Meng J, Zhang X, Zhang R, Zhang D, Zhang F, Mei Q. Paris saponin VII inhibits metastasis by modulating matrix metalloproteinases in colorectal cancer cells. Mol Med Rep. 2015;11:705–711. doi: 10.3892/mmr.2014.2728. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Zhang D, Ma X, Liu Z, Li F, Wu D. Paris saponin VII suppressed the growth of human cervical cancer Hela cells. Eur J Med Res. 2014;19:41. doi: 10.1186/2047-783X-19-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Fan L, Sun Y, Miao X, Zhang F, Meng J, Han J, Zhang D, Zhang R, Yue Z, Mei Q. Paris saponin VII from trillium tschonoskii reverses multidrug resistance of adriamycin-resistant MCF-7/ADR cells via P-glycoprotein inhibition and apoptosis augmentation. J Ethnopharmacol. 2014;154:728–734. doi: 10.1016/j.jep.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Fan L, Li Y, Sun Y, Han J, Yue Z, Meng J, Zhang X, Zhang F, Mei Q. Paris Saponin VII inhibits the migration and invasion in human A549 lung cancer cells. Phytother Res. 2015 Jun 24; doi: 10.1002/ptr.5389. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 24.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–3119. [PubMed] [Google Scholar]
- 25.Rajoria S, Suriano R, Wilson YL, Schantz SP, Moscatello A, Geliebter J, Tiwari RK. 3,3′-diindolylmethane inhibits migration and invasion of human cancer cells through combined suppression of ERK and AKT pathways. Oncol Rep. 2011;25:491–497. doi: 10.3892/or.2010.1076. [DOI] [PubMed] [Google Scholar]
- 26.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 27.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals-promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 28.Poudel B, Kim DK, Ki HH, Kwon YB, Lee YM, Kim DK. Downregulation of ERK signaling impairs U2OS osteosarcoma cell migration in collagen matrix by suppressing MMP9 production. Oncol Letters. 2014;7:215–218. doi: 10.3892/ol.2013.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Zhang Q. Questions about XY Wen et al. Entitled 'Matrix metalloproteinase 2 expression and survival of patients with osteosarcoma: A meta-analysis'. Tumour Biol. 2015;36:557–558. doi: 10.1007/s13277-015-3173-0. [DOI] [PubMed] [Google Scholar]
- 30.Wen X, Liu H, Yu K, Liu Y. Matrix metalloproteinase 2 expression and survival of patients with osteosarcoma: A meta-analysis. Tumour Biol. 2014;35:845–848. doi: 10.1007/s13277-013-1116-1. [DOI] [PubMed] [Google Scholar]
- 31.Thompson N, Lyons J. Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol. 2005;5:350–356. doi: 10.1016/j.coph.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Wang X, Wu T, Li B, Liu T, Wang R, Liu Q, Liu Z, Gong Y, Shao C. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci Rep. 2015;5:12579. doi: 10.1038/srep12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia P, Zhang R, Ge G. C/EBPβ mediates TNF-α-induced cancer cell migration by inducing MMP expression dependent on p38 MAPK. J Cell Biochem. 2015;116:2766–2777. doi: 10.1002/jcb.25219. [DOI] [PubMed] [Google Scholar]
- 34.Gupta J, Nebreda AR. Roles of p38α mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 2015;282:1841–1857. doi: 10.1111/febs.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]