Abstract
Objective
To establish executive function (EF) structure/organization and test a longitudinal developmental cascade model linking processing speed (PS) and EF skills at 8-years of age to academic achievement outcomes, both at 8- and 16-years, in a large sample of children/adolescents with surgically-repaired dextro-transposition of the great arteries (d-TGA).
Method
Data for this study come from the 8-(n = 155) and 16-year (n = 139) time points of the Boston Circulatory Arrest Study and included WISC-III, Trail Making Test, Test of Variables of Attention, and WIAT/WIAT-II tasks.
Results
A 2-factor model (Working Memory/Inhibition and Shifting) provided the best fit for the EF data, χ2(3) = 1.581, p = .66, RMSEA = 0, CFI = 1, NNFI = 1.044). Working Memory/Inhibition and Shifting factors were not correlated. In the structural equation model, PS was directly related to both EF factors and Reading at 8 years, and was indirectly related to Math and Reading achievement, both concurrently and longitudinally, via its effects on Working Memory/Inhibition. Shifting at 8 years was significantly associated with Math (but not Reading) at 16 years.
Conclusions
The academic difficulties experienced by children and adolescents with d-TGA may be driven, at least in part, by underlying deficits in processing speed and aspects of executive function. Intervention efforts aimed at bolstering these abilities, particularly if implemented early in development, may prove beneficial in improving academic outcomes and, perhaps by extension, in reducing the stress and diminished self-confidence often associated with academic underachievement.
Keywords: congenital heart disease, transposition of the great arteries, working memory, inhibition, cognitive flexibility
It is estimated that approximately 2 million people in the United States are living with congenital heart disease (CHD), and nearly 40,000 children are born with some form of CHD each year (Marelli et al., 2012; Hoffman & Kaplan, 2002; Reller, Strickland, Riehle-Colarusso, Mahle, & Correa, 2008). Medical and surgical advances over the past few decades have drastically increased CHD survival rates and, in so doing, brought into sharper relief the longer-range needs of this growing population of survivors (Marino et al., 2012). In particular, neurobehavioral deficits and academic underachievement threaten the successful navigation of stage-salient developmental tasks for children and adolescents with CHD. Approximately 17–50% require special educational supports and services (Bellinger et al., 2011, 2014; Calderon et al., 2013; Riehle-Colarusso et al., 2015; Shillingford et al., 2008, van Rijen et al., 2003); and, as adults, CHD survivors ultimately attain lower levels of education and experience higher rates of unemployment than healthy controls (van Rijen et al., 2003; Crossland, Jackson, Lyall, Burn, & O’Sullivan, 2005).
Despite growing recognition of the long-term adverse impact of CHD on behavior and brain development, few large-scale investigations have followed children with CHD over time to elucidate the mechanisms(s) underlying this increased neurodevelopmental risk. The Boston Circulatory Arrest Study (BCAS) is a notable exception, having followed a large cohort of children with dextro-transposition of the great arteries (d-TGA) longitudinally from infancy through early and later childhood and, most recently, into adolescence (Newburger et al., 1993; Bellinger et al., 1995, 1999, 2003, 2011). As such, the BCAS offers an unparalleled view on how children with CHD develop. In the current study, we use data from the 8- and 16-year time points of the BCAS to test a longitudinal developmental cascade model linking executive function and processing speed to concurrent and future math and reading achievement outcomes in children with d-TGA.
Executive Functions: Structure, Development, and Associations with Academic Achievement
Executive functions (EF) include a range of skills that facilitate purposeful, goal-directed, and socially-competent behavior (Diamond, 2013). In adults, EFs are generally conceptualized as an interconnected yet distinguishable set of three core abilities—inhibitory control, working memory, and cognitive flexibility (shifting)—that, in turn, contribute to higher-order reasoning, planning, and problem-solving (Diamond, 2013). Such a configuration is not static throughout the lifespan, however, but rather seems to emerge over a protracted time course from a single-factor structure during the preschool/early childhood years (Clark & Woodward, 2014; Hughes, Ensor, Wilson, & Graham, 2010; Wiebe et al., 2008, 2011; Willoughby et al., 2010, 2012), to a two-factor structure among school-age children and early adolescents (Lee, Bull, & Ho, 2013; van der Sluis, de Jong, & van der Leij, 2007), ultimately culminating in a mature three-factor structure later in adolescence (Lee et al., 2013), at least in typically-developing children and children born prematurely (Rose, Feldman, & Jankowski, 2011). No studies to date have investigated latent EF factor structure and organization in children with CHD.
Inhibitory control refers to the ability to resist the urge to act in a way that would likely prove disadvantageous. Lack of inhibitory control makes one vulnerable to responding too quickly, propelled by basic stimulus-response tendencies, appetitive drives, and/or overlearned habits rather than reason. Inhibitory control begins to develop early in childhood with adult-level performance on laboratory inhibition tasks typically achieved in late childhood or early adolescence (Bédard, Nichols, Barbosa, Schachar, Logan, & Tannock, 2002; Williams, Ponesse, Schachar, Logan, & Tannock, 1999). Evidence of associations between inhibition and academic outcomes exists but is fairly equivocal: whereas some studies have documented links to literacy and math achievement (see Allan, Hume, Allan, Farrington, & Lonigan, 2014 for recent meta-analysis; Blair & Razza, 2007; Bull & Scerif, 2001; Clark, Sheffield, Chevalier, Nelson, Wiebe, & Espy, 2013; Fulton, Yeates, Taylor, Walz, & Wade, 2012; Passolunghi & Siegel, 2004; St Clair-Thompson & Gathercole, 2006), others have failed to find significant correlations between inhibitory control processes and academic performance (e.g., Kim, Nordling, Yoon, Boldt, & Kochanska, 2013).
Working memory refers to the ability to actively maintain perceptual information in mind and use that information to achieve a goal (Baddeley & Hitch, 1974; D’Esposito & Postle, 2015). Unlike more basic short term memory processes, which enable one to repeat a string of numbers or parrot a sentence, for example, working memory involves the effortful mental manipulation of previously-encountered verbal or visual-spatial stimuli (Alloway et al., 2004, 2006) and, thus, serves as the foundation for creating meaningful connections between temporally-distinct experiences. Infants as young as 9 to 12 months show signs of rudimentary working memory ability (e.g., Diamond, 1985) with significant increases in working memory sophistication and refinement observed throughout childhood and adolescence (Luciana & Nelson, 1998; Conklin, Luciana, Hooper, & Yarger, 2007). Substantial evidence implicates working memory as critical for the development of academic competencies in reading and math (Alloway et al., 2009; Bull & Lee, 2014; Bull & Scerif, 2001; Christopher et al., 2012; Clark et al., 2013; Gathercole, Alloway, Willis, & Adams, 2006; Geary, 2012; Li & Geary, 2013; Monette, Bigras, & Guay, 2011; Nevo & Breznitz, 2011; Rose et al., 2011; St Clair-Thompson & Gathercole, 2006; van der Sluis et al., 2007; Zheng et al., 2011).
Cognitive flexibility or shifting refers to the ability to switch fluidly between tasks, rule sets, or perspectives, and to adjust effectively to optimize outcomes in the face of changing demands (Diamond, 2013). Cognitive flexibility undergoes a notably protracted developmental course that extends well into the second decade of life (Cepeda, Kramer, & Gonzalez de Sather, 2001; Clark et al., 2013). Like inhibitory control, relations between cognitive flexibility and academic outcomes have been relatively under-investigated (Rose et al., 2011) with mixed findings reported across studies (Agostino, Johnson, & Pascual-Leone, 2010; Espy, McDiarmid, Cwik, Stalets, Hamby, & Senn, 2004; van der Sluis et al., 2007).
Congenital Heart Disease and Implications for EF
Congenital heart disease is an umbrella term that includes a range of conditions affecting the structural integrity of the heart and/or its connecting vessels. In a typically-developing human heart, deoxygenated blood is pumped from the right ventricle via the pulmonary artery to the lungs, where it is oxygenated. The four pulmonary veins then carry the oxygen-rich blood back to the heart where it enters at the left atrium, progresses on to the left ventricle, and is ultimately distributed via the aorta to the body and brain. In d-TGA, one of several types of critical CHD, the aorta and pulmonary artery are transposed. Consequently, deoxygenated blood is pumped to the body and brain while oxygenated blood circulates to and from the lungs. Most infants born with d-TGA require surgical intervention (the arterial switch operation) within the first few days of life. From a developmental perspective, children with d-TGA are a particularly interesting group to study because, unlike other forms of critical CHD such as tetralogy of Fallot or hypoplastic left heart syndrome, rates of identified genetic syndromes are very low in d-TGA. In addition, definitive surgical intervention most often takes place very early in the neonatal period, and subsequent cardiac surgery is rarely needed.
Some posit that prematurity/preterm birth may serve as a useful model for conceptualizing neurologic risk in CHD (e.g., Licht et al., 2009). Brain development is delayed by approximately 4 to 5 weeks in fetuses with CHD (Licht et al., 2009) with reduced brain volumes relative to controls documented as early as 25 to 30 weeks gestation (Limperopoulos et al., 2010; Clouchoux et al., 2013). Moreover, both prematurity and CHD are independently associated with an increased risk for white matter injury, including periventricular leukomalacia, ostensibly stemming from damage to oligodendrocyte precursors during periods of particular vulnerability during fetal development (Galli et al., 2004; Kinney, Panigrahy, Newburger, Jonas, & Sleeper, 2005; Rivkin et al., 2013; Volpe, 2009).
It remains to be shown whether prematurity and CHD carry similar risks for neuropsychological functioning, although this too has been suggested (Licht et al., 2009). Like prematurity, CHD threatens neurodevelopment across several domains. Children with d-TGA, in particular, despite possessing age-appropriate intellectual abilities (Bellinger et al., 1999, 2003; Karsdorp et al., 2007), are at risk for EF deficits in early (Calderon et al., 2010, 2012, 2013, 2014) and middle childhood (Bellinger et al., 2003), as well as later in adolescence (Bellinger et al., 2011), particularly in cognitive flexibility (shifting), problem-solving, and verbally-mediated EF skills (Cassidy, White, DeMaso, Newburger, & Bellinger, 2015).
Processing Speed
Processing speed describes the efficiency with which an individual is able to perceive and act upon a stimulus (Kail & Salthouse, 1994). Lifespan studies document a gradual increase in speed of processing over infancy, childhood, and adolescence (Rose et al., 2012b) with a peak in young adulthood and subsequent decline with aging (Kail, 1991), in accordance with patterns of white matter development and organization (Ferrer et al., 2013; Soria-Pastor et al., 2008). Several studies document links between processing speed and complex cognition. Ferrer et al. (2013), for example, reported that increases in white matter microstructure over the course of childhood and adolescence contribute to more efficient speed of processing, which in turn correlates with better fluid reasoning abilities (see also Kail & Salthouse, 1994; Fry & Hale, 1996; Kail, 2007). Processing speed also mediates improved EF skills in both typically-developing and preterm children (Rose et al., 2011; Mulder, Pitchford, & Marlow, 2011). Indeed, even measured as early as 7 to 36 months of age, processing speed is a stronger predictor of working memory and cognitive flexibility (but not inhibitory control) than infant/toddler attention and memory abilities (Rose et al., 2012a). To date, no studies have examined directional associations between processing speed and EF in children with CHD.
Developmental Cascades
Developmental science has, from its inception, sought to understand the processes underlying growth and change over time. Whereas some events or experiences exert a direct effect on a particular function or set of functions, others are thought to operate indirectly, impacting distal outcomes via their effects on more proximal ones like a chain of dominoes. The concept of developmental cascades describes this latter scenario wherein “…the cumulative consequences for development of the many interactions and transactions occurring in developing systems…result in spreading effects across levels, among domains at the same level, and across different systems or generations” (Masten & Cicchetti, 2010, p. 491). From a clinical management perspective, knowledge of developmental cascades can, at least in theory, serve as the basis for powerful prevention and intervention efforts aimed at disrupting probabilistically maladaptive trajectories and redirecting at-risk individuals onto more positive pathways (Masten et al., 2005).
Fry and Hale (1996) were among the first to apply a developmental cascade model to the study of cognition, showing that processing speed is indirectly associated with fluid reasoning via its effects on working memory. More recently, Rose and her colleagues (2011) extended the concept of developmental cascades to look at academic achievement in 11-year-old typically-developing and preterm children. Using structural equation modeling, they found that processing speed is related directly to working memory, shifting, and inhibitory control, and indirectly to math and reading achievement via working memory. These findings suggest that attempts to improve downstream academic outcomes in high-risk children may benefit from early identification and remediation of processing speed and working memory vulnerabilities.
Present Aims
In the present study, we sought to test Rose et al.’s (2011) developmental cascade model in a large cohort of children with d-TGA followed longitudinally since infancy. We had two aims: 1) to test competing models of EF structure and organization at 8 years of age, to determine empirically which configuration provided the best fit to the available data, and 2) to examine directional associations between processing speed/EF and academic achievement (reading and math) both concurrently (at 8 years of age) and longitudinally (at 16-years of age).
We were interested in whether EF structure and organization within our sample of children with d-TGA would be similar qualitatively to patterns observed in previous studies of typically-developing children. On the basis of prior research with healthy 8-year-old children, we hypothesized that a two-factor model (Working Memory/Inhibition and Shifting) of distinguishable yet correlated EF factors would fit the data best (Shing, Lindenberger, Diamond, Li, & Davidson, 2010). We further predicted that processing speed would be directly and positively related to both EF factors, and indirectly, through Working Memory/Inhibition, to concurrent and future academic achievement outcomes.
Method
Recruitment and Procedure
The BCAS has been well-described in several previous reports (Newburger et al., 1993; Bellinger et al., 1995, 1997; 1999, 2003, 2011). Briefly, recruitment took place between April 1988 and February 1992. Eligible children included those with diagnosed d-TGA (with or without intact ventricular septum) who underwent surgical repair by 3 months of age and whose cardiac anatomy was appropriate for the arterial switch operation (ASO). Exclusion criteria included: birth weight less than 2.5 kg, a recognized genetic syndrome, extracardiac anomaly of greater than minor severity, prior heart surgery, or cardiovasculature requiring reconstruction of the aortic arch or other open-heart surgical procedures. Once enrolled, infants were then randomly assigned to receive the ASO with a method of vital organ support of either predominant deep hypothermic circulatory arrest or predominant low-flow bypass. These children have since been monitored with extensive medical, imaging, psychiatric, and neuropsychological follow-up assessments at 1-, 4-, 8-, and 16-years post-ASO. For the neuropsychological segment at 8- and 16-year time points, participants were invited to the hospital for a single evaluation session lasting approximately 4 hours, with rest breaks provided as needed. Tasks were administered in a fixed order by a licensed psychologist (DCB) or supervised research assistant.
This study was approved by the Institutional Review Board of Boston Children’s Hospital and conducted in accordance with American Psychological Association and Helsinki Declaration guidelines. Informed consent was obtained from parents of study participants. Adolescents provided assent to participate in the study.
Participants
A total of 171 infants participated in the initial BCAS. Of these, 155 children returned for follow-up assessment at 1-year; 158 at 4-years; 155 at 8-years; and, 139 at 16-years post-surgery. Approximately 81% of the original sample was retained throughout the study duration. The current sample consisted of 155 participants at the 8-year time point and 139 participants at the 16-year time point. Descriptive data are presented in Table 1 (see also Bellinger et al., 2003, 2011). No significant differences in 1-year SES, gestational age, birth weight, sex, race, 1- and 5-minute Apgar scores, and age at first operation were found between participants who were (n = 155) and who were not (n = 16) retained from the original sample; the Wilcoxon rank-sum and Fisher’s exact tests were used to evaluate differences for continuous and categorical variables, respectively. The total number of cardiac operations could not be compared as this measure was only collected at the 16-year assessment.
Table 1.
Sample demographic and medical/surgical characteristics
| N | Mean | SD | % | |
|---|---|---|---|---|
| Family SES at 8 years | 155 | 45.03 | 13.42 | |
| Family SES at 16 years | 138 | 45.83 | 12.22 | |
| Gestational age (weeks) | 154 | 39.77 | 1.21 | |
| Birth weight (kg) | 155 | 3.53 | 0.44 | |
| Sex (male) | 155 | 76.1 | ||
| Race (white) | 155 | 88.4 | ||
| Age at 8-year assessment (years) | 155 | 8.17 | 0.25 | |
| Age at 16-year assessment (years) | 139 | 16.08 | 0.51 | |
| One-minute Apgar score | 140 | 7.51 | 1.33 | |
| Five-minute Apgar score | 139 | 8.32 | 0.88 | |
| Age at first operation (days) | 155 | 9.90 | 11.46 | |
| Total cardiac operations | 138 | 1 (1–4)a |
median (minimum-maximum) Note.
SES = socioeconomic status
Measures
Neuropsychological protocols included a battery of well-standardized, developmentally-appropriate tests and rating scales, selected to assess a range of functional domains. The present investigation used a sub-set of data from the BCAS 8- and 16-year follow-up evaluations to test specific hypotheses related to processing speed, EF, and academic achievement. Detailed descriptions of our primary outcome variables can be found in Bellinger et al. (2003, 2011).
Processing Speed
Processing speed was assessed at 8-years using the Coding and Symbol Search subtests (age-referenced scaled scores) from the Wechsler Intelligence Scale for Children-Third Edition (WISC-III; Wechsler, 1991).
Executive Function
Inhibitory Control was assessed at 8-years using the Commission Errors score from the Test of Variables of Attention (TOVA, 11.5 minute version; Greenberg & Waldman, 1993). Working Memory was assessed at 8-years using the Digit Span [Longest Digit Span Backward (DSB) raw score] and Arithmetic (age-referenced scaled score) subtests from the WISC-III (“Freedom from Distractibility Index;” Wechsler, 1991). Cognitive Flexibility/Shifting was assessed at 8-years using the Trail Making Test-Intermediate Version (difference score: Trails B - Trails A; Reitan & Davison, 1974).
Academic Achievement
Academic achievement was assessed at 8-years using the Basic Reading, Reading Comprehension, Numerical Operations, and Mathematics Reasoning subtests from the Wechsler Individual Achievement Test (WIAT; Psychological Corporation, 1992) and at 16-years with the Word Reading, Reading Comprehension, Numerical Operations, and Math Reasoning subtests from the WIAT-Second Edition (WIAT-II; Psychological Corporation, 2002). Age-referenced standard scores were included in analyses.
Data Analysis
Descriptive data analyses were performed using IBM SPSS Statistics Version 21. Data were inspected for normality and outliers. Ten participants (about 6%) with missing data were excluded from analysis. Confirmatory factor analysis (CFA) and structural equation modeling were conducted using Mplus Version 7.3 (Muthén & Muthén, 2012). Model fit was determined using a range of statistics including chi-square, root mean square error of approximation (RMSEA), comparative fit index (CFI), and non-normed fit index (NNFI) estimates. As recommended by Hu and Bentler (1999), a good model fit was defined as RMSEA < .06, CFI > .95, and NNFI > .95. A non-significant chi-square (p > .05) would also suggest good model fit; however, it is widely recognized that chi-square estimates are overly sensitive to sample size making it a less useful indicator of fit than other indices (Brown, 2006).
A series of CFAs was performed to establish latent EF factor structure and organization. One-, two-, and three-factor models were specified. Akaike Information Criterion (AIC) estimates were compared and chi-square difference tests were performed to determine the best-fitting model. The preferred model was then retained for use in the subsequent structural equation model (SEM). Latent Reading and Math constructs were specified using 8- and 16-year variables from the WIAT and WIAT-II, respectively. The SEM was specified to test direct and indirect associations among processing speed, EF, and academic achievement outcomes. Socioeconomic status at 8- and 16-years was included as a time-varying covariate.
Results
Descriptive statistics and Pearson correlation coefficients are presented in Table 2.
Table 2.
Correlation matrix and descriptive statistics
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Coding | - | |||||||||||||||
| 2. Symbol Search | 0.59 | - | ||||||||||||||
| 3. Commission Errors | 0.21 | 0.22 | - | |||||||||||||
| 4. DS Backward | 0.40 | 0.37 | 0.02 | - | ||||||||||||
| 5. Arithmetic | 0.49 | 0.44 | 0.12 | 0.47 | - | |||||||||||
| 6. TMT B – A | 0.29 | 0.36 | 0.01 | 0.26 | 0.34 | - | ||||||||||
| 7. Basic Reading 8 | 0.44 | 0.55 | 0.19 | 0.38 | 0.61 | 0.29 | - | |||||||||
| 8. Reading Comp 8 | 0.49 | 0.56 | 0.21 | 0.34 | 0.61 | 0.36 | 0.82 | - | ||||||||
| 9. Math Reasoning 8 | 0.48 | 0.58 | 0.21 | 0.45 | 0.71 | 0.46 | 0.70 | 0.75 | - | |||||||
| 10. Numerical Ops 8 | 0.50 | 0.56 | 0.19 | 0.45 | 0.66 | 0.38 | 0.72 | 0.72 | 0.84 | - | ||||||
| 11. Word Reading 16 | 0.38 | 0.44 | 0.01 | 0.34 | 0.51 | 0.26 | 0.68 | 0.72 | 0.53 | 0.54 | - | |||||
| 12. Reading Comp 16 | 0.39 | 0.44 | 0.13 | 0.32 | 0.60 | 0.30 | 0.57 | 0.69 | 0.62 | 0.56 | 0.71 | - | ||||
| 13. Numerical Ops 16 | 0.51 | 0.54 | 0.11 | 0.36 | 0.66 | 0.45 | 0.61 | 0.71 | 0.71 | 0.66 | 0.69 | 0.73 | - | |||
| 14. Math Reasoning 16 | 0.49 | 0.52 | 0.11 | 0.38 | 0.66 | 0.48 | 0.56 | 0.64 | 0.73 | 0.67 | 0.63 | 0.70 | 0.83 | - | ||
| 15. SES 8 | 0.21 | 0.35 | 0.23 | 0.16 | 0.25 | 0.19 | 0.45 | 0.48 | 0.45 | 0.40 | 0.50 | 0.52 | 0.46 | 0.44 | - | |
| 16. SES 16 | 0.14 | 0.25 | 0.27 | 0.01 | 0.15 | 0.22 | 0.30 | 0.34 | 0.36 | 0.28 | 0.36 | 0.39 | 0.32 | 0.33 | 0.83 | - |
| MEAN | 9.55 | 9.56 | 61.45 | 3.14 | 9.45 | 283.58 | 96.69 | 97.10 | 98.27 | 96.21 | 97.60 | 96.63 | 96.79 | 97.46 | 44.95 | 45.81 |
| SD | 3.39 | 2.98 | 15.59 | 0.89 | 3.24 | 42.85 | 14.80 | 14.53 | 14.72 | 15.94 | 15.33 | 18.33 | 19.58 | 17.62 | 13.41 | 12.18 |
| n | 154 | 154 | 141 | 153 | 154 | 151 | 154 | 154 | 154 | 154 | 139 | 139 | 139 | 139 | 156 | 139 |
Note. Commission Errors = TOVA Commission Errors; DS = Digit Span; TMT B-A = Trail Making Test Trial B minus Trial A; Reading Comp = Reading Comprehension; Numerical Ops = Numerical Operations; SES = socioeconomic status (Hollingshead, 1975); 8 = data from the 8-year time point; 16 = data from the 16-year time point.
Confirmatory Factor Analysis (CFA): Executive Function Structure and Organization
CFA results are presented in Table 3. Two models fit the data very well: the three-factor model and the two-factor model (collapsing Working Memory and Inhibition). Models collapsing Shifting with another EF factor did not fit well, nor did a one-factor model collapsing all three EF constructs into a single latent factor.
Table 3.
Fit indices for executive function CFA models
| Fit Indices |
Comparison with 3 factor model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Models | χ2 | df | p-value | RMSEA (95% CI) | CFI | NNFI | AIC | Δχ2 | Δdf | p-value |
| Three factors: Working Memory, Inhibition, Shifting | 1.09 | 1 | 0.2963 | 0.024 (0, 0.217) | 0.999 | 0.992 | 3973.381 | |||
| Two factor models: | ||||||||||
| Collapsing Working Memory & Inhibition | 1.58 | 3 | 0.6637 | 0 (0, 0.106) | 1.000 | 1.044 | 3971.519 | 0.4 9 | 2 | 0.7827 |
| Collapsing Working Memory & Shifting | 19.37 | 3 | 0.0002 | 0.188 (0.114, 0.272) | 0.747 | 0.493 | 3987.660 | 18.279 | 2 | 0.0001 |
| Collapsing Inhibition & Shifting | 24.25 | 3 | <0.0001 | 0.214 (0.141, 0.297) | 0.671 | 0.342 | 3992.540 | 23.159 | 2 | 0.0000 |
| One factor: collapsing all three EF factors | 25.95 | 4 | <0.0001 | 0.189 (0.124, 0.261) | 0.660 | 0.490 | 3992.241 | 24.86 | 3 | 0.0000 |
Note. RMSEA = Root Mean Square Error of Approximation, CFI = Comparative Fit Index, NNFI = Non-Normed Fit Index/Tucker Lewis Index, AIC = Akaike Information Criterion. Accepted model is in bold.
In the two-factor model (Working Memory/Inhibition and Shifting), Shifting was identified by a single manifest variable. For Working Memory/Inhibition, standardized factor loadings for the DSB and Arithmetic indicators were .64 and .74, respectively (ps < .001); the loading of TOVA Commission Errors was .13 (p = .224). In the three-factor model, two of three EF constructs (i.e., Inhibition and Shifting) were each identified by a single manifest indicator. For Working Memory, standardized factor loadings for the DSB and Arithmetic indicators were .64 and .73, respectively (ps < .001).
Considering the high degree of similarity between three-factor and two-factor (collapsing Working Memory and Inhibition) models, neither of which fit appreciably better or worse than the other, the more parsimonious two-factor model was retained for subsequent analysis.
Structural Equation Model: PS, EF, and Academic Achievement
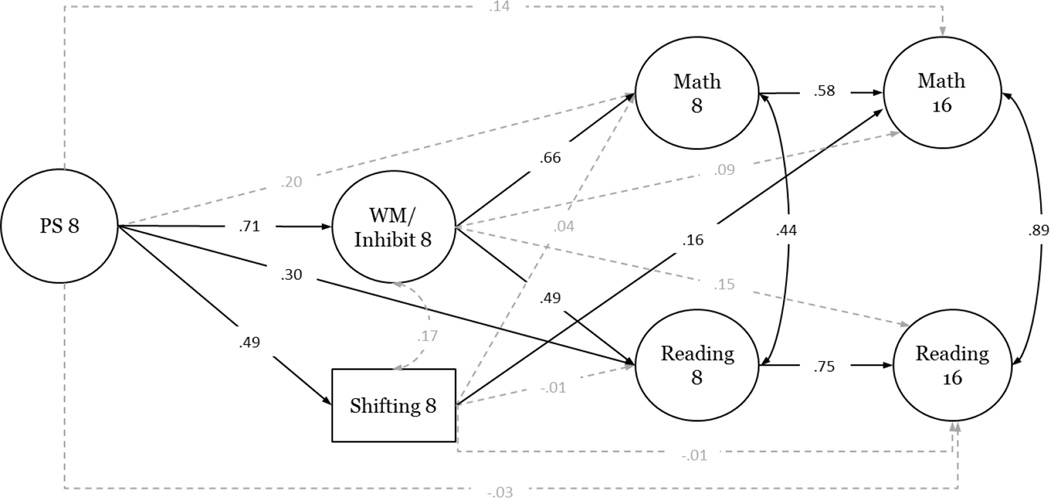
Structural equation modeling was used to test our hypothesis: processing speed → EF → academic achievement in math and reading. The proposed model demonstrated a good fit to the data, χ2(80) = 117.08, p < .001; RMSEA (90% CI) = .055 (.031 – .075), CFI = .978, NNFI = .967 (see Figure 1, Table 4). Processing Speed was directly related to both EF factors, as well as to Reading at 8-years. Processing speed was indirectly related to Reading and Math at 8 years via Working Memory/Inhibition (p = .002 and .003, respectively). Processing speed was also indirectly related to Math at 16 years via its effects on Shifting (p = .033). Intra-construct correlations between Reading and Math were significant at both time points, as were paths predicting 16-year Reading and Math from 8-year Reading and Math, respectively. Contrary to predictions, Working Memory/Inhibition and Shifting factors were not correlated.
Figure 1.
Longitudinal developmental cascade model linking processing speed, executive function, and academic achievement in children and adolescents with surgically-repaired dextro-transposition of the great arteries. Socioeconomic status at 8- and 16-years (not shown) was included as a time-varying covariate in this model. Values depicted are completely standardized path coefficients. Significant paths and bidirectional associations are represented by solid lines. Nonsignificant paths are represented by dashed lines. Factor loadings are omitted for ease of presentation. PS = Processing Speed; WM/Inhibit = Working Memory/Inhibition; 8 = data from the 8-year time point; 16 = data from the 16-year time point.
Table 4.
Standardized factor loadings and path coefficients for the accepted model
| Factor Loadings | Path Coefficients | ||||||
| Estimate | S.E. | P-Value | Estimate | S.E. | P-Value | ||
| PS 8 by | PS 8 on | ||||||
| Coding | 0.724 | 0.052 | <0.001 | SES 8 | 0.404 | 0.08 | <0.001 |
| Symbol Search | 0.82 | 0.048 | <0.001 | ||||
| WM/Inhibit 8 on | |||||||
| WM/Inhibit 8 by | SES 8 | 0.038 | 0.09 | 0.678 | |||
| DS Backward | 0.564 | 0.064 | <0.001 | PS 8 | 0.714 | 0.1 | <0.001 |
| Arithmetic | 0.868 | 0.055 | <0.001 | ||||
| Commission Errors | 0.18 | 0.094 | 0.055 | Shifting 8 | |||
| SES 8 | 0.036 | 0.084 | 0.665 | ||||
| Math 8 by | PS 8 | 0.491 | 0.087 | <0.001 | |||
| Math Reasoning 8 | 0.938 | 0.016 | <0.001 | ||||
| Numerical Ops 8 | 0.897 | 0.02 | <0.001 | Math 8 on | |||
| SES 8 | 0.191 | 0.058 | 0.001 | ||||
| Reading 8 by | PS 8 | 0.195 | 0.158 | 0.216 | |||
| Basic Reading 8 | 0.883 | 0.023 | <0.001 | WM/Inhibit 8 | 0.656 | 0.155 | <0.001 |
| Reading Comp 8 | 0.937 | 0.017 | <0.001 | Shifting 8 | 0.035 | 0.066 | 0.598 |
| Math 16 by | Reading 8 on | ||||||
| Math Reasoning 16 | 0.918 | 0.019 | <0.001 | SES 8 | 0.26 | 0.061 | <0.001 |
| Numerical Ops 16 | 0.916 | 0.019 | <0.001 | PS 8 | 0.299 | 0.14 | 0.033 |
| WM/Inhibit 8 | 0.489 | 0.136 | <0.001 | ||||
| Reading 16 by | Shifting 8 | −0.014 | 0.07 | 0.844 | |||
| Word Reading 16 | 0.839 | 0.032 | <0.001 | ||||
| Reading Comp 16 | 0.843 | 0.032 | <0.001 | SES 16 | |||
| SES 8 | 0.827 | 0.026 | <0.001 | ||||
| Math 16 on | |||||||
| SES 16 | 0.053 | 0.062 | 0.391 | ||||
| PS 8 | 0.139 | 0.128 | 0.28 | ||||
| WM/Inhibit 8 | 0.088 | 0.215 | 0.684 | ||||
| Shifting 8 | 0.155 | 0.069 | 0.024 | ||||
| Math 8 | 0.578 | 0.191 | 0.002 | ||||
| Reading 16 on | |||||||
| SES 16 | 0.138 | 0.065 | 0.034 | ||||
| PS 8 | −0.034 | 0.142 | 0.812 | ||||
| WM/Inhibit 8 | 0.146 | 0.171 | 0.393 | ||||
| Shifting 8 | −0.009 | 0.078 | 0.91 | ||||
| Reading 8 | 0.75 | 0.137 | <0.001 | ||||
| WM/Inhibit 8 with | |||||||
| Shifting 8 | 0.174 | 0.12 | 0.146 | ||||
| Math 8 with | |||||||
| Reading 8 | 0.439 | 0.139 | 0.002 | ||||
| Math 16 with | |||||||
| Reading 16 | 0.894 | 0.145 | <0.001 | ||||
Note. These estimates come from the final SEM model and therefore differ from those of the CFA. The left hand side of the table presents standardized loadings for the manifest variables within each factor. The keyword “by” indicates that the latent factor is observed by the indented manifest variables. The right hand side of the table presents the standardized path coefficients for the model. The keyword “on” indicates that there is a one-way directional arrow going into the “on” variable coming from the indented variable(s) below. The keyword “with” indicates that there is an association between the variables listed but no assumed directionality.
The structural equation model, as a whole, accounted for a large proportion of the variance in concurrent and future academic achievement. At 8-years, Processing Speed, EF, and socioeconomic status (SES) explained 74% of Reading and 84% of the variability in the Math outcomes. Processing Speed, EF, SES, and 8-year Reading/Math, in turn, explained 82% and 78% of the variability in the Reading and Math outcomes, respectively, at 16-years.
Discussion
This study had two primary aims: 1) to establish the factor structure and organization of three core EF components in a large cohort of children with critical congenital heart disease, and 2) to apply the resulting EF configuration to test a longitudinal developmental cascade model linking processing speed, EF, and academic achievement outcomes across childhood and into adolescence.
EF Structure and Organization
In our sample of 8-year olds with d-TGA repaired with the arterial switch operation, findings support a two-factor EF structure consisting of separable, uncorrelated Working Memory/Inhibition and Shifting factors. A three-factor model with distinct Working Memory, Inhibition, and Shifting factors also fit the data well but not better than the two-factor model collapsing Working Memory and Inhibition, and thus was rejected in favor of the more parsimonious two-factor structure.
These results are similar in some (but not all) respects to those from prior studies of EF development in healthy children; however, as with much prior EF research, conceptual ambiguity and differences in test batteries complicate direct comparisons. Shing and colleagues (2010), for example, reported that inhibitory control and working memory are not separable among children 4 to 9.5 years old, and only became distinguishable between 9.5 and 14.5 years of age. A similar differentiation of inhibitory control and working memory may be seen in children with d-TGA, as well, although appropriately-designed follow-up studies during later childhood and early adolescence would be necessary to test this hypothesis.
Alternatively, it is possible that EF development within the context of d-TGA may differ fundamentally from that of typically-developing children, in which case we might expect different organization patterns to obtain. In perhaps the best designed study of core EF skills development to date, Lee et al. (2013) also found that a two-factor model fit best among 8-year olds; however, in their study, Inhibition and Shifting factors were combined, while Working Memory (“Updating”) remained an independent, distinguishable factor that was correlated with the Inhibition/Shifting factor. A similar model (i.e., collapsing Inhibition and Shifting) did not fit well in our d-TGA sample, nor were our latent EF factors correlated significantly with each other. It seems, therefore, that prevailing models of EF development in typically-developing children may not adequately characterize the structure of EF in children with critical CHD, although this finding will need to be replicated in other CHD samples (including other d-TGA cohorts as well as groups of children with other cardiac lesions) and using comparable test batteries before strong conclusions may be drawn.
If prevailing models of EF are, in fact, less appropriate for children with CHD, what implications might atypical EF organization bear for real-world functional outcomes? Theoretically, if stronger correlations between EF abilities reflect a higher degree of network sophistication and neural synchrony (e.g., Uhlhaas, Roux, Rodriquez, Rotarska-Jaqiela, & Singer, 2010) among involved brain regions, then weaker or non-existent correlations may be a sign of relative network immaturity. In this way, a less coordinated repertoire of core EF components may result in less effective neural cost-sharing, as it were, making higher-order problem solving more effortful and laborious. This hypothesis, to our knowledge, has not been tested but would likely be of interest to clinicians working with medically complex children, particularly those presenting with concerns about “mental fatigue” that cannot be adequately explained by the outward physical manifestations of the medical condition itself.
Longitudinal Developmental Cascade Model
In our longitudinal developmental cascade model, Processing Speed was directly related to both EF factors and Reading at 8 years and was indirectly related to Math and Reading achievement, concurrently and at 16 years of age, via its effects on Working Memory/Inhibition. Shifting at 8 years was not significantly related to academic achievement at 8 years but directly predicted Math (but not Reading) at 16 years.
Findings provide further support for the critical importance of working memory to the acquisition and development of academic competencies. But why, among EF skills, might working memory be particularly important for learning? In many respects, working memory may be considered a foundational contributor to higher-order integration. Identifying commonalities across lessons, appreciating shared themes, linking new concepts with old—all of these inherently integrative tasks rely on the ability to maintain more than one piece of information in mind, which, in turn, affords one the opportunity to act upon that information in some purposeful way. Whether by visualizing the solution to a geometric proof, drawing contextual inferences from written materials, or simultaneously weighing the pros and cons of a multifaceted decision, working memory provides a mental stage for testing out ideas and getting a sense of how well they might work in the real world.
Framed in this way, it is not surprising that children with stronger working memories experience greater success in school. That the opposite tends to be true for children of more limited working memory capacities (e.g., Alloway et al., 2009) is also understandable, and in the case of children with CHD, raises the question of what may be done to mitigate this risk. One answer may be to intervene to increase working memory capacity, for example, using computerized working memory training programs (Calderon & Bellinger, 2015). Working memory training has not been attempted in children with CHD. Among other groups, while some studies have shown promising results (Holmes, Gathercole, & Dunning, 2009; Thorell, Lindqvist, Bergman Nutley, Bohlin, & Klingberg, 2009), others have found either no improvement or improvement but failure to generalize beyond working memory to critical academic or social outcomes (Dunning, Holmes, & Gathercole, 2013; Henry, Messer, & Nash, 2014; Melby-Lervag & Hulme, 2013; Shipstead, Redick, & Engell, 2012). On balance, the evidence would seem therefore to suggest both a need for further research to evaluate the efficacy and effectiveness of working memory training for children with CHD and an attitude of cautious skepticism and restraint among clinicians considering recommending these programs for children with CHD until more is known about how well they work.
Still another implication of this developmental cascade model is that there may, in fact, be multiple targets for intervention to promote later competence in children with CHD. Indeed, as Masten and her colleagues (2005) noted, “It is quite conceivable that the best way to prevent one kind of problem is to intervene earlier in another domain” (p. 743). For children with CHD, this other domain may be processing speed, or it may be something even earlier in development that either constrains or facilitates processing speed. Like computerized working memory training, and with many of the same caveats, there is some evidence to suggest that processing speed can be increased with training, presumably via repeated activation of underlying frontal-parietal systems (Mackey, Hill, Stone, & Bunge, 2011). That said, processing speed is also highly dependent on white matter integrity (e.g., Ferrer et al., 2013) which may be diminished in CHD (Rivkin et al., 2013; Rollins et al., 2014) and, thus, may be less amenable to remediation for children with CHD.
It is also possible that the experience of being an infant or young child with CHD carries with it a set of contextual risks that may, at least for some, undermine processing speed or EF or both. Clark and Woodward (2014), for example, showed that parents of children born very prematurely tend to engage in more intrusive and negative styles of parenting than those of full-term children. Moreover, parental intrusion and negativity—which the authors rightly discuss in terms of the fit between these typically harder-to-parent children and their respective caregivers—was then shown to undermine the development of executive control skills and increase risk for academic failure among preterm children. Parental overprotection—a construct related to some degree to intrusive parenting—is a concern among adolescents (Luyckx et al., 2011) and adults with CHD (Ong, Nolan, Irvine, & Kovacs, 2011), and during earlier childhood may contribute to restricted exploration of the environment (Bjarnason-Wehrens et al., 2007). How these and other early life experiences affect subsequent cognitive and self-regulatory development in children with CHD is not yet known but may provide important insights into targets for very early intervention, at the level of the family system, to maximize developmental potential.
Shifting ability in 8-year-old children with d-TGA accounted for very little variance in concurrent math skills but nonetheless was a significant predictor of how well those children would fare against more demanding high school mathematics at 16-years of age. Although this is certainly not the first investigation to highlight the importance of longitudinal study designs to uncover the longer-range, cross-domain implications of childhood cognitive status, it is, to our knowledge, the first to highlight this particular pathway between shifting and later math achievement among children with CHD. In so doing, this finding also reinforces the need for comprehensive neuropsychological assessment of children with CHD during childhood to identify areas of risk and provide individualized recommendations for management (Marino et al., 2012). More specifically, among children with CHD, deficits in shifting or cognitive flexibility, even in the absence of concurrent math failure, should be recognized to increase risk for future struggles in math. Educational supports and services may be appropriate under these circumstances, in addition to follow-up assessment, to promote effective progress in school.
Limitations
These findings should be interpreted in light of some limitations. First, as previously described, the data analyzed in this study were taken from the larger Boston Circulatory Arrest Study, which was not designed a priori to test the factor structure/organization of core EF skills or predictive associations between EF and academic achievement. Consequently, it was not possible to specify multi-indicator latent factors for Inhibition and Shifting. Moreover, in specifying our latent Working Memory factor with tasks that require the online maintenance and manipulation of numbers, it is possible that our model may be overestimating associations between working memory and mathematical abilities. Second, differences in EF factor conceptualization and test batteries across studies make it difficult to compare our findings with those from other groups. Third, because cardiac surgical techniques change over time, those techniques used in our sample of children/adolescents operated on more than 20 years ago are likely different than those used by many centers today. Finally, as a single-center study focusing on a particular form of severe CHD (i.e., d-TGA), it is possible that the findings obtained may not generalize to other settings or congenital heart lesions or operations.
Conclusion
Improving outcomes for individuals with congenital heart disease must begin with efforts to understand the processes and mechanisms underlying their increased risk for neurodevelopmental impairment. Findings from this study suggest that the academic difficulties experienced by children and adolescents with severe CHD may be driven, at least in part, by underlying deficits in processing speed and aspects of executive function. Intervention efforts aimed at bolstering these abilities, particularly if implemented early in development, may prove beneficial in improving academic outcomes and, perhaps by extension, in reducing the stress and diminished self-confidence often associated with academic underachievement.
Acknowledgments
This research was supported in part by grants HL41786, HL77681, and P30-HD18655 from the National Institutes of Health; the Farb Family Fund; and RR02172 from the National Center for Research Resources. The authors have no conflicts of interest to disclose. The authors wish to thank the children and families who participated in the Boston Circulatory Arrest Study.
Contributor Information
Adam R. Cassidy, Department of Psychiatry, Boston Children’s Hospital, Harvard Medical School
Matthew T. White, Department of Psychiatry, Boston Children’s Hospital, Harvard Medical School
David R. DeMaso, Department of Psychiatry, Boston Children’s Hospital, Harvard Medical School
Jane W. Newburger, Department of Cardiology, Boston Children’s Hospital, Harvard Medical School
David C. Bellinger, Departments of Psychiatry and Neurology, Boston Children’s Hospital, Harvard Medical School
References
- Agostino A, Johnson J, Pascual-Leone J. Executive functions underlying multiplicative reasoning: Problem type matters. Journal of Experimental Child Psychology. 2010;105:286–305. doi: 10.1016/j.jecp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Allan NP, Hume LE, Allan DM, Farrington AL, Lonigan CJ. Relations between inhibitory control and the development of academic skills in preschool and kindergarten: A meta-analysis. Developmental Psychology. 2014;50(10):2368–2379. doi: 10.1037/a0037493. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Willis C, Adams AM. A structural analysis of working memory and related cognitive skills in early childhood. Journal of Experimental Child Psychology. 2004;87:85–106. doi: 10.1016/j.jecp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Pickering SJ. Verbal and visual-spatial short-term and working memory in children: Are they separable? Child Development. 2006:1698–1716. doi: 10.1111/j.1467-8624.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Kirkwood H, Elliott J. The cognitive and behavioral characteristics of children with low working memory. Child Development. 2009;80(2):606–621. doi: 10.1111/j.1467-8624.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Bédard A-C, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Developmental Neuropsychology. 2002;21(1):93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, Strand RD. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. The New England Journal of Medicine. 1995;332(9):549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Rappaport LA, Wypij D, Wernovsky G, Newburger JW. Patterns of developmental dysfunction after surgery in infancy to correct transposition of the great arteries. Journal of Developmental and Behavioral Pediatrics. 1997;18:75–83. doi: 10.1097/00004703-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Wypij D, Kuban KCK, Rappaport LA, Hickey PR, Wernovsky G, Newburger JW. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. The Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Rivkin MJ, DeMaso D, Robertson RL, Stopp C, Dunbar-Masterson C, Wypij D, Newburger JW. Adolescents with tetralogy of Fallot: Neuropsychological assessment and structural brain imaging. Cardiology in the Young. 2014;11:1–10. doi: 10.1017/S1047951114000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Dunbar-Masterson C, Newburger JW. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason-Wehrens B, Dordel S, Schickendantz S, Krumm C, Borr D, Sreeram N, Brockmeier K. Motor development in children with congenital cardiac diseases compared to their healthy peers. Cardiology in the Young. 2007;17:487–498. doi: 10.1017/S1047951107001023. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false-belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Brown TA. Confirmatory factor analysis for applied research. New York: The Guilford Press; 2006. [Google Scholar]
- Bull R, Lee K. Executive functioning and mathematics achievement. Child Development Perspectives. 2014;8(1):36–41. [Google Scholar]
- Bull R, Scerif G. Executive functioning as a predictor of children’s mathematics ability: Inhibition, switching, and working memory. Developmental Neuropsychology. 2001;19(3):273–293. doi: 10.1207/S15326942DN1903_3. [DOI] [PubMed] [Google Scholar]
- Calderon J, Bellinger DC. Executive function deficits in congenital heart disease: Why is intervention important? Cardiology in the Young. 2015:1–9. doi: 10.1017/S1047951115001134. [DOI] [PubMed] [Google Scholar]
- Calderon J, Bonnet D, Courtin C, Concordet S, Plumet M-H, Angeard N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Developmental Medicine and Child Neurology. 2010;52(12):1139–1144. doi: 10.1111/j.1469-8749.2010.03735.x. [DOI] [PubMed] [Google Scholar]
- Calderon J, Angeard N, Moutier S, Plumet M-H, Jambaqué I, Bonnet D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. The Journal of Pediatrics. 2012;161(1):94.e1–98.e1. doi: 10.1016/j.jpeds.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Calderon J, Bonnet D, Pinabiaux C, Jambaque I, Angeard N. Use of early remedial services in children with transposition of the great arteries. Journal of Pediatrics. 2013;163(4):1105–1110. doi: 10.1016/j.jpeds.2013.04.065. [DOI] [PubMed] [Google Scholar]
- Calderon J, Jambaque I, Bonnet D, Angeard N. Executive functions development in 5- to 7-year-old children with transposition of the great arteries: A longitudinal study. Developmental Neuropsychology. 2014;39(5):365–384. doi: 10.1080/87565641.2014.916709. [DOI] [PubMed] [Google Scholar]
- Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive function in children and adolescents with critical cyanotic congenital heart disease. Journal of the International Neuropsychological Society. 2015;21:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JCM. Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37(5):715–730. [PubMed] [Google Scholar]
- Christopher ME, Miyake A, Keenan JM, Pennington B, DeFries JC, Wadsworth SJ, Olson RK. Predicting word reading and comprehension with executive function and speed measures across development: A latent variable analysis. Journal of Experimental Psychology General. 2012;141(3):470–488. doi: 10.1037/a0027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CA, Sheffield TD, Chevalier N, Nelson JM, Wiebe SA, Espy KA. Charting early trajectories of executive control with the shape school. Developmental Psychology. 2013;49(8):1481–1493. doi: 10.1037/a0030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Woodward LJ. Relation of perinatal risk and early parenting to executive control at the transition to school. Developmental Science. 2014:1–18. doi: 10.1111/desc.12232. [DOI] [PubMed] [Google Scholar]
- Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, Limperopoulos C. Delayed cortical development in fetuses with complex congenital heart disease. Cerebral Cortex. 2013;23(12):2932–2943. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31:103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Crossland DS, Jackson SP, Lyall R, Burn J, O’Sullivan JJ. Employment and advice regarding careers for adults with congenital heart disease. Cardiology in the Young. 2005;15:391–395. doi: 10.1017/S104795110500082X. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annual Review of Psychology. 2015;66:115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Development of the ability to use recall to guide actions, as indicated by infants’ performance on A-not-B. Child Development. 1985;56:868–883. [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning DL, Holmes J, Gathercole SE. Does working memory training lead to generalized improvements in children with low working memory? A randomized controlled trial. Developmental Science. 2013;16(6):915–925. doi: 10.1111/desc.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, McDiarmid MM, Cwik MF, Stalets MM, Hamby A, Senn TE. The contribution of executive functions to emergent mathematic skills in preschool children. Developmental Neuropsychology. 2004;26(1):465–486. doi: 10.1207/s15326942dn2601_6. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Whitaker KJ, Steele JS, Green CT, Wendelken C, Bunge SA. White matter maturation supports the development of reasoning ability through its influence on processing speed. Developmental Science. 2013;16(6):941–951. doi: 10.1111/desc.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JB, Yeates KO, Taylor HG, Walz NC, Wade SL. Cognitive predictors of academic achievement in young children 1 year after traumatic brain injury. Neuropsychology. 2012;26(3):314–322. doi: 10.1037/a0027973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AF, Hale S. Processing speed, working memory, and fluid intelligence: Evidence for a developmental cascade. Psychological Science. 1996;7(4):237–241. [Google Scholar]
- Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. Journal of Thoracic and Cardiovascular Surgery. 2004;127(3):692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Alloway TP, Willis C, Adams A. Working memory in children with reading disabilities. Journal of Experimental Child Psychology. 2006;93:265–281. doi: 10.1016/j.jecp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Geary DC. Cognitive predictors of achievement growth in mathematics: A five year longitudinal study. Developmental Psychology. 2012;47(6):1539–1552. doi: 10.1037/a0025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg LM, Waldman ID. Developmental normative data on the test of variables of attention (T. O. V. A.) Journal of Child Psychology and Psychiatry. 1993;34(6):1019–1030. doi: 10.1111/j.1469-7610.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Henry LA, Messer DJ, Nash G. Testing for near and far transfer effects with a short, face-to-face adaptive working memory training intervention in typical children. Infant and Child Development. 2014;23(1):84–103. [Google Scholar]
- Hoffman JL, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. Unpublished manuscript. New Haven, CT: Yale University; 1975. [Google Scholar]
- Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Developmental Science. 2009;12:F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Hughes C, Ensor R, Wilson A, Graham A. Tracking executive function across the transition to school: A latent variable approach. Developmental Neuropsychology. 2010;35(1):20–36. doi: 10.1080/87565640903325691. [DOI] [PubMed] [Google Scholar]
- Kail RV. Processing time declines exponentially during childhood and adolescence. Developmental Psychology. 1991;27:259–266. [Google Scholar]
- Kail RV. Longitudinal evidence that increases in processing speed and working memory enhance children’s reasoning. Psychological Science. 2007;18(4):312–313. doi: 10.1111/j.1467-9280.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychologica. 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Karsdorp PA, Everaerd W, Kindt M, Mulder BJM. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. Journal of Pediatric Psychology. 2007;32(5):527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- Kim S, Nordling JK, Yoon JE, Boldt LJ, Kochanska G. Effortful control in “hot” and “cool” tasks differentially predicts children’s behavior problems and academic performance. Journal of Abnormal Child Psychology. 2013;41(1):43–56. doi: 10.1007/s10802-012-9661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathologica. 2005;110:563–578. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- Lee K, Bull R, Ho RMH. Developmental changes in executive functioning. Child Development. 2013;84(6):1933–1953. doi: 10.1111/cdev.12096. [DOI] [PubMed] [Google Scholar]
- Li Y, Geary DC. Developmental gains in visuospatial memory predict gains in mathematics achievement. PLoS ONE. 2013;8(7):e70160. doi: 10.1371/journal.pone.0070160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2009;137(3):529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luyckx K, Goossens E, Missotten L, Moons P. Adolescents with congenital heart disease: The importance of perceived parenting for psychosocial and health outcomes. Journal of Developmental and Behavioral Pediatrics. 2011;32:651–659. doi: 10.1097/DBP.0b013e3182331e99. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Hill SS, Stone SI, Bunge SA. Differential effects of reasoning and speed training in children. Developmental Science. 2011;14(3):582–590. doi: 10.1111/j.1467-7687.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- Marelli A, Gilboa S, Devine O, Kucik J, Ionescu-Ittu R, Oster M, Jenkins K. Estimating the congenital heart disease population in the United States in 2010 - What are the numbers? Journal of the American College of Cardiology. 2012;59(13s1):E787. [Google Scholar]
- Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mahle WT. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- Masten AS, Roisman GI, Long JD, Burt KB, Obradovic J, Riley JR, Tellegen A. Developmental cascades: Linking academic achievement and externalizing and internalizing symptoms over 20 years. Developmental Psychology. 2005;41(5):733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M, Hulme C. Is working memory training effective? A meta-analytic review. Developmental Psychology. 2013;49(2):270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Monette S, Bigras M, Guay M-C. The role of executive functions in school achievement at the end of Grade 1. Journal of Experimental Child Psychology. 2011;113:353–371. doi: 10.1016/j.jecp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Marlow N. Processing speed mediates executive function difficulties in very preterm children in middle childhood. Journal of the International Neuropsychological Society. 2011;17:445–454. doi: 10.1017/S1355617711000373. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KCK, Ware JH. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. The New England Journal of Medicine. 1993;329(15):1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- Nevo E, Breznitz Z. Assessment of working memory components at 6 years of age as predictors of reading achievements a year later. Journal of Experimental Child Psychology. 2011;109:73–90. doi: 10.1016/j.jecp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Ong L, Nolan RP, Irvine J, Kovacs AH. Parental overprotection and heart-focused anxiety in adults with congenital heart disease. International Journal of Behavioral Medicine. 2011;18:260–267. doi: 10.1007/s12529-010-9112-y. [DOI] [PubMed] [Google Scholar]
- Passolunghi MC, Siegel LS. Working memory and access to numerical information in children with disabilities in mathematics. Journal of Experimental Child Psychology. 2004;88(4):348–367. doi: 10.1016/j.jecp.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler individual achievement test manual. San Antonio, TX: Harcourt, Brace, & Company; 1992. [Google Scholar]
- Psychological Corporation. Wechsler individual achievement test. 2nd. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- Reitan RM, Davison LA. Clinical neuropsychology: Current status and applications. New York: Hemisphere; 1974. [Google Scholar]
- Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in Atlanta, 1998–2005. The Journal of Pediatrics. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle-Colarusso T, Autry A, Razzaghi H, Boyle CA, Mahle WT, Van Naarden Braun K, Correa A. Congenital heart defects and receipt of special education services. Pediatrics. 2015;136(3):496–504. doi: 10.1542/peds.2015-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin MJ, Watson CG, Scoppettuolo La, Wypij D, Vajapeyam S, Bellinger DC, Newburger JW. Adolescents with D-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. The Journal of Thoracic and Cardiovascular Surgery. 2013;146(3):543–9e1. doi: 10.1016/j.jtcvs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins CK, Watson CG, Asaro LS, Wypij D, Vajapeyam S, Bellinger DC, Rivkin MJ. White matter microstructure and cognition in adolescents with congenital heart disease. The Journal of Pediatrics. 2014;165:936–944. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Modeling a cascade of effects: The role of speed and executive functioning in preterm/full-term differences in academic achievement. Developmental Science. 2011;14(5):1161–1175. doi: 10.1111/j.1467-7687.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Implications of infant cognition for executive functions at age 11. Psychological Science. 2012a;23(11):1345–1355. doi: 10.1177/0956797612444902. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ, Van Rossem R. Information processing from infancy to 11 years: Continuities and prediction of IQ. Intelligence. 2012b;40:445–457. doi: 10.1016/j.intell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121(4):e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- Shing YL, Lindenberger U, Diamond A, Li S-C, Davidson MC. Memory maintenance and inhibitory control differentiate from early childhood to adolescence. Developmental Neuropsychology. 2010;35(6):679–697. doi: 10.1080/87565641.2010.508546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, Engell RW. Is working memory training effective? Psychological Bulletin. 2012;138(4):628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Soria-Pastor S, Gimenez M, Narberhaus A, Falcon C, Botet N, Mercader JM, Junque C. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. International Journal of Developmental Neuroscience. 2008;26:647–654. doi: 10.1016/j.ijdevneu.2008.08.001. [DOI] [PubMed] [Google Scholar]
- St Clair-Thompson HL, Gathercole SE. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quarterly Journal of Experimental Psychology. 2006;59(4):745–759. doi: 10.1080/17470210500162854. [DOI] [PubMed] [Google Scholar]
- Thorell LB, Lindqvist S, Bergman Nutley S, Bohlin G, Klingberg T. Training and transfer effects of executive functions in preschool children. Developmental Science. 2009;12:106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriquez E, Rotarska-Jaqiela A, Singer W. Neural synchrony and the development of cortical networks. Trends in Cognitive Science. 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- van der Sluis S, de Jong PF, van der Leij A. Executive functioning in children, and its relations with reasoning, reading, and mathematics. Intelligence. 2007;35:427–449. [Google Scholar]
- van Rijen EHM, Utens EMWJ, Roos-Hesselink JW, Meijboom FJ, van Domburg RT, Roelandt JRTC, Verhulst FC. Psychosocial functioning of the adult with congenital heart disease: A 20–33 years follow-up. European Heart Journal. 2003;24:673–683. doi: 10.1016/s0195-668x(02)00749-2. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurology. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, Charak D. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Developmental Psychology. 2008;44(2):575–587. doi: 10.1037/0012-1649.44.2.575. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Sheffield T, Nelson JM, Clark CAC, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. Journal of Experimental Child Psychology. 2011;108:436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Blair CB, Wirth RJ, Greenberg M. The measurement of executive function at age 3 years: Psychometric properties and criterion validity of a new battery of tests. Psychological Assessment. 2010;22:306–317. doi: 10.1037/a0018708. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Blair CB, Wirth RJ, Greenberg M. The measurement of executive function at age 5 years: Psychometric properties and relationship to academic achievement. Psychological Assessment. 2012;24:226–239. doi: 10.1037/a0025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd. New York: Psychological Corporation; 1991. [Google Scholar]
- Zheng X, Swanson HL, Marcoulides GA. Working memory components as predictors of children’s mathematical word problem solving. Journal of Experimental Child Psychology. 2011;110:481–498. doi: 10.1016/j.jecp.2011.06.001. [DOI] [PubMed] [Google Scholar]