Abstract
Nerve regeneration remains a challenge to the treatment of peripheral nerve injury. Andrographolide (Andro) is the main active constituent of Andrographis paniculata, which has been applied in the treatment of several diseases, including inflammation, in ancient China. Andro has been reported to facilitate the reduction of edema and to exert analgesic effects in the treatment of various diseases. These findings suggest that Andro may be considered a promising anti-inflammatory agent that may suppress destruction and accelerate proliferation of Schwann cells following peripheral nerve injury. In the present study, the effects of Andro on RSC96 cells were investigated in vitro. The RSC96 cell line is a spontaneously immortalized rat Schwann cell line, which was originally derived from a long-term culture of rat primary Schwann cells. RSC96 cells were treated with a range of 0 to 50 µM Andro prior to the MTT assay. Cell proliferation, morphology, synthesis and nerve-specific gene expression were performed to detect the effect of Andro on RSC96 cells. The results of the present study demonstrated that the recommended doses of Andro ranged between 0.78 and 12.5 µM, among which the most obvious response was observed when used at 3.125 µM (P<0.05). DNA content was improved in Andro groups compared with the control group (P<0.05). In addition, Andro was able to promote the gene expression of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, ciliary neurotrophic factor, and the specific Schwann cell marker S100β (P<0.05). The results of a viability assay, hematoxylin-eosin staining, and immunohistochemistry were also improved in Andro groups. These results indicated that Andro may accelerate proliferation of RSC96 cells in vitro, whilst maintaining the Schwann cell phenotype; therefore, the present study may provide valuable evidence for the further exploration of the effects of Andro on peripheral nerves.
Keywords: peripheral nerve, andrographolide, RSC96 Schwann cells, proliferation
Introduction
Peripheral nerve injuries are considered one of the most challenging and difficult problems to treat with reconstructive surgery (1). Fractures, hematomas, contusions and compressions may induce peripheral nerve injury, which is characterized by the disruption of myelin sheaths and axons (2,3). Inflammation has been shown to have an important role in the pathogenesis of several neurodegenerative diseases, including Parkinson's disease, Alzheimer's disease, multiple sclerosis and amyotrophic lateral sclerosis (4–6). The ability of the mammalian peripheral nervous system (PNS) to regenerate axons following injury is well documented (7). Schwann cells have an important role in axon regeneration post-injury (8,9). Therefore, the identification of an effective anti-neuroinflammatory and neuroprotective agent, which is able to accelerate the proliferation of Schwann cells, thus maintaining the Schwann cell phenotype, is of great importance.
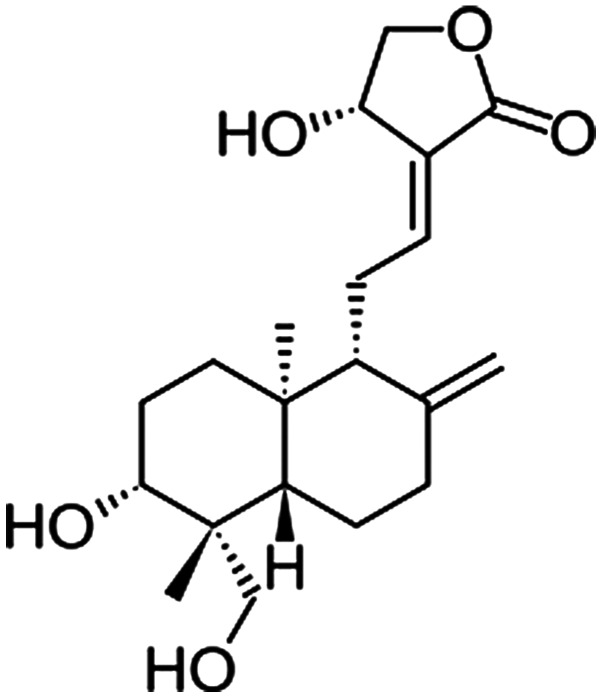
In traditional Chinese medicine, Andrographis paniculata is a traditional herb, which possesses immunological, antibacterial, antiviral, anti-inflammatory, antithrombotic, and lung and hepato-protective properties (10–13). Andrographolide (Andro; Fig. 1) is the primary active component of A. paniculata, which is widely used in South Asia and China for the treatment of inflammation-related diseases, due to its potent anti-inflammatory and antiviral properties (14–16). Andro and its derivatives, a group of diterpenes, have been reported to exert a protective effect against lipopolysaccharide-induced dopaminergic neurodegeneration in mesencephalic neuron-glia cultures (17). The anti-inflammatory role of Andro has been well-documented in several studies (18,19). Furthermore, Andro exerts proapoptotic effects on tumor cells (20,21). It has also been reported that Andro facilitates cell differentiation (22). These findings suggested that Andro may exert anti-neuroinflammatory and neuroprotective effects during peripheral nerve regeneration, which is a vital long-term strategy in the treatment of peripheral nerve injury.
Figure 1.
Chemical structure of andrographolide.
As myelin-forming cells in the PNS, Schwann cells have a crucial role in peripheral nerve regeneration (23). Schwann cells have been shown to be able to provide bioactive substrates for axonal migration, and release molecules that regulate axonal outgrowth (24). In addition, Schwann cells activate nonresident macrophages to the site of injury, in order to complete myelin phagocytosis, release cytokines, and secrete neurotrophic factors that guide the resultant regeneration (25,26). Schwann cells provide trophic support to axons via the expression of several neurotrophic factors, including brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and ciliary neurotrophic factor (CNTF), particularly following nerve injury (27). These findings indicate the specific nature of the relationship between Schwann cells and axons, and thus confirm our hypothesis.
Based on the hypothesis that Andro may be used as a potential anti-inflammatory agent to relieve the destruction and accelerate the proliferation of Schwann cells following peripheral nerve injury, the present study investigated its effects on the growth and phenotypic maintenance of RSC96 cells in vitro. Examination of cell proliferation, morphology, viability, and RSC96-specific gene expression was performed. The results suggested that Andro may exert effects on RSC96 cell attachment, survival and proliferation, and on the release of neurotrophic factors. The present study may provide evidence for the application of Andro in the clinical treatment of peripheral nerve injury.
Materials and methods
Reagents and instruments
Trypsin and antibiotics (100 U/ml penicillin and 100 U/ml streptomycin) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China); 6-well and 96-well cell culture plates were purchased from Costar (Corning Incorporated, Corning, NY, USA). Anti-S100β (S100β; cat. no. BA120; 1:200) antibody and the 3,3-diaminobenzidine tetrahydrochloride (DAB) kit were obtained from Wuhan Boster Biological Technology, Ltd. (Wuhan, China), Dulbecco's modified Eagle's medium/F-12 supplement (DMEM/F-12), fetal bovine serum (FBS) and 3–(4,5)-dimethylthiahiazol(-z-y1)-3,5-di-phenyltetrazo-lium-bromide (MTT) were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Dimethyl sulfoxide (DMSO), Hoechst 33258 and proteinase K were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Multiskan GO Microplate Spectrophotometer was obtained from Thermo Fisher Scientific, Inc. Other reagents and instruments used in the present study were purchased from the following companies: Hematoxylin-eosin (HE) kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China); RNeasy RNA extraction kit (Tiangen Biotech Co., Ltd., Beijing, China); reverse transcription (RT) kit (Fermentas; Thermo Fisher Scientific, Inc.); Fast-Start Universal SYBR Green Master Mix (Roche Diagnostics GmbH, Mannheim, Germany); quantitative polymerase chain reaction (qPCR) detection system (RealPlex4; Eppendorf, Hauppauge, NY, USA); LIVE/DEAD viability assay kit (Invitrogen; Thermo Fisher Scientific, Inc.); laser scanning confocal microscope (Nikon Corporation, Tokyo, Japan); and upright microscope (Olympus Corporation, Tokyo, Japan).
Cells culture
The RSC96 cell line consists of spontaneously immortalized rat Schwann cells, which are derived from the long-term culture of rat primary Schwann cells. RSC96 cells were purchased from the China Center for Type Culture Collection (Wuhan, China), and were cultured in DMEM/F-12 supplemented with 10% (v/v) FBS and 1% (v/v) antibiotics in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. The culture medium was replaced every 3 days after plating. RSC96 cells were passaged with 0.25% trypsin when cell confluence reached 80–90%. Confluent RSC96 cells were subsequently treated at the indicated times with the indicated concentrations of Andro.
Chemicals
Andro was purchased from Chengdu Must Bio-technology Co., Ltd. (Chengdu, China). Prior to experimentation, Andro was dissolved in DMSO in order to generate a 100 mM stock solution, and was stored at −4°C. The Andro stock solution was diluted with cultured medium to provided various concentrations and added to the cell culture for subsequent experiments. Prior to use, the culture medium contained 1.5625, 3.125 and 6.25 µM Andro was filtered using 0.22 µm filters for sterilization.
Cell cytotoxicity assay
Cell viability was estimated using a colorimetric assay based on the conversion of MTT into a blue formazan product. The cells were plated at 800 cells/well in 96-well cell culture plates and were pretreated with various concentrations of Andro (0–50 µM) for 3 days in a 5% CO2 humidified incubator at 37°C. MTT (5 mg/ml) was then added to each well and the plates were incubated in the dark at 37°C for 4 h. Subsequently, culture medium was removed and the cells were treated with 150 µl DMSO to dissolve the formazan product. The cells were incubated in DMSO with agitation for 10 min. Optical density of each sample was measured using a Multiskan GO Microplate Spectrophotometer at 570 nm. Five individual cultures were used for each test. The experiments were carried out in quintuplicate.
Cell proliferation analysis
Based on the results of the cytotoxicity assay, three doses of Andro, which exhibited a positive effect, were selected (1.5625, 3.125 and 6.25 µM), alongside a control group (0 µM Andro) for cell proliferation analysis. RSC96 cells in the various groups were cultured for 2, 4 and 6 days in a 5% CO2 humidified incubator at 37°C prior to subsequent experiments. Cells were digested with 0.25% trypsin and were resuspended in phosphate-buffered saline (PBS) containing 60 µg/ml proteinase K for 6 h at 60°C. After dyeing with Hoechst 33258, cell proliferation was determined by detecting DNA production using an ultraviolet spectrofluorometer; calf thymus DNA was used as a standard. The excitation wavelength was 346 nm and the emission wavelength was 460 nm. The experiments were carried out in quintuplicate.
Morphological examination
Cells were cultured for 2, 4 and 6 days, and were fixed in 4% paraformaldehyde for 40 min at room temperature for subsequent HE staining. Cells were incubated with a nuclear dye for 3 min, followed by a 10 sec incubation with HE. Subsequently, the cells were rinsed with PBS, naturally dried and sealed with neutral gum. Cells were then examined, and images were captured under an upright microscope.
Cell viability assay
Cell viability was determined using the LIVE/DEAD viability assay kit. Briefly, cells on coverslips were rinsed quickly with PBS (0.01 mol/l, pH 7.4) to remove the medium. Subsequently, 1 µM calcein-acetoxym-ethyl (calcein-AM) and 1 µM propidium iodide (PI) were added to the cell cultures and were incubated in the dark for 5 min at 37°C. Images were captured using a laser scanning confocal microscope.
Immunohistochemical staining
S100β protein expression was detected by immunohistochemical staining using anti-S100 (S100β), according to the manufacturer's protocol. Briefly, cells on coverslips were rinsed quickly with PBS (0.01 mol/l, pH 7.4) to remove the medium. Subsequently, the cells were fixed in 4% paraformaldehyde at room temperature for 40 min. After washing three times with PBS and permeabilizing with 3% Triton X-100 for 5 min, cells were incubated with 3% H2O2 for 10 min at room temperature, in order to suppress endogenous peroxidase activity. The cells were then treated with goat serum for 10 min at room temperature to block nonspecific staining. Subsequently, the cells were incubated with rat monoclonal anti-S100 antibody (S100β; 1:150 dilution) overnight at 4°C in a humidified chamber. After washing three times with PBS, secondary antibodies (cat. no. SP-9000; 1:50; OriGene Technologies, Inc., Beijing, China) and biotin-labeled horseradish peroxidase (OriGene Technologies, Inc.) were successively added for 15 and 10 min at room temperature. The chromogenic reaction of S100 was visualized using a DAB kit, and the slides were counterstained with hematoxylin. Finally, cells were gradually dehydrated, sealed with neutral gum, observed, and images were captured under an upright microscope.
RT-qPCR analysis
To further explore the effects of Andro on the expression of Schwann cell-specific genes, BDNF, GDNF and CNTF mRNA expression was analyzed by RT-qPCR. Total RNA was extracted from RSC96 cells using an RNeasy RNA extraction kit, according to the manufacturer's protocol. Reverse transcription of RNA was performed at 25°C for 5 min, 42°C 60 min and then 72°C for 5 min using a reverse transcription kit (Fermentas; Thermo Fisher Scientific, Inc.). The RT-qPCR reactions were performed using a qPCR detection system with a FastStart Universal SYBR Green Master Mix under the following conditions: 10 min at 95°C, 15 sec at 95°C and 1 min at 60°C for 35 cycles. The primer sequences (BGI, Shenzhen, China) for BDNF, GDNF, CNTF and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; internal control) are listed in Table I. The melting curve data were collected to verify PCR specificity. Each gene was analyzed in triplicate to diminish operation errors. Relative gene expression levels were calculated using the 2−ΔΔCq method (28), and were normalized to GAPDH gene expression. Each gene was analyzed in quintuplicate to reduce randomization error.
Table I.
Primer sequences used in quantitative polymerase chain reaction.
| Gene | Primer sequence (5′ to 3′) | Length (bp) | Amplicon size (bp) |
|---|---|---|---|
| GDNF | F: AGACCGGATCCGAGGTGC | 18 | 129 |
| R: TCGAGAAGCCTCTTACCGGC | 20 | ||
| BDNF | F: TACCTGGATGCCGCAAACAT | 20 | 182 |
| R: TGGCCTTTTGATACCGGGAC | 20 | ||
| CNTF | F: ATGGCTTTCGCAGAGCAAAC | 20 | 191 |
| R: CAACGATCAGTGCTTGCCAC | 20 | ||
| GAPDH | F: GTCATCATCTCAGCCCCCTC | 20 | 99 |
| R: GGATGCGTTGCTGACAATCT | 20 |
GDNF, glial cell-derived neurotrophic factor; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; F, forward primer; R, reverse primer; bp, base pairs.
Statistical analysis
Statistical analyses were conducted using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation. Statistical significance was determined using one-way analysis of variance followed by Dunnett's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxicity assay
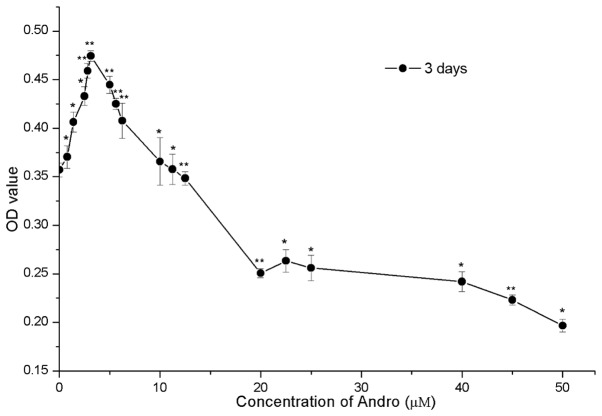
The present study examined the cytotoxicity of various concentrations of Andro on RSC96 cells using the MTT assay. Cells were treated with increasing concentrations of Andro (0–50 µM). As shown in Fig. 2, compared with the control group (0 µM), treatment with Andro between 0.78 and 12.5 µM exhibited low cytotoxicity. In addition, 0.78–12.5 µM Andro significantly accelerated cell growth (P<0.05) with the most obvious effect being observed when used at 3.125 µM (P<0.05). However, Andro exhibited a suppressive effect on RSC96 cells in vitro when used between 12.5 and 50 µM, as compared with the control group.
Figure 2.
Cytotoxicity of andrographolide (Andro) on RSC96 Schwann cells after 3 days. Data are presented as the mean ± standard deviation (n=5). *P<0.05, **P<0.01 vs. control group (0 µM). OD, optical density.
Cell proliferation
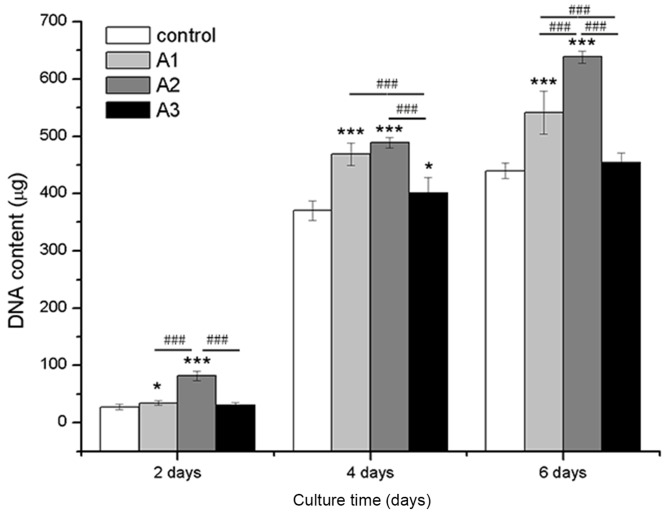
As presented in Fig. 3, RSC96 cells treated with 1.5625, 3.125 and 6.25 µM Andro exhibited increased proliferation compared with the control group (0 µM Andro). Proliferation was determined according to DNA content (P<0.05), which was markedly higher in the Andro groups compared with in the control group after the same culture period. Among the three concentrations, 3.125 µM Andro exhibited the strongest effect on cell growth at all time points.
Figure 3.
Quantification of cell proliferation by detection of DNA content. The RSC96 Schwann cells were cultured with 0 µM (control), 1.5625 µM (A1), 3.125 µM (A2) and 6.25 µM (A3) andrographolide for 2, 4 and 6 days. Data are presented as the mean ± standard deviation of five independent experiments. *P<0.05, ***P<0.001 vs. control; ###P<0.001 vs. A1, A2 and A3.
Cell morphology
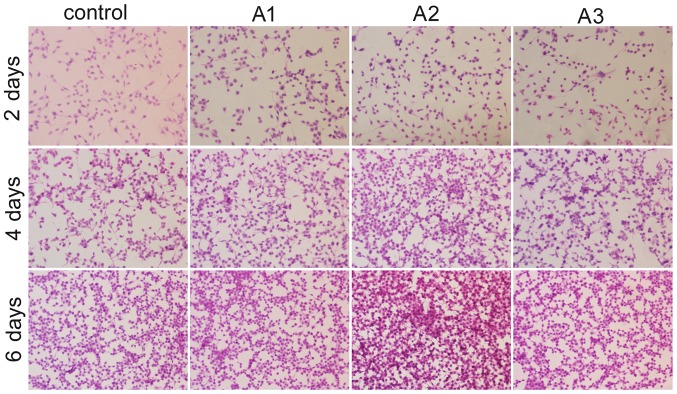
HE staining was conducted using an upright microscope to assess the morphology of RSC96 cells. The images indicated that the Andro groups exhibited increased cell growth compared with the control group at the same time point (Fig. 4). There were no marked differences in Schwann cell morphology between the groups after 6 days of culture. Compared with the control group, RSC96 cells in the presence of Andro grew better and had a distinctive proliferative tendency that gradually increased with time. In addition, when used at 3.125 µM, Andro was able to enhance the proliferation of RSC96 cells compared with the other two concentrations in vitro.
Figure 4.
Hematoxylin-eosin staining showing the morphology of RSC96 Schwann cells cultured with 0 µM (control), 1.5625 µM (A1), 3.125 µM (A2) and 6.25 µM (A3) andrographolide for 2, 4 and 6 days. Cell seeding density: 4×103/ml (original magnification, ×100).
Cell viability assay
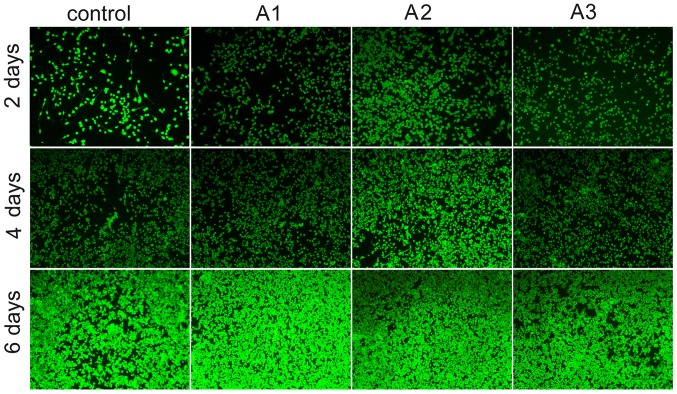
As presented in Fig. 5 viable cells and dead cells were stained with calcein-AM/PI. The results demonstrated that Andro exerted positive effects on survival. Images of calcein-AM/PI staining demonstrated that the survival of cells in the Andro groups was increased compared with in the control group. Consistent with the results of a cell proliferation assay (Fig. 4), more viable cells than dead cells were detected in the Andro groups, thus implying that Andro was able to better support cell growth compared with the control group. Among the Andro groups, treatment with 3.125 µM exhibited the best effects, as evidenced by an increase in the number of viable cells.
Figure 5.
Confocal laser scanning microscopy images showing the viability of RSC96 Schwann cells cultured with 0 µM (control), 1.5625 µM (A1), 3.125 µM (A2) and 6.25 µM (A3) andrographolide for 2, 4 and 6 days. Cell seeding density: 4×103/ml (original magnification, ×100).
S100β secretion
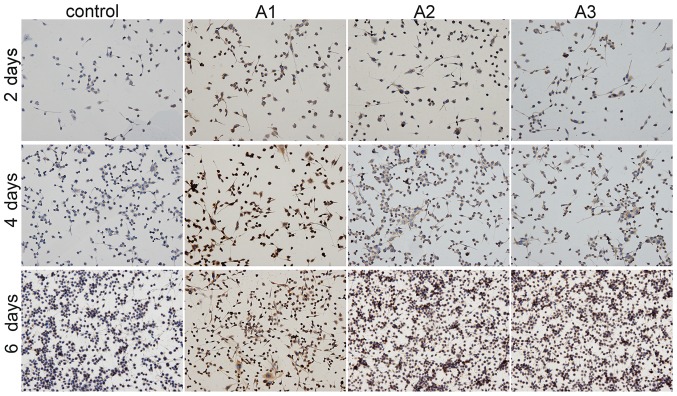
The present study detected Schwann cell-specific protein S100β expression using immunohistochemical staining (Fig. 6). Positive S100β staining was increased in the Andro groups compared with the control group at the same time points. Among the three doses of Andro tested, 3.125 µM was superior compared with the others in terms of phenotypic maintenance of Schwann cells.
Figure 6.
Immunohistochemical staining images showing the presence of S100β. RSC96 Schwann cells were cultured with 0 µM (control), 1.5625 µM (A1), 3.125 µM (A2) and 6.25 µM (A3) andrographolide for 2, 4 and 6 days. Cell seeding density: 4×103/ml (original magnification, ×200).
Gene expression
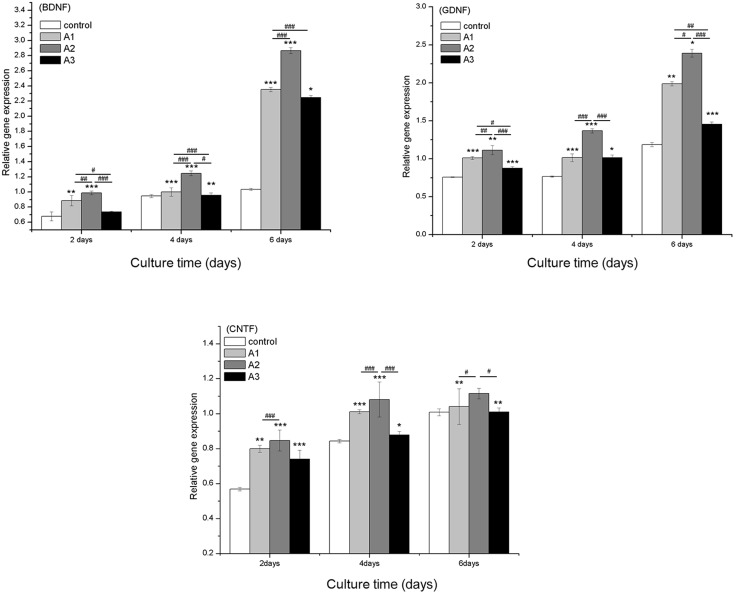
The mRNA expression levels of RSC96 cell-specific genes were determined by RT-qPCR analysis. Nerve growth factor (NGF) and several neurotrophic factors, including BDNF, GDNF and CNTF, have key roles in Schwann cells and the regeneration of peripheral nerves. The mRNA expression levels of BDNF, GDNF and CNTF were significantly increased in the Andro-treated groups compared with the control group (Fig. 7) except for BDNF levels at 6.25 µM concentratio. Furthermore, among all of the groups, 3.125 µM Andro exhibited the best effect on upregulation of BDNF, GDNF and CNTF.
Figure 7.
Quantitative comparison of neurotrophic-related gene expression by reverse transcription-quantitative polymerase chain reaction. The RSC96 Schwann cells were cultured with 0 µM (control), 1.5625 µM (A1), 3.125 µM (A2) and 6.25 µM (A3) andrographolide (Andro) for 2, 4 and 6 days. The gene expression levels in Andro-treated cells were compared with the control group using the 2−ΔΔCq method. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. Data are presented as the mean ± standard deviation of five independent experiments. *P<0.05, **P<0.01,***P<0.001 vs. control; #P<0.05, ##P<0.01, ###P<0.001 vs. A1, A2 and A3. BDNF, brain-derived neurotrophic factor; GDNF, glial cell-derived neuroptrophic factor; CTNF, ciliary neurotrophic factor.
Discussion
Andro is a diterpenoid lactone predominantly extracted from Andrographis paniculata, which is widely used in China and other regions of Asia for the treatment of inflammation-associated diseases. In addition, Andro has been reported to have neuroprotective properties (29–31). Previous studies demonstrated that Andro reduced inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation, thus indicating that Andro may have clinical use for the treatment of Parkinson's disease (17,32). The present study suggested that Andro enhanced neuroprotection and regeneration of peripheral nerves following injury, via its effect on the growth and phenotypic maintenance of RSC96 cells in vitro. The results indicated that Andro was able to promote RSC96 cell growth compared with the control group (Fig. 2). In addition, Andro markedly enhanced DNA synthesis and accelerated the proliferation of RSC96 cells (Figs. 3–5).
Consistent with the increased synthesis of DNA in RSC96 cells, Andro was able to upregulate the mRNA expression levels of BDNF, GDNF and CNTF (Fig. 7). NGF and several neurotrophic factors, including BDNF, GDNF and CNTF, have been reported to exert stimulatory effects on specific neuronal populations (33,34). Neurotrophic factor-based molecular therapies have potential for enhancing functional recovery, as well as for increasing nerve regeneration (35), since they affect several important aspects of regeneration, including axon growth, Schwann cell function and myelination (36). In addition, previous studies regarding molecular therapeutics have concentrated primarily on the creation of neurotrophin factor mimetics, particularly NGF, neurotrophin-3 and BDNF mimetics (37–39). It was reported that the secretion of neurotrophic factors by Schwann cells which were notably increased by Andro in the present study is necessary to promote axon growth and prevent neurons from initiating apoptosis (23,40). Therefore, the probable underlying mechanism is that Andro promoted RSC96 cell growth and neurotrophic factor secretion, thus inducing phenotypic maintenance via modulation of BDNF, GDNF and CNTF expression.
In the present study, the PCR, biochemical and immunohistochemical analyses demonstrated that S100β, a specific protein of Schwann cells, was effectively increased in Andro groups (Figs. 6 and 7). The S100β family, which consists of specific Schwann cell markers, is a family of low molecular weight proteins characterized by two calcium-binding sites, which is highly conserved among vertebrates (41). Furthermore, S100β, from the S100 protein family, has been identified as a potential important factor that contributes to neuronal development and differentiation (42,43). In addition, S100A4 is capable of stimulating neuronal differentiation in cultures of rat hippocampal neurons (44). In the present study, S100β protein expression was elevated in the Andro-treated cells. The modulation of S100β expression following treatment with Andro suggested that Andro may increase proliferation of RSC96 cells and maintain their phenotype.
The present study is a preliminary exploration regarding the effects of Andro on the proliferation and phenotype maintenance of RSC96 cells. Following treatment with the recommended concentrations of Andro (0.78–12.5 µM), the proliferation of RSC96 cells was accelerated in vitro. However, we cannot confirm whether Andro is suitable for the treatment of Schwann cells from other species, including humans. Further studies are required to elucidate the underlying mechanisms of the effects of Andro on Schwann cells, with the aim of identifying a promising anti-neuroinflammatory and neuroprotective agent. Furthermore, the application of Andro on peripheral nerve injury should be investigated.
In conclusion, Andro, which is the primary active component isolated from A. paniculata, exerted positive effects on the proliferation and phenotypic maintenance of RSC96 cells in vitro. These results suggested that Andro may serve as a promising therapeutic agent for peripheral nerve regeneration and neural tissue engineering. The present study may provide evidence for the clinical application of Andro.
Acknowledgments
The present study was financially supported by the Innovation Project of Guangxi Graduate Education of China (grant no. YCSZ2015124) and the National Natural Science Foundation of China (grant no. 81160221). The study was supported by the Research Center for Regenerative Medicine and Collaborative Innovation Center of Guangxi Biological Medicine.
References
- 1.Lundborg G. A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25:391–414. doi: 10.1053/jhsu.2000.4165. [DOI] [PubMed] [Google Scholar]
- 2.Robinson LR. Traumatic injury to peripheral nerves. Suppl Clin Neurophysiol. 2004;57:173–186. doi: 10.1016/S1567-424X(09)70355-1. [DOI] [PubMed] [Google Scholar]
- 3.Evans GR. Peripheral nerve injury: A review and approach to tissue engineered constructs. Anat Rec. 2001;263:396–404. doi: 10.1002/ar.1120. [DOI] [PubMed] [Google Scholar]
- 4.Raine CS. Multiple sclerosis: Immune system molecule expression in the central nervous system. J Neuropathol Exp Neurol. 1994;53:328–337. doi: 10.1097/00005072-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Rogers J, Shen Y. A perspective on inflammation in Alzheimer's disease. Ann N Y Acad Sci. 2000;924:132–135. doi: 10.1111/j.1749-6632.2000.tb05571.x. [DOI] [PubMed] [Google Scholar]
- 6.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 7.Zochodne DW. The microenvironment of injured and regenerating peripheral nerves. Muscle Nerve Suppl. 2000;9:S33–S38. doi: 10.1002/1097-4598(2000)999:9<::AID-MUS7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Dezawa M. Central and peripheral nerve regeneration by transplantation of Schwann cells and transdifferentiated bone marrow stromal cells. Anat Sci Int. 2002;77:12–25. doi: 10.1046/j.0022-7722.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Sanford MT, Xin Z, Lin G, Lue TF. Role of Schwann cells in the regeneration of penile and peripheral nerves. Asian J Androl. 2015;17:776–782. doi: 10.4103/1008-682X.154306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, Tandon JS. Immunostimulant agents from Andrographis paniculata. J Nat Prod. 1993;56:995–999. doi: 10.1021/np50097a002. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XF, Tan BK. Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats. Clin Exp Pharmacol Physiol. 2000;27:358–363. doi: 10.1046/j.1440-1681.2000.03253.x. [DOI] [PubMed] [Google Scholar]
- 12.Shen YC, Chen CF, Chiou WF. Andrographolide prevents oxygen radical production by human neutrophils: Possible mechanism(s) involved in its anti-inflammatory effect. Br J Pharmacol. 2002;135:399–406. doi: 10.1038/sj.bjp.0704493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu T, Wang DX, Zhang W, Liao XQ, Guan X, Bo H, Sun JY, Huang NW, He J, Zhang YK, et al. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-κB. PLoS One. 2013;8:e56407. doi: 10.1371/journal.pone.0056407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, Leung BP, Wong WS. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Respir Crit Care Med. 2009;179:657–665. doi: 10.1164/rccm.200809-1516OC. [DOI] [PubMed] [Google Scholar]
- 15.Chen JX, Xue HJ, Ye WC, Fang BH, Liu YH, Yuan SH, Yu P, Wang YQ. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol Pharm Bull. 2009;32:1385–1391. doi: 10.1248/bpb.32.1385. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Luo L, Wang X, Liao B, Li G. Inhibition of NF-kappaB expression and allergen-induced airway inflammation in a mouse allergic asthma model by andrographolide. Cell Mol Immunol. 2009;6:381–385. doi: 10.1038/cmi.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Liu B, Zhang W, Wilson B, Hong JS. Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. J Pharmacol Exp Ther. 2004;308:975–983. doi: 10.1124/jpet.103.059683. [DOI] [PubMed] [Google Scholar]
- 18.Wen L, Xia N, Chen X, Li Y, Hong Y, Liu Y, Wang Z, Liu Y. Activity of antibacterial, antiviral, anti-inflammatory in compounds andrographolide salt. Eur J Pharmacol. 2014;740:421–427. doi: 10.1016/j.ejphar.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 19.Ku CM, Lin JY. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013;141:1104–1113. doi: 10.1016/j.foodchem.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Chen YY, Hsu MJ, Sheu JR, Lee LW, Hsieh CY. Andrographolide, a novel NF- κB inhibitor, induces vascular smooth muscle cell apoptosis via a Ceramide-p47phox-ROS signaling cascade. Evid Based Complement Alternat Med. 2013;2013:821813. doi: 10.1155/2013/821813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talei D, Valdiani A, Maziah M, Sagineedu SR, Saad MS. Analysis of the anticancer phytochemicals in andrographis paniculata Nees. Under salinity stress. Biomed Res Int. 2013;2013:319047. doi: 10.1155/2013/319047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manikam SD, Stanslas J. Andrographolide inhibits growth of acute promyelocytic leukaemia cells by inducing retinoic acid receptor-independent cell differentiation and apoptosis. J Pharm Pharmacol. 2009;61:69–78. doi: 10.1211/jpp.61.01.0010. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann HC, Höke A. Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neurol Disord Drug Targets. 2010;9:801–806. doi: 10.2174/187152710793237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J Biomed Mater Res A. 2010;93:164–174. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 25.Rotshenker S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosse F. Extrinsic cellular and molecular mediators of peripheral axonal regeneration. Cell Tissue Res. 2012;349:5–14. doi: 10.1007/s00441-012-1389-5. [DOI] [PubMed] [Google Scholar]
- 27.Yuan H, Zhang J, Liu H, Li Z. The protective effects of resveratrol on Schwann cells with toxicity induced by ethanol in vitro. Neurochem Int. 2013;63:146–153. doi: 10.1016/j.neuint.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Chan SJ, Wong WS, Wong PT, Bian JS. Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischaemia. Br J Pharmacol. 2010;161:668–679. doi: 10.1111/j.1476-5381.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaitone SA, Abo-Elmatty DM, Shaalan AA. Acetyl-L-carnitine and α-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson's disease therapy. Pharmacol Biochem Behav. 2012;100:347–360. doi: 10.1016/j.pbb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Jalali-Nadoushan M, Roghani M. Alpha-lipoic acid protects against 6-hydroxydopamine-induced neurotoxicity in a rat model of hemi-parkinsonism. Brain Res. 2013;1505:68–74. doi: 10.1016/j.brainres.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Lai D, Wang L, Yu P, Zhu L, Guo B, Xu L, Zhou L, Sun Y, Lee SM, Wang Y. Neuroprotective effects of the andrographolide analogue AL-1 in the MPP+/MPTP-induced Parkinson's disease model in vitro and in mice. Pharmacol Biochem Behav. 2014;122:191–202. doi: 10.1016/j.pbb.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Aloe L, Rocco ML, Bianchi P, Manni L. Nerve growth factor: From the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bothwell M. NGF, BDNF, NT3, and NT4. Handb Exp Pharmacol. 2014;220:3–15. doi: 10.1007/978-3-642-45106-5_1. [DOI] [PubMed] [Google Scholar]
- 35.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klimaschewski L, Hausott B, Angelov DN. The pros and cons of growth factors and cytokines in peripheral axon regeneration. Int Rev Neurobiol. 2013;108:137–171. doi: 10.1016/B978-0-12-410499-0.00006-X. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Longo FM. Neurotrophin small-molecule mimetics. Prog Brain Res. 2000;128:333–347. doi: 10.1016/S0079-6123(00)28030-8. [DOI] [PubMed] [Google Scholar]
- 38.Peleshok J, Saragovi HU. Functional mimetics of neurotrophins and their receptors. Biochem Soc Trans. 2006;34:612–617. doi: 10.1042/BST0340612. [DOI] [PubMed] [Google Scholar]
- 39.Colangelo AM, Bianco MR, Vitagliano L, Cavaliere C, Cirillo G, De Gioia L, Diana D, Colombo D, Redaelli C, Zaccaro L, et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J Neurosci. 2008;28:2698–2709. doi: 10.1523/JNEUROSCI.5201-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Wang WJ, Ding WL, Li F, He J. Effect of panaxydol on hypoxia-induced cell death and expression and secretion of neurotrophic factors (NTFs) in hypoxic primary cultured Schwann cells. Chem Biol Interact. 2008;174:44–50. doi: 10.1016/j.cbi.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 42.Donato R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/S1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 43.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 44.Novitskaya V, Grigorian M, Kriajevska M, Tarabykina S, Bronstein I, Berezin V, Bock E, Lukanidin E. Oligomeric forms of the metastasis-related Mts1 (S100A4) protein stimulate neuronal differentiation in cultures of rat hippocampal neurons. J Biol Chem. 2000;275:41278–41286. doi: 10.1074/jbc.M007058200. [DOI] [PubMed] [Google Scholar]