Abstract
Racial/ethnic disparity in prostate cancer (PCa) is under studied in men with diabetes who are at higher risk for aggressive PCa. This study assessed the race/ethnic disparity in PCa incidence for men with type 2 diabetes (T2D), and whether the impact of metformin on PCa incidence varied by race/ethnicity. We conducted a retrospective study in 76,733 male veterans with T2D during 2003–2012. Cox proportional hazard model adjusting for covariates and propensity scores of metformin use and race/ethnic group membership were utilized to compute the hazard ratio (HR) of PCa incidence associated with race/ethnicity, and compare HR associated with metformin use between race/ethnic groups. Mean follow-up was 6.4±2.8 years; 7% were Hispanics; 17% were African American (AA); mean age was 67.8±9.8 years; 5.2% developed PCa; 38.9% used metformin. Among these diabetic men without metformin use, PCa incidence was higher in Hispanics and AA than in non-Hispanic White (NHW). Use of metformin alone or metformin+statins was associated with a greater PCa incidence reduction in Hispanics compared to NHW, but not between AA and NHW. Use of metformin+finasteride was associated with a greater PCa incidence reduction in Hispanics and AA compared to NHW. Our results suggested that metformin treatment could be a potential strategy to reduce PCa incidence in the minority populations who are at high risk for fatal PCa. It will be important to further examine the pleiotropic effects of metformin in multi-race/ethnic prospective studies to better inform clinical management, and potentially reduce racial/ethnic disparity in PCa incidence among diabetic men.
Introduction
Racial/ethnic disparity in prostate cancer (PCa) has been well established in the general US population. PCa incidence is greater among African-American (AA) yet lower among Hispanic men as compared to non-Hispanic white (NHW) men. (1) Whether diabetes status has an impact on the racial/ethnic disparity pattern observed in the general population is an important issue that is under studied. Although US men with diabetes are at an overall lower risk for PCa incidence than non-diabetic men, they are at a higher risk for aggressive PCa. (2,3) Given the continually rising epidemic of diabetes, aggressive PCa presents a significant public health concern especially among minority populations (e.g., AA and Hispanics) who are at high risk for diabetes. One large population-based study in men with diabetes reported an increased PCa incidence for both AA and Hispanics as compared to NHW, which is in contrast to reduced PCa incidence observed for Hispanic men when diabetes status is not considered. (4) Thus, racial/ethnic disparity in PCa incidence for men with diabetes warrants further investigation.
While a reduction in racial/ethnic disparity in PCa screening knowledge and improvement in quality of life for cancer survivors has been achieved through nonpharmacological interventions (such as informed decision-making or behavioral modification),(5) no pharmacological intervention has yet been identified that would reduce racial/ethnic disparity in PCa incidence or its mortality. Identifying PCa prevention medications that can alter disparity in PCa incidence and its related clinical outcomes is a research priority.
Metformin, the most commonly prescribed first-line glucose-lowering medication for patients with type 2 diabetes (T2D), has shown some promising yet variable results for PCa prevention. The beneficial impact of metformin on PCa reduction was found to vary by dose or concomitant use of statin or finasteride. (6–9) It is also likely that the variation of metformin’s PCa prevention effect could be attributed to individual pharmacokinetic differences. Among the polymorphisms in genes that are associated with metformin transport or glucose-lowering effects,(10–14) the allele frequency of one ethnic-specific MATE1 (multidrug and toxin compound extrusion-1) that is associated with reduced renal excretion of metformin,(11–13, 15) is strikingly high at 5% in Hispanics. Notably, the expression of metformin organic cation transporter 1 (OCT1) is more pronounced in obese subjects (16,17) who are at increased risk for metabolic syndrome and chronic inflammation. This implies that better metformin response could more likely be observed in populations with high prevalence of metabolic syndrome or chronic inflammation, such as Hispanics or AA. (18,19) In fact, Williams et al. observed a greater glycemic response to metformin in AA adults compared to NHW. (20) These data collectively suggest that metformin use could potentially modify race/ethnic disparity in PCa incidence. This hypothesis is yet to be tested in clinical studies.
The goal of this study is to assess (i) the race/ethnic disparity in PCa incidence among men with T2D who are non-users of metformin, and (ii) whether the impact of metformin on reducing PCa incidence is greater in the race/ethnic groups with a higher PCa incidence among men with T2D. To this end, we conducted a nationwide longitudinal cohort study (FY2003–FY2012) of insulin naïve male veterans with T2D to examine racial/ethnic disparity in PCa incidence and whether the impact of metformin on PCa incidence differed by race/ethnicity. To strengthen the causal interpretation of our finding, two inverse propensity score weighting techniques (21,22) were employed in our statistical analyses to minimize confounding due to baseline difference between metformin users and non-users, or difference in post-baseline clinical characteristics between race/ethnic groups.
Materials and Methods
Study Cohort
Our study cohort was derived from the electronic medical records (EMR) in the nationwide Veterans Administration Health Care System (VAHCS) databases. We began with 268,136 eligible beneficiaries who were 40–89 years of age in FY2003, and had a diagnosis of T2D (ICD-9 CM code: 250.00 or 250.02) but no cancer, cardiovascular diseases (CVD), or any glucose-lowering medication exposure during FY2001–FY2002. To obtain credible EMR for each patient, we limited the study cohort to patients with at least one visit to the general medicine, geriatric, or diabetes clinic each year. We further narrowed the study cohort to those who also met the following criteria during the study period: (i) having had prescription(s) of metformin as a glucose-lowering medication for ≥180 days or none; (ii) no prescription for insulin or any thiazolidinedione (TZD); (iii) no liver or renal diseases; and (iv) no missing covariates. We required criterion (ii) to eliminate potential effects associated with TZD or insulin use on cancer incidence as reported in the literature. (23,24) In addition, to account for the potential interactions between metformin with statin and finasteride,(6–9) we limited the cohort to 76,733 men who (i) have had prescription(s) of statins for ≥180 days or none; and (ii) have had prescription(s) of finasteride for ≥180 days or none. All study procedures were approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio and the Research and Development Committee of the South Texas Veterans Health Care System at San Antonio.
Data Sources
We used four linked VAHCS datasets for this study. VAHCS Inpatient and Outpatient Medical SAS Datasets were used to identify the cohort of men with T2D and their associated characteristics, including demographic variables and comorbidities. Additional clinical variables were extracted from the VA Decision Support System (medication prescription records, HbA1c, LDL, and prostate specific antigen (PSA) lab results and dates of measurements) and VAHCS Corporate Data Warehouse (height and weight values) (25).
Outcomes of Interest
The outcome of interest in this study is the incidence (rate) of the initial PCa diagnosis during the study period. The dependent variable used in our analyses is the time interval between the starting date and the initial PCa diagnosis observed during the study period. The starting date was October 1 2002 (the beginning of FY2003) for those without use of any glucose-lowering medication, the initiation of non-metformin glucose-lowering medication for users of non-metformin glucose-lowering medications, and the initiation of metformin for metformin users. A PCa event was defined as having an ICD-9 diagnosis of 185 during the study period. The study termination date for each patient was either the date of the initial PCa diagnosis, the date of death, or September 30, 2012 (the end of follow-up), whichever came first. Study subjects who did not have a PCa diagnosis during the study period were treated as censored.
Predictors and Measures
Medication Exposure
In the primary analysis, metformin use was defined as a minimum of 180 days of prescription at any dose, an exposure cut-point similar to that used in most clinical trials on metformin. (26) Non-users of metformin were patients who had no prescription for metformin during the study. Similarly, statin users consisted of patients who had any type of statin prescription at any dose for ≥180 days during the study, and non-users were those who had no statin prescription during the study. Finasteride users were patients who had any finasteride prescription for ≥180 days during the study period, and non-users were those who had no finasteride prescription during the study. These medication exposure variables are consistent with those in our prior study. (6,8)
In secondary analyses that assessed the impact of average daily dose of metformin use, patients with an average daily dose of ≥1000mg were compared with those with <1000 mg/day under ≥90, ≥120, or ≥180 days of prescription. That is, metformin users in the secondary analyses consisted of those in the primary analysis (all had prescription for ≥180 days) and the augmented users who had metformin prescription for 90–179 days.
Covariates
Covariates adjusted for in the analyses included age, race/ethnicity (Hispanic, AA, or NHW), and clinical characteristics of the patient: age-adjusted Charlson co-morbidity score,(27) and the mean change of body mass index (BMI), low-density lipoprotein (LDL) and hemoglobin A1c (HbA1c), and the maximum PSA level during the study period.
Statistical Analyses
Racial/Ethnic Disparity in PCa Incidence Among Non-users of Metformin
The Cox proportional hazard model was used to assess whether the covariate adjusted PCa incidence differed by race/ethnicity under no metformin use. Predictors in this Cox model included indicators of race/ethnicity groups (AA and Hispanics) with NHW being the referent, indicators of statin use (6,7,9) and finasteride use,(28) age, change in LDL, HbA1c, and BMI, and the maximum PSA level during the study period. Under this Cox regression model, the impact of race/ethnicity was assessed by the hazard ratio (HR) associated with AA or Hispanic group relative to the NHW group. The coefficient was assessed by the Wald test with p-value <0.05 being significant.
To enhance the causal interpretability of the racial/ethnic difference in PCa incidence, the inverse propensity scores (21,22) of race/ethnicity group membership were incorporated as the weights in the Cox regression model to minimize confounding due to imbalance in baseline covariates between race/ethnic groups. The propensity scores were calculated using logistic regression analysis, where the dependent variable was the indicator of being in a race/ethnic group and the independent variables included baseline age, HbA1c, PSA, BMI, LDL, and Charlson comorbidity score. Each propensity score was the likelihood of belonging to a race/ethnic group conditioned on each subject’s baseline characteristics.
Differential Metformin Impact on PCa Incidence Between Race/Ethnic Groups
To assess whether metformin could reduce the racial/ethnic difference in PCa incidence, we first conducted Cox regression analyses of the entire study cohort to compare the HR associated with metformin use between race/ethnic groups. Predictors in this Cox model included indicators of AA, Hispanics, metformin use, statin use, and finasteride use, two-way interactions of medication indicators (based on prior studies (6–9)), interactions between race/ethnicity with variables involving metformin use (i.e., indicator of metformin use, the product of the metformin use indicator and statin use indicator, and the product of metformin use indicator and finasteride use indicator), age, change in LDL, HbA1c, and BMI, and the maximum PSA level during the study period. To enhance the causal interpretability of metformin’s impacts, we also incorporated the propensity scores of metformin use as the inverse probability weights (IPW) in the Cox regression analyses. (21) Each propensity score was the likelihood of being treated with metformin for a patient calculated by the logistic regression analysis, where the dependent variable was the indicator of metformin use, and the independent variables included baseline HbA1c, age, and Charlson comorbidity score. Using these IPWs, individuals were weighted differently to achieve balance in baseline covariates between the metformin users and non-users, and therefore potential confounding due to imbalance in baseline characteristics was minimized. (21, 22) The interactions between race/ethnicity with metformin use were used to compare the HR associated with metformin use (empirically) between race/ethnic groups. Each model coefficient was assessed by the Wald test with p-value <0.05 being significant.
To gain further insight about the ‘potential biological’ variation of metformin impact between race/ethnic groups, we further employed the generalizability weighting technique (22) to predict whether the impact of metformin would have differed between race/ethnic groups should the post-baseline clinical characteristics be equalized between groups. The generalizability analysis involved two steps. First, we conducted stratified Cox proportional hazard model to assess the HR of PCa associated with metformin use for each race/ethnic group with covariates and propensity scores of metformin use being adjusted for as described above. Second, we derived the impact of metformin on PCa incidence for AA and Hispanics based on their respective likelihood functions such that the post-baseline clinical characteristics (HbA1c, BMI, LDL, and comorbidity) were calibrated between AA/Hispanics and NHW. The calibration was done by weighting the likelihood of the AA/Hispanics with the ratio of the proportion of the AA/Hispanic group in the cohort to the propensity score of being in the AA/Hispanic group conditioned on their clinical characteristics. (22) The impact of metformin derived from this generalizability analysis could be interpreted as the projected impact of metformin for AA or Hispanics should the clinical characteristics during the study period be calibrated between race/ethnic groups and the baseline covariates be balanced between metformin users and non-users.
The approach described above was modified for secondary analyses that examined the impact of the average metformin daily dose (≥1000mg vs. <1000mg) among metformin users. The modifications in the secondary analyses were: (i) the propensity scores of higher average metformin daily dose (≥1000mg per day) were incorporated as IPWs in the Cox regression analyses; (ii) the generalizability weights (to hypothetically equalize the post-baseline clinical characteristics between race/ethnic groups) were calculated only among metformin users; and (iii) three metformin exposure lengths, ≥90, ≥120, or ≥180 days of prescription, were analyzed separately.
All statistical analyses were conducted using SAS 9.1.
Results
In this study cohort of 76,733 men with T2D, the mean follow-up was 6.4±2.8 years, 59,906 (78.1%) were NHW, 12,593 (16.4%) were AA, 4,234 (5.5%) were Hispanics, mean age was 67.8±9.8 years, mean HbA1c was 6.5±1.0%, 3,983 (5.2%) had a PCa diagnosis, 19,805 (25.8%) used metformin for ≥180 days, 59,952 (78.1%) used statins, and 10,282 (13.4%) used finasteride. Detailed study cohort characteristics are shown in Table 1.
Table 1.
Cohort Characteristics
| Non-Users of Metformin | NHW | N=36776 | AA | N=7774 | Hispanic | N=2378 |
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Statin (%) | 74 | 70 | 73 | |||
| Finasteride (%) | 13 | 14 | 19 | |||
| PCa (%) | 6 | 9 | 7 | |||
| Follow up (days) | 2448.19 | 1085.44 | 2435.31 | 1091.94 | 2440.75 | 1053.11 |
| Age (years) | 70.17 | 9.48 | 65.14 | 11.02 | 68.75 | 10.02 |
| Charlson score | 4.18 | 2.80 | 4.54 | 3.06 | 4.28 | 3.01 |
| Baseline HbA1c (%) | 6.28 | 0.89 | 6.42 | 1.09 | 6.25 | 0.91 |
| HbA1c during study (%) | 6.34 | 0.81 | 6.32 | 0.89 | 6.32 | 0.78 |
| Baseline LDL (mg/dL) | 105.91 | 27.04 | 112.60 | 30.53 | 107.23 | 27.32 |
| LDL during study (mg/dL) | 90.02 | 24.27 | 95.44 | 28.05 | 89.03 | 23.49 |
| Baseline BMI (Kg/m2) | 30.57 | 5.47 | 31.41 | 5.66 | 28.89 | 4.97 |
| BMI during study (Kg/m2) | 29.57 | 5.50 | 30.40 | 5..84 | 27.89 | 5.13 |
| PSA during study (ng/mL) | 2.65 | 4.02 | 4.61 | 5.96 | 2.49 | 3.99 |
| Metformin User | NHW | N=23130 | AA | N=4819 | Hispanic | N=1856 |
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Statin (%) | 87 | 83 | 84 | 0.36 | ||
| Finasteride (%) | 13 | 12 | 17 | 0.37 | ||
| PCa (%) | 3 | 6 | 3 | 0.18 | ||
| Follow up (days) | 2167.48 | 868.22 | 2151.08 | 858.43 | 2102.30 | 829.90 |
| Age (years) | 64.42 | 9.31 | 59.02 | 9.50 | 63.08 | 9.25 |
| Charlson score | 3.49 | 2.39 | 3.42 | 2.48 | 3.25 | 2.36 |
| Baseline HbA1c (%) | 6.87 | 0.87 | 7.23 | 1.15 | 6.98 | 0.95 |
| HbA1c during study (%) | 6.94 | 0.84 | 7.16 | 1.06 | 7.04 | 0.92 |
| Baseline LDL (mg/dL) | 104.41 | 25.23 | 112.30 | 29.36 | 106.29 | 24.27 |
| LDL during study (mg/dL) | 84.87 | 22.89 | 92.64 | 32.55 | 86.87 | 22.59 |
| Baseline BMI (Kg/m2) | 31.82 | 5.50 | 31.45 | 5.70 | 30.11 | 4.83 |
| BMI during study (Kg/m2) | 30.69 | 5.62 | 30.53 | 5.83 | 29.03 | 5.01 |
| PSA during study (ng/mL) | 2.04 | 3.09 | 2.54 | 3.76 | 1.85 | 2.65 |
From Table 1, we observed imbalance in subjects’ characteristics at baseline between metformin users and non-users as well as between race/ethnic groups, and some of these variables (age, BMI, HbA1c, PSA and Charlson comorbidity score) were associated with PCa outcome. Thus in order to assess the impact of race/ethnicity or metformin use on PCa incidence it is necessary to balance potential confounders between the comparison groups, such as weighting subjects by the reciprocal (or inverse) of their propensity scores for race/ethnicity membership or metformin use.
Race/Ethnic Difference Under No Use of Metformin (see Table 2)
Table 2.
Covariate adjusted HR (95% CI) of PCa Incidence Associated Race/Ethnicity Among Non-Users of Metformin
|
|
||
|---|---|---|
| AA vs. NHW | Hispanic vs. NHW | |
| Without IPW adjustment | 1.88 (1.60–2.21) | 1.79 (1.37–2.33) |
| With IPW adjustment | 1.66 (1.50–1.83) | 2.11 (1.90–2.33) |
Under no use of metformin, the covariate adjusted Cox regression analysis without incorporating IPW (without balancing the covariates between race/ethnic groups) showed that HR associated with PCa for Hispanics vs. NHW was 1.79 (95% CI: 1.37–2.33, p<0.01), and HR for AA vs. NHW was1.88 (95% CI=(1.60,2.11), p<0.01). The increased PCa incidence in Hispanics and AA remained to be significant in the analysis that incorporated IPW: HR associated with PCa for Hispanics vs. NHW was 2.11 (95% CI=(1.90,2.33), p<0.01), and HR for AA vs. NHW was 1.66 (95% CI=(1.50,1.83), p<0.01). The reduction in the disparity between AA and NHW (from 1.88 to 1.66) associated with the IPW adjustment could be due to IPW calibration reducing the excessive PSA and LDL levels observed in AA relative to those in NHW. The increase in the disparity between Hispanics and NHW (from 1.79 to 2.11) associated with the IPW adjustment could be due to the IPW calibration putting more weight on individuals with older age and higher BMI in Hispanics to match those in NHW. The results above suggested that both AA and Hispanics were associated with an increased PCa incidence even if the difference in covariates between groups would have been eliminated.
Impact of Metformin
Table 3 contrasts the covariates-adjusted HR associated with metformin use (alone or in combination with statin or finasteride) among race/ethnic groups. We reported results under two scenarios: clinical variables (HbA1c, LDL, comorbidity, BMI) during the study period were i) calibrated between race/ethnic groups using generalizability weights or ii) without calibration.
Table 3.
HR (95% CI) of PCa Incidence associated with Metformin use by Race/Ethnicity
|
|
|||
|---|---|---|---|
| NHW | AA | Hispanics | |
|
|
|||
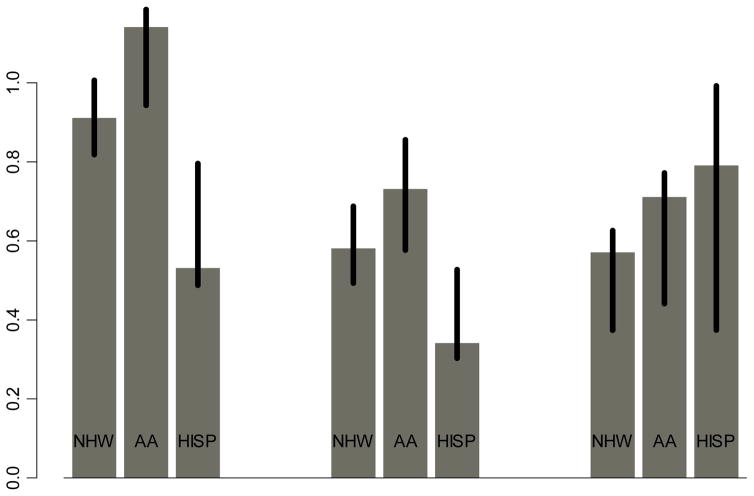
| Metformin | 0.91 (0.82–1.01) | 1.10 (0.94–1.27) | 0.63 (0.49–0.80) |
| 0.92§ (0.82–1.04) | 0.50*§ (0.47–0.52) | ||
| Metformin+Statin | 0.58 (0.49–0.69) | 0.70 (0.58–0.86) | 0.40 (0.30–0.53) |
| 0.59§ (0.48–0.72) | 0.32*§ (0.28–0.35) | ||
| Metformin+Finasteride | 0.48 (0.37–0.63) | 0.61 (0.44–0.77) | 0.58 (0.37–0.99) |
| 0.25*§ (0.16–0.39) | 0.25*§ (0.20–0.30) | ||
significantly different from NHW based on 95% CI of HR
generalizability weights were used to calibrate post-treatment clinical characteristics between comparison groups
Impact of Metformin Alone by Race/Ethnicity
In the analyses without adjusting for generalizability weights, metformin use alone was not associated with PCa incidence among NHW (HR=0.91, 95% CI=(.81,1.02), p=0.11) nor among AA (HR=1.10, 95% CI=(.94,1.27), p=0.23), while it was associated with a significant reduction in PCa incidence among Hispanics (HR=0.62, CI=(.49,.80), p<0.01). The testing of the interaction between metformin use alone and race/ethnicity indicators suggested that the impact of metformin alone on PCa incidence differed significantly by race/ethnicity: the HR associated with metformin alone was 31% less in Hispanics compared to NHW (p<0.01), and it was 21% greater in AA compared to NHW (p<0.01).
The differential impact of metformin use alone between NHW and Hispanics persisted even with the post-baseline clinical characteristics being calibrated between race/ethnic groups using IPW (see Table 3): metformin use alone was associated with a significant reduced PCa incidence in Hispanics (HR=0.50, p<.01) but not in NHW (HR=0.91, 95% CI=(0.82–1.01)) nor in AA (HR=0.92, 95%CI=(0.82–1.04)).
Metformin Dose Effect
Among metformin users who were not on statins or finasteride, for Hispanics, those who had an average daily dose of ≥1000 mg metformin (compared to <1000 mg) was associated with less PCa prevention benefit, while no differential dose effect was observed among AA. For AA, the IPW adjusted HR’s associated with an average daily dose of ≥1000 mg (vs. <1000 mg) were 1.34 (p=0.15), 0.91 (p=0.50), 1.06 (p=0.67) under ≥180, ≥120, and ≥90 days of prescription. In contrast, for Hispanics, the IPW adjusted HR’s associated with an average daily dose of ≥1000 mg were 1.35 (p=0.10), 1.75 (95% CI=(1.38,2.21), p<0.01), 1.98 (95% CI=(1.57,2.51), p<0.01) under ≥180, ≥120, and ≥90 days of prescription. For NHW, although metformin did not appear to have an overall impact on PCa incidence (see Table 3), there appeared to be some variation in dose effect among metformin users: the IPW adjusted HR’s associated with an average daily dose of ≥1000 mg (vs. <1000 mg) were 1.35 (95% CI=(1.13,1.61), p<0.01),1.52 (95% CI=(1.35,1.71), p<0.01), and 1.48 (95% CI=(1.31,1.66), p<0.01) under ≥180, ≥120, and ≥90 days of prescription.
In summary, metformin use alone could reduce the excess PCa incidence in Hispanics (compared to NHW) but not the excess PCa incidence in AA. However this beneficial effect of reducing ethic disparity in PCa could vary by daily dose of metformin use.
Impact of Combination Use of Metformin and Statins by Race/Ethnicity
In the analyses without adjusting for generalizability weights, the HR associated with combination use of metformin and statins relative to no use of either drug was 0.58 (95% CI=(0.49,0.69) p<0.01) for NHW, 0.70 (95% CI=(0.58,0.86, p<0.01) for AA, and 0.40 (95% CI=(0.30,0.53), p<0.01) for Hispanics. The beneficial impact of combination use of metformin and statin on reduced PCa incidence was greater in Hispanics compared to that in NHW (p<0.01), while it was less in AA compared to that in NHW (p<0.01). In the analyses adjusting for generalizability weights, the HR associated with combination use of metformin and statins relative to no use of either drug was 0.58 (95% CI=(0.49–0.69), p<0.01) for NHW, 0.59 (95% CI=(0.48–0.72), p<0.01) for AA, and 0.32 (95% CI=(0.28–0.35), p<0.01) for Hispanics. That is, the impact of combination use of metformin and statins differed significantly between NHW and Hispanics but not between NHW and AA. However, when (hypothetically) equalizing the clinical characteristics between the race/ethnic groups, the HR associated with the combination use of metformin and statins was similar between AA and NHW.
Metformin Dose Effect
The PCa prevention effect associated with the combination use of metformin and statins in NHW or AA did not differ by the average daily dose of metformin. For NHW who were on the combination of metformin and statins, the IPW adjusted HR’s associated with an average daily dose of ≥1000 mg (vs. <1000 mg) were 0.93 (p=0.86),1.03 (p=0.52), and 1.05 (p=0.65) under ≥180, ≥120, and ≥90 days of prescription of metformin; similarly, the corresponding IPW adjusted HR’s for AA were 1.02 (p=0.95), 0.75 (p=0.23), and 0.79 (p=0.36), respectively. In contrast, the PCa prevention effect associated with the combination use of metformin and statins for Hispanics was significantly reduced under a higher average daily dose of metformin use. The IPW adjusted HR’s associated with an average daily dose of ≥1000 mg among Hispanics were 1.92 (95% CI=1.00–3.66, p=0.05), 2.14 (95% CI=1.33–3.42, p<0.01), 2.22 (95% CI=1.36–3.63, p<0.01) under ≥180, ≥120, and ≥90 days of prescription.
In summary, the combination use of metformin and statins could reduce the disparity in PCa incidence between Hispanics and NHW. However this ethic disparity reduction effect could be attenuated by higher daily dose of metformin use.
Impact of Combination Use of Metformin and Finasteride by Race/Ethnicity
In the analyses without adjusting for generalizability weights, the HR associated with concurrent use of metformin and finasteride (compared to no use) was 0.48 (95% CI=0.37–0.63, p<0.01) for NHW, 0.58 (95% CI=0.44–0.77, p<0.01) for Hispanics, and 0.61 (95% CI=0.37–0.99, p=0.02) for AA. The impact of combination use of metformin and finasteride (compared to no use) on reduced PCa incidence did not differ significantly between NHW and Hispanics (p=0.27), while this impact was greater in NHW compared to that in AA (p<0.01).
In the analyses adjusting for generalizability weights, HR associated with the combination use of metformin and finasteride (compared to no use) was 0.48 (95% CI=0.37–0.63, p<0.01) for NHW, 0.25 (95% CI=0.16–0.39, p<0.01) for AA, and 0.25 (95% CI=0.20–0.30, p<0.01) for Hispanics.
Metformin Dose Effect
The PCa prevention effect associated with the combination use of metformin and finasteride did not vary by metformin daily dose in NHW nor Hispanics regardless of the length of metformin prescription. For NHW under the combination use of metformin and finasteride, the IPW adjusted HRs of PCa incidence associated with an average daily dose of ≥1000 mg metformin (vs. <1000 mg) were 1.11 (p=0.65), 1.43 (p=0.05), and 1.20 (p=0.32) under ≥180, ≥120, and ≥90 days of prescription, and the corresponding HRs for Hispanics were 1.78 (p=0.19), 1.07 (p=0.83), and 1.21 (p=0.60), respectively. In contrast, the effect of combination use of metformin and finasteride among AA varied by the average daily dose of metformin use under ≥90 or ≥120 days of metformin prescription, but not under longer period (≥180 days). The IPW adjusted HR’s associated with an average daily dose of ≥1000 mg (vs. <1000 mg) of metformin among AA were 1.77 (95% CI=0.82–3.80, p=0.14), 2.27 (95% CI=1.28–4.00, p<0.01), and 2.61 (95% CI=1.45–4.70, p<0.01) under ≥180, ≥120, and ≥90 days of prescription.
In summary, the combination use of metformin and finasteride could reduce the excess PCa incidence in both Hispanics and AA. This beneficial effect of reducing ethnic disparity in PCa did not vary by daily metformin dose. However, the racial disparity reduction effect could be attenuated by higher daily dose of metformin use.
Impact of Metformin on PCa Grade
We conducted further Cox regression analyses to examine the impact of metformin on high vs. low grade PCa among PCa patients. In this study of men with T2D, those with PCa and age similar to those in the PCPT, 50.4% had high grade cancer, while the PCPT, consisting of 8% diabetics, found 40% high grade cancer cases. The high grade PCa prevalence in our study is also consistent with Abdollah et al.’s study (2) where the prevalence of high grade PCa was 49% in patients with diabetes. Similar metformin effects were found in the high and low grade cases.
Covariates Associated with PCa Incidence by Race/Ethnicity
Covariates adjusted for in the Cox regression analyses and their associations with PCa incidence for each race/ethnic group are shown in Table 4. Older age was associated with increased PCa incidence, and the magnitude was similar between race/ethnic groups. Increased levels of PSA, BMI and LDL over time were also associated with increased PCa incidence for all race/ethnic groups. However, the magnitudes of these associations were stronger in Hispanics than those in non-Hispanics. Higher comorbidity score and decreased HbA1c level during the study period were associated with increased PCa incidence in NHW but not non-NHW.
Table 4.
HR of PCa Incidence Associated with Covariates by Race/Ethnicity
| NHW | AA | Hispanics | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | p-value | HR | p-value | HR | p-value | |
| Age | 1.026 | <.0001 | 1.026 | <.0001 | 1.021 | 0.0031 |
| Charlson comorbidity | 1.044 | <.0001 | 0.999 | 0.9564 | 0.995 | 0.8429 |
| Δ A1C | 0.942 | 0.0153 | 1.004 | 0.9115 | 0.957 | 0.6213 |
| Δ LDL | 1.003 | <.0001 | 1.002 | 0.0045 | 1.011 | 0.0006 |
| Δ BMI | 1.009 | <.0001 | 1.015 | <.0001 | 1.135 | 0.0009 |
| PSA | 1.002 | <.0001 | 1.003 | <.0001 | 1.048 | <.0001 |
Discussion
In this cohort of 76,733 male veterans with T2D who were free from cancer and CVD at baseline and remained insulin and TZD naïve during FY2003–FY2012, we found that under no use of metformin, both AA and Hispanics were associated with a higher PCa incidence than NHW men after adjusting for covariates (Table 2). Our finding of the higher PCa incidence in AA was consistent with the data in the general population, yet our observation of higher PCa incidence among Hispanic men contradicts the reported lower risk for Hispanic men in the general population. (1) However, our results are consistent with those reported by Waters et al. (4) The reason for the higher PCa risk in Hispanic men who are diabetic is not completely known. Based on prior studies in patients with T2D, one potential explanation for the impact of diabetes on PCa disparity between race/ethnic groups could be due to worse metabolic outcomes (e.g., higher HbA1c or cholesterol levels) among Hispanics or AA (compared to NHW),(18,19) that subsequently leads to chronic inflammation, hyperinsulinemia and dyslipidemia, and then increased the risk of PCa. In contrast, in our cohort, among non-users of metformin, glycemic control seemed similar between race/ethnic groups, and LDL was slightly higher in AA and Hispanics (see Table 1). Since no direct measures of chronic inflammation or hyperinsulinemia was available in our study, our IPW adjusted analyses implied that the racial/ethnic disparity could partly be due to chronic inflammation or hyperinsulinemia that was not mediated via glycemic control. The possibility of screening bias due to ethnic differences in PSA levels is not supported by our data as they were similar between NHW and Hispanic men.
Results from this study showing that a reduction in PCa incidence due to metformin use alone or in combination with statins was greater in Hispanics compared to NHW supports the hypothesis that metformin alone or in combination with statins could potentially reduce ethnic disparity in PCa incidence. Since the impact of statins did not differ by race/ethnicity, the superior impact of the combination use of metformin and statins in Hispanics could be driven by the superior impact of metformin use in Hispanics. In addition, the greater reduction in PCa incidence due to combination use of metformin and finasteride in Hispanics or AA (relative to NHW) suggests that metformin in combination with finasteride could reduce racial/ethnic disparity in PCa incidence. Like prior studies, (6–9) there appeared to be an add-on beneficial effect of finasteride or statins to metformin on reduced PCa incidence in this study (Figure 1) However, the heterogeneous impacts of metformin (dose and duration) seen in this study remains to be confirmed by prospective studies.
Figure 1.
HR Associated with Metformin Use for ≥180 days by Race/Ethnicity (Adjusting For Covariates, Propensity Score of Metformin Use But Without Generalizability Weights)
Our finding of a greater impact of metformin on PCa prevention in Hispanics or AA compared to NHW could be a reflection of the emerging evidence that approximately 30% of metformin response is heritable (29,30) Although drug response in these studies was measured in terms of metabolic measures such as HbA1c, preclinical studies have shown significant physiologic alterations in metformin transport that can affect its bioavailability in general and these genetic variants significantly differ in allele frequency by ethnic group. (10) Therefore pharmacogenomics of metformin response in terms of PCa prevention warrants further investigation as a plausible explanation for the differences observed.
Our study found that older age, increased PSA, BMI and LDL to be associated with increased PCa incidence across all race/ethnic groups (see Table 4),, suggesting that the management of PSA, BMI, and LDL could be crucial for PCa prevention in men with T2D. Since metformin could be associated with PSA, BMI, or LDL in some of the race/ethnic groups (Supplement Table), whether the greater PCa prevention benefit by metformin seen in AA and Hispanics with T2D was mediated through PSA, BMI or LDL remains to be seen. Increased HbA1c was associated with decreased PCa incidence only among NHW, which is consistent with the lower PCa prevalence in diabetics reported in the literature. (32) However it is not clear about the null association between HbA1c and PCa among non-NHW men with T2D. Comorbidity was associated with increased PCa incidence only among NHW but not non-NHW, which could be due to the larger sample size in NHW or the lower likelihood of aggressive tumors among NHW that intrinsically rendered comorbidities more prognostically important. (33)
The more favorable metformin impact in Hispanics vs. non-Hispanics found in this study is unlikely to be subject to PCa detection bias since we found no association between metformin use with PSA level or benign prostatic hyperplasia (BPH) diagnosis in any race/ethnic group. The null association between metformin use and PSA level was also reported in Randazzo et al.’s recent study (34) and the null association between metformin use and BPH diagnosis was seen in Murff et al.’s large retrospective VA study. (35)
There are limitations to this historical observational study. The duration of T2D and the starting dates of non-pharmacological treatments for T2D among patients who were non-users of glucose-lowering medications, two key variables for our analyses, were not available to us. However, all race/ethnic groups in our study cohort appeared to have similar T2D history to the extent that all patients were free from cancer and CVD at baseline, insulin naive, and with well glycemic control (with a slightly greater HbA1c level among the metformin users). Thus the interpretability of the impacts associated with race/ethnicity and metformin use derived from this study might depend on the similarity of the diabetes duration between the comparison groups and the accuracy of the starting date used in the analyses. In addition, due to the limited cancer grade data available in the VA cancer registry, our finding of metformin’s impact on PCa grade should be further validated. Lastly, to strengthen the causal interpretability of the result as in this non-randomized study, we employed inverse propensity score weighting methods to balance covariates between comparison groups. While these adjusted results could shed light on the biological explanation of variation in metformin response, further sensitivity analyses are necessary to assess the impact of unmeasured confounding on estimation bias. (36)
In conclusion, our study is the first to show that in men with T2D, metformin use could potentially reduce racial/ethnic disparity in PCa incidence. Given that metformin is currently a first-line choice to treat T2D, these results may appear inconsequential to clinical management. However, as with all anti-diabetic drugs, the use of metformin may gradually fade, especially as other glycemia-lowering drugs such as those in the incretin pathway come into favor. It will be important to thoroughly examine the potential pleiotropic effects of metformin in order to maximize the benefits given its well-defined safety profile and generic availability. It will also be important to confirm these results in clinical trials designed to examine ethnic-specific effects especially given the historical underrepresentation of the growing Hispanic population and the potential effect of ethnic-specific genetic variation on drug and/or therapeutic response. Likewise, investigating other new T2D treatments with respect to reducing race/ethnic disparity in PCa incidence will be beneficial for clinical management for diabetic and possibly pre-diabetic men.
Supplementary Material
Acknowledgments
Financial support. CP Wang, DM Lehman, J Hernandez receive NCI grant R21CA161180. IM Thompson receives NCI grant R21CA161180 and P30CA054174
Footnotes
Conflict of Interest. No conflict of interest for any author.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Abdollah F, Briganti A, Suardi N, Gallina A, Capitanio U, Salonia A, et al. Does diabetes mellitus increase the risk of high-grade prostate cancer in patients undergoing radical prostatectomy? Prostate Cancer Prostatic Dis. 2011;14:74–78. doi: 10.1038/pcan.2010.41. [DOI] [PubMed] [Google Scholar]
- 3.Mitin T, Chen M, Moran BJ, Dosoretz DE, Katin MJ, Braccioforte MH, et al. Diabetes mellitus, race, and the odds of high-grade prostate cancer in men diagnosed with prostate cancer in the United States. J Clin Oncol. 2011;29(suppl 7):abstr 180. doi: 10.1016/j.juro.2011.07.072. [DOI] [PubMed] [Google Scholar]
- 4.Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of Diabetes with Prostate Cancer Risk in the Multiethnic Cohort. Am J Epidemiol. 2009;169(8):937–945. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sajid S, Kotwal A, Dale W. Interventions to Improve Decision Making and Reduce Racial and Ethnic Disparities in the Management of Prostate Cancer: A Systematic Review. J Gen Intern Med. 2012;27(8):1068–1078. doi: 10.1007/s11606-012-2086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehman D, Lorenzo C, Hernandez J, Wang CP. Statin Use as a Moderator of Metformin Effect on Risk for Prostate Cancer Among Type 2 Diabetic Patients. Diabetes Care. 2012;35(5):1002–1007. doi: 10.2337/dc11-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70(6):2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 8.Wang CP, Hernandez J, Lorenzo C, Downs J, Thompson IM, Pollock BH, Lehman D. Statins and Finasteride Use Differentially Modifies the Impact of Metformin on Prostate Cancer Risk in Men with Type 2 Diabetes. Annals of Translational Medicine & Epidemiology. 2014;1(1):1004. [PMC free article] [PubMed] [Google Scholar]
- 9.Danzig MR, Kotamarti S, Ghandour RA, Rothberg MB, Dubow BP, Benson MC, et al. Synergism between metformin and statins in modifying the risk of biochemical recurrence following radical prostatectomy in men with diabetes. Prostate Cancer Prostatic Dis. 2014;18:63–68. doi: 10.1038/pcan.2014.47. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Teranishi K, Li S, Yee SW, Hesselson S, Stryke D, Johns SJ, Ferrin TE, Kwok P, Giacomini KM. Genetic Variants in Multidrug and Toxic Compound Extrusion 1, hMATE1, Alter Transport Function. Pharmacogenomics J. 2009;9(2):127–136. doi: 10.1038/tpj.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jablonski KA, McAteer JB, de Bakker PIW, Franks PW, Pollin TI, Hanson RL, et al. Common Variants in 40 Genes Assessed for Diabetes Incidence and Response to Metformin and Lifestyle Intervention in the Diabetes Prevention Program. Diabetes. 2010;59(10):2672–2681. doi: 10.2337/db10-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlyk AC, Giacomini KM, McKeon C, Shuldiner AR, Florez JC. Metformin pharmacogenomics: current status and future directions. Diabetes. 2014;63(8):2590–9. doi: 10.2337/db13-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58:745–749. doi: 10.2337/db08-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitman ML, Schadt EE. Pharmacogenetics of metformin response: a step in the path toward personalized medicine. Journal of Clinical Investigation. 2007;117(5):1226–1229. doi: 10.1172/JCI32133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2013;93:186. doi: 10.1038/clpt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang EH, Kim HK, Park CS, Kang JH. Increased expression of hepatic organic cation transporter 1 and hepatic distribution of metformin in high-fat diet-induced obese mice. Drug Metab Pharmacokinet. 2010;25:392–397. doi: 10.2133/dmpk.dmpk-10-nt-010. [DOI] [PubMed] [Google Scholar]
- 17.Moreno-Navarrete JM, Ortega FJ, Rodríguez-Hermosa JI, Sabater M, Pardo G, Ricart W, et al. OCT1 expression in adipocytes could contribute to increased metformin action in obese subjects. Diabetes. 2011;60:168–176. doi: 10.2337/db10-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanting LC, Joung IMA, Mackenbach JP, Lamberts SWJ, Bootsma AH. Ethnic Differences in Mortality, End-Stage Complications, and Quality of Care Among Diabetic Patients. Diabetes Care. 2005;28:2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Katzmarzyk PT, Horswell R, Zhao W, Li W, Johnson J, et al. Racial Disparities in the Control Status of Cardiovascular Risk Factors in an Underinsured Population with Type 2 Diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2014;31(10):1230–1236. doi: 10.1111/dme.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams LK, Padhukasahasram B, Ahmedani BK, Peterson EL, Wells KE, González Burchard E, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99(9):3160–3168. doi: 10.1210/jc.2014-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. The causal role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 22.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: the ACTG-320 trial. Am J Epidem. 2010;172:107–115. doi: 10.1093/aje/kwq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour M, Schwartz D, Judd R, Akingbemi B, Braden T, Morrison E, et al. Thiazolidinediones/PPARgamma agonists and fatty acid synthase inhibitors as an experimental combination therapy for prostate cancer. Int J Oncol. 2011;38(2):537–546. doi: 10.3892/ijo.2010.877. [DOI] [PubMed] [Google Scholar]
- 24.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25(12):1476–1481. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 25.http://www.virec.research.va.gov
- 26.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 28.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 29.Zhou K, Donnelly L, Yang J, Li M, Deshmukh H, Van Zuydam N, et al. Heritability of variation in glycaemic response to metformin: a genome-wide complex trait analysis. The Lancet Diabetes & Endocrinology. 2014;2(6):481–487. doi: 10.1016/S2213-8587(14)70050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd JN, Florez JC. An update on the pharmacogenomics of metformin: progress, problems and potential. Pharmacogenomics. 2014;15(4):529–539. doi: 10.2217/pgs.14.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol. 2013;31:3069–3075. doi: 10.1200/JCO.2012.46.7043. [DOI] [PubMed] [Google Scholar]
- 32.Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Diseases. 2013;16(2):151–158. doi: 10.1038/pcan.2012.40. [DOI] [PubMed] [Google Scholar]
- 33.Freeman VL, Durazo-Arvizu R, Keys LC, Johnson MP, Schafernak K, Patel VK. Racial Differences in Survival Among Men With Prostate Cancer and Comorbidity at Time of Diagnosis. American Journal of Public Health. 2004;94(5):803–808. doi: 10.2105/ajph.94.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randazzo M, Beatrice J, Huber A, Grobholz R, Manka L, Wyler SF, Chun FF, Recker F, Kwiatkowski M. Influence of metformin use on PSA values, free-to-total PSA, prostate cancer incidence and grade and overall survival in a prospective screening trial (ERSPC Aarau) World J Urol. 2015;33(8):1189–1196. doi: 10.1007/s00345-014-1426-y. [DOI] [PubMed] [Google Scholar]
- 35.Murff HJ, Roumie CL, Greevy RA, Grijalva CG, Hung AH, Liu X, Griffin MR. Thiazolidinedione and Metformin Use and the Risk of Benign Prostate Hyperplasia in Veterans with Diabetes Mellitus. Journal of Men’s Health. 2014;11(4):157–162. doi: 10.1089/jomh.2014.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan N, Meng XL, Lin JY, Chen CN, Alegria M. Disparities in Defining Disparities: Statistical Conceptual Frameworks. Statistics in Medicine. 2008;27(20):3941–3956. doi: 10.1002/sim.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.