Abstract
Objective
Breast cancer recurrence may be promoted by immunosuppression due to decreased immune surveillance. Among patients with immune-mediated disease and treated breast cancer, we examined rates of breast cancer recurrence with use of methotrexate, thiopurines, and anti-TNF therapy.
Methods
Three retrospective cohort studies within Medicare (2000-2012) included women with rheumatoid arthritis or inflammatory bowel disease who completed surgery for primary breast cancer. Recurrent or second primary breast cancers beyond 365 days from initial surgery were identified. Separate Cox regression models examined risk of cancer recurrence with use of methotrexate, thiopurines, and anti-TNF therapy after surgery, each compared to no use. Analyses were matched on type of breast surgery, and receipt and type of adjuvant therapy.
Results
Across all medication groups, 107 women developed breast cancer recurrence during 5,196 person-years. Incidence rates were 20.3 and 19.6 per 1,000 person-years in methotrexate users and nonusers, 32.3 and 17.6 in thiopurine users and nonusers, and 22.3 and 19.5 in anti-TNF users and nonusers, respectively. There was no significantly increased risk of breast cancer recurrence with use of methotrexate (adjusted hazard ratio [HR] 1.07, 95% CI 0.67-1.69), anti-TNF therapy (HR 1.13, 95% CI 0.65-1.97), or thiopurines (HR 2.10, 95% CI 0.62-7.14).
Conclusion
The risk of breast cancer recurrence with methotrexate, thiopurine, or anti-TNF therapy was not statistically significantly increased, although we cannot rule out a 2-fold or greater increased risk with thiopurines. These data provide reassurance to clinicians choosing to start methotrexate or anti-TNF therapy in RA or IBD patients with treated breast cancer.
Introduction
The incidence of rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) are increasing worldwide1,2. Treatment for these conditions is primarily with immunosuppression3,4 including thiopurines, methotrexate, anti-tumor necrosis factor (TNF) and other biologics. Some5,6,7-9 but not all studies10-12 have observed increased incidence of solid malignancies with these medications. Among patients with previous cancer, however, the risk of recurrent cancer after exposure to immunosuppressive therapy is even less clearly understood13,14. Prior studies in RA found no difference in cancer recurrence between the combination of anti-TNF therapy with methotrexate versus methotrexate alone15,16. Likewise, no association was observed between exposure to immunosuppressants and the risk of cancer recurrence in IBD17,18. However, these studies were small, did not distinguish recurrence of a prior malignancy from occurrence of a second malignancy, and combined many different cancers, thereby risking bias toward the null if the effect of immunosuppression is not universal across all solid cancers.
To address these limitations, we assessed the effect of immunosuppressive therapies on the risk of breast cancer recurrence after primary surgery for breast cancer among women with RA and IBD. Of the four most common solid cancers, there are several advantages to studying breast cancer. In contrast to colon cancer, screening results in earlier detection but is not preventative19; in contrast to prostate cancer, nearly all early-stage tumors receive treatment with intent to cure20; in contrast to lung cancer, there is a high survival rate overall21. Additionally, in patients with breast cancer treated with surgery, the presence of tumor-infiltrating lymphocytes (TILs) in breast tumor tissue is associated with a decreased risk of breast cancer recurrence and death22 which suggests that the immune system may be important in preventing recurrence.
Patients and Methods
Study design and population
We used data from Medicare (2000-2012) to conduct retrospective cohort studies among women with RA or IBD and a primary breast cancer treated with surgery. Medicare is a national health insurance program funded by the US government that covers more than 50 million elderly Americans (age 65 and above) and some individuals younger than 65 with disabilities (including RA or IBD). Medicare data were obtained from the Centers for Medicare and Medicaid Services (CMS)23.
Patients with primary breast cancer were identified using a validated algorithm with 99% specificity and 82% positive predictive value which combined a first breast cancer diagnosis with a related breast cancer surgery24. Patients with RA or IBD were identified using previously published methods25-28. Patients were included if they met the following criteria: 1) had a breast cancer diagnosis with related surgery (lumpectomy or mastectomy) code; 2) had a diagnosis of RA or IBD with a prescription for a disease modifying anti-rheumatic drug (DMARD) before or after the primary breast cancer surgery, but prior to the start of follow-up (described below in ‘Observation period’); and 3) had 6 months of continuous enrollment in Medicare parts A, B, and D preceding the first breast cancer diagnosis to avoid misclassification of prevalent breast cancers as incident.
Patients were excluded if they had a recurrent breast cancer event (i.e., recurrent breast cancer or a second primary breast cancer) prior to or within 365 days of the primary breast cancer surgery (when follow-up for the analysis began). Recurrent breast cancer was identified using a combination of diagnostic (secondary malignant neoplasm), surgical (resection of chest wall tumor), or therapy-related (use of selected chemotherapy, bone-modifying drugs, or palliative radiation) codes consistent with metastatic breast cancer, or codes consistent with a second primary breast cancer. Additionally, patients diagnosed with cancer other than breast or non-melanoma skin cancer in the 5 years prior to the breast cancer surgery were excluded. Finally, we excluded individuals with a gap in coverage between the first surgery and the start of follow-up (described below).
Primary outcome
The primary outcome was a recurrent breast cancer event occurring more than 365 days after the primary breast cancer surgery. A recurrent breast cancer event included distant recurrence of the original breast cancer or a second primary breast cancer, identified using a high specificity (97%) and high positive predictive value (83%) claims-based algorithm proposed by Chubak et al29.
Observation period
Follow-up started with the latest of 1) 365 days after the primary breast cancer surgery or 2) the first diagnosis of immune-mediated disease (RA or IBD) and the first treatment with a DMARD (Figure 1). Follow-up ended with the earliest of one of the following: 1) new initiation of the immunosuppressive drug of interest among non users at the start of follow-up, 2) a recurrent breast cancer event, 3) loss of enrollment, 4) death, or 5) December 31, 2012.
Figure 1.
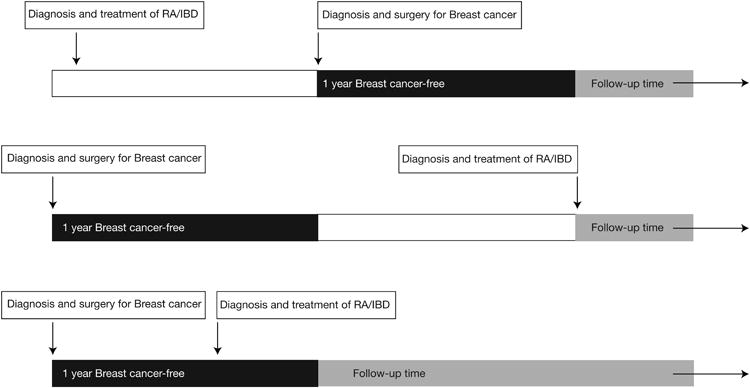
Examples of cohort entry and follow-up.
Cohort entry required a diagnosis of breast cancer with related surgery and a diagnosis and treatment of RA or IBD. Follow-up started with the latest of the following: date of the 1-year anniversary of the breast cancer surgery (top and bottom panels) or the date of first recorded diagnosis of RA or IBD and first treatment with a DMARD (middle panel).
Exposure definition
Medication exposures of interest included methotrexate, thiopurines (azathioprine or mercaptopurine), and the anti-TNF drugs (infliximab, adalimumab, certolizumab, golimumab, or etanercept). Patients were categorized as users versus nonusers at the start of follow-up. To be categorized as a user of a medication, a patient was required to have received at least one prescription prior to or on the date of the start of follow-up with an expected end date no later than 60 days prior to the start of follow-up. Nonusers included patients who had never used the medication and those who discontinued the medications at least 60 days prior to the start of follow-up.
Matching factors and potential confounders
Users of the medication of interest were matched to nonusers on risk factors for breast cancer recurrence at the start of follow-up, including surgery type (lumpectomy vs. mastectomy), and receipt and type of adjuvant therapy – post surgery radiotherapy and chemotherapy. These variables were measured within the 365 days after the first breast cancer surgery. In RA, methotrexate exposed patients were matched 1:1 to unexposed patients; in IBD, thiopurine exposed patients were matched 1:4 to unexposed patients; in RA and IBD, anti-TNF exposed patients were matched 1:4 to unexposed patients.
Potential confounders were measured on or before the start-up of follow up and included demographics such as age and race; inflammatory disease type (RA or IBD) and prior and concurrent use of immunosuppressive therapy (methotrexate, thiopurines, anti-TNF, or other biologic therapy [abatacept, rituximab, tocilizumab)]; use of non-steroid anti-inflammatory medications in the 90 days prior to start of follow-up; other comorbidities including history of chronic kidney disease, chronic liver disease, diabetes, coronary artery disease, or congestive heart failure; and breast cancer specific factors such as time from primary breast cancer surgery to follow-up start and receipt of post surgery endocrine and HER2 therapy.
Statistical analysis
Descriptive statistics compared characteristics between users and nonusers in each medication exposure groups. Incidence rates of breast cancer recurrence were computed. In the primary analysis, Cox regression models computed hazard ratios (HRs) for the association between breast cancer recurrence with use versus nonuse of methotrexate (in RA), thiopurines (in IBD), and anti-TNF therapy (in both), adjusted for potential confounders. Matched analyses were iteratively rerun 19 times; the iteration producing the median HR estimate was used as the primary model for confounder selection. This strategy avoids over- or under-estimation of the hazard ratio due to chance related to the selection of unexposed subjects for matching. Confounders were selected into the final multivariable model if inclusion modified HRs for the primary exposure by ≥10%30.
Subgroup and secondary analyses
The primary analysis was repeated among the subset with immune-mediated disease and documented exposure to immunosuppressive therapy in the Medicare files prior to the start of follow-up, thus comparing those who continued the medications versus those who discontinued it prior to the start of follow-up.
In secondary analyses, separate HRs were computed for each of the following secondary outcomes: a second primary breast cancer only, metastatic breast cancer only, and recurrent breast cancer using an alternate definition of recurrence. The latter included a prescription for a chemotherapy agent, identified with HCPCS, NDC, CPT, or ICD9 codes, used exclusively in metastatic disease as an additional method to identify patients with breast cancer recurrence.
Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Results where <11 patients were exposed to a therapy or experienced the outcome were not reported in adherence to the data use agreement with the Center for Medicare and Medicaid Services. The study protocol was approved by University of Alabama at Birmingham and University of Pennsylvania institutional review boards.
Results
Among 2,684 women with prior breast cancer and either RA or IBD, three matched cohorts were created including 892 users and 892 nonusers of methotrexate, 52 users and 208 nonusers of a thiopurine, and 291 users and 1164 nonusers of anti-TNF therapy (Table 1). The cohorts were not mutually exclusive. The median duration of follow-up for each matched pair of exposed and unexposed individuals ranged from 2.4 to 3.4 years. Overall, 85% of patients were aged 65 years or older. Within each medication exposure group, baseline demographics and comorbidities were generally similar between users and nonusers. Relative to nonusers, users of methotrexate, thiopurine, and anti-TNF were more likely to have used methotrexate (86% vs 64%), thiopurine (92% vs 7%), and anti-TNF therapy (97% vs 18%), respectively, prior to breast cancer surgery.
Table 1. Baseline characteristics of breast cancer and immune-mediated disease cohorts according to immunosuppressive therapy use.
| Characteristic | Group | Methotrexate | Thiopurine | Anti-TNF | |||
|---|---|---|---|---|---|---|---|
| User(n=892) | Nonuser(n=892) | User(n=52) | Nonuser(n=208) | User(n=291) | Nonuser(n=1164) | ||
| Age (y) | <65 y | 121 (13.6%) | 154 (17.3%) | <11a | 30 (14.4%) | 55 (18.9%) | 174 (14.9%) |
| 65 to <70 | 178 (20.0%) | 137 (15.4%) | 16 (30.8%) | 34 (16.3%) | 71 (24.4%) | 207 (17.8%) | |
| 70 to <75 | 206 (23.1%) | 229 (25.7%) | 11 (21.2%) | 55 (26.4%) | 71 (24.4%) | 285 (24.5%) | |
| 75 to <80 | 192 (21.5%) | 176 (19.7%) | 11 (21.2%) | 41 (19.7%) | 60 (20.6%) | 242 (20.8%) | |
| 80+ | 195 (21.9%) | 196 (22.0%) | <11 | 48 (23.1%) | 34 (11.7%) | 256 (22.0%) | |
| Raceb | White | 767 (86.0%) | 718 (80.5%) | 46 (88.5%) | 189 (90.9%) | 262 (90.0%) | 977 (83.9%) |
| Black | 89 (10.0%) | 124 (13.9%) | <11 | <11 | 16 (5.5%) | 132 (11.3%) | |
| Other | 36 (4.0%) | 50 (5.6%) | <11 | 11 (5.3%) | 13 (4.5%) | 55 (4.7%) | |
| Rheumatologic disease | RA | 892 (100.0%) | 892 (100.0%) | -- | -- | 273 (93.8%) | 1092 (93.8%) |
| IBD | -- | -- | 52 (100.0%) | 208 (100.0%) | 18 (6.2%) | 72 (6.2%) | |
| Methotrexate | Never | -- | 320 (35.9%) | 50 (96.2%) | 198 (95.2%) | 99 (34.0%) | 322 (27.7%) |
| New use at start of follow-up | 128 (14.3%) | -- | -- | -- | -- | -- | |
| Priorc | 23 (2.6%) | 569 (63.8%) | <11 | <11 | 64 (22.0%) | 387 (33.2%) | |
| Concurrentd | 741 (83.1%) | <11 | -- | -- | 128 (44.0%) | 455 (39.1%) | |
| Thiopurines | Never | 883 (99.0%) | 813 (91.1%) | -- | 193 (92.8%) | 269 (92.4%) | 1104 (94.8%) |
| New use at start of follow-up | -- | -- | <11 | -- | -- | -- | |
| Prior | <11 | 53 (5.9%) | <11 | 15 (7.2%) | 15 (5.2%) | 40 (3.4%) | |
| Concurrent | <11 | 26 (2.9%) | 48 (92.3%) | <11 | <11 | 20 (1.7%) | |
| Anti-TNF | Never | 664 (74.4%) | 595 (66.7%) | 43 (82.7%) | 189 (90.9%) | -- | 950 (81.6%) |
| New use at start of follow-up | -- | -- | -- | -- | <11 | -- | |
| Prior | 111 (12.4%) | 183 (20.5%) | <11 | 11 (5.3%) | <11 | 214 (18.4%) | |
| Concurrent | 117 (13.1%) | 114 (12.8%) | <11 | <11 | 280 (96.2%) | <11 | |
| Other biologicse | Never | 840 (94.2%) | 817 (91.6%) | 52 (100.0%) | 208 (100.0%) | 278 (95.5%) | 1078 (92.6%) |
| Prior | 32 (3.6%) | 41 (4.6%) | -- | -- | 12 (4.1%) | 41 (3.5%) | |
| Concurrent | 20 (2.2%) | 34 (3.8%) | -- | -- | <11 | 45 (3.9%) | |
| Breast cancer surgery | Bilateral mastectomy | 11 (1.2%) | 11 (1.2) | <11 | <11 | <11 | 20 (1.7%) |
| Mastectomy | 371 (41.6%) | 371 (32.7%) | 17 (32.7%) | 68 (32.7%) | 113 (38.8%) | 452 (38.8%) | |
| Lumpectomy | 510 (57.2%) | 510 (65.4%) | 34 (65.4%) | 136 (65.4%) | 173 (59.5%) | 692 (59.5%) | |
| Radiation therapyf | 420 (47.1%) | 420 (47.1%) | 31 (59.6%) | 124 (59.6%) | 147 (50.5%) | 588 (50.5%) | |
| Adjuvant chemotherapyf | 91 (10.2%) | 91 (10.2%) | <11 | 20 (9.6%) | 28 (9.6%) | 112 (9.6%) | |
| Hormonal therapyf | 518 (58.1%) | 446 (50.0%) | 32 (61.5%) | 136 (65.4%) | 164 (56.4%) | 645 (55.4%) | |
| HER2 therapyf | 26 (2.9%) | 23 (2.6%) | <11 | <11 | <11 | 34 (2.9%) | |
| Prior NSAIDsg | 295 (33.1%) | 227 (25.4%) | <11 | 31 (14.9%) | 90 (30.9%) | 320 (27.5%) | |
| Diabetes mellitus | 222 (24.9%) | 263 (39.5%) | <11 | 49 (23.6%) | 68 (23.4%) | 320 (27.5%) | |
| Chronic kidney disease | 43 (4.8%) | 72 (8.1%) | <11 | 23 (11.1%) | 16 (5.5%) | 75 (6.4%) | |
| Chronic liver disease | <11 | 40 (4.5%) | <11 | <11 | <11 | 31 (2.7%) | |
| Congestive heart failure | 98 (11.0%) | 146 (16.4%) | <11 | 24 (11.5%) | 35 (12.0%) | 158 (13.6%) | |
| Coronary artery disease | 188 (21.1%) | 278 (31.2%) | <11 | 44 (21.2%) | 61 (21.0%) | 305 (26.2%) | |
| Carotid artery disease | 21 (2.4%) | 18 (2.0%) | <11 | <11 | <11 | 29 (2.5%) | |
| Time from breast cancer surgery to follow-up start | 1 y | 752 (84.3%) | 834 (93.5%) | 48 (92.3%) | 186 (89.4%) | 283 (97.3%) | 1021 (87.7%) |
| 1 to 1.5 y | 35 (3.9%) | 12 (1.3%) | <11 | <11 | <11 | 38 (3.3%) | |
| 1.5 to 2 y | 28 (3.1%) | 18 (2.0%) | <11 | <11 | <11 | 28 (2.4%) | |
| 2+ y | 77 (8.6%) | 28 (3.1%) | <11 | <11 | <11 | 77 (6.6%) | |
| Follow-up time | Median (IQR) | 2.4 (1.6-3.1) | 2.5 (1.5-3.3) | 3.4 (1.7-4.8) | 3.2 (1.9-3.5) | 2.7 (1.7-3.7) | 2.5 (1.7-4.4) |
Abbreviations: y, years; RA, rheumatoid arthritis; IBD, inflammatory bowel disease; TNF, tumor necrosis factor, HER2, human epidermal growth factor receptor 2; NSAID, non-steroidal anti-inflammatory drug.
Results not reported where <11 subjects were exposed to a therapy
Other race includes Hispanics, Asian/Pacific Islander, Native American, unknown.
Use >90 days prior to the start of follow-up
Use in the 90 days prior to start of follow-up
Biologics assessed included abatacept, rituximab, tocilizumab
Within 365 days after breast surgery
Use in the 90 days prior to start of follow-up
In total, 107 women were diagnosed with recurrent breast cancer during 5,196 person-years (Table 2). The crude incidence rate of recurrent breast cancer was 20.3 and 19.6 per 1,000 person-years in methotrexate users and nonusers, 32.3 and 17.6 in thiopurine users and nonusers, and 22.3 and 19.5 in anti-TNF users and nonusers, respectively.
Table 2. Incidence rate and hazard ratios (HRs) for the association between methotrexate, thiopurines, and anti-TNF use and risk of breast cancer recurrence.
| Methotrexate | Thiopurines a | Anti-TNF | ||||
|---|---|---|---|---|---|---|
| User | Nonuser | User | Nonuser | User | Nonuser | |
| Cases of Recurrent breast cancer | 52 | 28 | <11 | <11 | 17 | 48 |
| Person-years of Follow-up | 2,557 | 1,425 | -- | -- | 764 | 2,466 |
| Crude incidence of Breast cancer recurrence (per 1000-py) | 20.3 (15.2-26.7) | 19.6 (13.1-28.4) | 32.3 (8.8-82.6) | 17.6 (7.6-34.6) | 22.3 (13.0-35.6) | 19.5 (14.4-25.8) |
| Adjusted (HR, 95%) | 1.07b (0.67-1.69) | Reference | 2.10c (0.62-7.14) | Reference | 1.13b (0.65-1.97) | Reference |
Person-years for thiopurine exposure are not shown to avoid calculation of absolute number of cases.
No covariates modified the HR by > 10%; covariates assessed included: age, race, calendar year, time from breast cancer surgery to start of follow-up, post surgery hormonal or HER2 therapy, use of non-steroid anti-inflammatory medications in the prior 90 days, prior or concurrent use of immunosuppressive therapy (methotrexate, thiopurines, anti-TNF, or other biologic therapy [abatacept, rituximab, tocilizumab], and histories of chronic kidney disease, chronic liver disease, diabetes mellitus, coronary artery disease, and congestive heart failure.
Adjusted for histories of coronary artery disease and congestive heart failure; no other covariates modified the HR by >10%
Adjusted hazard ratios for the association between recurrent breast cancer with each of the medication exposures are presented in Table 2. In the methotrexate analysis, there was no statistically significant association between use of methotrexate and the risk of breast cancer recurrence (HR 1.07, 95% CI 0.67-1.69). Similarly, in the anti-TNF analysis, use of anti-TNF therapy was not associated with breast cancer recurrence (HR 1.13, 95% CI 0.65-1.97). Repeating the anti-TNF analysis only among patients with RA (>90% of the cohort) yielded nearly identical results (HR 1.11, 95% CI 0.64-1.95). In the thiopurine analysis, use of thiopurines was associated with an elevated but not statistically significant risk of breast cancer recurrence (HR 2.10, 95% CI 0.62-7.14).
In a subgroup analysis, we repeated the primary analysis among the subset with immune-mediated disease and immunosuppressive therapy prior to breast cancer surgery (Table 3). Among prior users of methotrexate, there was no increased risk of breast cancer recurrence in methotrexate continuers (i.e., were prevalent users at the start of follow-up) relative to discontinuers (i.e., were not prevalent users at the start of follow-up) (HR 1.15, 95% CI 0.63-2.08). Similarly, among prior users of anti-TNF therapy, there was not a statistically significantly increased risk of breast cancer recurrence in anti-TNF continuers relative to discontinuers (HR 1.37, 95% CI 0.57-3.30). There were too few patients who had prior therapy with thiopurines to produce stable estimates of hazard ratios in this subgroup.
Table 3. Analysis of breast cancer recurrence in subgroup of methotrexate, thiopurine, or anti-TNF users prior to start of follow-up who continued or discontinued their therapy.
| Methotrexate | Thiopurinesa | Anti-TNF | ||||
|---|---|---|---|---|---|---|
| Continuerb | Discontinuer | Continuer | Discontinuer | Continuer | Discontinuer | |
| Cases of Recurrent breast cancer | 42 | 15 | <11 | 0 | 17 | <11 |
| Person-years of Follow-up | 1,858 | 726 | -- | -- | 725 | -- |
| Crude incidence of breast cancer recurrence (per 1000-py) | 22.6 (16.3-30.6) | 20.7 (11.6-34.1) | 34.6 (9.4-88.5) | -- | 23.5 (13.7-37.6) | 14.1 (5.7-29.1) |
| Adjusted (HR, 95%) | 1.15c (0.63-2.08) | Reference | -- | Reference | 1.37d (0.57-3.30) | Reference |
Person-years for thiopurine exposure are not shown to avoid calculation of absolute number of cases
Prevalent users of methotrexate, thiopurines, or an anti-TNF at the start of follow-up
No covariates modified the HR by > 10%; covariates assessed included: age, race, calendar year, time from breast cancer surgery to start of follow-up, post surgery hormonal or HER2 therapy, use of non-steroid anti-inflammatory medications in the prior 90 days, prior or concurrent use of immunosuppressive therapy (methotrexate, thiopurines, anti-TNF, or other biologic therapy [abatacept, rituximab, tocilizumab], and histories of chronic kidney disease, chronic liver disease, diabetes mellitus, coronary artery disease, and congestive heart failure.
Adjusted for prior or concurrent other biologic use (none, within 90 days, > 90 days prior to start of follow-up); no other covariates modified the HR by > 10%.
In secondary analyses, we repeated the primary analysis with the outcomes of metastatic disease only, a second breast cancer primary only, and an alternate definition of metastatic disease using chemotherapy agents only used for metastatic disease. These results are shown in Supplementary Table 1. Use of methotrexate, thiopurines, or anti-TNF therapy were not statistically significantly associated with any of the secondary outcomes (Supplementary Table 1). Of note, results from the analysis of a second primary breast cancer produced lower hazard ratios that were not statistically significant (ever use of methotrexate HR 0.82, 95% CI 0.38-1.75; ever use of thiopurines HR 0.88, 95% CI 0.10-7.86; ever use of anti-TNF HR 0.62, 95% CI 0.21-1.83). In contrast, the risk of metastatic disease among patients treated with thiopurines was nearly 4-fold higher than among nonusers, although this was not statistically significant (HR 3.87, 95% CI 0.97-15.51).
Discussion
The risk of cancer recurrence must be considered when selecting a treatment regimen for patients with active symptoms of RA or IBD and a history of cancer. For patients with a solid cancer within the preceding 5 years, the safety of starting or resuming biologic therapy is uncertain14. This issue is particularly relevant for patients with breast cancer, since it is common (over 230,000 new diagnoses in 2015) and has a high 5-year survival rate31. In these cohort studies of women with immune-mediated disease and prior breast cancer, we observed no statistically significant association between use of methotrexate, thiopurines or anti-TNF medications and breast cancer recurrence risk.
A few prior observational studies have addressed the question of solid cancer recurrence in DMARD users15-18. Similar to our study, these studies showed no increased risk in solid cancer recurrence with any immunosuppression17,18 and found no difference in risk of recurrence between anti-TNF treated and biologics-naïve patients15,16. However, these studies did not specifically investigate the recurrence risk of prior breast cancer. Rather, the prior studies combined all types of cancer, an approach that may bias results toward the null if the effect of immunosuppression on the risk of recurrence differs by tumor type, as has been observed in some studies32.
We studied breast cancer as this is a common cancer in women, has a high 5-year survival rate, but also has a high risk of recurrence after the first year, either as metastatic disease or a second primary cancer, and lymphocyte-mediated tumor surveillance is associated with decreased risk of metastatic disease. A prior small study by Raaschou et al. included 18 women with breast cancer recurrence33. Similar to our study, no increased risk was observed in those using anti-TNF therapy (HR 1.1, 95% CI 0.4-2.8) relative to biologics-naïve individuals.
Our study, with > 100 breast cancer recurrences and 5,196 person-years of follow-up, provides important reassurance to rheumatologists and gastroenterologists choosing to start immunosuppressive therapy in patients with RA or IBD and recently treated breast cancer. Our data, which suggest that anti-TNF users with prior breast cancer were not at higher risk of recurrence compared with nonusers, do not support current clinical practice which generally avoids anti-TNF therapy in this population14. Furthermore, there was no statistically significantly increased risk of breast cancer recurrence among the subset of patients with anti-TNF use prior to breast cancer surgery who continued anti-TNF therapy after surgery. Taken together, results from this study provide some of the strongest evidence to date to support new treatment guidelines recommending that RA patients with previously treated solid malignancies should not be treated differently than RA patients without this condition 34.
To our knowledge, this is the first study to specifically examine the risk of breast cancer recurrence with methotrexate and thiopurines. These drugs have historically been first line immunosuppressant agents for RA and IBD, respectively. However, both of these have been linked to increased risk of cancer. Thiopurines are associated with an increased risk of lymphoma, non-melanoma skin cancer and possibly other cancers35-37. Methotexate has been associated with an increased risk of recurrent non-melanoma skin cancer38. The data from the current study suggest that methotrexate does not increase the risk of a second breast cancer event. Although the association between thiopurines and a second breast cancer event was not statistically significant, the hazard ratio was greater than 2.0. Thus additional studies addressing the risk with thiopurines are needed.
The decision to start or resume immunosuppression in patients with RA or IBD and a prior breast cancer should take into consideration both the severity of the underlying immune disease, potential alternative therapies, and biological factors of the primary breast cancer. For example, some patients with IBD can be managed with non-immunosuppressive drugs such as mesalamine. Likewise, a major concern for patients with curable breast cancer and treating oncologists is whether treatment with immunosuppression regimens convert occult metastases or dormant cells to clinically apparent metastases or cause a local recurrence. This is particularly relevant for those with triple negative breast cancer (TNBC), which is more aggressive and, if it recurs, will do so within 5 years of initial diagnosis. Additionally, TILs in the stroma surrounding TNBC primary tumor predicts for an improvement in survival and decreased risk of recurrence22. Thus, there is theoretical concern that immunosuppressive medications may counteract the benefit of the immune response represented by TILs. Unfortunately, we were not able to perform subgroup analyses to examine TNBC in this study.
There were several strengths of this study. A matched cohort design allowed us to control for breast cancer specific factors known to influence the risk of breast cancer recurrence after surgery. Notably, follow-up time began on the 1 year anniversary of the primary surgery in > 90% of cohort members and median follow-up time was similar across exposure groups. A validated, claims-based algorithm with high specificity (97%) and positive predictive value (83%) was used to identify breast cancer recurrence29. In secondary analyses, the study also provided separate estimates for risks of a second primary and metastatic breast cancer. Furthermore, Medicare is a geographically diverse patient population and breast cancer incidence rates are highest among women aged older than 65, most of whom are eligible for Medicare21. Medicare also covers younger patients for reasons such as disability, which RA and IBD patients may qualify for on the basis of their condition. Notably, 15-20% of our cohort were younger than 65.
The study also has several potential limitations. As with all observational studies, there is the risk of unmeasured confounding. We were unable to measure RA or IBD disease activity. Higher disease activity almost certainly is associated with receipt of immunosuppressant therapy and perhaps could lead to an increased risk of cancer. As such, failure to adjust for disease activity would be expected to bias the association away from the null. Given that no association was observed with methotrexate or anti-TNF therapy, such bias would not be expected to change the conclusions of this study. Furthermore, an unmeasured confounder would need to be strongly associated with both treatment and the risk of a second breast cancer event to have biased a clinically meaningful association between anti-TNF or methotrexate to the results that we observed. There is also the potential for surveillance bias if users of immunosuppressive therapy were more frequently surveyed by clinicians than nonusers, but this would bias toward an elevated risks for breast cancer recurrence, which we did not observe.
Additionally, there was limited statistical power for analyses related to the thiopurines. For example, in the primary analysis, the HR for breast cancer recurrence with thiopurine ever use was 2.10, but confidence intervals were wide (95% CI 0.62-7.14). Thus, we cannot confidently exclude a meaningful increased risk of breast cancer recurrence among users of this class of medications. Likewise, even in our large cohort there was limited statistical power for subgroup analyses related to duration of immunosuppressive therapy. Results from this study cannot be generalized to women with active breast cancer undergoing treatment as we focused only on subjects with presumed cured breast cancer. Finally, the small number of users prevented us from studying rituximab, which has been recommended by some for patients with RA in this setting.
In summary, among women with immune-mediated disease and treated breast cancer, there was no statistically significant increase in the risk of breast cancer recurrence with use of methotrexate, thiopurine, or anti-TNF therapy, although we cannot rule out a 2-fold or greater increased risk with thiopurines. The data from our study may help rheumatologists and gastroenterologists to better assess the risk-benefit relationship when choosing between commonly used immunosuppressant therapies for patients with a history of cancer.
Supplementary Material
Acknowledgments
We thank Mrs. Stephanie I. Scott (Stephanie I. Scott Designs) for technical assistance with Figure 1.
Funding: This study was supported in part by the Agency for Healthcare Research & Quality (grants R01 HS018517 and U19 HS021110) and the NIH (grants K08-DK095951, K23-CA187185, and K24-DK078228). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Ms. Brensinger, Drs. Clark, Boursi, Chen, and Xie report no potential conflicts of interest.
Author Contributions: Drs. Curtis and Lewis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Mamtani, Clark, Scott, Lewis, Curtis. Acquisition, analysis, and interpretation of data: Mamtani, Clark, Scott, Lewis, Curtis, Brensinger. Drafting of the manuscript: Mamtani, Clark. Critical revision of the manuscript for important intellectual content: Mamtani, Clark, Scott, Boursi, Chen, Xie, Yun, Osterman, Brensinger, Lewis, Curtis. Statistical analysis: Brensinger, Chen, Lewis. Obtained funding: Lewis, Curtis, Scott, Mamtani. Administrative, technical, or material support: Brensinger, Lewis, Curtis. Study supervision: Lewis, Curtis.
Disclosures: Dr. Lewis has served as a consultant for Takeda, Amgen, Millennium Pharmaceuticals, Prometheus, Lilly, Shire, AstraZeneca, Janssen Pharmaceuticals, Merck, and AbbVie. He has served on a Data and Safety Monitoring Board for clinical trials sponsored by Pfizer. He has received research support from Bayer, Shire, Centocor, Nestle, and Takeda.
Dr. Curtis has served as a consultant for Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo and AbbVie. He has received research support from Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, and AbbVie.
Dr. Osterman has served as a consultant for Janssen, Abbott, and UCB. He has received research support from UCB.
Drs. Mamtani and Scott have served as consultants for Takeda.
Dr. Yun has received research support from Amgen.
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54 e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis and rheumatism. 2010 Jun;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis and rheumatism. 2000 Jan;43(1):22–29. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.D'Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008 Feb 23;371(9613):660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 5.Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012 Aug;143(2):390–399 e391. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchbinder R, Barber M, Heuzenroeder L, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis and rheumatism. 2008 Jun 15;59(6):794–799. doi: 10.1002/art.23716. [DOI] [PubMed] [Google Scholar]
- 7.Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflammatory bowel diseases. 2015 May;21(5):1089–1097. doi: 10.1097/MIB.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasternak B, Svanstrom H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. American journal of epidemiology. 2013 Jun 1;177(11):1296–1305. doi: 10.1093/aje/kws375. [DOI] [PubMed] [Google Scholar]
- 9.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. Jama. 2006 May 17;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 10.Askling J, van Vollenhoven RF, Granath F, et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: does the risk change with the time since start of treatment? Arthritis and rheumatism. 2009 Nov;60(11):3180–3189. doi: 10.1002/art.24941. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. Jama. 2012 Sep 5;308(9):898–908. doi: 10.1001/2012.jama.10857. [DOI] [PubMed] [Google Scholar]
- 12.Nyboe Andersen N, Pasternak B, Basit S, et al. Association between tumor necrosis factor-alpha antagonists and risk of cancer in patients with inflammatory bowel disease. Jama. 2014 Jun 18;311(23):2406–2413. doi: 10.1001/jama.2014.5613. [DOI] [PubMed] [Google Scholar]
- 13.Bernheim O, Colombel JF, Ullman TA, Laharie D, Beaugerie L, Itzkowitz SH. The management of immunosuppression in patients with inflammatory bowel disease and cancer. Gut. 2013 Nov;62(11):1523–1528. doi: 10.1136/gutjnl-2013-305300. [DOI] [PubMed] [Google Scholar]
- 14.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis care & research. 2012 May;64(5):625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon WG, Watson KD, Lunt M, et al. Influence of anti-tumor necrosis factor therapy on cancer incidence in patients with rheumatoid arthritis who have had a prior malignancy: results from the British Society for Rheumatology Biologics Register. Arthritis care & research. 2010 Jun;62(6):755–763. doi: 10.1002/acr.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strangfeld A, Hierse F, Rau R, et al. Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis research & therapy. 2010;12(1):R5. doi: 10.1186/ar2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaugerie L, Carrat F, Colombel JF, et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2014 Sep;63(9):1416–1423. doi: 10.1136/gutjnl-2013-305763. [DOI] [PubMed] [Google Scholar]
- 18.Axelrad J, Bernheim O, Colombel JF, et al. Risk of New or Recurrent Cancer in Patients With Inflammatory Bowel Disease and Previous Cancer Exposed to Immunosuppressive and Anti-Tumor Necrosis Factor Agents. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015 Aug 3; doi: 10.1016/j.cgh.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Harding C, Pompei F, Burmistrov D, Welch HG, Abebe R, Wilson R. Breast Cancer Screening, Incidence, and Mortality Across US Counties. JAMA internal medicine. 2015 Sep 1;175(9):1483–1489. doi: 10.1001/jamainternmed.2015.3043. [DOI] [PubMed] [Google Scholar]
- 20.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. The New England journal of medicine. 2012 Jul 19;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SEER Cancer Statistics Review, 1975-2012. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 22.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Sep 20;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennessy S FC, Cunningham F. U.S. government claims databases. In: Strom BL KS, Hennessy S, editors. Pharmacoepidemiology. 5th. Sussex: John Wiley and Sons; 2012. pp. 209–223. [Google Scholar]
- 24.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer causes & control : CCC. 2007 Jun;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 25.Herrinton LJ, Curtis JR, Chen L, et al. Study design for a comprehensive assessment of biologic safety using multiple healthcare data systems. Pharmacoepidemiology and drug safety. 2011 Nov;20(11):1199–1209. doi: 10.1002/pds.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor alpha inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis and rheumatism. 2013 Jan;65(1):48–58. doi: 10.1002/art.37740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. Jama. 2012 Jul 4;308(1):43–49. doi: 10.1001/jama.2012.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn's disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014 May;12(5):811–817 e813. doi: 10.1016/j.cgh.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chubak J, Yu O, Pocobelli G, et al. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. Journal of the National Cancer Institute. 2012 Jun 20;104(12):931–940. doi: 10.1093/jnci/djs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American journal of epidemiology. 1989 Jan;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 31.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015 Jan-Feb;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 32.Penn I. Effect of immunosuppression on preexisting cancers. Transplantation proceedings. 1993 Feb;25(1 Pt 2):1380–1382. [PubMed] [Google Scholar]
- 33.Raaschou P, Frisell T, Askling J for the ASG, for the ASG. TNF inhibitor therapy and risk of breast cancer recurrence in patients with rheumatoid arthritis: a nationwide cohort study. Annals of the rheumatic diseases. 2014 Aug 8; doi: 10.1136/annrheumdis-2014-205745. [DOI] [PubMed] [Google Scholar]
- 34.Singh JA, Saag KG, Bridges SL, Jr, et al. American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis care & research. 2015 doi: 10.1002/acr.22783. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015 May;13(5):847–858 e844. doi: 10.1016/j.cgh.2014.05.015. quiz e848-850. [DOI] [PubMed] [Google Scholar]
- 36.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011 Nov;141(5):1621–1628. e1621–1625. doi: 10.1053/j.gastro.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 37.Abbas AM, Almukhtar RM, Loftus EV, Jr, Lichtenstein GR, Khan N. Risk of melanoma and non-melanoma skin cancer in ulcerative colitis patients treated with thiopurines: a nationwide retrospective cohort. The American journal of gastroenterology. 2014 Nov;109(11):1781–1793. doi: 10.1038/ajg.2014.298. [DOI] [PubMed] [Google Scholar]
- 38.Scott FI MR, Bresinger CM, Haynes K, Chiesa-Fuxench ZC, Zhang J, Lang C, Fenglong X, Yun H, Osterman MT, Beukelman T, Margolis DJ, Curtis JR, Lewis JD. Risk of non-melanoma skin cancer in patients with a history of NMSC with the use of immunosuppressant and biologic agents in autoimmune disease. JAMA Dermatology (In Press) 2015 doi: 10.1001/jamadermatol.2015.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


