Abstract
The aim of the present study was to determine the effects of curcumin on the osteoclastogenic potential of peripheral blood mononuclear cells (PBMCs) obtained from patients with rheumatoid arthritis (RA), and to investigate the underlying molecular mechanisms. PBMCs from patients with RA (n=12) and healthy controls (n=10) were cultured to assess osteoclastogenic potential. The number of tartrate-resistant acid phosphatase-positive osteoclasts differentiated from PBMCs isolated from patients with RA was significantly increased compared with that of the healthy controls. In addition, the osteoclast number in patients with RA was correlated with the clinical indicators, Sharp score (r=0.810; P=0.001) and lumbar T-score (r=−0.685; P=0.014). Furthermore, the resorption area was increased in the RA group compared with the healthy controls. The mRNA and protein expression levels in PBMC-derived osteoclasts treated with curcumin were measured by reverse transcription-quantitative polymerase chain reaction and western blotting, respectively. Curcumin inhibited the osteoclastogenic potential of PBMCs, potentially by suppressing activation of extracellular signal-regulated kinases 1 and 2, p38 and c-Jun N-terminal kinase, and inhibiting receptor activator of nuclear factor κB (RANK), c-Fos and nuclear factor of activated T cells (NFATc1) expression. The results of the present study demonstrated that curcumin may inhibit the osteoclastogenic potential of PBMCs from patients with RA through the suppression of the mitogen-activated protein kinase/RANK/c-Fos/NFATc1 signaling pathways, and that curcumin may be a potential novel therapeutic agent for the treatment of bone deterioration in inflammatory diseases such as RA.
Keywords: rheumatoid arthritis, osteoclasts, peripheral blood mononuclear cells, curcumin, receptor activator of nuclear factor κB
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by abnormal immune responses, chronic inflammation of peripheral joints and progressive bone destruction (1,2). Under the pathological conditions of RA, bone homeostasis is in persistent disequilibrium, resulting in uncoordinated bone formation and degradation, and is mediated by abnormal osteoclast formation (3). Osteoclasts are derived from osteoclast precursor cells (OPCs), which originate from the monocyte/macrophage lineage. Previous studies have demonstrated that the osteoblast-associated synthesis and secretion of receptor activator of nuclear factor κB ligand (RANKL) and osteoclast-associated RANK are essential in osteoclastogenesis, and that RANK on monocytes binds to RANKL, initiating osteoclast differentiation (4,5). Furthermore, macrophage-derived proinflammatory mediators in RA directly contribute to the degradation of articular cartilage and subchondral bone, and the activation of RA monocytes/macrophages occurs locally and in the peripheral circulation (6,7). Therefore, the present study investigated the osteoclastogenic potential of peripheral blood mononuclear cells (PBMCs) from rheumatoid arthritis patients.
Curcumin is the primary active ingredient of turmeric (Curcuma longa) and has been demonstrated to possess anti-inflammatory and anti-arthritic properties (8). Previous studies have revealed its underlying mechanisms of action as the induction of apoptosis in human fibroblast-like synoviocytes and protection against collagen-induced arthritis (9,10). A randomized pilot study in patients with RA indicated that curcumin treatment is safe and is not associated with any adverse events (11). Accumulating evidence suggests that signaling pathways malfunction in RA (12). Curcumin has been demonstrated to mediate the suppression of mitogen-activated protein kinases (MAPKs)/RANK and extracellular signal-regulated kinase 1 and 2 (ERK1/2) signaling pathways and thus promotes chondrogenic differentiation (13,14). Furthermore, curcumin inhibits osteoclast differentiation and function via the inhibition of the signalosome-associated kinase inhibitor of κB in a dose-dependent manner (15). Therefore, the present study aimed to investigate the effect of curcumin on osteoclastogenesis of PBMCs from patients with RA via the suppression of the MAPK/RANK/c-Fos/nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) signaling pathways.
Materials and methods
Patients and PBMC culture
A total of 12 patients and 10 healthy controls were recruited from the Department of Integrated Traditional Chinese and Western Medicine, Jinling Hospital (Nanjing, China), and written informed consent obtained. The study was approved by the Ethics Committee of the Department of Integrated Traditional Chinese and Western Medicine, Jinling Hospital. Human blood samples from the patients with RA and healthy controls were collected between January and December 2014.
PBMCs were separated from erythrocytes by density centrifugation at 650 × g, 18°C for 20 min, using Ficoll® PM 400 Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO, USA) of blood samples, and were maintained in α-minimal essential medium (α-MEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a humidified 5% CO2 incubator (Thermo Fisher Scientific, Inc.). The medium was replenished every second day.
Osteoclast differentiation
Isolated PBMCs were seeded onto plates. Non-adherent cells were harvested after 24 h and were seeded (3.5×105 cells/well in 96-well plates) onto glass coverslips in the presence of 50 ng/ml recombinant human macrophage colony-stimulating factor (rhM-CSF; R&D Systems China Co., Ltd., Shanghai, China) and 100 ng/ml rhRANKL (R&D Systems China Co., Ltd.) for 14 days. Osteoclasts with ≥3 nuclei/cell were identified as tartrate-resistant acid phosphatase (TRAP) -positive cells. Osteoclast differentiation was additionally confirmed by the expression of RANK using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). To investigate the effect of curcumin treatment, 0–10 µM curcumin was added to wells for the 14 days of osteoclast differentiation.
Cell viability detection by Cell Counting kit-8 (CCK-8)
PBMCs (1×105/well) were seeded in 96-well plates (three wells per group) and treated with with curcumin (0–40 µM) for 48 h. Subsequently, 10 µl CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well, and cell viability was measured at 490 nm using an enzyme-linked immunosorbent assay reader (BioTek Instruments, Inc., Winooski, VT, USA), according to the manufacturer's instructions.
TRAP staining and bone resorption pit assay
Cells were washed with phosphate-buffered saline, fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100. Fixed cells were subjected to an assay for TRAP activity using an Acid Phosphatase, Leukocyte (TRAP) kit (Sigma-Aldrich) according to the manufacturer's instructions. Images were captured with a digital camera attached to the microscope (Leica DM 2500; Leica Microsystems GmbH, Wetzlar, Germany). TRAP positive multinucleated cells (≥3 nuclei) were identified as osteoclasts, and the number of osteoclasts was counted in 10 fields per sample.
PBMCs were seeded onto 100-µm thick bovine bone slices and incubated with α-MEM containing 50 ng/ml M-CSF and100 ng/ml RANKL. After 21 days, cells were removed by sonication and the bovine bone slices were stained with 0.25% toluidine blue (Sigma-Aldrich) to identify resorption pits. In addition, resorption lacunae were visualized using a Hitachi S-3400N scanning electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan).
RT-qPCR
RNA was extracted from the PBMCs using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Synthesis of cDNA was performed on 2 µg total RNA using Moloney Murine Leukemia Virus reverse transcriptase (Promega Corporation, Madison, WI, USA) and oligo dT 15 primers (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. qPCR was performed using the Applied Biosystems 7300 Real-Time PCR System (Thermo Fisher Scientific, Inc.). Reaction mixtures (20 µl) were prepared using the TaqMan® Universal PCR Master Mix (Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. Following initial denaturation at 95°C for 3 min, 40 cycles were performed of denaturation at 95°C for 15 sec, annealing at 56°C for 20 sec and extension at 72°C for 20 sec. The Cq (quantification cycle fluorescence value) was calculated using SDS software, version 2.1 (Applied Biosystems; Thermo Fisher Scientific, Inc.), and the relative expression levels of RANK mRNA were calculated using the 2−ΔΔCq method (16) and normalized to the internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The following primer sequences were used: Forward, 5′-CCTCGGGGTcTGGGAGTTCG-3′ and reverse, 5′-CGTACACCACGATGATGTCACCCT-3′ for RANK; and forward, 5′-ACAGGGGAGGTGATAGCATT-3′ and reverse, 5′-GACCAAAAGCCTTCATACATCTC-3′ for GAPDH.
Western blotting
PBMCs were homogenized and lysed in NP-40 buffer (Beyotime Institute of Biotechnology, Haimen, China). Following 5–10 min boiling, cells were centrifuged at 10,000 × g, 4°C for 10 min to obtain the supernatant. Protein samples (50 µg) were separated by 10% sodium dodecyl sulfate-polyacrylimide gel electrophoresis and transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% (w/v) non-fat milk powder in Tris-buffered saline and 0.1% (w/v) Tween 20 (TBST), and incubated with the following primary antibodies: Rabbit anti-RANK (1:500; sc-9072), mouse anti-c-Fos (1:1,000; sc-271243), mouse anti-NFATc1 (1:1,000; sc-17834), mouse anti-phosphorylated (p)-ERK1/2 (1:1,000; sc-136521), mouse anti-ERK1/2 (1:1,000; sc-514302), rabbit anti-p-p38 (1:1,000; sc-17852-R), mouse anti-p38 (1:1,000; sc-81621), goat anti-p-c-Jun N-terminal kinase (JNK; 1:1,000; sc-12882) and mouse anti-JNK (1:1,000; sc-137019) all from Santa Cruz Biotechnoogy, Inc. (Dallas, TX, USA), and rabbit anti-β-actin antibody (1:2,000; catalog no. AP0060; Bioworld Technology, Inc., St. Louis Park, MN, USA), at 4°C overnight. Following three washes with TBST, membranes were incubated with the following secondary antibodies conjugated to horseradish peroxidase: Donkey anti-goat IgG (1:10,000; sc-2020), donkey anti-mouse IgG (1:10,000; sc-2096) and goat anti-rabbit IgG (1:10,000; catalog no. sc-2004) from Santa Cruz Biotechnoogy, Inc. at a dilution of 1:10,000–1:20,000. Following a 1-h incubation at 37°C, membranes were washed three times with TBST. Blots were visualized using an enhanced chemiluminescence system (Amersham; GE Healthcare Life Sciences, Chalfont, UK). Signals were densitometrically assessed using Quantity One® software version 4.5 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and normalized to the β-actin signals to correct for unequal loading.
Clinical assessments
The Sharp score of patients with RA was calculated using Multix Select DR X-ray (Siemens AG, Munich, Germany), as described previously (17), from the measurements of arthritis (scale, 0–5) and joint space abnormality (scale, 0–4).
The T-score of Patients with RA was calculated as described previously (18), from the measurement of the bone mineral density of lumbar vertebra L1-L4 by dual-energy X-ray absorptiometry.
Statistical analysis
Data are expressed as the mean ± standard deviation. All statistical analyses were performed using GraphPad Prism software, version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Correlations were assessed by Spearman's coefficient rho (ρ) with a 95% confidence interval. Groups were compared using one-way analysis of variance, followed by Tukey's multiple comparison test as a post hoc test to compare the mean values of each group. P<0.05 was considered to indicate a statistically significant difference.
Results
Enhanced osteoclastogenic potential in patients with RA
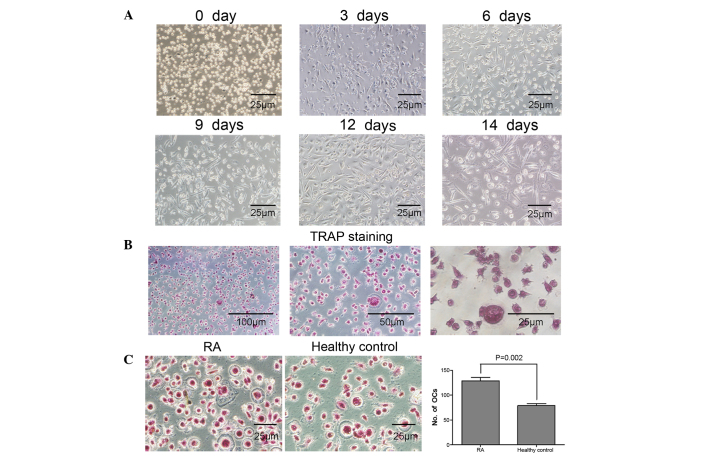
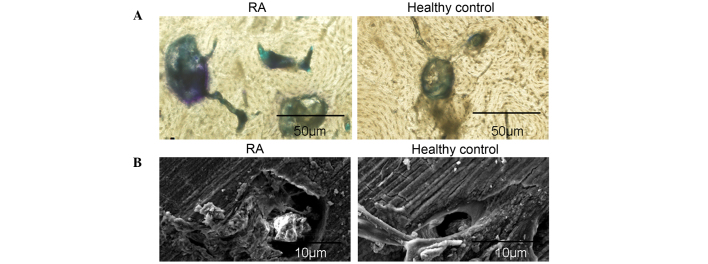
The osteoclastogenic potential of PBMCs from patients with RA was induced in the presence of 50 ng/ml rhM-CSF and 100 ng/ml rhRANKL. A total of three days later, PBMCs were adhesive and had narrowly elongated morphologies. Following six days of culture, fibroblast-like cells were significantly increased, and PBMCs were in cell condensation following nine days of culture. Notably, a large number of large polynuclear giant cells were observed at day 14 (Fig. 1A). Osteoclasts with ≥3 nuclei/cell were identified as TRAP-positive cells, confirming that osteoclast differentiation was induced by rhM-CSF and rhRANKL (Fig. 1B). Furthermore, the number of TRAP-positive osteoclasts differentiated from PBMCs from patients with RA was significantly increased compared with healthy controls (P=0.002; Fig. 1C). The resorption area was measured as a separate indicator of osteoclast formation. The bone resorption pits on bone slices were stained with toluidine blue, and resorption areas were analyzed by light and electron microcopy. The results demonstrated that the lacunar number and area of bone resorption were increased in the RA group compared with the healthy control group (Fig. 2A and B). These results confirmed the increased osteoclastogenic potential of PBMCs isolated from patients with RA.
Figure 1.
Enhanced osteoclastogenic potential of PBMCs from patients with RA. (A) The morphology of PBMCs following culture with 50 ng/ml M-CSF and 100 ng/ml RANKL. Following three days in culture, PBMCs were adhesive and narrowly elongated, following six days fibroblast-like cells were significantly increased, and PBMCs were in cell condensation following nine days of culture. Polynuclear giant cells were observed at day 14. (B) Observation of osteoclast morphology in PBMCs from RA patients using TRAP staining following 14 days in culture. Cells were fixed in 4% formalin, permeabilized with 0.1% Triton X-100 and stained with TRAP solution. (C) PBMCs isolated from RA patients and healthy controls were cultured with 50 ng/ml M-CSF and 100 ng/ml RANKL for 14 days and TRAP staining performed. The number of osteoclasts (TRAP-positive cells containing ≥3 nuclei/cell counted in 10 fields of each sample) differentiated from PBMCs from patients with RA was significantly increased compared with healthy controls. Data are expressed as the mean ± standard deviation, n=10 per group. PBMCs, peripheral blood mononuclear cells; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor κB ligand; TRAP, tartrate-resistant acid phosphatase; RA, rheumatoid arthritis; OC, osteoclasts.
Figure 2.
Enhanced osteoclastic bone-resorption potential in PBMCs from patients with RA. PBMCs seeded on bovine bone slices were incubated with 50 ng/ml M-CSF and 100 ng/ml RANKL for 21 days. PBMCs were then removed, and the bone slices were (A) stained using toluidine blue or (B) analyzed by scanning electron microscopy, to identify resorption pits. The lacunar number and area of bone resorption were increased in the RA group compared with the healthy control group. PBMCs, peripheral blood mononuclear cells; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor κB ligand; RA, rheumatoid arthritis.
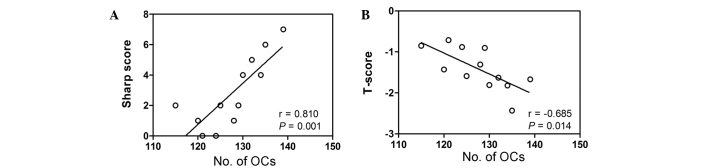
Correlation between osteoclastogenic potential and clinical indicators
To determine whether there was a correlation between the number of osteoclasts and a clinical indicator of disease, the Sharp score was calculated by assessing articulation in the two hands of patients with RA. This Sharp score was correlated with the number of osteoclasts counted in TRAP-stained PBMCs isolated from patients with RA and healthy controls. As presented in Fig. 3A, the Sharp score was significantly positively correlated with the number of osteoclasts in patients with RA (r=0.810; P=0.001). In addition, a significant negative correlation was detected between the osteoclast number and the lumbar T-score in patients with RA (r=−0.685; P=0.014; Fig. 3B).
Figure 3.
Osteoclastogenic potential in PBMCs from RA patients was closely associated with the clinical parameters, Sharp score and T-score, in RA patients. (A) Linear correlation plot of the osteoclast number and Sharp score in patients with RA. The Sharp score was calculated by assessing articulation in the two hands of the patients with RA and correlating this value with the number of osteoclasts counted in TRAP-stained PBMCs isolated from patients with RA and healthy controls. The Sharp score was significantly positively correlated with the number of osteoclasts in patients with RA. (B) Linear correlation plot of the osteoclast number and lumbar T-score in patients with RA. A significant negative correlation was detected between the osteoclast number and the lumbar T-score in patients with RA. RA, rheumatoid arthritis; TRAP, tartrate-resistant acid phosphatase; PBMCs, peripheral blood mononuclear cells; OC, osteoclasts.
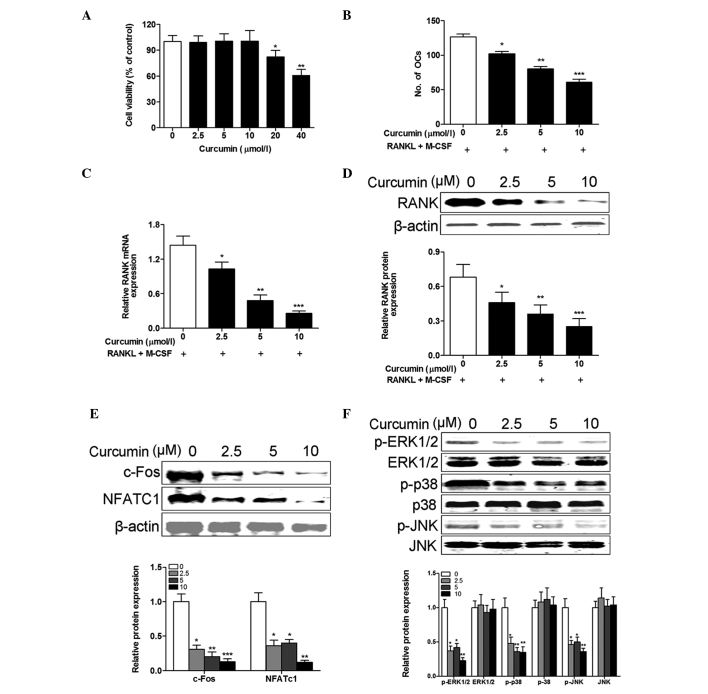
Curcumin inhibits osteoclastogenic potential of PBMCs from patients with RA
To investigate the cytotoxicity of curcumin, PBMCs isolated from healthy controls were incubated with various concentrations of curcumin for 48 h. The CCK-8 assay revealed that PBMCs had similar viability following treatment with 0–10 µM curcumin for 48 h. However, viability was significantly reduced at concentrations of ≥20 µM (20 µM, P= 0.031; 40 µM, P= 0.006; Fig. 4A). To evaluate the inhibitory effects of curcumin on the osteoclastogenesis of PBMCs isolated from RA patients, M-CSF and RANKL-treated cells were exposed to concentrations of curcumin <20 µM. The results demonstrated that curcumin treatment inhibited the number of osteoclasts generated in a dose-dependent manner (2.5 µM, P=0.012; 5 µM, P=0.003; 10 µM, P<0.001; Fig. 4B). This reduction in osteoclast differentiation following curcumin treatment was confirmed by the dose-dependent reduction in RANK mRNA (2.5 µM, P=0.018; 5 µM, P=0.008; 10 µM, P<0.001; Fig. 4C) and protein (2.5 µM, P=0.035; 5 µM, P=0.009; 10 µM, P<0.001; Fig. 4D) expression levels. c-Fos and NFATc1 are crucial for osteoclast differentiation (5,19). Therefore, it was examined whether curcumin inhibited the osteoclastogenic potential of PBMCs from patients with RA through regulation of the expression of c-Fos and NFATc1 in response to M-CSF and RANKL stimulation. The western blotting results demonstrated that the protein expression levels of c-Fos and NFATc1 were significantly suppressed in PBMCs from RA patients by curcumin in a dose-dependent manner (P<0.001 at all curcumin concentrations; Fig. 4E). To determine the involvement of signaling pathways and the molecular mechanisms underlying the effects of curcumin on M-CSF and RANKL-stimulated osteoclast differentiation of PBMCs from patients with RA, the activation of MAPKs in PBMCs from RA patients was evaluated. The results indicated that curcumin inhibited the protein expression levels of p-ERK1/2, p-p38 and p-JNK in PBMCs from RA patients in a dose-dependent manner (P<0.001 at all curcumin concentrations; Fig. 4F). These results suggest that curcumin inhibited M-CSF and RANKL-stimulated osteoclast differentiation via intracellular MAPK signaling pathways.
Figure 4.
Curcumin inhibits the osteoclastogenic potential of PBMCs from patients with RA. (A) PBMCs from healthy controls were incubated with various concentrations of curcumin (0–40 µM) in the presence of M-CSF (50 ng/ml) and RANKL (100 ng/ml) for 48 h, and the cell viability was examined using the Cell Counting kit-8 assay. Cell viability was not affected by <20 µM curcumin. PBMCs from patients with RA were incubated with various concentrations of curcumin (0–10 µM) in the presence of 50 ng/ml M-CSF and 100 ng/ml RANKL for 14 days. (B) The osteoclast differentiation was measured by TRAP staining and the number of osteoclasts (TRAP-positive cells containing ≥3 nuclei/cell) was counted in 10 fields of each sample. Curcumin treatment inhibited the number of osteoclasts generated in a dose-dependent manner. The (C) mRNA and (D) protein expression levels of RANK were measured by reverse transcription-quantitative polymerase chain reaction and western blotting, respectively, and were reduced by curcumin treatment. (E) The protein expression levels of (E) c-Fos and NFATc1, and (F) p-ERK1/2, ERK1/2, p-p38, P-38, p-JNK and JNK were measured and normalized to β-actin. Curcumin treatment decreased the protein expression levels of c-Fos, NFATc1, p-ERK1/2, p-p38 and p-JNK in a dose-dependent manner. Data are expressed as the mean ± standard deviation, n=3 per group. *P<0.05, **P<0.01 and ***P<0.001 vs. 0 µmol/l curcumin treatment. PBMCs, peripheral blood mononuclear cells; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor κB ligand; RA, rheumatoid arthritis; RANK, receptor activator of nuclear factor κB; TRAP, tartrate-resistant acid phosphatase; NFATc1, nuclear factor of activated T cells, cytoplasmic 1; p, phosphorylated; ERK1/2, extracellular signal-regulated kinases 1 and 2; JNK, c-Jun N-terminal kinase.
Discussion
Curcumin has been demonstrated to possess anti-inflammatory activities in interleukin (IL)-1β-stimulated human chondrocytes (14) and murine macrophages (3). In RA, proinflammatory cytokines, including IL-1, -6, -8 and -11 and tumor necrosis factor α, have been reported to be osteoclastogenic (3). However, the role of curcumin in RA and its contribution to the inhibition of the osteoclastogenic potential of PBMCs remains unclear. In the present study, the anti-osteoclastogenic effect of curcumin and its underlying mechanisms were investigated.
Monocytes migrate out of the peripheral blood and into inflammatory tissue where they differentiate into resident macrophages and dendritic cells, which secrete a variety of inflammatory cytokines involved in the pathogenesis of RA (20,21). In the present study, PBMCs from patients with RA were isolated to investigate the systemic enhancement of osteoclastogenesis in RA. The results confirmed the increased osteoclastogenic potential of PBMCs isolated from patients with RA compared with healthy controls. Furthermore, increased numbers of mature osteoclasts were observed in PBMC cultures from patients with RA compared with healthy controls. These results suggest that PBMCs may contribute to osteoclast formation in the presence of osteoclastogenic cytokines or RANKL in RA patients. Therefore, RA may have direct effects on bone metabolism. Ikic et al (7) demonstrated that PBMC-derived OPCs trans-differentiate into osteoclasts in the presence of M-CSF and RANKL in vitro, suggesting that inflammatory factors may directly contribute to osteoclastogenesis. The results of the present study confirm a number of previous studies (22,23), simultaneously, the Sharp score was positively correlated with osteoclast number, and lumbar T-score was negatively correlated with osteoclast number.
Previous studies indicate that inflammatory cytokines enhance osteoclastogenesis via a RANKL-RANK dependent mechanism, upregulating the expression levels of RANK on osteoclast precursors and increasing their sensitivity to RANKL, which may result in bone erosion in RA (3,24,25). In the present study, curcumin inhibited the number of osteoclasts generated from PBMCs in a dose-dependent manner, and reduced the RANK mRNA and protein expression levels in PBMCs from patients with RA. It has been previously demonstrated that RANKL activates multiple signaling pathways in osteoclast precursors via RANK and stimulates critical transcription factors for osteoclast differentiation (26,27). NFATc1 is a crucial transcription factor that is expressed in osteoclast precursors through Ca2+ oscillation, MAPKs and c-Fos or RANK in response to RANKL (28). In the present study, curcumin inhibited the osteoclastogenic potential of PBMCs from patients with RA, potentially via the suppression of ERK1/2, p38 and JNK activation, and the inhibition of c-Fos and NFATc1 expression. In conclusion, the results of the present study suggest that curcumin inhibits osteoclast formation via preventing the phosphorylation of components of the MAPK signaling pathways, and that it may be a potential novel therapeutic agent for managing osteoporosis or bone deterioration in inflammatory diseases including RA. However, future studies are required to demonstrate the anti-osteoclastogenic potential of curcumin in animal models of osteoporosis and RA.
References
- 1.Wendling D, Abbas W, Godfrin-Valnet M, Kumar A, Guillot X, Khan KA, Vidon C, Coquard L, Toussirot E, Prati C, Herbein G. Dysregulated serum IL-23 and SIRT1 activity in peripheral blood mononuclear cells of patients with rheumatoid arthritis. PloS One. 2015;10:e0119981. doi: 10.1371/journal.pone.0119981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han M, Sung YK, Cho SK, Kim D, Won S, Choi CB, Bang SY, Cha HS, Choe JY, Chung WT, et al. Factors associated with the use of complementary and alternative medicine for Korean patients with rheumatoid arthritis. J Rheumatol. 2015;42:2075–2081. doi: 10.3899/jrheum.141447. [DOI] [PubMed] [Google Scholar]
- 3.Jung SM, Kim KW, Yang CW, Park SH, Ju JH. Cytokine-mediated bone destruction in rheumatoid arthritis. J Immunol Res. 2014;2014:263625. doi: 10.1155/2014/263625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jules J, Wang S, Shi Z, Liu J, Wei S, Feng X. The IVVY motif and tumor necrosis factor receptor associated factor (TRAF) sites in the cytoplasmic domain of the receptor activator of nuclear factor κB (RANK) cooperate to induce osteoclastogenesis. J Biol Chem. 2015;290:23738–23750. doi: 10.1074/jbc.M115.667535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong X, Yang Y, Wu W, Wan H, Li X, Zhong M, Su X, Jia S, Lin N. Triterpenoid Saponin W3 from Anemone flaccida suppresses osteoclast differentiation through inhibiting activation of MAPKs and NF-κB pathways. Int J Biol Sci. 2015;11:1204–1214. doi: 10.7150/ijbs.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze-Koops H, Davis LS, Kavanaugh AF, Lipsky PE. Elevated cytokine messenger RNA levels in the peripheral blood of patients with rheumatoid arthritis suggest different degrees of myeloid cell activation. Arthritis Rheum. 1997;40:639–647. doi: 10.1002/art.1780400408. [DOI] [PubMed] [Google Scholar]
- 7.Ikić M, Jajić Z, Lazić E, Ivčević S, Grubišić F, Marušić A, Kovačić N, Grčević D. Association of systemic and intra-articular osteoclastogenic potential, proinflammatory mediators and disease activity with the form of inflammatory arthritis. Int Orthop. 2014;38:183–192. doi: 10.1007/s00264-013-2121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Wu X, Wei Z, Dou Y, Zhao D, Wang T, Bian D, Tong B, Xia Y, Xia Y, Dai Y. Oral curcumin has anti-arthritic efficacy through somatostatin generation via cAMP/PKA and Ca(2+)/CaMKII signaling pathways in the small intestine. Pharmacol Res. 2015;95–96:71–81. doi: 10.1016/j.phrs.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Kloesch B, Becker T, Dietersdorfer E, Kiener H, Steiner G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int Immunopharmacol. 2013;15:400–405. doi: 10.1016/j.intimp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Huang G, Xu Z, Huang Y, Duan X, Gong W, Zhang Y, Fan J, He F. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J Clin Immunol. 2013;33:550–557. doi: 10.1007/s10875-012-9839-0. [DOI] [PubMed] [Google Scholar]
- 11.Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–1725. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- 12.Niu X, Lu C, Xiao C, Zhang Z, Jiang M, He D, Bian Y, Zhang G, Bian Z, Lu A. The shared crosstalk of multiple pathways involved in the inflammation between rheumatoid arthritis and coronary artery disease based on a digital gene expression profile. PloS One. 2014;9:e113659. doi: 10.1371/journal.pone.0113659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther. 2010;12:R127. doi: 10.1186/ar3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakibaei M, Mobasheri A, Buhrmann C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011;6:171–179. doi: 10.1007/s12263-010-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Metzler I, Krebbel H, Kuckelkorn U, Heider U, Jakob C, Kaiser M, Fleissner C, Terpos E, Sezer O. Curcumin diminishes human osteoclastogenesis by inhibition of the signalosome-associated I kappaB kinase. J Cancer Res Clin Oncol. 2009;135:173–179. doi: 10.1007/s00432-008-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Ravindran J, Cavill C, Balakrishnan C, Jones SM, Korendowych E, McHugh NJ. A modified Sharp score demonstrates disease progression in established psoriatic arthritis. Arthritis Care Res (Hoboken) 2010;62:86–91. doi: 10.1002/acr.20018. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, Miller PD, Binkley NC, Kendler DL, Wong M, Krohn K. Use of lowest single lumbar spine vertebra bone mineral density T-score and other T-score approaches for diagnosing osteoporosis and relationships with vertebral fracture status. J Clin Densitom. 2008;11:525–531. doi: 10.1016/j.jocd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Min JY, Baek JM, Ahn SJ, Jun HY, Yoon KH, Choi MK, Lee MS, Oh J. CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo. Bone. 2015;79:242–251. doi: 10.1016/j.bone.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 22.Pathak JL, Bravenboer N, Verschueren P, Lems WF, Luyten FP, Klein-Nulend J, Bakker AD. Inflammatory factors in the circulation of patients with active rheumatoid arthritis stimulate osteoclastogenesis via endogenous cytokine production by osteoblasts. Osteoporos Int. 2014;25:2453–2463. doi: 10.1007/s00198-014-2779-1. [DOI] [PubMed] [Google Scholar]
- 23.Izawa T, Mori H, Shinohara T, Mino-Oka A, Hutami IR, Iwasa A, Tanaka E. Rebamipide attenuates mandibular condylar degeneration in a murine model of TMJ-OA by mediating a chondroprotective effect and by downregulating RANKL-mediated osteoclastogenesis. PloS One. 2016;11:e0154107. doi: 10.1371/journal.pone.0154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, McClanahan T, Bowman EP. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther. 2010;12:R29. doi: 10.1186/ar2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EG, Yun HJ, Lee SI, Yoo WH. Ethyl acetate fraction from Cudrania tricuspidata inhibits IL-1beta-stimulated osteoclast differentiation through downregulation of MAPKs, c-Fos and NFATc1. Korean J Intern Med. 2010;25:93–100. doi: 10.3904/kjim.2010.25.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappaB in osteoclasts. Biochem Biophys Res Commun. 2003;305:211–214. doi: 10.1016/S0006-291X(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 28.Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med (Berl) 2005;83:170–179. doi: 10.1007/s00109-004-0612-6. [DOI] [PubMed] [Google Scholar]