Abstract
Objective
Scleroderma patients with autoantibodies to centromere proteins (CENPs) and/or interferon-inducible protein 16 (IFI16) are at increased risk of severe vascular complications. We set out to define whether these autoantigens are enriched in cells of the vasculature.
Methods
Successive stages of embryoid bodies (EBs) as well as vascular progenitors were used to evaluate the expression of scleroderma autoantigens IFI16 and CENP by immunoblotting. CD31 was included to mark early blood vessels. IFI16 and CD31 expression were defined in skin paraffin sections from scleroderma patients and from healthy controls. IFI16 expression was determined by flow cytometry in circulating endothelial cells (CECs) and circulating progenitor cells (CPCs).
Results
Expression of CENP-A, IFI16 and CD31 was enriched in EBs at days 10 and 12 of differentiation, and particularly in cultures enriched in vascular progenitors (IFI16, CD31, CENPs A and-B). This pattern was distinct from that of comparator autoantigens. Immunohistochemical staining of skin paraffin sections showed enrichment of IFI16 in CD31-positive vascular endothelial cells in biopsies from scleroderma patients and normal controls. Flow cytometry analysis revealed IFI16 expression in CPCs, but minimal expression in CECs.
Conclusion
Expression of scleroderma autoantigens IFI16 and CENPs, which are associated with severe vascular disease, is increased in vascular progenitors and mature endothelial cells. High level, lineage-enriched expression of autoantigens may explain the striking association between clinical phenotypes and the immune targeting of specific autoantigens.
Introduction
Scleroderma is a systemic disease characterized by pathologic features of autoimmunity, non-inflammatory vasculopathy, and tissue fibrosis (1). Most scleroderma patients produce scleroderma-specific autoantibodies that are associated with distinct clinical phenotypes. For example, anti-topoisomerase-1 antibodies are associated with interstitial lung disease (ILD) and digital ulcers (2-5). In contrast, anti-centromere (CENP) and anti-interferon-inducible protein 16 (IFI16) antibodies are frequently found in scleroderma patients with severe vascular complications, with digital loss significantly more common in patients with anti-CENP autoantibodies (6, 7), and digital gangrene significantly more common in the anti-IFI16 antibody positive group (8).
Anti-centromere antibodies were first discovered in patients with the CREST variant of scleroderma (calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, and telangectasias) using human epithelial cell line 2 (HEp-2) cell substrates (9). These patients have limited skin disease, a better overall survival, increased cutaneous telangiectasia, and less ILD; however this group is at a significantly increased risk of ischemic digital loss when compared to scleroderma patients with other serotypes (6). Anti-centromere antibodies target mainly CENP-A, CENP-B, and CENP-C, which are the major components of the active centromere. Studies demonstrate a high concordance in clinical phenotypes of patients with anti-CENP-A and anti-CENP-B antibodies (10).
Although many autoantigens in systemic autoimmune diseases are believed to be ubiquitously expressed, the phenotypes associated with specific autoantibody responses are distinct, suggesting that localized tissue autoantigen expression may direct immune-mediated injury toward specific targets. Whether the targets of the immune response associated with scleroderma vascular complications (IFI16 and CENP proteins) are preferentially expressed in cells of the developing and mature blood vessel remains unknown. To answer questions about autoantigen expression across cell lineages in an open-ended way, we established a novel system to examine autoantigen expression in different lineages during differentiation of embryoid bodies (EBs). Human embryonic stem (ES) cells, when differentiating in vitro, form EBs. These cellular clusters have the potential to develop into all tissue types. The three germ layers (ectoderm, mesoderm, and endoderm) develop over the 12 day time course, with each progressing on a distinct timeline. We initially found that the time-course of IFI16 and CENP expression coincided with that of CD31, a marker of the vascular endothelial lineage, and confirmed that high level expression of IFI16 and CENPs occurred in CD31–positive cells in EBs. We also differentiated human ES cells into cultures enriched for vascular progenitors (11), and showed that these cells express particularly high levels of IFI16 and CENPs A and B. IFI16 was also expressed at high levels in mature endothelial cells in normal and scleroderma skin biopsies.
Taken together, our data demonstrates that the autoantigens associated with vascular injury in scleroderma are expressed at high levels across the continuum of endothelial cell development (progenitors to mature cells), potentially contributing to the striking association of phenotype and immune response in this important disease subset.
Materials and Methods
Human ES cell and EB cell culture and differentiation
Human ES and EB cells were obtained from the Johns Hopkins Stem Cell Core Facility. ES cell culture and EB differentiation were performed as described (12). Briefly, the human embryonic stem cell (ESC) line H9 (National Institutes of Health [NIH] code: WA09) was cultured on primary mouse embryonic fibroblasts (PMEFs) in Medium A (Dulbecco's modified Eagle's medium (DMEM)/F12 (GIBCO), supplemented with 20% KnockOut Serum Replacement, 0.1 mM nonessential amino acids, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol (all from Life Technology), and 10 ng/ml of FGF-2 (R&D)). For EB differentiation, undifferentiated ESC colonies were re-suspended in DMEM/F12 with 20% FBS, 0.1 mM nonessential amino acids, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol, plated on Ultra-Low attachment surface plates (Corning) and differentiated for 1, 4, 7, 10, and 12 days. Lysates were prepared at 4°C from the ES and EB cultures after washing the cells with ice cold PBS and lysing with Buffer B (1% Nonident P40, 20 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA containing a protease inhibitor cocktail).
Vascular progenitor cell culture and differentiation
hESC line H9 (National Institutes of Health [NIH] code: WA09) was used for the generation of human EBs differentiated towards vascular progenitor cells as previously described (13, 14). Briefly, hESC/PMEF co-cultures in gelatinized plates were grown up to 80% confluence. Twenty-four hours prior to EB formation, standard ES cell medium was switched to “adaptation medium” containing StemSpan SFEM (StemCell Technologies), 15% fetal calf serum (FCS, StemCell Technologies), 50 μg/mL ascorbic acid (Sigma Aldrich), 1% ExCyte (Millipore), 1% Penicillin/Streptomycin (Life Technologies), and 0.5% insulin-transferrin-selenium (ITS, Life Technologies). hESC colonies were washed and treated in collagenase type IV (1 mg/mL, Life Technologies) for 5 min at 37°C. Cells were collected using a cell scraper, washed and resuspended in adaptation medium (0.5 mL from 2 wells of hESC). 0.5 mL of cells were deposited on 2.5 mL/well of “methycellulose medium” (containing Methocult SF H4236 (StemCell Technologies) 15% FCS, 50 μg/mL ascorbic acid, 3.5 mL of PFHMII (Life Technologies), and 0.5% ExCyte) in a 6-well ultra low adherent plate (Corning). These cultures were harvested after 2 days and washed twice in PBS. Formed EBs were replated in “liquid differentiation medium” consisting of StemPro34 SFM (Life Technologies) supplemented with L-glutamine (1%, Life Technologies), 1-Thioglycerol (3.5 μL/100 mL medium, Sigma Aldrich), BMP4, VEGF-A165, FGF2 (each 50 ng/mL, all from R&D system), and heparin sulfate (5 μg/mL, Sigma Aldrich). Medium was replaced every 2 to 3 days. Cells were harvested at day 8 in PBS for washing and centrifugation (100 g, 2 min). Collagenase type IV treated EB clumps were moved into a tube with PMEF medium, centrifuged, and resuspended in EGM2 (Lonza) supplemented with additional 25 ng/mL VEGF-A165. Clumps were re-plated onto fibronectin (10 μg/mL) coated plates and cultured for an additional 4-5 days in EGM2 with 25 ng/mL VEGF-A165. Cells were treated in accutase or trypsin for fluorescent activated cell sorting (FACS) to purify vascular progenitor cells using CD31+CD146+ expression (15). Vascular progenitor cells were plated onto fibronectin-coated 6-well plates in EGM2 (100,000-200,000 cells/10 cm2) and expanded for 7-10 days prior to lysing as described above.
Immunoblotting ES/EB lysates
Equal protein amounts of each lysate were electrophoresed on SDS-polyacrylamide gels and subsequently transferred to nitrocellulose or immobilon membranes. Immunoblots were performed with the following markers of stem cell differentiation: Octamer binding transcription factor 4 (Oct 4) (rabbit polyclonal, Abcam), a marker of undifferentiated progenitors, Nanog (rabbit monoclonal, Cell Signaling), N Cadherin (NCAD), a marker of mesoendoderm from which early blood vessels are derived (rabbit polyclonal, Abcam), and CD31, a specific marker of early blood vessels (mouse monoclonal, DAKO). As a loading control, immunoblots were performed with monoclonal antibodies against α-smooth muscle actin (mouse monoclonal, Sigma-Aldrich). Autoantigens were detected by immunoblotting with the following commercial antibodies: RNA polymerase 3, (POLR3A, rabbit polyclonal, Novus), IFI16 (mouse monoclonal, Santa Cruz), CENP-A (rabbit polyclonal, Cell Signaling), CENP-B (rabbit polyclonal, Millipore), topoisomerase 1 (rabbit polyclonal, Novus), TIF1γ (mouse monoclonal, Novus) or patient sera with high titer antibodies against PARP or CENP-A (15, 16). Identical CENP-A blotting results were obtained when using the rabbit polyclonal antibody and the patient serum. Time course experiments for CENP-A, IFI16, topoisomerase I and POLR3A were performed at least three times each, with similar results in each experiment. Time course experiments were repeated on 2 occasions for each of the other autoantigens (CENP-B, TIF1γ, PARP), with consistent results. All immunoblots were performed as described previously (17), with detection of blotted bands using enhanced chemiluminesence (Pierce).
Embedding and cryosectioning of EBs
Day 12 EBs in suspension were pelleted by gentle centrifugation, washed and then fixed in 2% PLP (2% paraformaldehyde, 0.075 M L-Lysine, 0.0375 M PO4, 0.01 M Na-m-periodate). After incubating for 30 min at room temperature, PLP was removed by gentle centrifugation, and NH4Cl was added to quench. The EBs were then combined with low melt agarose and allowed to solidify on ice. Cold 0.5 M sucrose in 0.1M NaPO4, pH 7.4 was added to the EBs, followed by overnight incubation at 4°C. The pellet was then embedded in OCT, flash frozen in 2-methylbutane and stored at -80°C. 16 micron sections were cut for use in the immunofluorescence studies described below.
Immunofluorescence staining of EBs
Slides with EB sections were immersed in ice-cold acetone for 30 seconds, washed with PBS and then blocked with 5% goat serum for one hour at room temperature. Primary antibodies CD31 (mouse monoclonal, DAKO, 10 μg/ml or rabbit polyclonal, Abcam 0.2 μg/ml), IFI16 (mouse monoclonal, Sigma, 5 μg/ml) and CENP-A (patient serum diluted 1:1000) were subsequently added in 5% goat serum and incubated overnight at 4°C. After washing with PBS, secondary antibodies (donkey anti-mouse 488, goat anti-human 594, goat anti-mouse 488, and donkey anti-rabbit 594; all from Invitrogen and diluted 1:200) were subsequently added for one hour at room temperature. Washed, stained EBs were mounted with Prolong Gold with DAPI (Molecular Probes). Images were captured using a Zeiss Axioskop 50 microscope fitted with a Zeiss AxioCam HRc camera and AxioVision 4 software.
Human tissue, peripheral blood mononuclear cells (PBMCs) and disease status
All studies on human materials were approved by the Johns Hopkins Institutional Review Board and Health Insurance Portability and Accountability Act, and patient and control samples were de-identified. Scleroderma patients were followed in the Johns Hopkins Scleroderma Center for routine clinical care and met ACR/EULAR 2013 criteria (18/20) or had three of five CREST criteria (2/20) (18, 19). To study IFI16 expression in vivo in skin, three millimeter punch biopsies from the dorsal forearm were obtained at the time of the clinic visit. Patients were classified as having diffuse (n=11) or limited (n=9) cutaneous scleroderma based on the extent of skin involvement (19, 20). Control skin biopsies (n=8) were obtained from non-scleroderma family members or friends of the patient. Skin biopsies were embedded in paraffin and 6 micron sections were cut for immunohistochemistry studies. To study IFI16 expression in circulating endothelial cells and circulating progenitor cells, PBMCs were prepared from blood obtained from 5 limited and 6 diffuse scleroderma patients, and 5 healthy donors.
Immunohistochemistry
Skin paraffin sections were rehydrated, then soaked in target retrieval solution (DAKO) for 30 mins at 95°C. After blocking, the sections were incubated overnight at 4°C with antibodies against anti-IFI16 (mouse monoclonal, 13 μg/ml, Sigma) or anti-CD31 (rabbit polyclonal from Invitrogen, 0.2 μg/ml). The sections were subsequently incubated with donkey anti-mouse IgG Alexa Fluor 594 and donkey anti-rabbit IgG Alexa Fluor 488 (Abcam, both at 1:200 dilution). After extensive washing, the sections were mounted, viewed and photographed as described above.
Flow cytometry
PBMCs were isolated from whole blood using Ficoll-plaque plus (GE Healthcare). They were subsequently fixed and permeabilized using the Transcription Factor buffer set per the manufacturer's instructions (BD Biosciences). Permeabilized PBMCs were stained for 30 minutes at room temperature with monoclonal antibodies against CD3 (BV510, clone UCHT1, BD Biosciences), CD31 (APC, clone WM59, Biolegend), CD45 (PE-Dazzle 594, clone H130, Biolegend) and IFI16 (FITC, clone 1G7, Santa Cruz Biotechnology). Data were acquired with a FACSAria I SORP cell sorter (Becton Dickinson) and subsequently analyzed with FCS Express 4 (De Novo Software). Since CD34 was lost during the permeabilization and fixation protocol, circulating hematopoietic progenitor cells were identified by the expression of CD31 and CD45.
Results
Biochemical levels of CENP-A and IFI16 expression increase over time in the progenitor cell differentiation system, with patterns similar to that of CD31
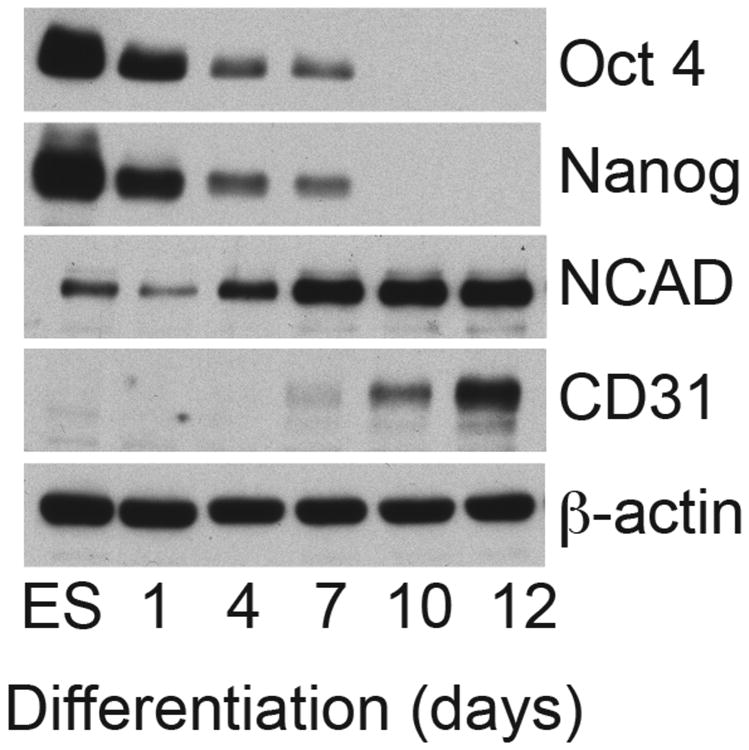
We utilized a human ES/EB progenitor cell differentiation system to define whether CENP and IFI16 expression vary as progenitor cells differentiate. We first validated the timeline of differentiation in our ES/EB system as follows. Biochemical levels of well-described differentiation markers were immunoblotted using equal protein amounts of progenitor lysates from different days of progenitor differentiation (Fig 1). Equivalent protein loading in each lane was confirmed by immunoblotting the same lysates with β-actin as a loading control. As expected, high levels of both Oct 4 and Nanog, transcription factors required to maintain ES pluripotency and self-renewal, were seen in undifferentiated progenitors (ES); levels dropped rapidly thereafter, with >50% loss at day 4 and detection of these markers being absent by day 10.
Figure 1. Biochemical validation of the human ES/EB progenitor cell differentiation system.
Lysates made from human ES cells and EBs harvested at different times of differentiation over a 12-day period were immunoblotted with antibodies against specific differentiation markers (Oct4, Nanog, NCAD and CD31). Equal protein amounts were electrophoresed in each gel lane and β-actin was immunoblotted as a loading control.
The development of the early vasculature arises from the mesoendoderm, which is marked by NCAD in our system. NCAD expression was detectable by immunoblotting around day 4 and levels progressively increased thereafter through day 12. We also determined the expression of CD31 to mark developing blood vessels (derived from mesoendoderm). CD31 was absent in the early stages of EB differentiation, with robust levels detected at day 10 and thereafter (Fig 1).
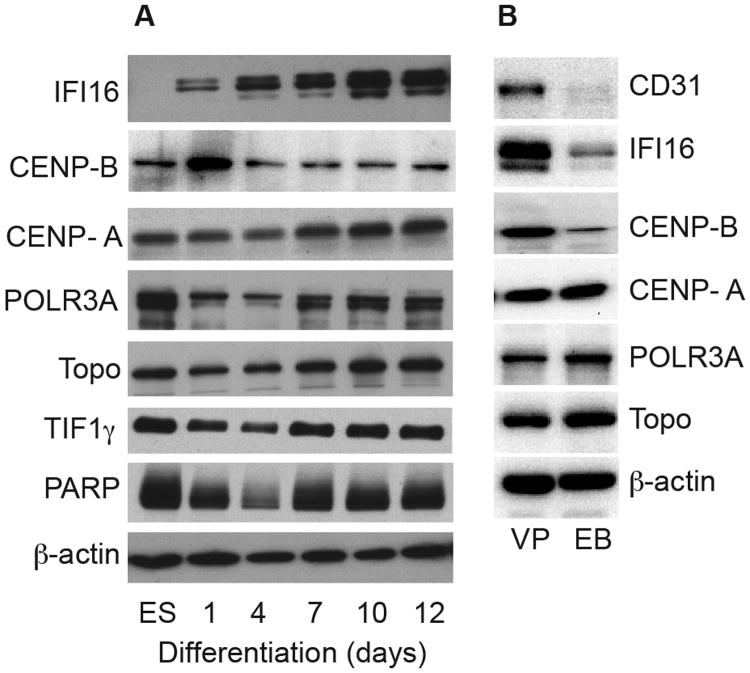
Having validated the ES/EB system, we used it to define whether IFI16 and CENPs are expressed uniformly during EB differentiation, or whether they appear at times when specific lineages are being formed. Biochemical levels of IFI16 and CENPs that are present in equal protein amounts of lysates were determined by immunoblotting (Fig 2A). Interestingly, both CENP-A and IFI16 expression were enriched at days 10-12, relative to more immature EBs. This pattern of enrichment in the later days of differentiation is similar to that of CD31 expression (Fig 1). Similar data were obtained for the CENP-A blots using patient serum (shown in Fig 2A) or using a rabbit polyclonal anti-CENP-A antibody (not shown). While IFI16, CENP-A, and CD31, were all distinctly enriched on days 10 and 12 of EB differentiation, CENP-A and IFI16 expression patterns differed slightly earlier in the differentiation time course. Similar to NCAD, CENP-A expression was detected by blotting initially in ES, then progressively increasing through day 12. In contrast, IFI16 was not detected in ES cells. Very low IFI16 levels were blotted on day 1, after which they increased progressively. In contrast, we noted a unique expression pattern with CENP-B: levels were low in ES and remained unchanged through day 12.
Figure 2. Scleroderma autoantigen expression levels in the progenitor cell differentiation system.
(A) Lysates made from human ES cells and EBs harvested at different times of differentiation over a 12-day period were immunoblotted with commercial antibodies against IFI16, CENP-B, POLR3A, topoisomerase I, and TIF1γ, or patient sera with high titer antibodies against PARP or CENP-A. (B) Lysates made from day 12 EBs and vascular progenitors (“VP”) were immunoblotted as above; a rabbit polyclonal antibody was used for the CENP-A blot. (A & B): Equal protein amounts were electrophoresed in each gel lane. The same lysates were immunoblotted with an antibody against β–actin as a loading control (lowest panels of each set).
Of note, the progressive enrichment patterns seen with IFI16 and CENP-A were unique to these autoantigens and were not observed for any other comparator autoantigens we tested (POLR3A and topoisomerase I, both prominent specificities in scleroderma, and TIF1γ and PARP which are targeted in dermatomyositis and SLE, respectively – see Fig 2A). Typically, most autoantigens were expressed robustly in ES, then progressively decreased through day 4, followed by a subsequent increase (Fig 2A, lower panels).
Previous studies demonstrate that both hematopoietic and blood vessel lineages arise from a bipotential hemangioblast that generates hematopoietic and endothelial cells (21-24). These studies have successfully demonstrated differentiation of human embryonic stem cells (hESCs) into hemangioblasts (11, 24). Additional, recent studies have identified alternative transitional states called hemogenic endothelium as the direct developmental ancestors of adult-type hematopoietic stem cells (25). We therefore immunoblotted lysates made from these vascular progenitors and day 12 EBs to compare the levels of CD31, IFI16 and CENPs-A and B. IFI16 and CENP-B were both enriched in the vascular progenitors (in the case of IFI16, this enrichment was striking). CENP-A was expressed at similar levels in day 12 EBs and the vascular progenitors (Fig 2B). Note that the exposure shown in Fig 2B was optimized to visualize the CD31 and IFI16 blotting in vascular progenitor levels in the linear range; longer exposures show IFI16 and CD31 day 12 EB levels similar to those in Fig 2A (IFI16) and Fig 1 (CD31).
CENP-A and IFI16 are enriched in EB cells expressing CD31
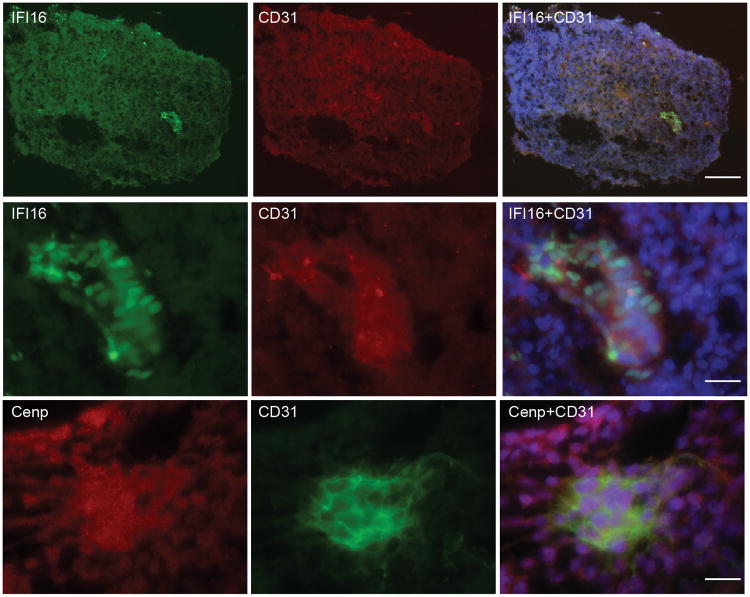
While immunoblotting reads out enrichment of IFI16/CENP-A and CD31 in day 12 EBs, it does not provide definitive information regarding whether these autoantigens are enriched in CD31 positive cells. To address this directly, we examined IFI16 and CENP-A expression in day 12 EBs by immunofluorescence. Day 12 EBs were selected as this was the time point when expression of these autoantigens and CD31 were highest as determined by immunoblotting (CENP-B was not examined since expression levels in day 12 EBs were low - Fig 2A). After optimizing antibody dilutions for immunofluorescence staining, day 12 EBs were double-stained with a commercial antibody to CD31 and a high-titer anti-CENP-A antibody positive scleroderma patient serum (defined by immunoblotting), or CD31 and a monoclonal antibody against IFI16. Expression of both IFI16 and CENP-A was enriched in CD31-positive cells relative to other cells in the day 12 EBs (Fig 3).
Figure 3. Progenitors expressing CD31, a marker of early blood vessels, have high levels of IFI16 and CENP-A expression.
EB day 12 cultures were embedded, cryosectioned and double stained with CD31 and antibodies against either IFI16 or CENP-A as described in the Methods section. Top panel (10×): IFI16 (green), CD31 (red), and a merged image with DAPI (blue nuclear stain) shows enrichment of IFI16 in cells expressing CD31 in day 12 EB's. Middle panel (63×): Higher magnification of the image shown in the top panel. Bottom panel (63×): CENP (red) is enriched in CD31 expressing cells (green). A merged image with DAPI (blue nuclear stain) is also shown. Scale bar represents 100 microns (top panel) and 20 microns (middle and lowest panels).
IFI16 expression is enriched in vascular endothelial cells in skin biopsies from both normal individuals and scleroderma patients
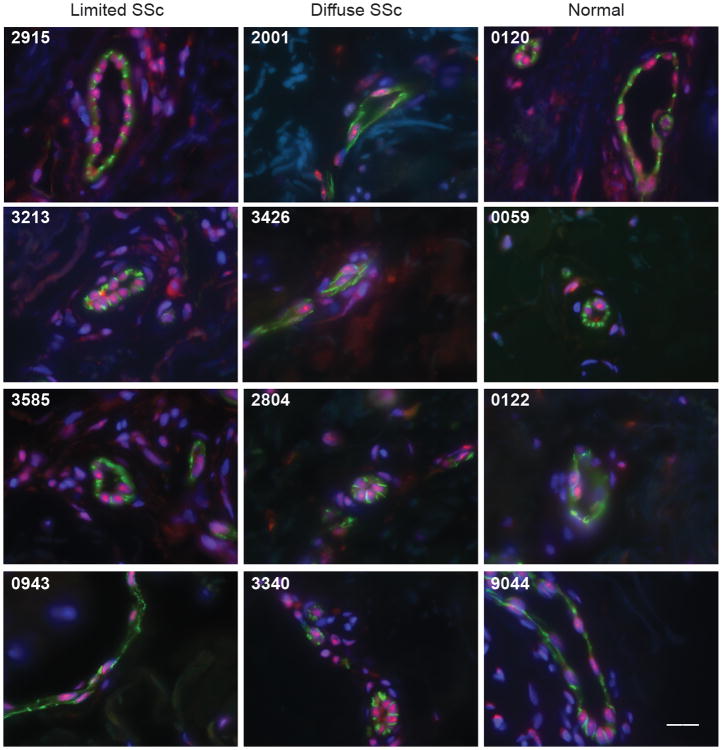
The observations above showing high levels of IFI16 expression in vascular progenitor cells (Fig 2B) and co-localization of IFI16 and CD31 in vessels developing in EBs (day 12 EBs, Fig 3), prompted us to examine expression of both IFI16 and CD31 in human skin biopsies. Serial skin paraffin sections from 20 scleroderma patients (11 diffuse and 9 limited) and 8 control subjects were studied. Three of the nine biopsied limited scleroderma patients, and five of the eleven biopsied diffuse scleroderma patients had a history of severe vascular events (digital ulcers, pits or gangrene). Of these, four had digital ischemic ulcers present at the time of the skin biopsy (2 limited, 2 diffuse). The paraffin sections were double-stained with antibodies against CD31 and IFI16. Prominent nuclear IFI16 staining in the absence of CD31 was noted in the epidermis, consistent with previous reports (26, 27). When we focused specifically on CD31-positive vascular endothelial cells lining vessels, we noted that they were enriched in IFI16 expression relative to other cells, with prominent nucleolar staining observed with some frequency. Representative data from four control subjects, four scleroderma patients with diffuse disease and 4 scleroderma patients with limited disease is shown in Fig 4. No staining was detected when sections were incubated with equivalent amounts of rabbit and mouse IgG, followed by fluorescently labeled secondary antibodies (not shown).
Figure 4. IFI16 expression is enriched in vascular endothelial cells in skin biopsies from both normal individuals and scleroderma patients.
Skin paraffin sections from 20 scleroderma patients (9 with limited disease and 11 with diffuse disease) and 8 normal controls were double stained with a rabbit polyclonal antibody against CD31 (depicted in green) and a mouse monoclonal anti-IFI16 antibody (depicted in red). All sections were counterstained with DAPI (represented in blue). Representative data (merged images) from 4 scleroderma patients with limited disease (tissue numbers 2915, 3213, 3585 and 0943), 4 scleroderma patients with diffuse disease (tissue numbers 2001, 3426, 2804, and 3340) and 4 normal controls (tissue numbers 0120, 0059, 0122 and 9044) is shown. Robust levels of IFI16 staining are found in vascular endothelial cells in both normal controls and scleroderma patients. Scale bar represents 20 microns. Tissues 2915, 3213, 2001 and 3426 are from patients with digital ulcers or digital gangrene; all other scleroderma tissue was from patients with Raynaud's phenomoneon without digital ulcers.
Interestingly, enrichment of IFI16 in CD31-positive endothelial cells lining vessels was a feature common to both control and scleroderma skin in all 28 skin biopsies examined. Furthermore, the levels and staining pattern of IFI16 in CD31-positive skin vessels in tissue from scleroderma patients with limited and diffuse disease was similar. Amongst the scleroderma tissue, there was no difference in IFI16 levels between those biopsies obtained from patients with active digital ischemic events at the time of biopsy (Fig 4, biopsy numbers 2915, 3213, 2001 and 3426) compared to those without (biopsy numbers 3585, 0943, 2804 and 3340). Interestingly, in 2 scleroderma patients with digital ulcers, staining of IFI16 was prominent in the cytoplasm of endothelial cells rather than in the nucleus. This pattern was not observed in controls or other scleroderma patients. Future studies will examine whether this distinct cytoplasmic distribution of IFI16 is observed more commonly in blood vessels from scleroderma patients with active vascular symptoms. These studies demonstrate that the cutaneous vessels known to be a target in scleroderma clearly express the autoantigen irrespective of disease/control status, but our small sample size prevented us from detecting differences in expression in patient subtypes or from normal controls.
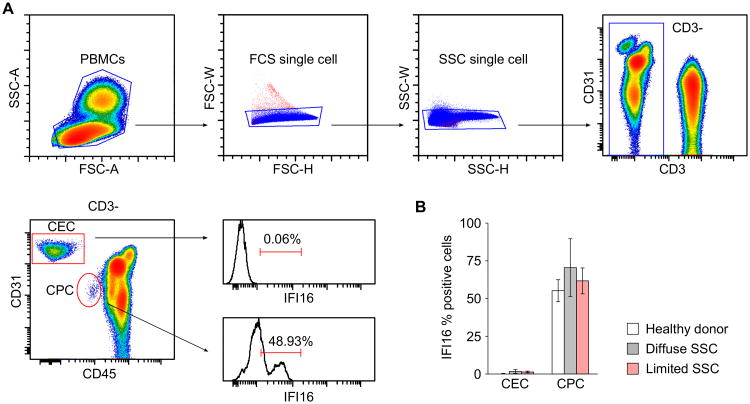
IFI16 expression is enriched in circulating CPCs
Our immunohistochemical staining of skin biopsies (Fig 4) revealed an enrichment of IFI16 in CD31-positive cells in endothelial cells lining vessels. We also investigated IFI16 expression in circulating endothelial cells (CECs) and circulating hematopoietic progenitor cells (CPCs) in scleroderma patients and healthy controls, using flow cytometry. CPCs and CECs were identified in purified PBMCs by their differential expression of CD31 and CD45, with CD31 expression higher in CECs than CPCs (Fig 5A). In all the populations we studied, IFI16 was expressed in a large proportion of CPCs but was virtually absent in CECs (Fig 5). Of note, the percentage of IFI16-positive cells was comparable in controls and scleroderma patients, and there was no difference between scleroderma cutaneous subtypes (Figure 5B). The origin of CECs is not yet entirely clear, but they appear to originate from vascular walls and have very limited or no replicative potential, indicating that this population might represent endothelial cells after their residence lining the vessel (28, 29). Consistent with the limited role of CECs in the vascular repair process, no major abnormalities have been reported for CECs in scleroderma. Although not believed to primarily contain endothelial cell progenitors, CPCs are believed to be actively recruited during vascular disease to enhance repair of damaged blood vessels (30-34). An immune response against IFI16 might therefore affect vessel repair through negative effects on these IFI16-positive CPCs.
Figure 5. IFI16 expression is enriched in circulating hematopoietic progenitor cells.
(A) Gating strategy of a representative PBMC flow cytometry staining. Live cells were defined by FSC-A and SSC-Area. FSC-Height vs FSC-Width and SSC-Height vs SSC-Width were used to exclude cell aggregates from the analysis. Within the CD3 negative gate circulating endothelial cells (CEC) and circulating progenitor cells (CPC) were identified by their differential expression of CD31 and CD45. Histogram representations of the percentage of IFI16 positive cells for CEC and CPC are shown. (B) Percentage of IFI16 positive cells (± standard deviation) in CEC and CPC in PBMCs prepared from five healthy donors, 6 patients with diffuse scleroderma and 5 patients with limited scleroderma.
Discussion
Autoantibodies against IFI16 were recently reported to associate with digital gangrene in scleroderma (8). These autoantibodies were enriched in those with limited skin disease, and were associated with a low DLCO. High levels of anti-IFI16 antibodies were associated with digital gangrene. Patients with limited scleroderma and digital loss are also more likely to have anti-CENP antibodies (35). In these studies, we examined whether autoantigens associated with vascular disease in scleroderma are enriched in the vascular cell lineage. To obtain some indication of the specificity of any findings, we performed our initial screens of expression levels on developing EBs, which contain all 3 germ layers, and provide some context for interpretation. Those studies demonstrated that expression of IFI16 is very low in ES cells, and only appears in significant amounts in EBs at the time of early blood vessel formation (coinciding with expression of CD31, a marker of the vascular lineage). Interestingly, IFI16 expression also coincided with enhanced CENP-A levels in d12 EBs. By immunofluorescence, these antigens were both expressed in EBs at high levels in CD31-positive cells. When ES cells were more specifically differentiated towards vascular progenitors, expression of IFI16 was further enhanced, and CENP-B expression (not increased in d12 EBs) was also increased.
In vivo expression of IFI16 was enriched in mature endothelial cells in normal and scleroderma skin biopsies (Fig 4). Interestingly, IFI16 was expressed in CPCs in normal and scleroderma patients, but was not expressed in CECs. These data demonstrate that IFI16, a specificity more frequently targeted in scleroderma patients with digital gangrene, is enriched in vascular progenitors and mature endothelial cells. While CENP-B is expressed at enhanced levels in ES-derived vascular progenitors differentiated in vitro, it is not possible to conclusively define its expression in vivo. We propose that the striking enrichment in IFI16 expression in vascular progenitors and endothelial cells might focus anti-IFI16 immune responses (possibly induced by a proimmune event like infection or cancer) and effector pathways onto blood vessels. This may play a role in generating the digital ischemia phenotype in these patients. Our results do not address whether endothelial cells in other vascular beds express enhanced levels of IFI16 and CENPs. Since the presence of pulmonary hypertension is a risk factor associated with digital ischemia in scleroderma (36), defining the expression in other vascular beds will be of interest.
In scleroderma (and other autoimmune diseases), ongoing tissue injury and immune system activation can also be associated with a failure of homeostatic tissue repair. Tissue-specific progenitor cells are present in mature adults and have a role in tissue maintenance and repair (37-39). Enrichment of IFI16 and CENP proteins in less mature vascular cells may therefore have implications for vessel homeostasis, potentially rendering scleroderma patients with this immune response more susceptible to severe vascular complications after exposure to other vascular injuries (e.g. hypoxia, or interferons).
It is noteworthy that while IFI16 is also a frequent target in other autoimmune rheumatic diseases, including Sjogren's syndrome and SLE, the clinical associations in these other diseases are distinct (8, 40). Thus, digital gangrene is not a feature of Sjogren's syndrome (SS), and it is uncommon in SLE. IFI16 antibodies in SS are associated with markers of more severe disease (including hypergammaglobulinemia, higher focus scores, and germinal centers)(40). In SLE, IFI16 antibodies are associated with anti-DNA antibodies, and a higher prevalence of pericarditis (40, 41). The lack of similar vascular damage in these other rheumatic syndromes in the presence of IFI16 autoantibodies raises several possible mechanisms: (i) A different form of IFI16 may be present in different tissues. There are known IFI16 splice variants (42). It is possible that the IFI16 immune response in scleroderma is directed against a form of the antigen more likely to be found in vessels; (ii) Expression of IFI16 is known to be constitutive or induced, notably by type I and type II interferons (43). IFI16 expression can also be induced by other stimuli, including hypoxia. For example, a 2005 study showed IFI16 upregulation in human endothelial cells in the setting of oxidative stress after human endothelial cells were exposed to sublethal concentrations of hydrogen peroxide in vitro (44). It is possible that different stimuli induce the distinct expression or modification of IFI16 in different tissues, accounting for associations with different phenotypic expression in different diseases; (iii) It is possible that the immune response to IFI16 is an amplifier of pathology, but cannot initiate damage itself. The vascular damage in scleroderma patients with IFI16 immune responses might therefore be initiated by another vascular insult (e.g. hypoxia, granzyme) which occurs in scleroderma but not SS or SLE – potentially leading to a secondary amplifying injury; (iv) the IFI16 immune response may not cause tissue damage, but may instead prevent the vessel from responding effectively to other insults. The observation that CPCs have very high levels of IFI16 could be consistent with that model. It is also of interest that the pattern of IFI16 expression in vessels of 2 patients with active vascular events appeared distinct, with a striking cytoplasmic pattern not seen in normal endothelial cells. Future studies will attempt to collect additional samples from this subgroup to more fully evaluate this.
Taken together, our findings suggest strongly that IFI16 (and likely some of the CENP targets) are expressed preferentially in vascular lineage cells, across the spectrum of maturity from progenitor through mature endothelial cells, but not in circulating endothelial cells. These studies raise important questions about the specificity of the association of the IFI16 immune response with vessel phenotype in scleroderma but not other rheumatic syndromes, and strongly suggest that additional understanding of the antigen expression and structure in the target tissue during active injury will provide important insights into the mechanisms underlying specific patterns of tissue injury.
Acknowledgments
We are grateful to the Johns Hopkins Stem Cell Core Facility for culturing the differentiating EBs. We thank Professor Ann Hubbard and Lydia Nyasae for their critical input with embedding the EBs.
Dr. McMahan's work was supported by the Maryland Stem Cell Research Fund, by the Jerome L. Greene Foundation, the Scleroderma Research Foundation, and by a Rheumatology Research Foundation Scientist Development Award. Dr. Wigley was supported by the Martha McCrory Professorship and the Scleroderma Research Foundation. Dr. Antiochos is supported by the Jerome L. Greene Foundation. Dr. Zambidis was funded by NIH/NHLBI U01HL099775, MSCRF 2013-MSCRFII-0032-00 and NIH/NICHD R01HD082098. Dr. Park was supported by the MSCRF (2014-MSCRFE-118153). Dr. Halushka was supported by the American Heart Association (13GRNT16420015). Dr Rosen is supported by RO1 DE 12354-15A1. Dr Casciola-Rosen's work was supported by the NIH (R56-AR-062615). The Johns Hopkins Rheumatic Disease Research Core Center, where the immunohistochemistry and flow cytometry were performed, is supported by the NIH (grant P30-AR-053503).
References
- 1.Varga JA, Trojanowska M. Fibrosis in systemic sclerosis. Rheum Dis Clin North Am. 2008 Feb;34(1):115–43. vii. doi: 10.1016/j.rdc.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greidinger EL, Flaherty KT, White B, Rosen A, Wigley FM, Wise RA. African-American race and antibodies to topoisomerase I are associated with increased severity of scleroderma lung disease. Chest. 1998 Sep;114(3):801–7. doi: 10.1378/chest.114.3.801. [DOI] [PubMed] [Google Scholar]
- 3.Khimdas S, Harding S, Bonner A, Zummer B, Baron M, Pope J, et al. Associations with digital ulcers in a large cohort of systemic sclerosis: results from the Canadian Scleroderma Research Group registry. Arthritis Care Res (Hoboken) 2011 Jan;63(1):142–9. doi: 10.1002/acr.20336. [DOI] [PubMed] [Google Scholar]
- 4.Tiev KP, Diot E, Clerson P, Dupuis-Simeon F, Hachulla E, Hatron PY, et al. Clinical features of scleroderma patients with or without prior or current ischemic digital ulcers: post-hoc analysis of a nationwide multicenter cohort (ItinerAIR-Sclerodermie) J Rheumatol. 2009 Jul;36(7):1470–6. doi: 10.3899/jrheum.081044. [DOI] [PubMed] [Google Scholar]
- 5.Domsic RT. Scleroderma: the role of serum autoantibodies in defining specific clinical phenotypes and organ system involvement. Curr Opin Rheumatol. 2014 Nov;26(6):646–52. doi: 10.1097/BOR.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wigley FM, Wise RA, Miller R, Needleman BW, Spence RJ. Anticentromere antibody as a predictor of digital ischemic loss in patients with systemic sclerosis. Arthritis Rheum. 1992 Jun;35(6):688–93. doi: 10.1002/art.1780350614. [DOI] [PubMed] [Google Scholar]
- 7.Weiner ES, Earnshaw WC, Senecal JL, Bordwell B, Johnson P, Rothfield NF. Clinical associations of anticentromere antibodies and antibodies to topoisomerase I. A study of 355 patients. Arthritis Rheum. 1988 Mar;31(3):378–85. doi: 10.1002/art.1780310309. [DOI] [PubMed] [Google Scholar]
- 8.McMahan Z, Shah A, Vaidya D, Wigley F, Rosen A, Casciola-Rosen L. Anti-IFI16 antibodies in scleroderma are associated with digital gangrene. 2015 [Google Scholar]
- 9.Earnshaw WC. Discovering centromere proteins: from cold white hands to the A, B, C of CENPs. Nat Rev Mol Cell Biol. 2015 Jul;16(7):443–9. doi: 10.1038/nrm4001. [DOI] [PubMed] [Google Scholar]
- 10.Hudson M, Mahler M, Pope J, You D, Tatibouet S, Steele R, et al. Clinical correlates of CENP-A and CENP-B antibodies in a large cohort of patients with systemic sclerosis. J Rheumatol. 2012 Apr;39(4):787–94. doi: 10.3899/rheum.111133. [DOI] [PubMed] [Google Scholar]
- 11.Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008 Nov 1;112(9):3601–14. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009 Sep 17;461(7262):402–6. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park TS, Bhutto I, Zimmerlin L, Huo JS, Nagaria P, Miller D, et al. Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature. Circulation. 2014 Jan 21;129(3):359–72. doi: 10.1161/CIRCULATIONAHA.113.003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park TS, Zimmerlin L, Zambidis ET. Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells. Cytometry A. 2013 Jan;83(1):114–26. doi: 10.1002/cyto.a.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995 Dec 1;182(6):1625–34. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall JC, Casciola-Rosen L, Berger AE, Kapsogeorgou EK, Cheadle C, Tzioufas AG, et al. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17609–14. doi: 10.1073/pnas.1209724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013 Nov;65(11):2737–47. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001 Jul;28(7):1573–6. [PubMed] [Google Scholar]
- 20.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980 May;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 21.Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002 Sep;129(17):4147–57. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 22.Tavian M, Hallais MF, Peault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development. 1999 Feb;126(4):793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- 23.Cortes F, Debacker C, Peault B, Labastie MC. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech Dev. 1999 May;83(1-2):161–4. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 24.Peters A, Burridge PW, Pryzhkova MV, Levine MA, Park TS, Roxbury C, et al. Challenges and strategies for generating therapeutic patient-specific hemangioblasts and hematopoietic stem cells from human pluripotent stem cells. Int J Dev Biol. 2010;54(6-7):965–90. doi: 10.1387/ijdb.093043ap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschi KK. Hemogenic endothelium during development and beyond. Blood. 2012 May 24;119(21):4823–7. doi: 10.1182/blood-2011-12-353466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gariglio M, Azzimonti B, Pagano M, Palestro G, De Andrea M, Valente G, et al. Immunohistochemical expression analysis of the human interferon-inducible gene IFI16, a member of the HIN200 family, not restricted to hematopoietic cells. J Interferon Cytokine Res. 2002 Jul;22(7):815–21. doi: 10.1089/107999002320271413. [DOI] [PubMed] [Google Scholar]
- 27.Mondini M, Vidali M, De Andrea M, Azzimonti B, Airo P, D'Ambrosio R, et al. A novel autoantigen to differentiate limited cutaneous systemic sclerosis from diffuse cutaneous systemic sclerosis: the interferon-inducible gene IFI16. Arthritis Rheum. 2006 Dec;54(12):3939–44. doi: 10.1002/art.22266. [DOI] [PubMed] [Google Scholar]
- 28.Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet. 2004 Aug 14-20;364(9434):603–10. doi: 10.1016/S0140-6736(04)16853-0. [DOI] [PubMed] [Google Scholar]
- 29.Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007 Jan-Feb;25(1):60–6. [PubMed] [Google Scholar]
- 30.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997 Feb 14;275(5302):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 31.Sata M. Circulating vascular progenitor cells contribute to vascular repair, remodeling, and lesion formation. Trends Cardiovasc Med. 2003 Aug;13(6):249–53. doi: 10.1016/s1050-1738(03)00106-3. [DOI] [PubMed] [Google Scholar]
- 32.Iwata H, Sata M. Potential contribution of bone marrow-derived precursors to vascular repair and lesion formation: lessons from animal models of vascular diseases. Front Biosci. 2007 May 1;12:4157–67. doi: 10.2741/2377. [DOI] [PubMed] [Google Scholar]
- 33.Sata M. Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arterioscler Thromb Vasc Biol. 2006 May;26(5):1008–14. doi: 10.1161/01.ATV.0000206123.94140.f3. [DOI] [PubMed] [Google Scholar]
- 34.Sata M, Fukuda D, Tanaka K, Kaneda Y, Yashiro H, Shirakawa I. The role of circulating precursors in vascular repair and lesion formation. J Cell Mol Med. 2005 Jul-Sep;9(3):557–68. doi: 10.1111/j.1582-4934.2005.tb00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wigley FM, Wise RA, Miller R, Needleman BW, Spence RJ. Anticentromere antibody as a predictor of digital ischemic loss in patients with systemic sclerosis. Arthritis Rheum. 1992 Jun;35(6):688–93. doi: 10.1002/art.1780350614. [DOI] [PubMed] [Google Scholar]
- 36.Sunderkotter C, Herrgott I, Bruckner C, Moinzadeh P, Pfeiffer C, Gerss J, et al. Comparison of patients with and without digital ulcers in systemic sclerosis: detection of possible risk factors. Br J Dermatol. 2009 Apr;160(4):835–43. doi: 10.1111/j.1365-2133.2008.09004.x. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010 Jan;77(1):22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002 Aug 15;35(4):657–69. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung Y, Kandyba E, Chen YB, Ruffins S, Chuong CM, Kobielak K. Bifunctional ectodermal stem cells around the nail display dual fate homeostasis and adaptive wounding response toward nail regeneration. Proc Natl Acad Sci U S A. 2014 Oct 21;111(42):15114–9. doi: 10.1073/pnas.1318848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baer AN, Petri M, Sohn J, Rosen A, Casciola-Rosen L. Association of Antibodies to Interferon-Inducible Protein-16 With Markers of More Severe Disease in Primary Sjogren's Syndrome. Arthritis Care Res (Hoboken) 2016 Feb;68(2):254–60. doi: 10.1002/acr.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seelig HP, Ehrfeld H, Renz M. Interferon-gamma-inducible protein p16. A new target of antinuclear antibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 1994 Nov;37(11):1672–83. doi: 10.1002/art.1780371117. [DOI] [PubMed] [Google Scholar]
- 42.Johnstone RW, Kershaw MH, Trapani JA. Isotypic variants of the interferon-inducible transcriptional repressor IFI 16 arise through differential mRNA splicing. Biochemistry. 1998 Aug 25;37(34):11924–31. doi: 10.1021/bi981069a. [DOI] [PubMed] [Google Scholar]
- 43.Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS One. 2011;6(10):e27040. doi: 10.1371/journal.pone.0027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gugliesi F, Mondini M, Ravera R, Robotti A, de Andrea M, Gribaudo G, et al. Up-regulation of the interferon-inducible IFI16 gene by oxidative stress triggers p53 transcriptional activity in endothelial cells. J Leukoc Biol. 2005 May;77(5):820–9. doi: 10.1189/jlb.0904507. [DOI] [PubMed] [Google Scholar]