Abstract
Objective
Alterations in the microbiome, including the periodontal microbiome, may be a risk factor for rheumatoid arthritis (RA). Most studies that have analyzed this association are relatively small, focus primarily on a single periodontal pathogen (Porphyromonas gingivalis), and are not population-based. We investigated the association between elevated serum IgG antibodies to 19 periodontal species and the prevalence of rheumatoid factor (RF) in a large nationally representative sample of adults.
Methods
The Third National Health and Nutrition Examination Survey is a cross-sectional sample of the non-institutionalized US population (n=33,994). Our study population included all dentate participants ≥60 years, who did not have RA as defined by a modified version of the American College of Rheumatology 1987 criteria, and had complete data for both serum IgG antibodies against periodontal bacteria and serum RF antibody titer (n=2461).
Results
Adjusted odds ratios (ORs) (95% CI) summarizing the relationship between the 19 periodontal serum IgGs and RF seropositivity ranged from 0.53 (0.29, 0.97) to 1.27 (0.79, 2.06), and 17 of the 19 observed ORs were < 1.0. The ORs for RF seropositivity among participants with elevated Prevotella intermedia [0.53 (0.29, 0.97)] and Capnocytophaga ochracea [0.54 (0.31, 0.95)] IgG were statistically significant.
Conclusion
We have found elevated periodontal IgGs to be mostly unassociated with RF seropositivity in the nationally representative NHANES III. Elevated antibody levels to P. intermedia and C. ochracea were associated with lower odds of RF seropositivity.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation and pain in the joints, and varying degrees of systemic involvement. It affects 0.5–1.0% of the U.S population, and results in disability and increased mortality (1). While genetic factors are the best understood risk factors for RA, they explain only 50–60% of the total risk for RA (2–4). Other known environmental risk factors, of which tobacco use has shown the strongest evidence (1), have been identified but they do not fully explain the remaining risk (4).
Proposed environmental risks for RA include adverse microbial exposures common in periodontitis. Periodontal pathogens might contribute to the development of RA in susceptible individuals, possibly by triggering a loss of immune tolerance (5). Accordingly, several studies have demonstrated higher rates of periodontitis in patients with RA (6–8). Periodontitis is also associated with increased incidence of RA longitudinally (9–11), and anti-infective periodontal therapy might reduce RA severity (12). A small number of studies have also explored the periodontitis-RA link by examining the associations between antibody levels to periodontal pathogens – a marker of chronic adverse microbial exposure – and RA, with mixed results. Antibodies to Porphyromonas gingivalis, a well-studied periodontal pathogen, are elevated in RA cases compared to controls in some studies (13–15), while others found no difference between RA cases and controls (16–18).
Previous studies have helped identify important considerations for unbiased investigation of the relationship between periodontitis and RA. While periodontitis is an attractive candidate environmental risk factor for rheumatoid arthritis, untangling whether periodontitis represents a risk for RA or is simply a comorbid condition has been problematic because periodontitis and RA share common risk factors. For example, the strongest known genetic risk for RA, the HLA-DRB1 ‘shared epitope’ alleles, has also been shown to be a risk factor for periodontal disease (19, 20). Furthermore, tobacco use is also a strong risk factor for RA and periodontitis.
Most previous investigations into the relationship between periodontitis and RA have been cross-sectional and studied only existing RA, increasing the potential for reverse causation through RA-induced host changes due to medications and behavioral modifications. It is therefore of interest to study these relationships prior to the onset of clinically manifested RA when the risk of reverse causality is minimized. Moreover, as early treatment of RA reduces the severity of the disease course (21), identification of risk factors for preclinical RA could spur interventions that improve the trajectory of disease outcomes or potentially allow prevention of disease.
Two serum autoantibodies associated with preclinical RA are anti-citrullinated peptide antibodies (ACPA) and rheumatoid factor (RF). ACPA and RF appear to develop prior to the onset of clinically apparent synovitis (22–25) and most RA patients are characterized by the presence of serum antibodies of at least these two distinct types, and are thus denoted as being seropositive. ACPAs are highly specific biomarkers of preclinical RA and are associated with severity of RA (22–25). Similarly, RF has long been known to be present prior to clinical RA and to predict future RA development, particularly when present in high titers (22, 26). Both RF and ACPA however, have been shown to be present in very low titers for long periods preclinically prior to the onset of RA, at which point titers of both types of antibodies tend to rise dramatically (23–25).
The biological rationale supporting periodontal pathogens as a trigger for the development of ACPAs is particularly noteworthy as P. gingivalis, a well-studied periodontal pathogen, is the only bacteria currently known to contain an enzyme peptidyl arginine deiminase (PAD) able to citrullinate arginine residues in human proteins common to both periodontal and joint tissues (27). Previous studies report conflicting results with some studies observing a positive relationship between P. gingivalis antibodies and only ACPA (13, 16), or RF (28, 29), while others show a positive relationship between P. gingivalis antibodies and both ACPA and RF (17, 30), or a combined outcome measure of either ACPA or RF (31). One recent study found no relationship between P. gingivalis antibodies and ACPA or RF (18).
There are several possible explanations for previous inconsistent findings. First, the bacterial etiology of periodontitis is polymicrobial but the majority of previous serological studies only measure P. gingivalis antibodies (13, 16, 18, 28, 30); and more robust microbial exposure assessments might be important as other periodontal pathogens have been linked with RA (32, 33). Furthermore, many prior studies examining the relationship between antibodies to periodontal bacteria and RA relevant outcomes are small, conducted in samples of fewer than 200 participants (13–15, 28, 30), and none are population-based. To our knowledge, no study has investigated whether the levels of serum antibodies to several periodontal bacteria, measured in a population-based sample, are related to preclinical RA risk.
The Third National Health and Nutrition Examination survey (NHANES III) provides an opportunity to investigate the association between IgG antibody levels to periodontal bacteria and RF seropositivity in a large, population-based sample. We hypothesized that elevated serum antibodies would be associated with increased risk of RF seropositivity. In order to establish consistency with previous research, we also evaluated the association between clinical periodontal measures and RF seropositivity.
MATERIALS & METHODS
Study population
Details on the design and methodology of NHANES III have been previously published (34). Briefly, the Third National Health and Nutritional Examination Survey (NHANES III) is a nationally representative cross-sectional sample of the non-institutionalized US population (n=33,994) aged 2 months and older. The NHANES III is a multi-stage, stratified and clustered sample and was conducted in two phases, Phase 1 from 1988–1990 and Phase 2 from 1991–1994. Survey interviews and examinations were conducted, and the data contains information on sociodemographic characteristics, health behaviors, clinical periodontal measures, physical examinations of the joints of the upper and lower extremities, as well as serum analysis for rheumatoid factor (RF) antibody and antibodies to periodontal pathogens.
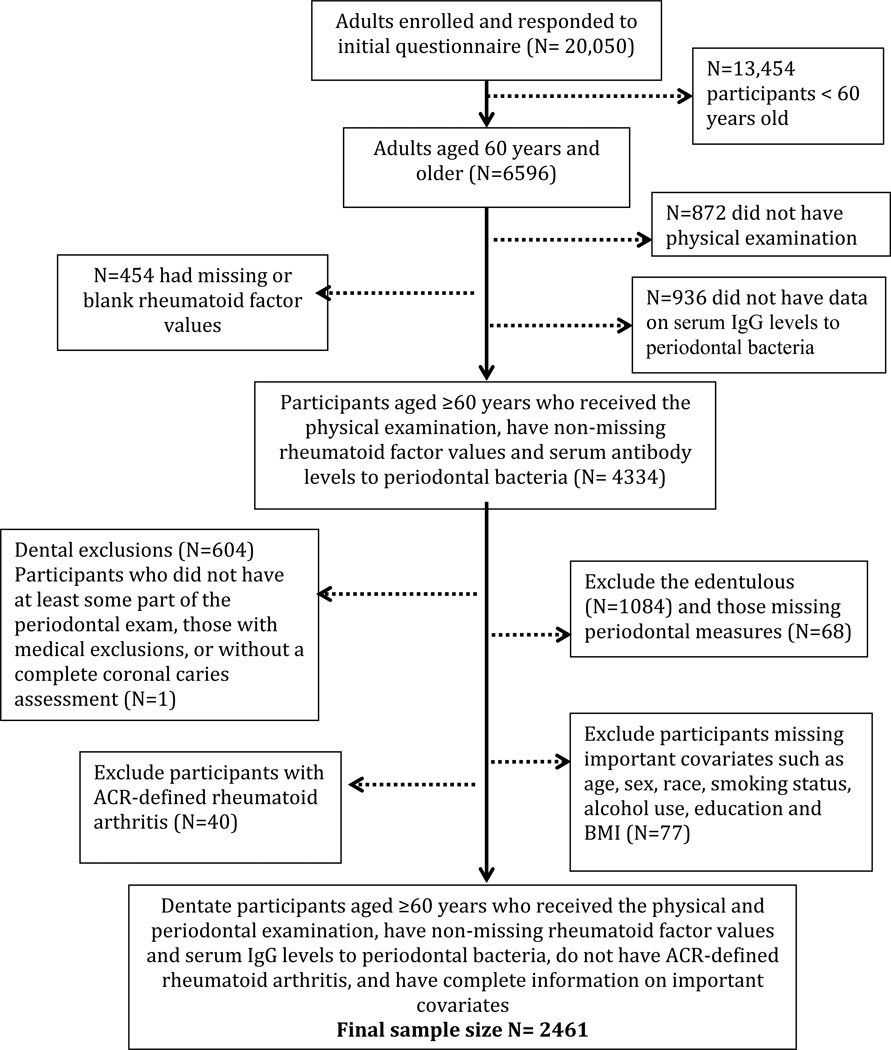
The current analysis includes all dentate participants without ACR-defined rheumatoid arthritis, with a non-missing value for the outcome rheumatoid factor antibody titer, and who had complete serum IgG periodontal antibody data. Because musculoskeletal examinations and serum analysis for RF antibody were only carried out for those 60 years and older, our study only includes participants ≥60 years. Individuals were excluded if they did not have a periodontal examination or coronal caries examination, or were missing important sociodemographic or health behavior variables related to periodontal disease and rheumatoid factor seropositivity such as age, sex, race, education, smoking status, alcohol use and body mass index (BMI). The final sample size was n=2461.
Immunoglobulin G (IgG) Antibody Assessment
The presence and level of IgG antibodies against 19 periodontal bacteria were measured from serum using a checkerboard immunoblotting technique and the results were reported in gravimetric units (35, 36). Optimal antibody IgG thresholds corresponding to moderate/severe periodontitis (according to the CDC/AAP definition) have previously been published for the 19 bacteria in the same NHANES III dataset (37). Because differing lots of protein A preparations were used in the laboratory analysis of Phase 1 and Phase 2 samples, there was a systematic difference in antibody levels between Phase 1 and 2 (35, 37). To take into account the differences, the antibody titers thresholds for elevated titer status were calculated separately for Phase 1 and 2. In the present analysis, “elevated” antibody thresholds are defined using these a priori Dye et al. cutoffs that maximized the association of antibody levels and clinical periodontal status.
For our sensitivity analysis in the Supplemental Results, we also created bacteria antibody clusters in an a priori fashion utilizing previous groupings derived from hierarchical cluster analysis (an unsupervised method) of these same antibody levels from the NHANES III dataset (38). The authors identified the following four mutually exclusive clusters and named them using the commonly used color coding for periodontal bacteria (39): i) orange-blue (Eubacterium nodatum, Actinomyces naeslundii), ii) yellow-orange ( Streptococcus intermedius, Streptococcus oralis, Streptococcus mutans, Fusobacterium nucleatum, Parvimonas micros (previously Micromonas micros), Capnocytophaga ochracea), iii) red-green (Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans mix, Eikenella corrodens, Selenomonas noxia, Veillonella parvula, Campylobacter rectus), and iv) orange-red (Prevotella melaninogenica, Prevotella intermedia, Prevotella nigrescens, Porphyromonas gingivalis mix) clusters (38). Cluster scores were created from the sum of the natural log transformed values of antibody levels for each species in the cluster to form continuous measures. We then dichotomized the scores of each cluster by the ≥ 75th percentile versus the < 75th percentile to indicate “high” versus “low” cluster scores, creating a binary measure of cluster burden.
Rheumatoid factor antibody positivity and rheumatoid arthritis assessment
The main outcome in the present analysis was rheumatoid factor seropositivity. Blood samples were first screened using latex-enhanced nephelometry (Behring Nephelometer Analyzer System) and the presence and levels of serum RF antibodies then determined using the Singer-Plotz latex agglutination test (40). A negative measurement was defined in NHANES III as ≤ 13 IU/ml, and the lowest non-zero recorded RF titer level in the NHANES III dataset was 20 IU/ml. For our analysis we classified all non-zero, non-missing values for RF as being RF seropositive, and zero values were coded as being RF negative, creating a dichotomous outcome.
We used clinical RA status as a secondary outcome for our sensitivity analyses. We defined clinical RA according to a modified version of the American College of Rheumatology (formerly the American Rheumatoid Association) (ACR) 1987 revised criteria for the classification of rheumatoid arthritis (41). The original ACR criteria required 4 out of 7 criteria to be met for the diagnosis of RA, but hand radiographs were not available in NHANES III. Hence in the modified version, participants who met 3 or more of the 6 available criteria were classified as having RA. These modified criteria have been used previously by other authors (6), and have shown good agreement with other classification methods used to classify RA in the NHANES III dataset (42).
Data drawing from the questionnaire, physical examination and laboratory files were used to fulfill our modified ACR criteria as follows: 1) self-reported presence of morning stiffness in the hands or knees for at least 1 hour for at least 6 weeks; 2) physical examination findings of the presence of soft tissue swelling in 3 or more of the following joint areas: left and right proximal interphalangeal joint (PIP), metacarpophalangeal (MCP), wrist, knee and first metatarsophalangeal (MTP) (physical examination of elbow and ankle soft tissue swelling, one of the joint areas included in the original ACR criteria was not available in NHANES III); 3) examination findings of soft tissue swelling in at least one hand joint (PIP, MCP or wrist); 4) symmetric arthritis as defined as simultaneous soft tissue swelling of the same joint (PIP or MCP or wrist or knee or first MTP) on both sides of the body; 5) presence of subcutaneous nodules observed on the shaft of either forearm; 6) presence of serum RF. Participants who had missing values on some criteria but still met 3 or more criteria were classified as having RA.
Risk factor assessment
Confounding variables associated with rheumatoid arthritis and periodontitis were also collected. Age, gender, race ethnicity (Non Hispanic White, Non Hispanic Black, Mexican American, Other), education level (< 12th grade, completed 12th grade, > 12th grade), self-reported smoking status (never, current, former), alcohol use (never, former, current drinker), and body mass index (BMI, measured weight in kilograms divided by height in meters squared) were measured.
Periodontal disease assessment
The oral health examination of the NHANES III has been described previously (43). It consisted of a visual and tactile oral and dental examination performed by a licensed dentist specially trained in the use of specific epidemiologic indices for oral health.
Periodontal measures were done in two randomly assigned quadrants. The mid-buccal and mesial-buccal sites of each fully erupted tooth, excluding the third molars, were assessed for gingival bleeding on probing (BOP), calculus, gingival recession, and pocket depth. Loss of attachment was calculated from measurements of the free gingival margin to the cemento-enamel junction and the probing depth (free gingival margin to base of the sulcus).
Statistical Analysis
Analysis of variance and categorical analysis methods were used for descriptive statistics, accounting for the weights used in the complex survey design. Multivariable logistic regression models were used to analyze the relationship between clinical periodontal measures and RF seropositivity. We also examined the association between elevated periodontal bacteria antibody levels and RF seropositivity, controlling for confounders such as age, gender, race, ethnicity, education, smoking status, alcohol use and BMI. As the main outcome of interest is preclinical RA, defined by RF seropositivity, the sample for the main analysis excluded those with ACR-defined RA (n=40). In sensitivity analyses, we explored the associations between elevated periodontal bacteria antibody levels with ACR-defined RA as the outcome so comparisons could be made with other publications. We also examined the association between the high versus low periodontal bacteria antibody clusters scores with RF seropositivity in the Supplemental Results. NHANES III uses a complex multi-stage probability sample survey design and oversamples young children, older adults, Blacks and Mexican Americans. The NHANES III Analytic and Reporting Guidelines recommends appropriate sample weights and the sample design to be taken into account in all analyses to avoid misinterpretation (44). Therefore the SURVEYLOGISTIC (SAS) procedure in SAS was used to account for the complex survey design and used with sample weights (WTPFEX6), cluster and strata variables (SDPPSU6 and SDPSTRA6 respectively) provided by the Center for Disease Control and Prevention (45). All statistical analyses were carried out using SAS 9.3 software (SAS, Cary, North Carolina, USA). Based on our final analysis sample of N=2461, we had only 57% power for detecting an odds ratio (OR) of either 0.7 or lower or 1.4 or higher.
RESULTS
Study participants
The mean age of the participants was 70 years; 19% of the participants were Non-Hispanic Black and 23% were Mexican-American. Approximately 50% had less than a 12th grade education, and 49% reported never smoking. Table 1 summarizes additional participant characteristics. The prevalence of rheumatoid factor seropositivity (RF+) was 4.9% (n=143). Having rheumatoid factor seropositivity was associated with having higher self-reported RA, lower diastolic blood pressure, lower white blood cell counts and lower BMI. The mean ± SD probing depth was 1.6 ± 0.6, and the mean ± SD attachment loss was 2.2 ± 1.5. The percent of individuals with elevated serum IgG to specific periodontal microbiota ranged from 28% – 40% across the different bacteria species. The highest prevalence of elevated serum IgG was for V. parvula while the lowest was for E. nodatum (Table 3).
Table 1.
General Characteristics of Participants according to prevalent rheumatoid factor seropositivity (RF+): Dentate participants aged ≥ 60 years, without ACR-defined rheumatoid arthritis, enrolled in NHANES III 1988–1992. (N=2461)
| Characteristic | Unweighted All (n=2461) |
Weighted All |
RF+ n= 143 (4.9%) |
RF− n= 2318 (95.1%) |
P value |
|---|---|---|---|---|---|
| Self Report RA | 7 | 6 | 16 | 5 | 0.01 |
| Age (years) | 70.2 ± 7.5 | 69.4 ± 0.3 | 70.2 ± 0.7 | 69.4 ± 0.4 | 0.24 |
| Female (%) | 51 | 57 | 61 | 57 | 0.53 |
| Race/Ethnicity (%) | 0.25 | ||||
| Non-Hispanic White | 56 | 85 | 79 | 85 | |
| Non-Hispanic Black | 19 | 7 | 12 | 7 | |
| Mexican-American | 23 | 3 | 3 | 3 | |
| Other | 3 | 5 | 6 | 5 | |
| Education (%) | 0.64 | ||||
| < 12th grade | 50 | 32 | 33 | 32 | |
| 12th grade | 26 | 34 | 37 | 33 | |
| >12th grade | 24 | 34 | 30 | 35 | |
| Smoking Status (%) | 0.54 | ||||
| Never | 49 | 49 | 45 | 49 | |
| Former | 37 | 39 | 38 | 39 | |
| Current | 14 | 12 | 17 | 12 | |
| Pack Years of Smoking | 15.9 ±29.4 | 17.5 ± 1.0 | 18.8 ± 3.8 | 17.5 ± 1.0 | 0.73 |
| Alcohol use (%) | 0.38 | ||||
| Never | 21 | 18 | 17 | 18 | |
| Former | 44 | 41 | 49 | 41 | |
| Current | 35 | 40 | 34 | 41 | |
| Systolic Blood Pressure (mmHg) |
137.9 ± 22.5 | 134.8 ± 0.7 | 131.5 ± 2.4 | 134.8 ± 0.7 | 0.17 |
| Diastolic Blood Pressure (mmHg) |
72.8 ± 12.0 | 72.4 ± 0.4 | 68.1 ± 1.7 | 72.6 ± 0.4 | 0.02 |
| Hypertension | 42 | 37 | 36 | 37 | 0.77 |
| White Blood Cell Count (cells/mm3) |
7.1 ± 2.6 | 7.0 ± 0.1 | 6.6 ± 0.2 | 7.0 ± 0.1 | 0.02 |
| Mean probing depth (mm) | 1.6 ± 0.6 | 1.5 ± 0.03 | 1.6 ± 0.06 | 1.5 ± 0.02 | 0.16 |
| Percentage of probing depths ≥ 4mm (%) |
4.5 ± 11.0 | 2.9 ± 0.3 | 3.7 ± 0.9 | 2.8 ± 0.3 | 0.38 |
| Percentage of sites with bleeding on probing (%) |
14.2 ± 21.1 | 11.3 ± 0.7 | 12.0 ± 1.7 | 11.1 ± 0.7 | 0.55 |
| Mean attachment loss (mm) | 2.2 ±1.5 | 1.9 ± 0.1 | 2.1 ± 0.2 | 1.9 ± 0.1 | 0.15 |
| Percentage of site with attachment loss ≥ 5mm (%) |
11.7 ± 21.3 | 8.4 ± 0.7 | 10.9 ± 2.1 | 8.2 ± 0.7 | 0.22 |
| C-reactive Protein (mg/dl) | 0.55 ± 1.1 | 0.49 ± 0.02 | 0.51 ± 0.06 | 0.48 ± 0.02 | 0.69 |
| LDL- cholesterol | 140.9 ± 37.6 | 142.5 ± 1.4 | 141.7 ± 6.0 | 142.4 ± 1.5 | 0.91 |
| HDL- cholesterol | 51.3 ± 16.1 | 51.8 ± 0.5 | 49.7 ± 2.3 | 51.9 ± 0.5 | 0.34 |
| Body Mass Index BMI (kg/m2) |
27.4 ± 5.0 | 27.0 ± 0.1 | 26.0 ± 0.5 | 27.1 ± 0.1 | 0.02 |
| Lymphocytes (percent of 100 cells) |
31.7 ±11.3 | 31.6 ± 0.5 | 32.8 ± 2.0 | 31.5 ± 0.5 | 0.53 |
| Monocytes (percent of 100 cells) |
5.8 ± 3.3 | 5.9 ± 0.2 | 6.3 ± 0.6 | 5.9 ± 0.2 | 0.46 |
| Log-transformed P.gingivalis antibody level (fg/ml) |
6.2 ± 2.2 | 5.7 ± 0.08 | 5.7 ± 0.2 | 5.7 ± 0.09 | 0.82 |
Note: Values shown are either percent or mean (SD). Weighted results incorporate survey weights making the results generalizable to the civilian non-institutionalized US population aged ≥ 60 years and RA free. Results presented among RF subgroups are weighted including the prevalence of RF+ and Rao-Scott Modified Chi square and T-tests were used as appropriate to test for differences across RF positivity.
Subsamples due to missing data: n = 2393 with pack years data; n = 2392 with systolic blood pressure, diastolic blood pressure and hypertension; n = 2434 with white blood cell count; n = 1020 with LDL counts; n = 2441 with HDL counts; n = 747 with lymphocytes and monocytes counts.
Self Report RA represents patients who self-reported a doctor diagnosis of RA in an interviewer administered questionnaire regardless of physical exam results or serology.
Table 3.
Odds ratios for prevalent rheumatoid factor seropositivity (RF+) by Serum Antibody Levels for 19 Periodontal Bacteria: Dentate participants aged ≥ 60 years and without ACR-defined rheumatoid arthritis, enrolled in NHANES III 1988–1992. (N=2461)
|
Periodontal bacteria antibody levels |
Phase 1* cut point |
Phase 2* cut point |
% Elevated Antibody levels based on cut points |
Crude OR |
p- value |
Adjusted OR |
p- value |
|---|---|---|---|---|---|---|---|
| P. gingivalis (Pg) mix antibody | 713 | 1149 | 39% | 0.89 (0.55, 1.45) | 0.65 | 0.80 (0.47, 1.33) | 0.38 |
| P. intermedia (Pi) antibody | 457 | 502 | 38% | 0.57 (0.33, 1.00) | 0.05 | 0.53 (0.29, 0.97) | 0.04 |
| P. nigrescens (Pn) antibody | 310 | 392 | 38% | 0.70 (0.41, 1.20) | 0.20 | 0.70 (0.41, 1.21) | 0.20 |
| T. forsythia (Tf) antibody | 128 | 276 | 38% | 0.81 (0.49, 1.34) | 0.41 | 0.82 (0.51, 1.34) | 0.44 |
|
A. actinomycetemcomitans (Aa) mix |
766 | 2612 | 33% | 0.84 (0.45, 1.58) | 0.59 | 0.82 (0.43, 1.55) | 0.54 |
| F. nucleatum (Fn) antibody | 154 | 164 | 34% | 0.62 (0.37, 1.05) | 0.07 | 0.61 (0.35, 1.05) | 0.08 |
| S. oralis (So) antibody | 83 | 189 | 35% | 0.85 (0.51, 1.41) | 0.52 | 0.80 (0.48, 1.35) | 0.40 |
| P. micra (Pmi) antibody | 235 | 337 | 33% | 0.92 (0.58, 1.46) | 0.73 | 0.92 (0.59, 1.44) | 0.73 |
| C. rectus (Cr) antibody | 112 | 219 | 38% | 0.91 (0.60 1.39) | 0.68 | 0.87 (0.55, 1.39) | 0.56 |
| E. corrodens (Ec) antibody | 198 | 450 | 38% | 0.65 (0.36, 1.17) | 0.12 | 0.62 (0.35, 1.12) | 0.11 |
| E. nodatum (En) antibody | 1755 | 3897 | 28% | 1.08 (0.58, 2.00) | 0.82 | 1.15 (0.61, 2.17) | 0.67 |
| S. intermedius (Si) antibody | 201 | 459 | 33% | 0.87 (0.48, 1.57) | 0.77 | 0.88 (0.48, 1.61) | 0.67 |
| C.ochracea (Co) antibody | 285 | 206 | 30% | 0.56 (0.32, 0.97) | 0.04 | 0.54 (0.31, 0.95) | 0.03 |
| V. Parvula (Vp) antibody | 51 | 74 | 40% | 0.93 (0.54, 1.60) | 0.79 | 0.98 (0.57, 1.69) | 0.95 |
| A. naeslundii (An) antibody | 1097 | 640 | 30% | 1.14 (0.70, 1.87) | 0.60 | 1.27 (0.79, 2.06) | 0.32 |
|
P. melaninogenica (Pm) antibody |
290 | 354 | 38% | 0.97 (0.56, 1.70) | 0.91 | 0.97 (0.56, 1.65) | 0.90 |
| S. noxia (Sn) antibody | 38 | 83 | 35% | 0.92 (0.51, 1.65) | 0.78 | 0.91 (0.50, 1.66) | 0.76 |
| T. denticola (Td) antibody | 136 | 403 | 33% | 0.64 (0.35, 1.18) | 0.15 | 0.63 (0.35, 1.15) | 0.13 |
| S. mutans (Sm) antibody | 117 | 201 | 35% | 0.88 (0.54, 1.46) | 0.63 | 0.84 (0.51, 1.39) | 0.50 |
Based on calculated threshold values for elevated antibody status for the CDC/AAP definition of moderate to severe periodontitis in Dye et al. “Serum Antibodies to Periodontal Bacteria as Diagnostic Markers of Periodontitis” (2009)
Multivariable model adjusted for age, sex, race, education, smoking, BMI, alcohol
Clinical Periodontal Measures and prevalent RF seropositivity
After multivariable adjustment for age, sex, education, smoking, alcohol use and BMI, the OR [95% CI] for RF seropositivity for every 1 mm increase in either mean probing depth or mean attachment loss were 1.34 [0.92, 1.97]; p=0.13 and 1.09 [0.93, 1.28]; p=0.29, respectively (Table 2). Findings were weaker for bleeding on probing and tooth loss (Table 2). Sensitivity analyses controlling for the presence of both probing depth and attachment loss also show similar results. In supplemental analyses, results for the ACR-defined RA outcome (prevalence 1.6%) were similar (Table S1).
Table 2.
Odds ratios for prevalent rheumatoid factor seropositivity (RF+) by clinical periodontal measures: Dentate participants aged ≥ 60 years and without ACR-defined rheumatoid arthritis, enrolled in NHANES III 1988–1992. (N=2461)
| Crude RF+ prevalence= 5.8 % (143/2461) |
p-value | Adjusted* RF+ prevalence= 5.8 % (143/2461) |
p-value | |
|---|---|---|---|---|
| Mean probing depth | 1.34 (0.97, 1.87) | 0.08 | 1.34 (0.92, 1.97) | 0.13 |
| Mean attachment loss | 1.14 (0.98, 1.33) | 0.10 | 1.09 (0.93, 1.28) | 0.29 |
| Total missing teeth | 1.01 (0.98, 1.04) | 0.64 | 1.00 (0.97, 1.03) | 0.80 |
| Percent of sites bleeding on probing |
1.03 (0.94, 1.11) | 0.53 | 1.02 (0.93, 1.12) | 0.63 |
Mean probing depths, attachment loss and percent of bleeding on probing sites are based on the mesiobuccal and midbuccal sites of all fully erupted teeth, excluding 3rd molars, in 2 randomly selected quadrants.
Odds ratios are for either a 1 mm increase in probing depth or attachment loss; 1 additional missing tooth; or a 10% increase in BOP.
Adjusted for age, sex, race, education, smoking, BMI, alcohol.
Association between elevated serum IgG to periodontal bacteria and prevalent RF seropositivity
The IgG levels to the 19 periodontal bacteria were all positively correlated (p <0.001) with moderate to strong correlations (Figure S1). Odds ratios summarizing the relationship between 19 serum IgG levels to periodontal bacteria and RF seropositivity are shown in Table 3. Adjusted odds ratios ranged from 0.53 to 1.27, controlling for the age, gender, race, education, smoking status, alcohol use and BMI, and 17 of the 19 observed ORs were < 1.0 suggesting inverse relationships, although only two ORs were statistically significant. P.intermedia antibody was inversely associated with RF+ : OR [95% CI] =0.53 [0.29, 0.97]; p=0.04. Similarly, the OR [95% CI] for C. ochracea was 0.54 [0.31, 0.95]; p=0.03. The observed ORs summarizing the relationship between antibody levels and ACR-defined RA were weaker and none were statistically significant (Table S2). The aforementioned findings were also unchanged in sensitivity analyses stratified by either smoking status or clinical periodontal status (data not shown).
In further sensitivity analyses using a priori antibody clusters as the main exposure and mutually adjusting for the clusters, the results were not meaningfully changed from the species specific analyses, and none of the observed ORs were statistically significant (Table S3). When considering RA as the outcome in the sensitivity analysis, there were no clear patterns observed for the analyses based on the cluster groupings (Table S4). Results for the association between antibody titers to periodontal bacteria and RF+ when using different RF titer thresholds for positivity (>25th percentile, >50th percentile, 75th percentile) also demonstrate no meaningful difference in the pattern of findings (Table S5).
DISCUSSION
We have found elevated serum IgG antibodies to P. intermedia and C. ochracea to be associated with lower prevalence of RF seropositivity. While the remaining antibody levels were not associated with prevalent RF seropositivity, it was noteworthy that ~75% of the antibodies to periodontal bacteria considered, including the two statistically significant findings, demonstrated ORs <1.0. In contrast, the ORs observed for clinical measures of periodontal disease (probing depth, attachment loss and bleeding) with RF seropositivity were >1.0. The trend for positive relationships (i.e., ORs >1.0) between periodontal disease and both RF seropositivity and ACR-defined clinical RA were consistent with several previous reports (6, 17). This observed consistency, particularly when using the same data as previous findings (6), gives support to the internal validity of our current unexpected results for the overall inverse relationships between IgG antibody levels to periodontal microbiota and RF seropositivity.
In a study utilizing the same NHANES III dataset (37), serum IgG levels to periodontal bacteria were found to be moderately accurate proxies for clinical periodontal status. Thus we expected that the serological markers of periodontitis would show a similar pattern to the positive relationship between periodontitis and prevalent RA previously found in other studies. For example, Mikuls and colleagues found a significant association (OR 1.56) between IgG antibodies to P. gingivalis and the presence of RA-related autoantibody positivity (31). However the outcome “autoantibody positivity” in their study was defined as the presence of at least one of the autoantibodies measured (ACPA, RF measured by nephelometry, or ELISA for RF isotypes), and it thus cannot be determined if the observed associations were due to a stronger positive relationship between P.gingivalis antibodies and ACPA. Furthermore, their study population consisted of individuals at high risk of RA (subjects with HLA-DR4 and first-degree relatives of RA patients), whereas our study was population-based, had higher mean age, lower extent and severity of periodontal disease, but included more ever-smokers. Lastly, our methods of assessing RF positivity were different.
Interestingly, while non-significant the same study by Mikuls et al. (2012) also found an inverse association similar to our study of OR 0.76 (0.47, 1.23) for P.intermedia with autoantibody positivity, controlling for confounders and P.gingivalis antibodies (31). Inverse associations between serum antibodies to periodontal bacteria and RA have also been observed in other studies. Scher et al. observed a higher percentage of healthy participants testing positive for antibodies against P.gingivalis when compared to patients with new onset RA and chronic RA (32). They also identified Prevotella as one of the only two organisms found with increased prevalence in new-onset RA, regardless of periodontal disease status, versus healthy controls (32), and called for further exploration into the role of Prevotella.
Thus while our findings of inverse associations have some precedence and may warrant further investigation, they may also reflect the poor correlation and mixed findings between antibodies to periodontal bacteria and RF seropositivity seen in the literature. A recent study on a cohort of patients with early arthritis found no significant difference in P.gingivalis antibody levels by RF positivity status (18). The authors proposed that the strong effect of smoking on RA could have obscured the association between P.gingivalis and early RA. As with RA risk, smoking has also been associated with the development of both rheumatoid factor (46, 47) and anti-citrullinated peptide antibodies (48). Therefore, the potential for smoking–related confounding or causal preemption exists even in preclinical studies. Nevertheless, in our sample of older adults with no clinical diagnosis of RA, we found no meaningful difference in results even among never smokers.
The interrelationship between RF, ACPA and periodontal bacteria is complex and the nature of the interaction between ACPA and RF in the development of RA is unresolved. Furthermore, RF has been associated with a number of other chronic infectious disease states in individuals who do not appear to be developing RA, and its use as a prodromal indicator of incipient RA may be problematic. ACPAs are more sensitive and more specific for the presence of rheumatoid arthritis than rheumatoid factor (49). Synergy between the development of ACPA and RF has also been observed (50), indicating that development of both of these types of antibodies is the strongest predictor for incipient rheumatoid arthritis. Whether antibodies to periodontal bacteria are related to the presence of ACPA would thus be of interest. Unfortunately, ACPAs were not measured in the NHANES III cohort and cannot be analyzed in our study.
Rather than indicating a null association between periodontitis and RA, we believe that our findings draw attention to the potentially problematic use of serum antibodies to periodontal pathogens as proxies of bacterial exposure in cross-sectional studies of RA risk, particularly in population-based samples. The use of serological markers to periodontal bacteria exposure has some important advantages, including the ability to be measured from stored or fresh serum, allowing the study of large retrospective cohorts, and requiring much less participant burden and examiner time than a clinical periodontal examination or microbial sampling. However, the dual connotation of high antibody levels complicates the interpretation of results from studies using serum antibodies to mark exposure to adverse microbial exposures. While elevated antibodies likely reflect increased bacterial burden, they simultaneously signify a presumably protective immune response to the microbial challenge that may in turn control or reduce lifetime bacterial exposures.
Due to the cross-sectional nature of our data, we cannot establish temporality and the presence of RF antibodies could be concomitant with a reduced immune response to periodontal bacteria, secondary to a dysregulated immune response. This raises the potential for misclassification of exposure status (i.e., classifying individuals with high levels of bacterial exposure as having low levels of exposure and vice-versa) that is differential by outcome status. If the misclassification rate is high enough, the bias induced can be substantial and might even produce measures of association that are opposite to the true direction. This may have been especially important due to an increasingly senescent immune response in the individuals included in our sample, who were all over age 60 (younger individuals were not included in this analysis because of the lack of musculoskeletal exams and RF serum analysis). Similarly, age-related exclusions might have created a selection bias due to the exclusion of individuals with HLA-DRB1 shared epitope rheumatoid arthritis risk alleles who tend to experience rapid progression of periodontal disease and rheumatoid arthritis at younger ages.
We did not exclude patients based on the use of immunosuppressive or disease-modifying RA medications, and our study sample could have included a number of participants using such medications with conceivably altered immune responses to periodontal bacteria, resulting in the inverse association seen. Because of our exclusion of edentulous individuals and those without complete data on all periodontal antibodies, we had very few RF seropositive participants with RA (n=8) and were unable to perform further subgroup analyses on seropositive RA subjects only as previous studies have done. An alternative explanation for our inverse findings could be incidence-prevalence bias. If individuals with elevated (vs. low) serum antibodies to periodontal bacteria are more likely to die or progress rapidly from RF+ asymptomatic status to overt RA, this can induce an association that appears inverse because the frequency of the exposure is underestimated among the cases studied (51).
Finally, we were only able to consider serum antibodies to a small number of microorganisms available in the NHANES III dataset, among the hundreds of known periodontal bacteria. Recent literature suggests a complex periodontal microbiome with synergistic effects on health and disease that this study cannot address (52). We also did not have data on RA risk genotypes, such as the HLA-DRB1 shared epitope strongly associated with RA. It is possible that periodontal antibody levels might demonstrate stronger associations in genetically susceptible individuals, as previously seen in the gene-environment interactions between the HLA genotype and smoking (48).
This study adds to the periodontitis-RA literature by exploring the associations between serological markers of exposure to periodontal bacteria and a preclinical marker of RA, RF seropositivity. We found ORs to be predominantly inverse though largely not statistically significant between antibodies to periodontal bacteria and RF seropositivity in the nationally representative NHANES III sample. The strengths of our study include extending the investigation beyond the commonly studied P.gingivalis association to the antibodies to 19 common periodontal bacteria. To the best of our knowledge, our study is the first to examine the relationship between antibodies to periodontal bacteria and RF seropositivity in a population-based sample. Our findings were consistent after controlling for important confounders such as smoking and alcohol. Future studies examining the association between periodontal infection and RA would be improved by including assessments of the periodontal microbiota and measuring genetic susceptibility. In addition, the use of prospective cohort designs starting at ages younger than those of the known peak incidence of RA, and data on the future development of RA in these subjects and the subsequent associations between RA, RF and antibodies to periodontal pathogens would be of great interest.
Supplementary Material
Figure 1.
Flowchart of sample population derivation for this study of the association between serum IgG levels to 19 periodontal bacteria and rheumatoid factor (RF) in dentate participants aged ≥ 60 years from the NHANES III (1988–1994) dataset.
Acknowledgments
This research was partially supported by NIH grants R00 DE018739 and R01 DK 102932 to Dr. Demmer.
Footnotes
We have no financial conflict of interest to report.
REFERENCES
- 1.Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J Autoimmun. 2010;35:10–14. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43:30–37. doi: 10.1002/1529-0131(200001)43:1<30::AID-ANR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Frisell T, Holmqvist M, Kallberg H, Klareskog L, Alfredsson L, Askling J. Familial risks and heritability of rheumatoid arthritis: role of rheumatoid factor/anti-citrullinated protein antibody status, number and type of affected relatives, sex, and age. Arthritis Rheum. 2013;65:2773–2782. doi: 10.1002/art.38097. [DOI] [PubMed] [Google Scholar]
- 4.Yarwood A, Huizinga TWJ, Worthington J. The genetics of rheumatoid arthritis: risk and protection in different stages of the evolution of RA. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–318. doi: 10.1007/s10753-004-6641-z. [DOI] [PubMed] [Google Scholar]
- 6.Pablo P de, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–76. [PubMed] [Google Scholar]
- 7.Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013;92:399–408. doi: 10.1177/0022034513483142. [DOI] [PubMed] [Google Scholar]
- 8.Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP, et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–986. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Yamazaki T, Hamaguchi M, Morimoto T, Yamori M, Asai K, et al. Periodontitis and Porphyromonas gingivalis in Preclinical Stage of Arthritis Patients. PLoS One. 2015;10:e0122121. doi: 10.1371/journal.pone.0122121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demmer RT, Molitor JA, Jacobs DR, Jr, Michalowicz BS. Periodontal disease, tooth loss and incident rheumatoid arthritis: results from the First National Health and Nutrition Examination Survey and its epidemiological follow-up study. J Clin Periodontol. 2011;38:998–1006. doi: 10.1111/j.1600-051X.2011.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molitor JA, Alonso A, Wener MH, Mich-alowicz BS, Beck JD, Gersuk VH, et al. Moderate to severe adult periodontitis increases risk of rheumatoid arthritis in non-smokers and is associated with elevated ACPA titers: The ARIC Study. Arthritis Rheum. 2009;60:1160. [Google Scholar]
- 12.Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol. 2007;13:134–137. doi: 10.1097/RHU.0b013e3180690616. [DOI] [PubMed] [Google Scholar]
- 13.Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9:38–42. doi: 10.1016/j.intimp.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogrendik M, Kokino S, Ozdemir F, Bird PS, Hamlet S. Serum antibodies to oral anaerobic bacteria in patients with rheumatoid arthritis. MedGenMed. 2005;7:2. [PMC free article] [PubMed] [Google Scholar]
- 15.Okada M, Kobayashi T, Ito S, Yokoyama T, Komatsu Y, Abe A, et al. Antibody responses to periodontopathic bacteria in relation to rheumatoid arthritis in Japanese adults. J Periodontol. 2011;82:1433–1441. doi: 10.1902/jop.2011.110020. [DOI] [PubMed] [Google Scholar]
- 16.Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, Woude D van der, et al. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010;37:1105–1112. doi: 10.3899/jrheum.091323. [DOI] [PubMed] [Google Scholar]
- 17.Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090–1100. doi: 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seror R, Gall-David S Le, Bonnaure-Mallet M, Schaeverbeke T, Cantagrel A, Minet J, et al. Anti-Porphyromonas gingivalis antibodies titres are associated with non-smoking status in early rheumatoid arthritis: Results from the ESPOIR cohort. Arthritis Rheumatol. 2015 doi: 10.1002/art.39118. [DOI] [PubMed] [Google Scholar]
- 19.Bonfil JJ, Dillier FL, Mercier P, Reviron D, Foti B, Sambuc R, et al. A “case control” study on the role of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO) J Clin Periodontol. 1999;26:77–84. doi: 10.1034/j.1600-051x.1999.260203.x. [DOI] [PubMed] [Google Scholar]
- 20.Marotte H, Farge P, Gaudin P, Alexandre C, Mougin B, Miossec P. The association between periodontal disease and joint destruction in rheumatoid arthritis extends the link between the HLA-DR shared epitope and severity of bone destruction. Ann Rheum Dis. 2006;65:905–909. doi: 10.1136/ard.2005.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finckh A, Liang MH, Herckenrode CM van, Pablo P de. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006;55:864–872. doi: 10.1002/art.22353. [DOI] [PubMed] [Google Scholar]
- 22.Rantapaa-Dahlqvist S, Jong BAW de, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 23.Nielen MMJ, Schaardenburg D van, Reesink HW, Twisk JWR, Stadt RJ van de, Horst-Bruinsma IE van der, et al. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann Rheum Dis. 2006;65:535–537. doi: 10.1136/ard.2005.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67:801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Ronnelid J, et al. Antibodies of IgG IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puente A del, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum. 1988;31:1239–1244. doi: 10.1002/art.1780311004. [DOI] [PubMed] [Google Scholar]
- 27.Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smit M de, Westra J, Vissink A, Doornbos-van der Meer B, Brouwer E, Winkelhoff AJ van. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012;14:R222. doi: 10.1186/ar4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada M, Kobayashi T, Ito S, Yokoyama T, Komatsu Y, Abe A, et al. Antibody Responses to Periodontopathic Bacteria in Relation to Rheumatoid Arthritis in Japanese Adults. J Periodontol. 2011;82:1433–1441. doi: 10.1902/jop.2011.110020. [DOI] [PubMed] [Google Scholar]
- 30.Arvikar SL, Collier DS, Fisher MC, Unizony S, Cohen GL, McHugh G, et al. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther. 2013;15:R109. doi: 10.1186/ar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikuls TR, Thiele GM, Deane KD, Payne JB, O’Dell JR, Yu F, et al. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arthritis Rheum. 2012;64:3522–3530. doi: 10.1002/art.34595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64:3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff B, Berger T, Frese C, Max R, Blank N, Lorenz HM, et al. Oral status in patients with early rheumatoid arthritis: a prospective, case-control study. Rheumatol. 2014;53:526–531. doi: 10.1093/rheumatology/ket362. [DOI] [PubMed] [Google Scholar]
- 34.Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Heal Stat 2. 1992:1–35. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Third National Health and Nutrition Examination Data 1988–1994 (NHANES III) Documentation, Codebook, and Frequencies?: Antibodies to Periodontal Pathogens Laboratory Surplus Sera Survey Years: 1988 to 1994. 2008
- 36.Papapanou PN, Neiderud AM, Sandros J, Dahlen G. Checkerboard assessments of serum antibodies to oral microbiota as surrogate markers of clinical periodontal status. J Clin Periodontol. 2001;28:103–106. doi: 10.1034/j.1600-051x.2001.280116.x. [DOI] [PubMed] [Google Scholar]
- 37.Dye BA, Herrera-Abreu M, Lerche-Sehm J, Vlachojannis C, Pikdoken L, Pretzl B, et al. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol. 2009;80:634–647. doi: 10.1902/jop.2009.080474. [DOI] [PubMed] [Google Scholar]
- 38.Merchant AT, Shrestha D, Chaisson C, Choi YH, Hazlett LJ, Zhang J. Association between Serum Antibodies to Oral Microorganisms and Hyperglycemia in Adults. J Dent Res. 2014;93:752–759. doi: 10.1177/0022034514538451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 40.Third National Health and Nutrition Examination Survey, 1988–1994: NHANES III reference manuals and reports: Laboratory procedures used for NHANES III. Hyattsville, MD: Centers for Disease Control and Prevention; 1996. U.S. Department of Health and Human Services, National Center for Health Statistics. [Google Scholar]
- 41.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 42.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48:917–926. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 43.Drury TF, Winn DM, Snowden CB, Kingman A, Kleinman DV, Lewis B. An overview of the oral health component of the 1988–1991 National Health and Nutrition Examination Survey (NHANES III-Phase 1) J Dent Res. 1996;75:620–630. doi: 10.1177/002203459607502S02. Spec No. [DOI] [PubMed] [Google Scholar]
- 44.National Center for Health Statistics. Analytic and reporting guidelines. The Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) Hyattsville, MD: 1996. Centers for Disease Control and Prevention. [Google Scholar]
- 45.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Third National Health and Nutrition Examination Survey, 1988–1994: NHANES III reference manuals and reports: Examination Data File Documentation. 1996
- 46.Masdottir B, Jonsson T, Manfredsdottir V, Vikingsson A, Brekkan A, Valdimarsson H. Smoking, rheumatoid factor isotypes and severity of rheumatoid arthritis. Rheumatol. 2000;39:1202–1205. doi: 10.1093/rheumatology/39.11.1202. [DOI] [PubMed] [Google Scholar]
- 47.Mattey DL, Dawes PT, Clarke S, Fisher J, Brownfield A, Thomson W, et al. Relationship among the HLA-DRB1 shared epitope, smoking, and rheumatoid factor production in rheumatoid arthritis. Arthritis Rheum. 2002;47:403–407. doi: 10.1002/art.10514. [DOI] [PubMed] [Google Scholar]
- 48.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 50.Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol (Hoboken, NJ) 2014;66:813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Heal. 2004;58:635–641. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162:22–38. doi: 10.1016/j.imlet.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.