Abstract
Artemisia argyi Folium has been used to treat skin diseases, including eczema and dermatitis, in South Korean medicine. The present study investigated the curative effects of Artemisia argyi Folium extract (AAFE) on 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis (AD)-like skin lesions in a BALB/c mouse model. Briefly, the dorsal skin of the BALB/c mice was sensitized three times with DNCB, whereas the ears were challenged twice. Repeated treatment with DNCB induced AD-like lesions. The effects of AAFE on AD-like lesions were evaluated by clinical observation, histopathological analysis, immunohistochemistry and enzyme-linked immunosorbent assay. In addition, reverse transcription-polymerase chain reaction and western blotting were performed. Treatment with AAFE reduced AD-like lesions, as determined by clinical observation, histopathological analysis, and detection of the serum levels of histamine, immunoglobulin E and cytokines. With regards to its mechanism of action, AAFE inhibited the phosphorylation of Lck/yes-related novel tyrosine kinase (Lyn), spleen tyrosine kinase (Syk), mitogen-activated protein kinases (MAPKs), phosphoinositide 3-kinase (PI3K)/Akt and IκBα, which have essential roles in the production of various cytokines in lymph nodes. The suppressive activity of AAFE may be due to the inhibition of a series of immunopathological events, including the release of proinflammatory cytokines. The results of the present study strongly suggest that AAFE exerts an anti-AD effect by inhibiting the Lyn, Syk, MAPKs, PI3K/Akt and IκBα pathways. Therefore, AAFE may be considered an effective herbal remedy for the treatment of AD.
Keywords: Artemisia argyi Folium; 2,4-dinitrochlorobenzene; atopic dermatitis
Introduction
Artemisia argyi
Folium has long been used as an herbal treatment or moxibustion in the traditional medicine of East Asian countries. It is used widely to treat various chronic diseases, including osteoarthritis, asthma, gastrointestinal disorders, dysmenorrhea and insomnia (1–5). Several studies have reported that compounds isolated from Artemisia argyi Folium have antitumor, anti-inflammatory and anti-allergic effects (6–14); however, to the best of our knowledge, a study using whole Artemisia argyi Folium extract (AAFE) has not yet been performed.
Atopic dermatitis (AD) is a common relapsing inflammatory skin disease, which is associated with the following symptoms: Erythema, eczema, pruritus, xerosis and lichenification (15). AD is characterized by several immune disorders, and patients with AD present high levels of histamine and immunoglobulin (Ig)E. The cytokine milieu consists of T helper (Th)2 cytokines, including interleukin (IL)-4, IL-6 and IL-13; Th1 cytokines, including transforming growth factor (TGF)-β and interferon (IFN)-γ; and non-Th proinflammatory cytokines, including IL-1β and tumor necrosis factor (TNF)-α throughout the acute and chronic phases of AD (16–18).
Overproduction of soluble mediators, including histamine, IgE and cytokines, is associated with activation of cell signaling molecules, including Lck/yes-related novel tyrosine kinase (Lyn), spleen tyrosine kinase (Syk), mitogen-activated protein kinases (MAPKs), phosphoinositide 3-kinase (PI3K)/AKT and IκB/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in AD pathogenesis (19–22). The present study aimed to investigate whether AAFE is able to alleviate the pathological symptoms of multiplex immune disorders through the regulation of intracellular signaling pathways in an animal model of 2,4-dinitrochlorobenzene (DNCB)-induced AD.
Materials and methods
Animals
Female BALB/c mice were purchased from Hyochang Science (Daegu, South Korea) and were 8 weeks old at the initiation of the present study. Mice were maintained in a temperature-controlled room (23±1°C) with relative humidity (50±10%) and underwent a 12 h light/dark cycle. The mice were housed in polystyrene cages at Dong-Eui University (Busan, South Korea) and were given ad libitum access to standard rodent chow and water. The mice used in the present study were cared for according to the Guide for the Care and Use of Laboratory Animals (23). The experimental protocol was approved by the Institutional Animal Research Committee of Dong-Eui University on Animal Care and Use (Approval number: DEU-R2014-015), and all efforts were made to minimize animal suffering and reduce the number of animals used in the experiments.
Preparation of AAFE
AAFE was isolated from Artemisia argyi Folium purchased from Omniherb Co., Ltd. (Daegu, South Korea). A total of 100 g Artemisia argyi Folium was mixed with 1 L 75% ethanol at 60°C, and was incubated for 24 h with agitation (90 rpm). The extract was filtered and evaporated using a rotary evaporator under a reduced pressure. The extract was subsequently lyophilized, and the extract yield was ~20.5%. A voucher specimen (DKMP-201203-AAFE) was deposited at Korean Medical Physiology Laboratory, Dong-Eui University. The extracted powder was stored at −20°C until further use.
Induction of AD-like skin lesions and administration of AAFE
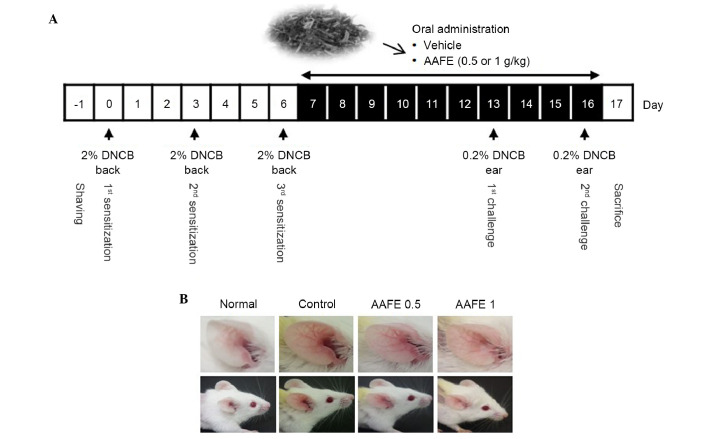
The backs of the BALB/c mice were shaved using an electric clipper and depilatory cream, and were washed with sterilized phosphate-buffered saline (PBS)-gauze 1 day prior to sensitization. During the sensitization process, 100 μ1 2% DNCB (dissolved in 100% ethanol; Sigma-Aldrich, St. Louis, MO, USA) or vehicle (100% ethanol) was applied to the shaved backs of the DNCB-immunized or non-immunized mice on days 0, 3 and 6. On days 13 and 16, 20 μ1 0.2% DNCB or vehicle was used to challenge the ears of the mice.
The BALB/c mice (age, 8 weeks) were randomized into four groups (n=5/group): Non-immunized (normal), DNCB-immunized (control), and DNCB-immunized AAFE (AAFE 0.5 and AAFE 1)-treated groups. Mice in the AAFE 0.5 and AAFE 1 groups were orally administered 0.5 or 1 g/kg AAFE dissolved in saline, respectively, once daily for 10 days. Normal and control groups were administered the same volume of saline for 10 days (Fig. 1A). Following completion of the experiments, the mice were anesthetized with diethyl ether (2 g/kg) and whole blood samples were collected from the mice by cardiac puncture. The mice were sacrificed by extended diethyl ether (2 g/kg) inhalation, and the ear tissues and lymph nodes were removed and stored at −70°C until further analysis.
Figure 1.
Experimental protocols and inhibition of DNCB-induced AD progression by topical application of AAFE in BALB/c mice. (A) Experimental schedule for the induction of AD-like skin lesions and oral administration of AAFE. (B) Representative images of the ear skin from each group are shown. DNCB, 2,4-dinitrochlorobenzene; AD, atopic dermatitis; AAFE, Artemisia argyi Folium extract.
Histopathology
A total of 16 days after AD induction, the mice were anesthetized with diethyl ether, and the ear tissues were excised. Part of the excised tissue was fixed in 4% paraformaldehyde (Sigma-Aldrich) for 24 h, embedded in paraffin, and used to prepare 5-μm sections. The sections were subsequently deparaffinized in xylene, rehydrated, and stained with hematoxylin and eosin (H&E). The sections were examined under a light microscope (Olympus Corporation, Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry was performed using the floating method. The deparaffinized sections were then treated with 10% normal goat serum (ab138478; Abcam, Cambridge, UK) for 1 h at 4°C, in order to prevent unspecified immune reactions, followed by incubation with mouse anti-TGF-β1 (cat. no. ab9758; 1:100 dilution; rabbit polyclonal; Abcam) overnight at 4°C. The sections were then incubated for 1 h at room temperature with a biotinylated anti-mouse IgG antibody (Vectastatin ABC kit; Vector Laboratories, Inc., Burlingame, CA, USA), and were finally incubated with the ABC complex (1:100) for 1 h. Peroxidase was visualized using 0.05% 3,3′-diaminobenzidine and 0.01% hydrogen peroxide in Tris-buffered saline (TBS). The specificity of the immunoreaction was tested by incubating sections without primary antibody. The expression was calculated from the percentage of immunopositive cells in 105 μm2.
Histamine assay
Serum histamine levels were measured according to the o-phthaldialdehyde spectrofluorometric method (24). Blood samples from the mice were centrifuged for 10 min at 4°C and 400 × g, and the extracted serum was used to measure histamine levels. Briefly, a mixture of 22.5 μl 0.1 N HCl and 2.5 μl 70% HClO4 was added to 25 μl serum and centrifuged at 350 × g for 10 min at 4°C. The upper phase (40 μl) was then transferred to tubes containing 25 μl 5 N NaOH, 0.06 g NaCl and 500 μl n-butanol, and the sample was centrifuged at 350 × g for 10 min at 4°C to remove contaminated materials from the upper phase. The lower phase (400 μl) was transferred to tubes containing 150 μl 0.1 N HCl and 500 μl n-heptane, and the sample was centrifuged at 350 × g for 10 min at 4°C. The lower phase (100 μl) was subsequently transferred to tubes containing 200 μl 1 N NaOH and 5 μl 1% ο-phthaldialdehyde, and incubated for 3 min at room temperature. The reaction was terminated by the addition of 10 μl 3 N HCl, and fluorescence intensity was measured at an excitation wavelength of 360 nm and an emission wavelength of 450 nm using the Fluorescence Spectrometer Spectramax M2 (Molecular Devices, LLC, Sunnyvale, CA, USA). The percentage of histamine release was calculated as follows: Histamine release (%) = (AAFE or Control / Normal) × 100.
Enzyme-linked immunosorbent assay (ELISA)
Total serum IgE and cytokine (IL-1β, IL-4, IL-6 and IFN-γ) levels were quantified using the mouse IL-1β, (cat. no. 559603) IL-4 (cat. no. 555232), IL-6 (cat. no. 555240) and IFN-γ (cat. no. 551866) OptEIA™ sandwich ELISA Quantitation kits (BD Biosciences, San Diego, CA, USA) according to the manufacturer's protocol. The total levels in the blood serum were calculated using a linear regression equation obtained from standard absorbance values.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the skin-draining lymph nodes using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. The primers used to amplify IL-1β, IL-4, IL-6, IL-13, TNF-α, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are shown in Table I and were purchased from Bioneer, Inc. (Seoul, Korea). Total RNA (1 μg) and total reagents (20 μl) were used with One-step RT-PCR PreMix (iNtRON Biotechnology, Inc., Seongnam, Korea) for RT-PCR. The reverse transcription reaction was performed at 45°C for 30 min and 94°C for 2 min, then the PCR reaction was performed at 94°C for 30 sec (denaturation), 55-56°C for 30 sec (annealing), and 72°C (extention) for 1 min, and repeated for 30 cycles followed by incubation at 72°C for 7 min. The PCR reaction was performed using a GeneAmp PCR system 9700 (Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR products were subsequently separated by 2% (w/v) agarose gel electrophoresis and were stained with ethidium bromide. GAPDH was used as a housekeeping gene for each experimental condition.
Table I.
Oligonucleotide reverse transcription-polymerase chain reaction primers used in the present study.
| Gene | Sequences (5′→3′) | Size (bp) | Accession no. |
|---|---|---|---|
| IL-1β | Sense AAG CTC TCC ACC TCA ATG GAC A | 453 | NM_008361.3 |
| Anti-sense GTC TGC TCA TTC ACG AAA ABB GAG | |||
| IL-4 | Sense ACC TTG CTG TCA CCC TCT TC | 351 | NM_021283 |
| Anti-sense TTG TGA GCG TGG ACT CAT TC | |||
| IL-6 | Sense TCC AGT TGC CTT CTT GGG AC | 100 | NM_031168.1 |
| Anti-sense GTG TAA TTA AGC CTC CGA CTT G | |||
| IL-13 | Sense GCT CTC GCT TGC CTT GGT GGT C | 218 | NM_008355.3 |
| Anti-sense CAT CCG AGG CCT TTT GGT TAC AG | |||
| TNF-α | Sense GCG ACG TGG AAC TGG CAG AAG | 340 | NM_013693.3 |
| Anti-sense TCC ATG CCG TTG GTT AGG AGG | |||
| IFN-γ | Sense TCA AGT GGC ATA GAT GTC GAA GAA | 92 | NM_008337.3 |
| Anti-sense TGG CTC TGC AGG ATT TTC ATG | |||
| GM-CSF | Sense GCA TGT AGA TGC CAT CAA AGA AGC | 342 | X03019.1 |
| Anti-sense CAT TTC TGG ACC GGC TTC CAG C | |||
| GAPDH | Sense CCA CAG TCC ATG CCA TCA C | 568 | NM_008084.3 |
| Anti-sense TCC ACC ACC CTG TTG CTG TA |
IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; GM-CSF, granulocyte-macrophage colony-stimulating factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; bp, base pairs.
Western blotting
The skin-draining lymph nodes were homogenized with ice-cold lysis buffer, which consisted of 20 mmol/l Tris-HCl (pH 8.0), 150 mmol/l NaCl, 2 mmol/l ethylenediaminetetraacetic acid, 1 mmol/l NaF, 1% Igepal CA-630, 1 mmol/l phenylmethylsulfonyl fluoride, 1 mmol/l Na3VO4, and protease inhibitor cocktail. Following a 10 min incubation on ice, the homogenized suspension was centrifuged at 2,000 × g for 15 min at 4°C, and the supernatant was used to determine protein concentrations. The protein concentration in the tissue lysates was determined using a protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total proteins (25 μg from each sample) were then separated by l0% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose transfer membranes (Whatman; GE Healthcare Europe GmbH, Freiburg, Germany). The membranes were blocked with 5% skim milk in TBS-Tween (TBST) buffer [10 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl and 0.05% Tween 20) for 1 h, and were then incubated with phospho-specific or total antibodies to Lyn; Syk; MAPK family members: Extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and p38; PI3K; Akt; IκBα; and β-actin. The membranes were incubated with the primary antibodies (diluted in 5% skim milk in TBST) overnight at 4°C and were then washed. The membranes were subsequently incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG antibodies (diluted in 5% skim milk in TBST). Immunoreactive bands were developed using enhanced chemiluminescence (ECL) regents (Pierce ECL Western Blotting Substrate; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The primary and secondary antibodies used in the present study and the dilutions used are listed in Table II.
Table II.
Primary and secondary antibodies used in the present study.
| Antibody | Cat. no. | Source | Dilution |
|---|---|---|---|
| Primary antibodies | |||
| TGF-β1 | ab9758 | Abcam, Cambridge, UK | 1:100 |
| Lyn/p-Lyn | #2732S/2731S | Cell Signaling Technologies, Inc., Beverly, MA, USA | 1:1,000 |
| Syk/p-Syk | sc-1077/sc293118 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:1,000 |
| ERK/p-ERK | #9102/#9101S | Cell Signaling Technologies, Inc. | 1:1,000 |
| JNK/p-JNK | #9151S/#9251S | Cell Signaling Technologies, Inc., | 1:1,000 |
| p38/p-p38 | #9212/#9211S | Cell Signaling Technologies, Inc., | 1:1,000 |
| PI3K/p-PI3K | #4292S/#4228S | Cell Signaling Technologies, Inc., | 1:1,000 |
| Akt/p-Akt | #9272/#9271S | Cell Signaling Technologies, Inc., | 1:1,000 |
| IκBα/p-IκBα | #9242/#9246S | Cell Signaling Technologies, Inc., | 1:1,000 |
| β-actin | sc-47778 | Santa Cruz Biotechnology, Inc. | 1:1,000 |
| Secondary antibodies | |||
| Goat anti-rabbit IgG, pAb | ADI-SAB-300-J | Enzo Life Sciences, Inc., Farmingdale, NY, USA | 1:5,000 |
| Goat anti-mouse IgG, pAb | ADI-SAB-100-J | Enzo Life Sciences, Inc. | 1:2,500 |
TGF-β1, transforming growth factor-β1; Lyn, Lck/yes-related novel tyrosine kinase; Syk, spleen tyrosine kinase; ERK, extracellular signal-regulated kinase; JNK, c-jun N-terminal kinase; PI3K, phosphatidylinositol 3-kinase; p-, phosphorylated; IgG, immunoglobulin G; pAb, polyclonal antibody.
Statistical analysis
Data from the control or drug-treated groups are presented as the mean ± standard deviation of three independent experiments and each experiment includes triplicate sets in vitro and of five animals per group in vivo. Data were analyzed using one-way analysis of variance followed by Dunnett's post-hoc test in the GraphPad Prism 5 package (GraphPad Software Inc., San Diego, CA, USA). P<0.05 were considered to indicate a statistically significant difference.
Results
Progression of AD-like skin lesions in BALB/c mice
The representative clinical features of the treatment groups are presented in Fig. 1B. Repeated application of DNCB induced skin dryness, followed by erythema, edema and hyperemia in the ear of the control group. However, the AAFE 0.5 and AAFE 1 groups exhibited inhibition of these symptoms of AD (Fig. 1B).
Histopathological and immunohistochemical features
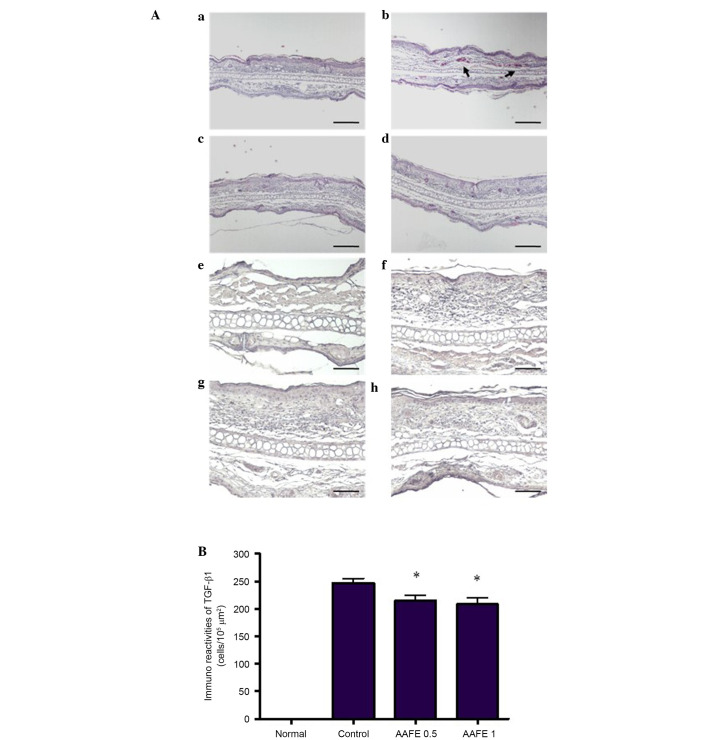
Histopathological features of the ear skin lesions are presented in Fig. 2A. In the normal group, inflammatory cell infiltration was not observed following H&E staining. However, in the control group inflammatory cell infiltration of the dermis was observed. Inhibition of these histopathological alterations was observed in the AAFE 0.5 and AAFE 1 groups compared with the control group (Fig. 2Aa–d). Immunohistochemistry was performed to evaluate DNCB-induced TGF-β1 expression in the AD-like lesions. TGF-β1 was not detected in the epidermis and dermis of the normal group; however, marked TGF-β1 expression was detected in the epidermis and dermis of the control group. Conversely, the number of TGF-β1-expressing cells was markedly lower in the AAFE 0.5 and AAFE 1 groups compared with that of the control group (Fig. 2Ae–h and 2B).
Figure 2.
Hematoxylin and eosin staining and TGF-β1 immunohistochemical staining of ear skin tissue in BALB/c mice. (A) (a) Inflammatory responses were not observed in the normal group. (b) The control group exhibited marked increases in inflammatory response, and the (c) AAFE 0.5 g/kg and (d) AAFE 1 g/kg groups exhibited relative decreases compared with the control group. Markedly increased protein levels of TGF-β1 were detected in the (f) control group compared with the (e) normal group. Protein expression levels of TGF-β1 were decreased in the (g) AAFE 0.5 g/kg and (h) AAFE 1 g/kg groups compared with the control group. Scale Bar: a-d=50 μm; e-h=100 μm. (B) Immunohistochemical staining of ear skin tissue for TGF-β1. The number of TGF-β1-positive cells was significantly decreased in the AAFE 0.5 g/kg and AAFE 1 g/kg groups compared with the control group. Data are presented as the mean ± standard deviation from five animals. Data were analyzed using one-way analysis of variance followed by Dunnett's post-hoc multiple comparison test. *P<0.05 vs. the control group. AAFE, Artemisia argyi Folium extract; TGF-β1, transforming growth factor-β1.
Effects of AAFE on serum levels of histamine and IgE
To assess the inhibitory effects of AAFE on histamine and IgE production by DNCB-induced AD-like lesions, histamine and IgE levels were measured in the serum. The control group displayed significant increases in histamine and IgE levels compared with the normal group, whereas the AAFE 0.5 and AAFE 1 groups exhibited suppressed histamine and IgE levels compared with the control group (Fig. 3).
Figure 3.
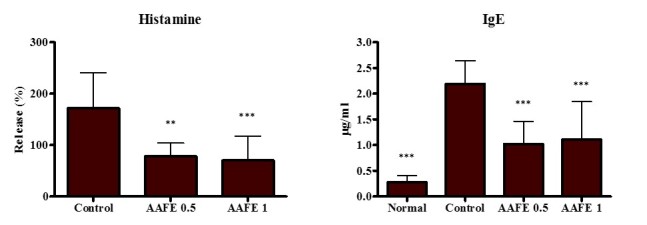
Effects of AAFE on serum levels of histamine and IgE. Data are presented as the mean ± standard deviation from five animals. Histamine levels are presented as a percentage relative to the normal levels of histamine release. Data were analyzed using one-way analysis of variance followed by Dunnett's post-hoc multiple comparison test. **P<0.01, ***P<0.001 vs. the control group. AAFE, Artemisia argyi Folium extract; IgE, immunoglobulin E.
Effects of AAFE on serum cytokine production and mRNA expression of cytokines in skin-draining lymph nodes
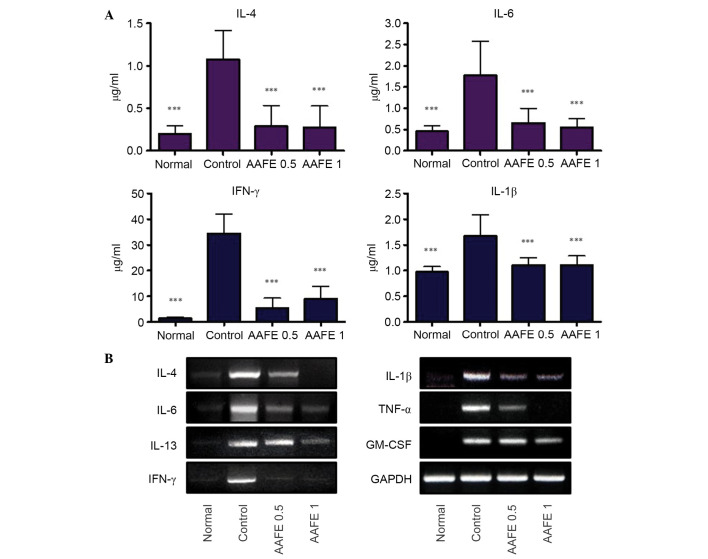
To assess the suppressive effects of AAFE on the production and gene expression of cytokines in DNCB-induced AD-like lesions, the serum levels of IL-1β, IL-4, IL-6 and IFN-γ cytokines, and the mRNA expression levels of diverse cytokines were determined in the skin-draining lymph nodes. The control group exhibited significant increases in serum levels of IL-1β, IL-4, IL-6 and IFN-γ compared with the normal group, whereas the AAFE 0.5 and AAFE 1 groups exhibited suppressed serum levels of IL-1β, IL-4, IL-6 and IFN-γ compared with the control group (Fig. 4A). Furthermore, the skin-draining lymph nodes of the control group exhibited higher expression levels of IL-1β, IL-4, IL-6 and IFN-γ compared with in the normal group. In the AAFE 0.5 and AAFE 1 groups the mRNA expression levels of IL-4 and IL-6, which are produced by Th2 cells, and IFN-γ, which is produced by Th1 cells, were suppressed in a dose-dependent manner. In addition, the AAFE 0.5 and AAFE 1 groups exhibited reduced mRNA expression levels of IL-1β, TNF-α and GM-CSF (Fig. 4B).
Figure 4.
Effects of AAFE on serum levels of IL-4, IL-6, IFN-γ and IL-1β, and mRNA expression levels of IL-4, IL-6, IL-13, IFN-γ, IL-1β, TNF-α and GM-CSF in skin-derived lymph nodes from a mouse model of AD-like lesions. (A) Serum IL-4, IL-6, IFN-γ and IL-1β levels. Data presented as the mean ± standard deviation from five animals. Data were analyzed using one-way analysis of variance followed by Dunnett's post-hoc multiple comparison test. ***P<0.001 vs. the control group. (B) Representative IL-4, IL-6, IL-13, IFN-γ, IL-1β, TNF-α and GM-CSF mRNA expression levels. AAFE, Artemisia argyi Folium extract; IL, interleukin; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; GM-CSF, granulocyte-macrophage colony-stimulating factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Effects of AAFE on activation of Lyn, Syk, MAPKs, PI3K/Akt and IκBα
Lyn, Syk, MAPKs, PI3K/Akt and IκBα/NF-κB signaling pathways have previously been implicated in the pathogenesis of allergic inflammatory skin diseases, and proinflammatory cytokines are known to induce activation of these signaling pathways (25,26). Therefore, the present study investigated whether AAFE regulates Lyn, Syk, MAPKs, PI3K/Akt and IκBα signaling associated with AD-like lesions. As shown in Fig. 5, in the skin-draining lymph nodes of the control group the phosphorylation of Lyn, Syk, MAPKs (ERK, JNK and p38), PI3K/Akt and IκBα were overexpressed compared with the normal group; however, in the AAFE 0.5 and AAFE 1 groups the phosphorylation of Lyn, Syk, MAPKs, (ERK, JNK and p38), PI3K/Akt and IκBα were suppressed in a dose-dependent manner (Fig. 5).
Figure 5.
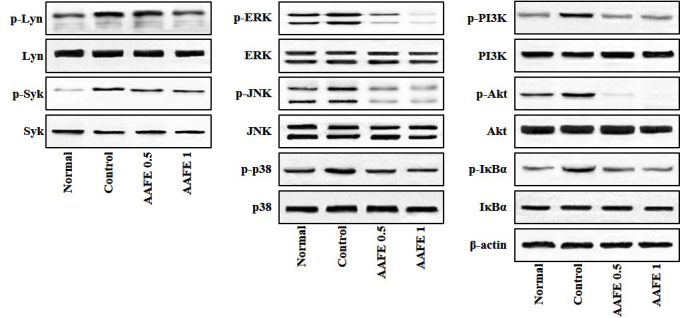
Effects of AAFE on the activation of Lyn, Syk, MAPKs, PI3K/Akt and IκBα in skin-derived lymph nodes from a mouse model of AD-like lesions. Representative Lyn, Syk, MAPKs, PI3K/Akt and IκBα expression levels are presented. Total proteins were analyzed by western blotting using phospho-specific or total antibodies against Lyn, Syk, ERK, JNK, p38, PI3K, Akt, IκBα and β-actin. p-, phosphorylated; AAFE, Artemisia argyi Folium extract; Lyn, Lck/yes-related novel tyrosine kinase; Syk, spleen tyrosine kinase; ERK, extracellular signal-regulated kinase; JNK, c-jun N-terminal kinase; PI3K, phosphatidylinositol 3-kinase.
Discussion
AD is an inflammatory, relapsing and itchy skin disorder, which is divided into two phases. Acute AD is characterized by erythema, eczema, excoriation, exudation and pruritus, and represents a Th2-biased immune response. Chronic AD is characterized by dry and thickened skin, lichenification and fibrotic papules, and represents a Th1/Th2/non Thcomplexed immune response.
The present study determined the effects of AAFE on DNCB-induced AD-like lesions. Macroscopic observations indicated that AAFE prevented the progression of AD-like pathogenesis through a reduction of clinical symptoms, including eczema and erythema (Fig. 1B).
TGF-β1 is a multifunctional cytokine that is implicated in skin dendritic cell homeostasis, fibroblast growth and tissue remodeling. TGF-β1 promotes severe skin inflammation, the formation of lichenification and fibrotic papules in AD lesions, and also has an important role in structural changes, such as fibrosis, in chronic lung diseases (27). In the present study, histopathological analysis indicated that AAFE attenuated inflammatory symptoms and TGF-β1 immunoreactivities in ear skin tissue (Fig. 2).
IgE-mediated hypersensitive immune responses to generally harmless environmental antigens contribute to the pathogenesis of AD. IgE binds to allergens and induces the aggregation of FcεRI on the surface of mast cells/basophils, triggering an allergic cascade through the release of inflammatory mediators, including histamine, prostaglandins, chemokines and cytokines (28,29). The results of the present study suggested that AAFE exerted an inhibitory effect on histamine release and IgE production in the serum (Fig. 3).
The cytokine milieu of patients with AD is known to consist of Th2 cytokines, Th1 cytokines and non-Th proinflammatory cytokines throughout the acute and chronic phases of AD (16–18). The Th2 cytokines, which include IL-4, IL-6 and IL-13, are systemically upregulated in AD, and the typical Th1 cytokine, IFN-γ, is upregulated in chronic AD-skin lesions (30). Non-Th proinflammatory cytokines, including IL-1β and TNF-α, are produced from resident cells such as keratinocytes, mast cells and dendritic cells, thus implying their roles in the initiation and maintenance of atopic inflammation (30,31). In the present study, treatment with AAFE attenuated serum levels of IL-4, IL-6, IFN-γ and IL-1β (Fig. 4A), and the mRNA expression levels of IL-4, IL-6, IL-13, IL-1β, TNF-α and GM-CSF in skin-derived lymph nodes (Fig. 4B). These results suggested that oral administration of AAFE may attenuate the excessive cytokine milieu and inflammation in a model of AD-like skin lesions.
The Src-family kinase, Lyn, and the protein tyrosine kinase, Syk, are key regulators in the allergic proximal signaling pathway; these tyrosine kinases lead to the activation and tyrosine phosphorylation of MAPKs, PI3K/Akt and IκBα downstream of Syk (25,26). MAPKs (ERK1/2, p38 and JNK) are involved in the immune response and transduce extracellular signals, including inflammatory cytokines, growth factors and stress stimuli, into intracellular responses such as inflammation, differentiation and apoptosis (32). The PI3K/Akt pathway regulates the mast cell response, which is related to allergic diseases (33). Activation of NF-κB signaling by proinflammatory cytokines mediates the degradation of IκBα and the nuclear translocation of NF-κB, which consequently induces inflammation and tissue damage (22). In the present study, AAFE inhibited DNCB-induced phosphorylation of Lyn/Syk, MAPKs (ERK, JNK and p38), PI3K/Akt and IκBα (Fig. 5).
In conclusion, the findings of the present study demonstrated that AAFE exerted ameliorative effects on DNCB-induced AD-like lesions, by exerting anti-allergenic skin inflammatory effects related to the recovery of skin barrier dysfunction. These effects may be associated with suppression of cytokine abundance via the regulation of crucial factors, including Lyn, Syk, MAPKs, PI3K/Akt, and IκBα, during the process of AD pathogenesis.
The present study aimed to determine whether AAFE exerts therapeutic effects in AD. The anti-AD effects of AAFE were identified, and were shown to be related to suppression of inflammatory actions and skin barrier damage in a mouse model of AD-like skin lesions. Therefore, the effects of AAFE may help improve treatment for various allergic diseases and skin diseases.
Acknowledgments
The present study was supported by a grant from the Dong-Eui University Foundation (grant no. 2013AA108).
References
- 1.Ren X, Yao C, Wu F, Li Z, Xing J, Zhang H. Effectiveness of moxibustion treatment in quality of life in patients with knee osteoarthritis: A randomized, double-blinded, placebo-controlled trial. Evid Based Complement Alternat Med. 2015;2015:569523. doi: 10.1155/2015/569523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R, Chen M, Xiong J, Chi Z, Zhang B, Tian N, Xu Z, Zhang T, Li W, Zhang W, et al. Curative effect of heat-sensitive moxibustion on chronic persistent asthma: A multicenter randomized controlled trial. J Tradit Chin Med. 2013;33:584–591. doi: 10.1016/S0254-6272(14)60025-X. [DOI] [PubMed] [Google Scholar]
- 3.Park JW, Lee BH, Lee H. Moxibustion in the management of irritable bowel syndrome: Systematic review and meta-analysis. BMC Complement Altern Med. 2013;13:247. doi: 10.1186/1472-6882-13-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Yu S, Lao L, Yang M, Chen J, Luo X, Wang Y, Chen X, Li J, Zhu L, et al. Use of moxibustion to treat primary dysmenorrhea at two interventional times: Study protocol for a randomized controlled trial. Trials. 2015;16:35. doi: 10.1186/s13063-015-0552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J, Wang LP, Liu CZ, Zhang J, Wang GL, Yi JH, Cheng JL. Efficacy of acupuncture for primary insomnia: A randomized controlled clinical trial. Evid Based Complement Alternat Med. 2013;2013:163850. doi: 10.1155/2013/163850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong MA, Lee KW, Yoon DY, Lee HJ. Jaceosidin, a pharmacologically active flavone derived from Artemisia argyi, inhibits phorbolester-induced upregulation of COX-2 and MMP-9 by blocking phosphorylation of ERK-1 and -2 in cultured human mammary epithelial cells. Ann N Y Acad Sci. 2007;1095:458–466. doi: 10.1196/annals.1397.049. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Bae EA, Park EK, Shin YW, Baek NI, Han EJ, Chung HG, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int Immunopharmacol. 2007;7:1678–1684. doi: 10.1016/j.intimp.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Han JM, Jin YY, Baek NI, Bang MH, Chung HG, Choi MS, Lee KT, Sok DE, Jeong TS. In vitro antioxidant and anti-inflammatory activities of Jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch Pharm Res. 2008;31:429–437. doi: 10.1007/s12272-001-1175-8. [DOI] [PubMed] [Google Scholar]
- 9.Min SW, Kim NJ, Baek NI, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009;125:497–500. doi: 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Yin Y, Sun Y, Gu L, Zheng W, Gong F, Wu X, Shen Y, Xu Q. Jaceosidin inhibits contact hypersensitivity in mice via down-regulating IFN-γ/STAT1/T-bet signaling in T cells. Eur J Pharmacol. 2011;651:205–211. doi: 10.1016/j.ejphar.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 11.Bao X, Yuan H, Wang C, Liu J, Lan M. Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi. Carbohydr Polym. 2013;98:1236–1243. doi: 10.1016/j.carbpol.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Li J, Sun J, Zeng KW, Cui JR, Jiang Y, Tu PF. NO inhibitory guaianolide-derived terpenoids from Artemisia argyi. Fitoterapia. 2013;85:169–175. doi: 10.1016/j.fitote.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Nam Y, Choi M, Hwang H, Lee MG, Kwon BM, Lee WH, Suk K. Natural flavone jaceosidin is a neuroinflammation inhibitor. Phytother Res. 2013;27:404–411. doi: 10.1002/ptr.4737. [DOI] [PubMed] [Google Scholar]
- 14.Zeng KW, Wang S, Dong X, Jiang Y, Tu PF. Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits microglia-mediated neuroinflammation via suppression of NF-κB, JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine. 2014;21:298–306. doi: 10.1016/j.phymed.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(Suppl 6):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Akdis CA, Akdis M, Trautmann A, Blaser K. Immune regulation in atopic dermatitis. Curr Opin Immunol. 2000;12:641–646. doi: 10.1016/S0952-7915(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 17.Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015;45:566–574. doi: 10.1111/cea.12495. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Martinez O, Overbergh L, Mathieu C, Prabhakar BS, Chan LS. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin Exp Immunol. 2004;138:375–387. doi: 10.1111/j.1365-2249.2004.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 20.Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014;26:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 22.Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington (DC), USA: 2011. [Google Scholar]
- 24.Shore PA, Burkhalter A, Cohn VH., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959;127:182–186. [PubMed] [Google Scholar]
- 25.Sanderson MP, Wex E, Kono T, Uto K, Schnapp A. Syk and Lyn mediate distinct Syk phosphorylation events in Fcε RI-signal transduction: Implications for regulation of IgE-mediated degranulation. Mol Immunol. 2010;48:171–178. doi: 10.1016/j.molimm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Siraganian RP, de Castro RO, Barbu EA, Zhang J. Mast cell signaling: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KS, Park SJ, Kim SR, Min KH, Lee KY, Choe YH, Hong SH, Lee YR, Kim JS, Hong SJ, et al. Inhibition of VEGF blocks TGF-beta1 production through a PI3K/Akt signalling pathway. Eur Respir J. 2008;31:523–531. doi: 10.1183/09031936.00125007. [DOI] [PubMed] [Google Scholar]
- 28.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31:475–529. doi: 10.1615/CritRevImmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuepbach-Mallepell S, Philippe V, Brüggen MC, Watanabe H, Roques S, Baldeschi C, Gaide O. Antagonistic effect of the inflammasome on thymic stromal lymphopoietin expression in the skin. J Allergy Clin Immunol. 2013;132:1348–1357. doi: 10.1016/j.jaci.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 32.Peti W, Page R. Molecular basis of MAP kinase regulation. Protein Sci. 2013;22:1698–1710. doi: 10.1002/pro.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MS, Rådinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]