Abstract
Allicin is a major component of garlic, extracted as an oily liquid. The present study was designed to investigate the beneficial effects of allicin on traumatic spinal cord injury (TSCI) in mice, and whether the effects are mediated via regulation of the heat shock protein 70 (HSP70), v-akt murine thymoma viral oncogene homolog 1 (Akt) and inducible nitric oxide synthase (iNOS) pathways. Adult BALB/c mice (30–40 g) received a laminectomy at the T9 vertebral level as a model of TSCI. In the present study, treatment of the TSCI mice with allicin significantly increased their Basso, Beattie and Bresnahan (BBB) scores (P<0.01) and reduced the spinal cord water content (P<0.01). This protective effect was associated with the inhibition of oxidative stress and inflammatory responses in TSCI mice. Western blot analysis demonstrated that allicin increased the protein levels of HSP70, increased the phosphorylation of Akt and reduced the iNOS protein expression levels in TSCI mice. Additionally, treatment with allicin significantly reduced the levels of ROS and enhanced the NADH levels in TSCI mice. Collectively, these data demonstrate that the effects of allicin on TSCI are mediated via regulation of the HSP70, Akt and iNOS pathways in mice.
Keywords: allicin, traumatic spinal cord injury, HSP-70, Akt, iNOS
Introduction
With high incidences of disability and other serious consequences, traumatic spinal cord injury (TSCI) can result in catastrophic damage to patients (1). Direct or indirect insult to the backbone and spinal cord can lead to TSCI, which subsequently affects the daily life of the patient and their family members (2). TSCI morbidity is higher in developed countries compared with developing countries. The USA mortality rate is 20–45/100,000, and the rate of TSCI occurrence is 900/100,000 (3). Advancements to the transportation, construction and mining industries have increased the incidence of TSCI in China. The morbidity rate of TSCI caused by earthquake disasters has reached 10%. Survey data from the population of Beijing in 2005 indicated that the rate of TSCI was 60.2/100,000 (4).
Due to serious complications, patients with TSCI may require long periods of hospitalization. The TSCI clinical pathway, a standardized method of patient care following TSCI, has achieved great success (5). Multidisciplinary investigation was used to develop a clinical pathway for a standardized model of TSCI treatment. Following a 6-month trial period, the clinical pathway reduced the length of patient hospitalization and the total costs (6). The TSCI clinical pathway has various advantages and lasting influences on the overall quality of TSCI treatment. The pathway reinforces to health professionals that rehabilitation and nursing at the acute phase of TSCI are inseparable (7). Additionally, the diagnosis of TSCI is clear, the surgical invention is uniformed and the complications are predictable. It is important to formulate a similar standardized clinical pathway for the treatment of patients with TSCI in China (8).
Allicin is a major component of garlic. Previous studies have demonstrated that allicin has important health benefits and medicinal effects (9). Allicin has been demonstrated to inhibit the growth of pathogenic microorganisms, including certain bacteria, viruses and fungi, and to inhibit tumor growth (10,11). Additionally, it can prevent arteriosclerosis, stenocardia, cerebral infarction, arrhythmia and hydrargyrism. Allicin can also can reduce cholesterol and blood pressure, regulate blood sugar levels, enhance the immune system and reduce oxidation (12). Therefore, the aim of the present study was to investigate the use of allicin in TSCI, and to elucidate whether the effects of allicin are mediated by the heat shock protein 70 (HSP70), v-akt murine thymoma viral oncogene homolog 1 (Akt) and inducible nitric oxide synthase (iNOS) pathways.
Materials and methods
Animals, surgery and experimental groups
Adult BALB/c mice (n=40; 30–40 g) were obtained from The First Central Hospital of Baoding (Baoding, China). The mice were housed at 24±1°C and maintained on a 12:12 h light/dark cycle. The mice were provided with ad libitum access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of The First Central Hospital of Baoding and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
All experimental mice were anesthetized by ketamine (100 mg/kg; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and xylazine (10 mg/kg; Sinopharm Chemical Reagent Co., Ltd.) injection via the caudal vein. The mice were randomly assigned into five experimental groups (n=8 in each group), as follows: Sham group; TSCI group; 1 mg/kg allicin group; 10 mg/kg allicin group; and 50 mg/kg allicin group. Allicin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) and saline were delivered via intraperitoneal injection. The anesthetized mice, excluding the sham group, received a laminectomy at the T9 vertebral level using the Infinite Horizons impactor (75 kdyn; Precision Systems and Instrumentation, LLC, Fairfax Station, VA, USA). Following TSCI, all mice were housed in warmed cages at 35–37°C. The mice received either 1, 5 or 10 mg/kg allicin. Sham and TSCI mice received 2 ml sterile saline.
Locomotor recovery and the spinal cord water content
Mice were relocated to the test environment (90×4 cm) where Basso, Beattie and Bresnahan (BBB) testing was performed prior to and 1 week following TSCI. BBB scores range from 0 (no observable hind-limb movements) to 21 (normal gait). Following sacrifice by cervical dislocation under anesthesia, the spinal cord samples were collected, weighed, recorded and dried at 80°C for 48–72 h. The spinal cord water content was calculated using the following formula: [(Wet weight - dry weight)/wet weight] × 100.
Enzyme-linked immunosorbent assay for markers of oxidative stress and inflammation
Briefly, the spinal cord samples were collected and homogenized, and the supernatant was collected by centrifugation at 12,000 × g and resuspended in lysis buffer containing protease inhibitors (Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China). The supernatant was collected and the protein concentration was measured using a bicinchoninic acid (BCA) assay kit (Cell Biolabs, Inc., San Diego, CA, USA). The activities of catalase (CAT; #707002), superoxide dismutase (SOD; #706002), nuclear factor-κB (NF-κB; #10007889) and tumor necrosis factor-α (TNF-α; #500850) were measured using commercially available assay kits (Cayman Chemical Company, Ann Arbor, MI, USA) following the manufacturer's protocol.
Western blot analysis
Briefly, the spinal cord samples were homogenized, and the supernatant was collected and harvested in lysis buffer containing protease inhibitors. The protein concentration was measured using the BCA protein assay kit. Protein samples (~60 μg) were separated by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was blocked with 5% non-fat milk and incubated with the following primary antibodies, purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), at 4°C for 10–12 h: Rabbit polyclonal phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K; 1:800; cat. no. sc-67306); rabbit polyclonal Akt (1:500; cat. no. sc-8312); rabbit polyclonal phospho-Akt (1:500; cat. no. sc-135650); rabbit polyclonal iNOS (1:500; cat. no. sc-8309); and rabbit polyclonal β-actin (1:600; cat. no. sc-130656). Membranes were then washed with Tris-buffered saline-Tween 20 and incubated for 2 h at room temperature with goat anti-rabbit secondary antibodies (Wuhan Boster Biological Technology, Co., Ltd., Wuhan, China; cat. no. BA1054). The experiment was repeated in triplicate. Membranes were exposed with enhanced chemiluminescence reagent (Beyotime Institute of Biotechnology, Haimen, China) and quantified with Image J version 3.0 analysis software (imagej.nih.gov/ij).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) of HSP70
Total RNA was isolated from spinal cord samples of each group using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total RNA (1 μl) was used to synthesize cDNA using a TaqMan miRNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.), following treatment with DNase I for 10 min at 37°C. A SYBR Green PCR Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and Bio-Rad iQ5 Gradient Real-Time PCR system (Bio-Rad Laboratories, Inc.) were used to perform qPCR and analyze the gene expression levels of HSP70. The thermocycling conditions were as follows: 95°C for 15 min; followed by 40 cycles of 94°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The HSP70 primers used for all RT-qPCR experiments were as follows: Forward, 5′-ACCAGGACACTGTTGAGTTC-3′; and reverse, 5′-ACTCATCTCCGAGTTCACAC-3′. GAPDH was used as the reference gene, with primers as follows: Forward, 5′-AAGGTGAAGGTCGGAGTCAA-3′; and reverse, 5′-AATGAAGGGGTCATTGATGG-3′. The qPCR data was analyzed using the 2−ΔΔCq method (13).
Mitochondrial reactive oxygen species (ROS) and nicotinamide adenine dinucleotide (NADH) production
Briefly, the spinal cord samples were homogenized, and the supernatant was collected, and ROS and NADH levels measured using 10 μM dichlorofluorescein diacetate (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 37°C in the dark. Mitochondrial ROS levels were detected by fluorescence measurements at 480 nm excitation and 530 nm emission wavelengths (Opsys MR; Dynex Technologies, Chantilly, VA, USA). Mitochondrial NADH levels were detected by measuring fluorescence at 360 nm excitation and 460 nm emission wavelengths.
Statistical analysis
Statistical analysis was performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance tests were performed to compare differences between all groups, followed by least significant difference post-hoc tests. Data are presented as the mean ± standard error. P<0.05 was considered to indicate a statistically significant difference.
Results
Protective effects of allicin on locomotor recovery in TSCI mice
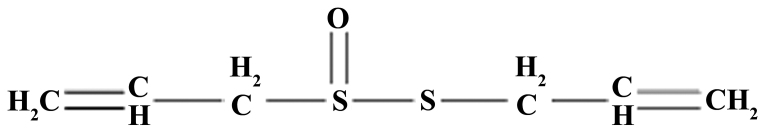
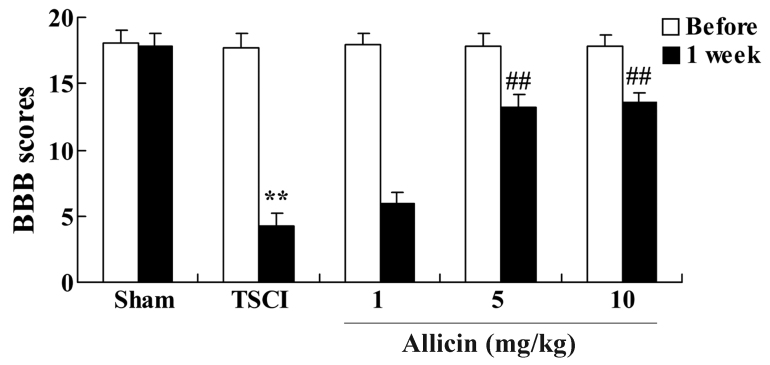
The present study investigated the effect of allicin on locomotor recovery in TSCI mice. The chemical structure of allicin is presented in Fig. 1. As demonstrated in Fig. 2, 1 week after TSCI, the BBB scores of TSCI mice were significantly reduced compared with the sham group (P<0.001). Additionally, there was a significant increase in the BBB scores following treatment with 5 or 10 mg/kg allicin compared with the TSCI model group (P=0.0023 and P=0.0017, respectively).
Figure 1.
The chemical structure of allicin.
Figure 2.
Protective effect of allicin on locomotor recovery in TSCI mice. BBB scores of mice in the sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) groups. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. BBB scores, Basso, Beattie and Bresnahan scores; TSCI, traumatic spinal cord injury.
Protective effect of allicin on the spinal cord water content in TSCI mice
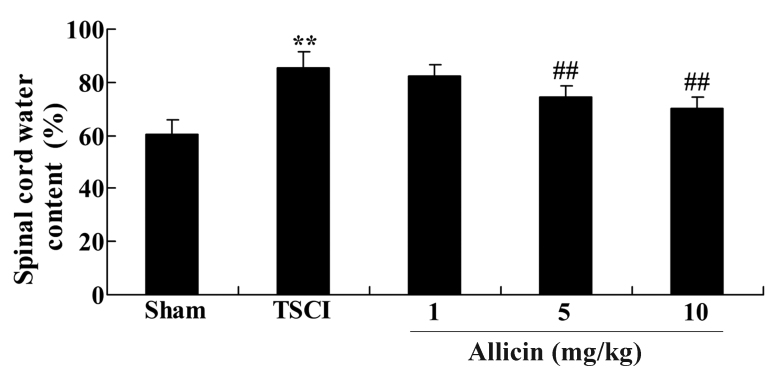
To confirm the effect of allicin on the spinal cord water content in TSCI mice, the current study measured the spinal cord water content following 1 week of treatment with allicin. The results are presented in Fig. 3; the spinal cord water content of TSCI mice was significantly increased compared with the sham group (P=0.0041). After 1 week of allicin treatment, the mice treated with 5 or 10 mg/kg allicin exhibited a significant reduction in the spinal cord water content when compared with the percentage water content in the TSCI model group (P=0.0057 and P=0.0032, respectively).
Figure 3.
Protective effect of allicin on the spinal cord water content in TSCI mice. Spinal cord water content in the sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) groups. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. TSCI, traumatic spinal cord injury.
Protective effect of allicin on oxidative stress in TSCI mice
To determine whether the protective effect induced by allicin was mediated by anti-oxidative activity, the enzymatic activities of CAT and SOD were measured following allicin treatment. As demonstrated in Fig. 4, the enzymatic activities of CAT and SOD were significantly reduced in the TSCI group compared with the sham group (P<0.0001 and P=0.0001, respectively). CAT and SOD enzymatic activities were increased by allicin treatment (5 or 10 mg/kg) compared with the TSCI group (P=0.0023, P=0.0014, P=0.0005 and P=0.0003, respectively).
Figure 4.
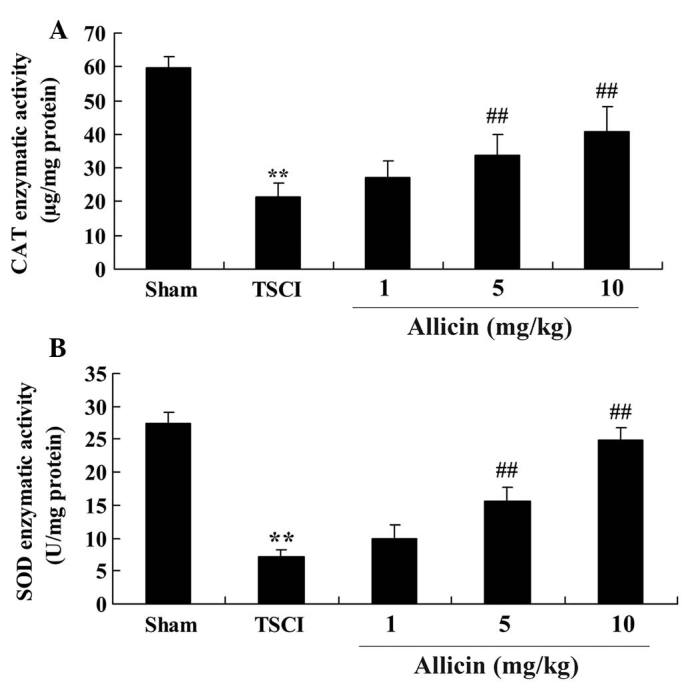
Protective effect of allicin on oxidative stress in TSCI mice. Protective effect of allicin on the enzymatic activities of (A) CAT and (B) SOD in the sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) groups. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. TSCI, traumatic spinal cord injury; SOD, superoxide dismutase; CAT, catalase.
Protective effect of allicin on inflammation in TSCI mice
The current study also investigated the effect of allicin on the anti-inflammatory signaling pathways. The results demonstrated that the levels of NF-κB and TNF-α were increased in the TSCI group compared with the sham group (each P<0.0001; Fig. 5). Additionally, allicin treatment, at all concentrations, significantly reduced the levels of NF-κB and TNF-α compared with the TSCI model rats (P=0.0071, P=0.0042, P=0.0052, P=0.0021, P=0.0030 and P=0.0016, respectively; Fig. 5).
Figure 5.
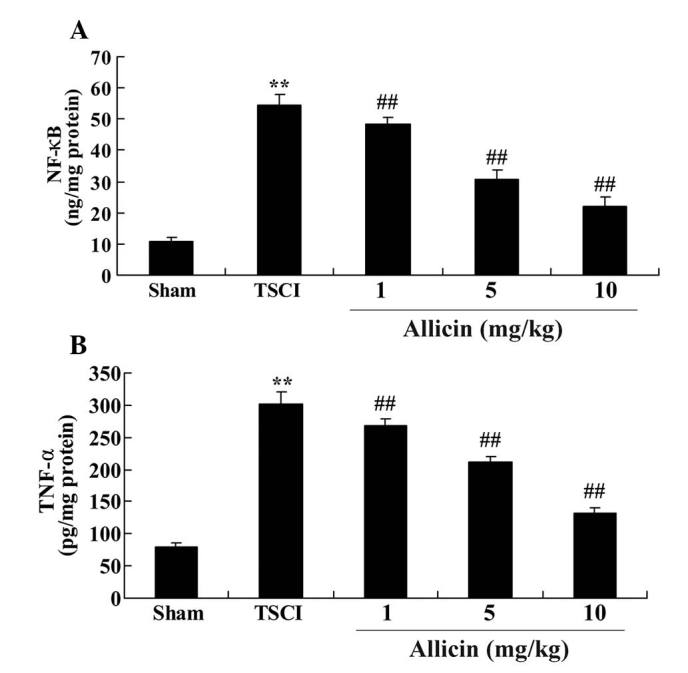
Protective effect of allicin on inflammation in TSCI mice. Protective effect of allicin on levels of (A) NF-κB and (B) TNF-α in the sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) groups. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. TSCI, traumatic spinal cord injury; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α.
Protective effect of allicin on PI3K/Akt in TSCI mice
To further investigate the protective effects of allicin on TSCI, the current study investigated changes to the PI3K/Akt pathways following allicin treatment of TSCI rats. As demonstrated in Fig. 6, the protein expression levels of PI3K and the phosphorylation of Akt in TSCI mice were reduced compared with the sham group (P=0.0019 and P=0.0006, respectively). However, treatment with 5 or 10 mg/kg allicin significantly increased the protein expression levels of PI3K and the phosphorylation of Akt compared with the TSCI mice (P=0.0053, P=0.0022, P=0.0036 and P=0.0017, respectively).
Figure 6.
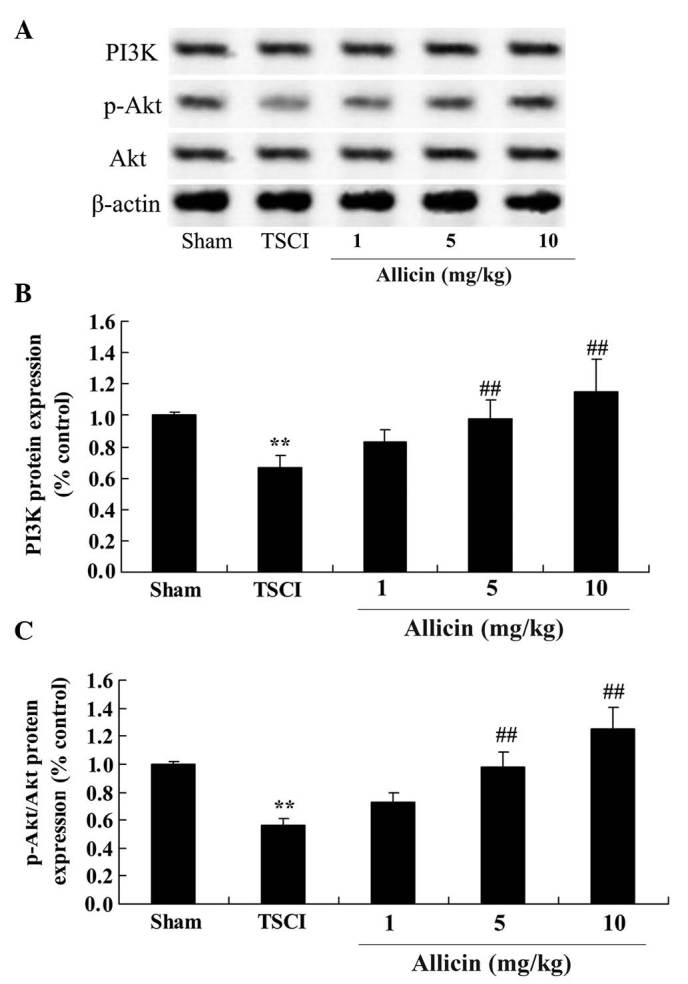
Protective effect of allicin on PI3K/Akt protein and phosphorylation levels in TSCI mice. (A) The effects of allicin on the PI3K/Akt and p-Akt levels were measured using western blotting. (B) Analysis of PI3K levels and (C) p-Akt/Akt in samples from the sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) groups. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. TSCI, traumatic spinal cord injury; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; Akt, v-akt murine thymoma viral oncogene homolog 1; p-Akt, phospho-Akt.
Protective effect of allicin on iNOS in TSCI mice
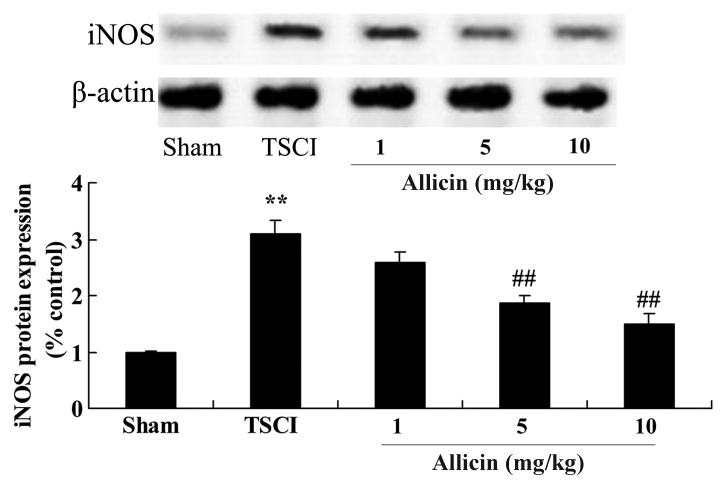
To investigate the mechanisms that mediate the protective effects of allicin against TSCI, iNOS protein expression in TSCI mice was measured using western blot analysis. As presented in Fig. 7, TSCI significantly increased iNOS protein expression compared with the sham group (P=0.0007). Administration of 5 and 10 mg/kg allicin significantly inhibited the promotion of iNOS protein expression compared with the TSCI model mice (P=0.0012 and P=0.0003, respectively).
Figure 7.
Effect of allicin on iNOS in TSCI mice. iNOS levels were measured in sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) groups. using western blot analysis. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. iNOS, nitric oxide synthase; TSCI, traumatic spinal cord injury.
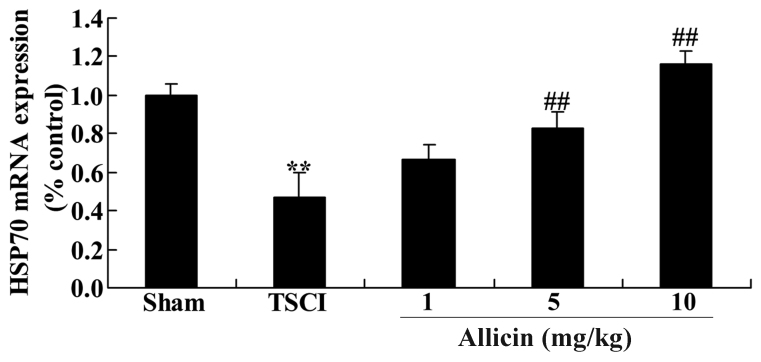
Protective effect of allicin on HSP70 in TSCI mice
The present study also measured the HSP70 mRNA expression levels to investigate the mechanisms that mediate the effects of allicin on TSCI. As presented in Fig. 8, the HSP70 mRNA expression levels were significantly reduced in the TSCI group compared with the sham mice (P=0.0022). Furthermore, treatment with allicin (5 or 10 mg/kg) significantly increased the HSP70 mRNA expression levels compared with the TSCI mice (P=0.0025 and P=0.0018, respectively; Fig. 8).
Figure 8.
Effect of allicin on HSP70 mRNA levels in TSCI mice. Sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) groups. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. HSP70, heat shock protein family A member 4, TSCI, traumatic spinal cord injury.
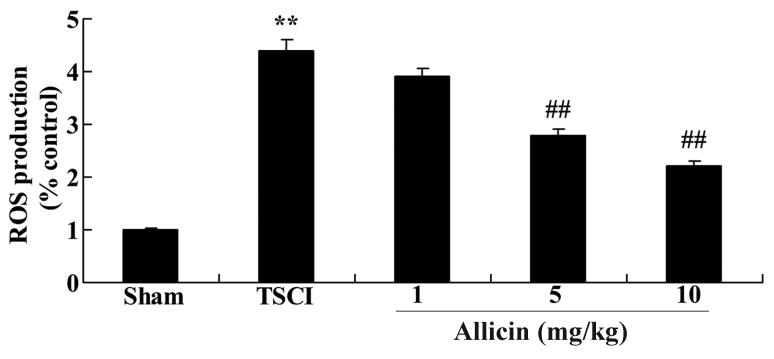
Protective effect of allicin on ROS production in TSCI mice
To evaluate whether ROS are associated with the protective effect of allicin on TSCI, ROS production was measured in the experimental groups. The results of Fig. 9 demonstrate a significant increase in ROS levels following TSCI compared with the sham group (P<0.0001). Notably, treatment with 5 or 10 mg/kg allicin significantly reduced the levels of ROS compared with the TSCI group (P=0.0030 and P=0.0013, respectively).
Figure 9.
Effect of allicin on ROS levels in sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) mice. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. ROS, reactive oxygen species; TSCI, traumatic spinal cord injury.
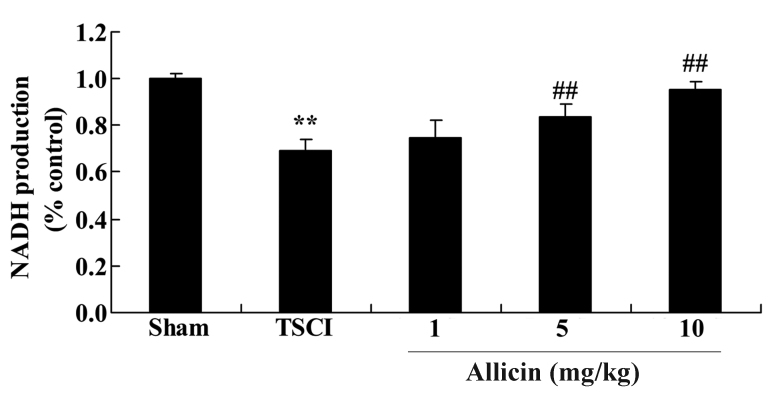
Protective effect of allicin on NADH production in TSCI mice
To investigate the effects of allicin on NADH production in TSCI mice, the NADH levels were analyzed following TSCI and treatment with allicin. As demonstrated in Fig. 10, NADH levels were reduced in the TSCI group compared with the sham group (P=0.0007). However, allicin treatment (5 or 10 mg/kg) significantly elevated the NADH levels compared with TSCI mice (P=0.0032 and P=0.0021).
Figure 10.
Effect of allicin on NADH production in sham, TSCI model and allicin-treated (1, 5 or 10 mg/kg) mice. Data are presented as the mean ± standard error. **P<0.01 vs. sham group, ##P<0.01 vs. TSCI model group. NADH, nicotinamide adenine dinucleotide; TSCI, traumatic spinal cord injury.
Discussion
The development of the transportation and construction industries has led to an increase in the number of patients suffering from traumatic TSCI (14). TSCI places a heavy burden on the families of patients and society, as the condition leads to high disability rates, high costs, loss of labor forces and other serious complications, including infection, bedsores and deep venous thrombosis (15). Certain countries have conducted numerous detailed studies on TSCI, however, few have been performed in China (15). The current study confirms the protective effect of allicin. Allicin treatment increased locomotor recovery, and reduced the spinal cord water content, oxidative stress and inflammation in TSCI mice. Liu et al (9) reported that allicin protects spinal cord neurons via suppression of the oxidative stress pathway. Li et al (16) indicated that allicin alleviates inflammation in Caco-2 cells.
Akt is considered a vital regulatory factor for signal transduction, and together with purine signals is important in nervous physiological and pathological processes (17). Akt signaling facilitates the growth, differentiation, survival and regeneration of neurons via the P2 receptor following injury of the central nervous system, and mediates the remodeling of impaired neural tissues (18). As a crucial mediator of protein kinase cascades, following impairment of the central nervous system, ATP can activate the PI3K/Akt pathways, which are important for the survival and repair of neurons (19). In the present study, treatment with allicin significantly increased the levels of PI3K and phospho-Akt in TSCI rats. Liu et al (20) demonstrated that allicin protects against cardiac hypertrophy and fibrosis through the activation of the ROS-dependent Akt signaling pathways.
Numerous studies have suggested that injury of the central nervous system caused by ischemia and other damage increases the expression levels of NOS (21,22). Excessive NO synthesis occurs, which directly impairs the function of the nervous system. As a micromolecule and free radical, NO possesses various bioactivities, including vasodilatation, neural information transmission and cytotoxic effects (23). Excessive NO can induce cell apoptosis, which is characteristic of the secondary changes exhibited following TSCI (24). In the current study, administration of allicin significantly inhibited the promotion of iNOS protein expression in TSCI mice. Liu et al (9) reported that allicin protects spinal cord neurons through suppression of the NOS pathway. Zhou et al (25) also demonstrated that allicin protects against mechanical trauma injury via Akt- and mitogen-activated protein kinase 1-mediated regulation of the NOS pathway.
HSPs are a widely expressed, evolutionarily conserved family of proteins. They function as molecular chaperones, and are important for the transport and folding of proteins during cellular stress. HSP70 proteins are considered to be the principle family members (26). HSP70 protein expression is stimulated following stress, including heat shock, ischemia, oxygen deficit, viral infection and mechanical injuries (27). Previous studies on HSP70 in TSCI have observed that, following injury, the HSP70 protein expression levels were increased (26). In the present study, treatment with allicin significantly increased the levels of HSP70 mRNA expression in TSCI mice. Liu et al (9) previously reported that allicin protects spinal cord neurons through the regulation of the HSP70 pathway.
Numerous studies have investigated the secondary damage mechanisms following TSCI. It was previously demonstrated that ROS are a crucial mediator of the secondary damages caused by SCI. Following TSCI, a series of pathological changes occur in the spinal cord tissue, including edema, bleeding, anoxia and ischemia (28). These changes lead to mitochondrial dysfunction and increased of ROS production (29). In the case of the rate of ROS production exceeding the scavenging activity of the defense system, ROS accumulates in cells. In the present study, allicin significantly reduced the levels of ROS following TSCI. A previous study demonstrated that allicin protects spinal cord neurons via suppression of ROS production (9). Chan et al (30) demonstrated that allicin protects rat cardiomyoblasts by inhibiting the generation of ROS.
NADH can protect cells from injury via oxidation and peroxidation of heavy metal chromate-induced hemoglobin (31). Previous research has demonstrated that NADH can effectively eliminate oxygen free radicals, inhibit the production of ROS, stabilize the cytomembrane, activate multi-enzyme systems, and enhance the synthesis and metabolism of nucleic acids, proteins and polysaccharides. Furthermore, NADH can regulate and improve metabolism (32). Additionally, NADH is important for the production of the materials required for regeneration, repair and protection. These results demonstrate the importance of NADH as a mediator of the allicin-induced locomotor recovery of TSCI mice. The present study demonstrated that allicin significantly increased NADH production, which had been reduced by TSCI. Rabinkov et al (33) reported that the predominant biological action of allicin may be attributed to its effect on NADH levels.
In conclusion, the findings of the current study demonstrated that the beneficial effects of allicin following TSCI are mediated via the regulation of oxidative stress and inflammation. The protective effects of allicin were dependent on the promotion of HSP70 protein expression and NADH levels, and the inhibition of iNOS and ROS levels following TSCI. The results of the present study indicate that the protective effects of allicin may be useful in the treatment of TSCI.
References
- 1.Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol. 2014;258:121–129. doi: 10.1016/j.expneurol.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konya D, Gercek A, Akakin A, Akakin D, Tural S, Cetinel S, Ozgen S, Pamir MN. The effects of inflammatory response associated with traumatic spinal cord injury in cutaneous wound healing and on expression of transforming growth factor-beta1 (TGF-beta1) and platelet-derived growth factor (PDGF)-A at the wound site in rats. Growth Factors. 2008;26:74–79. doi: 10.1080/08977190802025339. [DOI] [PubMed] [Google Scholar]
- 3.Yin KJ, Kim GM, Lee JM, He YY, Xu J, Hsu CY. JNK activation contributes to DP5 induction and apoptosis following traumatic spinal cord injury. Neurobiol Dis. 2005;20:881–889. doi: 10.1016/j.nbd.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Chan SC, Chan AP. Rehabilitation outcomes following traumatic spinal cord injury in a tertiary spinal cord injury centre: A comparison with an international standard. Spinal Cord. 2005;43:489–498. doi: 10.1038/sj.sc.3101743. [DOI] [PubMed] [Google Scholar]
- 5.Franceschini M, Di Clemente B, Citterio A, Pagliacci MC. Follow-up in persons with traumatic spinal cord injury: Questionnaire reliability. Eura Medicophys. 2006;42:211–218. [PubMed] [Google Scholar]
- 6.Dobkin BH, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair. 2003;17:153–167. doi: 10.1177/0888439003255508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Weert KC, Schouten EJ, Hofstede J, van de Meent H, Holtslag HR, van den Berg-Emons RJ. Acute phase complications following traumatic spinal cord injury in Dutch level 1 trauma centres. J Rehabil Med. 2014;46:882–885. doi: 10.2340/16501977-1858. [DOI] [PubMed] [Google Scholar]
- 8.Wong AM, Leong CP, Su TY, Yu SW, Tsai WC, Chen CP. Clinical trial of acupuncture for patients with spinal cord injuries. Am J Phys Med Rehabil. 2003;82:21–27. doi: 10.1097/00002060-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Liu SG, Ren PY, Wang GY, Yao SX, He XJ. Allicin protects spinal cord neurons from glutamate-induced oxidative stress through regulating the heat shock protein 70/inducible nitric oxide synthase pathway. Food Funct. 2015;6:321–330. doi: 10.1039/C4FO00761A. [DOI] [PubMed] [Google Scholar]
- 10.Adetumbi MA, Lau BH. Allium sativum (garlic)–a natural antibiotic. Med Hypotheses. 1983;12:227–237. doi: 10.1016/0306-9877(83)90040-3. [DOI] [PubMed] [Google Scholar]
- 11.Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ. Allicin: Chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Wang Y, Cao YG, Qi HP, Li L, Bai B, Liu Y, Sun HL. Antiarrhythmic effects and ionic mechanisms of allicin on myocardial injury of diabetic rats induced by streptozotocin. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:697–704. doi: 10.1007/s00210-013-0872-1. [DOI] [PubMed] [Google Scholar]
- 13.Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PLoS One. 2011;6:e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q, Ning GZ, Li YL, Feng HY, Feng SQ. Factors affecting the length of stay of patients with traumatic spinal cord injury in Tianjin, China. J Spinal Cord Med. 2013;36:237–242. doi: 10.1179/2045772313Y.0000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Xiang Q, Li C, Zhou Y. Epidemiology of traumatic cervical spinal fractures and risk factors for traumatic cervical spinal cord injury in China. J Spinal Disord Tech. 2013;26:E306–E313. doi: 10.1097/BSD.0b013e3182886db9. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Lun W, Zhao X, Lei S, Guo Y, Ma J, Zhi F. Allicin alleviates inflammation of trinitrobenzenesulfonic acid-induced rats and suppresses P38 and JNK pathways in Caco-2 cells. Mediators Inflamm. 2015;2015:434692. doi: 10.1155/2015/434692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L, Xie J, Xin N, Wang Z. Panax notoginseng saponins promote wound repair of anterior cruciate ligament through phosphorylation of PI3K, AKT and ERK. Int J Clin Exp Pathol. 2015;8:441–449. [PMC free article] [PubMed] [Google Scholar]
- 18.Yune TY, Park HG, Lee JY, Oh TH. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J Neurotrauma. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]
- 19.Jung SY, Kim DY, Yune TY, Shin DH, Baek SB, Kim CJ. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med. 2014;7:587–593. doi: 10.3892/etm.2013.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Cao F, Tang QZ, Yan L, Dong YG, Zhu LH, Wang L, Bian ZY, Li H. Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J Nutr Biochem. 2010;21:1238–1250. doi: 10.1016/j.jnutbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Gong FL, Zhao GB, Li J. Chrysin suppressed inflammatory responses and the inducible nitric oxide synthase pathway after spinal cord injury in rats. Int J Mol Sci. 2014;15:12270–12279. doi: 10.3390/ijms150712270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbasi Habashi S, Sabouni F, Moghimi A, Ansari Majd S. Modulation of lipopolysaccharide stimulated nuclear factor kappa B mediated iNOS/NO production by bromelain in rat primary microglial cells. Iran Biomed J. 2016;20:33–40. doi: 10.7508/ibj.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren B, Zhang YX, Zhou HX, Sun FW, Zhang ZF, Wei Z, Zhang CY, Si DW. Tanshinone IIA prevents the loss of nigrostriatal dopaminergic neurons by inhibiting NADPH oxidase and iNOS in the MPTP model of Parkinson's disease. J Neurol Sci. 2015;348:142–152. doi: 10.1016/j.jns.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Xia X, Zhu X, Cao J, Xu D, Ni Y, Liu Y, Yan S, Cheng X, Liu Y, Wang Y. Expression of SGTA correlates with neuronal apoptosis and reactive gliosis after spinal cord injury. Cell Tissue Res. 2014;358:277–288. doi: 10.1007/s00441-014-1946-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhou YF, Li WT, Han HC, Gao DK, He XS, Li L, Song JN, Fei Z. Allicin protects rat cortical neurons against mechanical trauma injury by regulating nitric oxide synthase pathways. Brain Res Bull. 2014;100:14–21. doi: 10.1016/j.brainresbull.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Sharma HS, Olsson Y, Westman J. A serotonin synthesis inhibitor, p-chlorophenylalanine reduces the heat shock protein response following trauma to the spinal cord: An immunohistochemical and ultrastructural study in the rat. Neurosci Res. 1995;21:241–249. doi: 10.1016/0168-0102(94)00855-A. [DOI] [PubMed] [Google Scholar]
- 27.Iguchi M, Littmann AE, Chang SH, Wester LA, Knipper JS, Shields RK. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athl Train. 2012;47:184–190. doi: 10.4085/1062-6050-47.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta. 2012;1822:675–684. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeo JE, Kim JH, Kang SK. Selenium attenuates ROS-mediated apoptotic cell death of injured spinal cord through prevention of mitochondria dysfunction; in vitro and in vivo study. Cell Physiol Biochem. 2008;21:225–238. doi: 10.1159/000113764. [DOI] [PubMed] [Google Scholar]
- 30.Chan JY, Tsui HT, Chung IY, Chan RY, Kwan YW, Chan SW. Allicin protects rat cardiomyoblasts (H9c2 cells) from hydrogen peroxide-induced oxidative injury through inhibiting the generation of intracellular reactive oxygen species. Int J Food Sci Nutr. 2014;65:868–873. doi: 10.3109/09637486.2014.925428. [DOI] [PubMed] [Google Scholar]
- 31.Jin W, Ming X, Hou X, Zhu T, Yuan B, Wang J, Ni H, Jiang J, Wang H, Liang W. Protective effects of erythropoietin in traumatic spinal cord injury by inducing the Nrf2 signaling pathway activation. J Trauma Acute Care Surg. 2014;76:1228–1234. doi: 10.1097/TA.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 32.Lee YS, Sindhu RK, Lin CY, Ehdaie A, Lin VW, Vaziri ND. Effects of nerve graft on nitric oxide synthase, NAD(P)H oxidase, and antioxidant enzymes in chronic spinal cord injury. Free Radic Biol Med. 2004;36:330–339. doi: 10.1016/j.freeradbiomed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Rabinkov A, Miron T, Konstantinovski L, Wilchek M, Mirelman D, Weiner L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim Biophys Acta. 1998;1379:233–244. doi: 10.1016/S0304-4165(97)00104-9. [DOI] [PubMed] [Google Scholar]