Abstract
Background
Children with chronic kidney disease (CKD) and hypertension have increased blood pressure variability (BPV). Increased BPV has been associated with lower neurocognitive test scores in adults. Children with CKD are at risk for decreased neurocognitive function. Our objective was to determine if children with CKD and increased BPV had worse performance on neurocognitive testing compared with children with CKD and lower BPV.
Methods
This was a cross-sectional and longitudinal analysis of the relation between BPV and neurocognitive test performance in children 6 years and older enrolled in the Chronic Kidney Disease in Children (CKiD) study. Visit-to-visit BPV was assessed by the standard deviation of visit BPs (BPV-SD) and average real variability (ARV). Ambulatory BPV was assessed by SD of wake and sleep periods on 24hr ambulatory BP monitoring.
Results
650 subjects had a mean follow-up period of 4.0 years. Subjects with systolic visit-to-visit BPV in the upper tertile had lower scores on Delis–Kaplan Executive Function System (D-KEFS) Verbal Category Switching than those with BPV in the lower tertile (BPV-SD, 8.3 vs. 9.5, p = 0.006; ARV, 8.5 vs. 9.6, p = 0.02). On multivariate analysis, the association between lower Category Switching score and increased BPV remained significant after controlling for mean BP, demographic characteristics, and disease-related variables (BPV-SD, β = −0.7, 95% confidence interval [CI]: −1.28 to −0.12; ARV, CI: −1.05 to −0.02). Ambulatory BPV was not independently associated with any cognitive measure.
Conclusions
Higher systolic visit-to-visit BPV was independently associated with decreased D-KEFS Category Switching scores in children with mild-to-moderate CKD.
Keywords: Hypertension, pediatric, neuropsychological testing, kidney, CKD
INTRODUCTION
In adults, alterations in blood pressure (BP) can have negative cardiovascular effects, not only through elevations of mean BP, but also through increases in blood pressure variability (BPV). BPV can be defined either in the short term, as measured by variability in measurements within a 24 hour period during ambulatory BP monitoring (ambulatory BPV), or in the long term as variability in office visit measurements over time (visit-to-visit BPV) [1]. Studies show that increases in both short term and long term BPV are associated with increases in hypertensive target organ damage, cardiovascular events, all-cause mortality, and faster progression of chronic kidney disease (CKD) [2–6].
Increased BPV may also affect neurocognitive function. In adults, visit-to-visit BPV and ambulatory BPV have both been associated with lower scores in global cognition, executive function, attention, processing speed and memory in the elderly [7–10]. Long term visit-to-visit BPV was evaluated over 25 years in a young adult cohort and was associated with worse psychomotor speed and verbal memory in midlife, independent of cumulative BP exposure [11].
Recently, ambulatory BPV was evaluated in children with mild-to-moderate CKD. Children with CKD who were hypertensive were found to have increased BPV compared with those who were normotensive [12]. Children with CKD are frequently hypertensive and are at risk for decreased neurocognitive function [13]. To our knowledge, the potential impact of increased BPV on cognition in children with CKD has not been previously evaluated. The objective of the current study was to evaluate the impact of BPV on cognition in children with mild-to-moderate CKD.
METHODS
Participants
Subjects were participants in the Chronic Kidney Disease in Children Study (CKiD), a longitudinal, observational cohort study of children with CKD being conducted at 48 pediatric nephrology centers in North America. Enrollment criteria for CKiD have been published and include age 1 to 16 years and Schwartz-estimated GFR (eGFR) of 30 to 90 ml/min per 1.73 m2, without solid organ, bone marrow, or stem cell transplant; dialysis treatment within 3 months before enrollment; cancer/leukemia or HIV treatment; and pregnancy [14]. This study is an analysis of CKiD subjects age 6 years and above for whom both neurocognitive data and BP measurements were available and for whom there was at least 2 years of BP data (3 visits). Subjects younger than 6 years were administered a different neurocognitive test battery and were therefore excluded. CKiD study visits occurred at study entry, at 6 months, and then annually from the time of initial enrollment, with neurocognitive assessments occurring 6 months after enrollment, at 24 months, and then every two years thereafter.
Measurements
Casual Blood Pressure Measurement
Casual BP measurements were obtained in the right arm by auscultation at each study visit by trained examiners. Training was repeated annually. Three BP measurements were obtained by auscultation of the brachial artery using the first Korotkoff sound for systolic BP (SBP) and the fifth Korotkoff sound for diastolic BP (DBP). The average of the three BP measurements was recorded as the participant’s BP for the study visit [15].
Ambulatory BP Monitoring
Twenty-four hour ambulatory BP monitoring (ABPM) was performed at 6 months and then every 2 years. For each 24hr recording, measurements were obtained every 20 minutes through the day and night at a bleed step of 8 mm/Hg. A diary was kept during the monitoring to record sleep and wake times. ABPM studies were considered successful only when at least one successful BP measurement was obtained during each hour of monitoring and the overall success rate was at least 75 %. The means of SBP and DBP were determined for wake and sleep periods. BP index was defined as the mean BP divided by the 95th percentile for that subject. Normative data for ABPM were based on American Heart Association recommendations on ABPM for children and adolescents [16].
Blood Pressure Variability
Long-term BPV was assessed by visit-to-visit variability, using the mean casual BP measurement from each study visit leading up to and including the visit of the most recent neurocognitive assessment for each subject. Because of subject age and study vintage, the number of BP observations differed between subjects. Visit-to-visit BPV was assessed by two methods: 1) the standard deviation of visit BPs (BPV-SD), and 2) average real variability (ARV), with all BPs indexed to the 95th percentile for age, gender, and height for that subject [17]. The ARV method quantifies variability between adjacent readings and is defined by the following formula [6];
Short term BPV was evaluated using the ABPM session done prior to the most recent neurocognitive testing. ABPM and neurocognitive assessments were not done concurrently at any point in the CKiD study; by study design ABPM sessions and neurocognitive assessments were separated by 6 to 12 months. Ambulatory variability was measured as the standard deviation of the mean SBP and DBP for the wake and sleep periods [12]. Standard deviation of the 24hr mean BP was not used since that time period includes the nocturnal dip. All ambulatory BP measures were indexed to the 95th percentile for gender and height according to ambulatory norms [18].
Neurocognitive Assessments
This analysis evaluated subject performance on selected measures from the CKiD neurocognitive test battery, including Wechsler Abbreviated Scales of Intelligence (WASI) Matrix Reasoning subtest, Conners’ Continuous Performance Test-II (CPT-II) commission errors, and parent-rated Behavior Rating Inventory of Executive Function Global Executive Composite (BRIEF GEC). These measures were chosen because it was previously shown that CKiD participants were most likely to score more than 1 to 1.5 SD below the normative mean on these measures, and they are measures that have been used throughout all the years of the CKiD study [13]. In addition, based on the literature on hypertension and cognition, Wechsler Intelligence Scale for Children-IV (WISC-IV) Digit Span and Delis–Kaplan Executive Function System (D-KEFS) Verbal Fluency (Letter Fluency, Category Fluency, Category Switching) were included as measures of working memory and cognitive speed/efficiency. All of the assessments were administered or supervised by a licensed psychologist who was not aware of the subject’s BP status. To ensure quality control, neurocognitive data for the first two cases completed at each clinical site were reviewed by the Clinical Coordinating Center neuropsychologists. Thereafter, 25 % of the assessments were reviewed to verify administration fidelity, scoring accuracy, accuracy of translation of raw scores into standardized scores, and accuracy of transcription. All of the measures in the neurocognitive battery have good to excellent ratings of reliability [19].
Analysis
The data in this report are reported as the means ± SD, medians with interquartile ranges, or frequencies and percentages, as appropriate. To address the primary research question, whether children with increased BPV have poorer performance in measures of neurocognitive function, test scores of the subjects with BPV in the upper tertile were compared to those in the lower tertile in unadjusted analysis using 2-sided t tests. Systolic BPV and diastolic BPV were analyzed separately. For each neurocognitive measure, the score at the most recent neurocognitive assessment was evaluated so that the complete observed history of BP measurements could be utilized. For cognitive measures with 3 or more values over time, the annual change in score was also evaluated. This was not the case for D-KEFS Verbal Fluency as it was added to the neurocognitive battery relatively recently, thus limiting our ability to assess the annual change over time for that task. The D-KEFS Category Switching scores were reported as Switching Total score, the average of Accuracy and Total Correct scaled scores, since these scores correlated closely in our sample, indicating overlap of the cognitive abilities being tested.
To assess the association between BPV and neurocognitive performance, multiple linear regression was performed for each neurocognitive measure found to be different between the BPV groups. In the models for visit-to-visit variability, the main predictor was log2-transformed (BPV-SD or ARV), so the estimate can be interpreted as the change in neurocognitive outcome per doubling of BPV-SD or ARV. In the models for ambulatory variability, the main predictor is log2(ABPM SD), so the estimate can be interpreted as the change in neurocognitive outcome per doubling of SD, adjusted for the covariates. The multivariate analyses used all available BPV data, including the middle tertile, and evaluated the log linear relation between BPV and neurocognitive measures. Other variables controlled for in the regression models included mean corresponding BP index, sex, age, maternal education, African-American race, body mass index (BMI) percentile, eGFR [20], percentage of life with CKD, nephrotic proteinuria, and low birth weight. The significance level for all data analyses was set at p < 0.05. SAS 9.2 (SAS Institute, Cary, NC) and S-Plus 8.2 (TIBCO Software, Palo Alto, CA) were used for analysis and figure generation.
RESULTS
Sample Characteristics
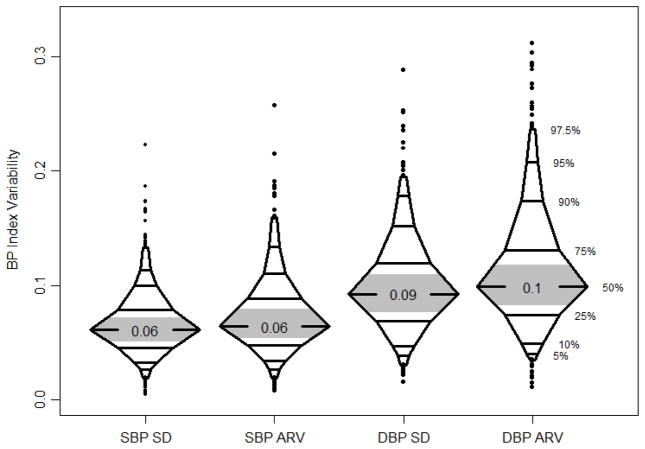
The study sample comprised 650 participants. At the time of the most recent neurocognitive assessment, the median age was 15.4 years, median duration of study follow-up was 4.0 years, and the median number of visits with casual BP measurement was 6. The sample was 62 % male, 22 % African-American, and the median eGFR was 47 ml/min/1.73m2. Table 1 shows sociodemographic and kidney disease-related characteristics of the sample. Figure 1 shows the median values and distribution of visit-to-visit systolic and diastolic BPV by both the BP-SD and ARV methods.
Table 1.
Demographic and kidney disease-related characteristics
| Characteristic | Value |
|---|---|
|
| |
| Age, y (median, IQR) | 15.4 [12.1, 18] |
|
| |
| Male (%, n) | 62% (403) |
|
| |
| African-American race (%, n) | 22% (140) |
|
| |
| Low birth weight (<2500 g) (%, n) | 19% (117) |
|
| |
| BMI percentile (median, IQR) | 64 [31, 90] |
|
| |
| eGFR (median, IQR) | 47 [30, 64] |
|
| |
| Nephrotic proteinuria (uP/C > 2) (%, n) | 18% (107) |
|
| |
| Glomerular etiology (%, n) | 26% (167) |
|
| |
| Percent of life with CKD (median, IQR) | 100% [82%, 100%] |
|
| |
| Years in study (median, IQR) | 4.0 [2.1, 6.1] |
|
| |
| Number of study visits (median, IQR) | 6 [4, 8] |
|
| |
| SBP Index (median, IQR) | 0.87 [0.83, 0.92] |
|
| |
| DBP Index (median, IQR) | 0.82 [0.76, 0.88] |
|
| |
| SBP or DBP ≥ 90th percentile | |
| at most recent visit | 152 (24%) |
| at any study visit | 385 (59%) |
|
| |
| Antihypertensive medication use (any) | 463 (71%) |
| ACE inhibitor/ARB | 363 (56%) |
| Calcium Channel Blocker | 119 (18%) |
| Other | 102 (16%) |
ACE: Angiotensin converting enzyme, ARB: Angiotensin receptor blocker, BMI: Body mass index, CKD: chronic kidney disease, DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, IQR: interquartile range, SBP: systolic blood pressure
Figure 1.
Median values and distribution of visit-to-visit systolic and diastolic BPV for both BP-SD and ARV. All BPs were indexed to the 95th percentile for age, gender, and height for that subject. The middle tertile is shaded in grey.
BPV: Blood pressure variability, BPV-SD: standard deviation of visit BPs, ARV: average real variability, DBP: diastolic blood pressure, SBP: systolic blood pressure
Visit-to-visit BPV and Neurocognitive Test Performance - Analysis of the most recent neurocognitive assessment
The number of subjects in each analysis varied by the particular neurocognitive test, ranging from 279 for D-KEFS Category Switching to 622 for parent BRIEF GEC. Bivariate analysis using the most recent neurocognitive assessment revealed that subjects with systolic visit-to-visit BPV in the upper tertile had lower scores (worse performance) on D-KEFS Verbal Category Fluency and Category Switching compared with subjects with BPV in the lower tertile, by both BPV-SD and ARV. In contrast, there was no significant difference between systolic visit-to-visit BPV groups in any other neurocognitive measure (Table 2 and 3). Multivariate linear regression analysis showed that increased systolic visit-to-visit BPV remained independently associated with lower scores on the D-KEFS Verbal Category Switching task for both BPV-SD and ARV, but not for Category Fluency (Table 4). Because Category Switching was performed in only 279 subjects, we examined the difference in demographics between those subjects with Category Switching and those who did not have Category Switching performed (n = 371). Subjects with Category Switching had less nephrotic proteinuria (11 % vs. 23 %, p <0.001), slightly shorter follow-up (3.8 vs. 4.1 years, p = 0.03), slightly lower average SBP index (0.87 vs. 0.87, p = 0.04), higher eGFR (56 vs. 36, p <0.001), and less percentage of life with CKD (100 vs. 100 %, p = 0.04). We adjusted for all of these demographic characteristics in the multivariate analyses. The group with Category Switching also had a lower proportion of subjects with systolic BPV in the upper tertile (BPV-SD, 27 vs 38 %, p = 0.02; ARV, 29 vs 36 %, p = 0.02).
Table 2.
Bivariate analysis comparing subjects with visit-to-visit systolic BPV-SD in the 3rd tertile to subjects in the 1st tertile
| Neurocognitive Variable (at most recent visit) | N | Systolic BP standard deviation | p-value (1st vs. 3rd) | ||
|---|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | |||
| Matrix Reasoning | 535 | 49.7 ± 10.5 | 48.7 ± 10.7 | 48.6 ± 10.1 | 0.31 |
| GEC (parent or self) | 622 | 53.1 ± 11.1 | 53.4 ± 12.4 | 52.5 ± 11.8 | 0.58 |
| Errors of Commission | 487 | 51.2 ± 12.2 | 51.2 ± 12.0 | 52.1 ± 11.3 | 0.46 |
| Digit Span Forward | 475 | 8.7 ± 2.9 | 8.4 ± 3.1 | 8.3 ± 2.9 | 0.28 |
| Digit Span Reverse | 475 | 9.5 ± 3.1 | 9.8 ± 3.3 | 9.1 ± 3.1 | 0.36 |
| Letter Fluency | 281 | 9.2 ± 3.4 | 9.4 ± 3.6 | 8.9 ± 3.2 | 0.55 |
| Category Fluency | 281 | 10.5 ± 3.4 | 10.6 ± 3.4 | 9.3 ± 3.9 | 0.03 |
| Category Switching* | 279 | 9.5 ± 2.8 | 9.4 ± 3.3 | 8.3 ± 3.0 | 0.006 |
| Category Contrast** | 279 | −1.1 ± 3.4 | −1.2 ± 3.4 | −1.0 ± 3.5 | 0.92 |
Note. Matrix Reasoning, GEC, and Errors of Commission are reported in T-Scores with a Mean = 50 and a Standard Deviation of 10. For Matrix Reasoning higher scores reflect a better performance; for GEC and Errors of Commission higher scores reflect a worse performance. Digit Span and D-KEF tasks are reported in scaled scores with a Mean = 10 and a Standard Deviation of 3, with higher scores reflecting a more intact performance.
average of Accuracy and Total scaled scores.
Contrast = Switching Scaled Score – Fluency Scaled Score. BP: blood pressure, BPV-SD: standard deviation of visit BPs, GEC: Global Executive Composite
Table 3.
Bivariate analysis comparing subjects with visit-to-visit systolic ARV in the 3rd tertile to subjects in the 1st tertile.
| Neurocognitive Variable (at most recent visit) | N | Systolic BP ARV | p-value (1st vs. 3rd) | ||
|---|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | |||
| Matrix Reasoning | 535 | 49.4 ± 10.9 | 50.1 ± 9.4 | 47.7 ± 10.7 | 0.13 |
| GEC (parent or self) | 622 | 52.7 ± 11.5 | 52.7 ± 11.5 | 53.6 ± 12.2 | 0.44 |
| Errors of Commission | 487 | 50.7 ± 12.4 | 51.2 ± 12.1 | 52.6 ± 10.9 | 0.16 |
| Digit Span Forward | 475 | 8.6 ± 2.9 | 8.6 ± 3.2 | 8.2 ± 2.8 | 0.17 |
| Digit Span Reverse | 475 | 9.6 ± 3.1 | 9.8 ± 3.4 | 9.0 ± 3.1 | 0.09 |
| Letter Fluency | 281 | 9.4 ± 3.0 | 9.5 ± 3.8 | 8.6 ± 3.5 | 0.08 |
| Category Fluency | 281 | 10.6 ± 3.5 | 10.3 ± 3.4 | 9.5 ± 3.8 | 0.03 |
| Category Switching | 279 | 9.6 ± 2.6 | 9.2 ± 3.4 | 8.5 ± 3.2 | 0.02 |
| Category Contrast | 279 | −1.2 ± 3.7 | −1.1 ± 3.4 | −1.0 ± 3.3 | 0.70 |
Note. Matrix Reasoning, GEC, and Errors of Commission are reported in T-Scores with a Mean = 50 and a Standard Deviation of 10. For Matrix Reasoning higher scores reflect a better performance; for GEC and Errors of Commission higher scores reflect a worse performance. Digit Span and D-KEF tasks are reported in scaled scores with a Mean = 10 and a Standard Deviation of 3, with higher scores reflecting a more intact performance.
average of Accuracy and Total scaled scores.
Contrast = Switching – Fluency.
BP ARV: blood pressure average real variability, GEC: Global Executive Composite
Table 4.
The association of increased indexed visit-to-visit BPV and Verbal Category Fluency and Category Switching scores in separate multiple linear regression analyses for systolic BPV-SD and ARV.
| Neurocognitive Variable (at most recent visit) | N | Visit-to-visit systolic BPV
|
||
|---|---|---|---|---|
| Estimate | 95% CI | p-value | ||
|
| ||||
| Category Fluency | 222 | |||
| BPV-SD | −0.21 | (−0.92, 0.51) | 0.57 | |
| ARV | −0.32 | (−0.96, 0.32) | 0.33 | |
|
| ||||
| Category Switching | 220 | |||
| BPV-SD | −0.70 | (−1.28, −0.12) | 0.02 | |
| ARV | −0.54 | (−1.05, −0.02) | 0.04 | |
All regression models adjusted for mean corresponding BP index, sex, age, maternal education, African-American race, BMI percentile, eGFR, percent of life with CKD, nephrotic proteinuria, and low birth weight.
ARV: average real variability, BMI: body mass index, BP: blood pressure, BPV: blood pressure variability, BPV-SD: standard deviation of visit BPs, CI: confidence interval, CKD: chronic kidney disease, eGFR: estimated glomerular filtration rate
With regard to diastolic visit-to-visit BPV, bivariate analysis showed no difference between groups for most measures, except a small difference in CPT-II commission errors (1st tertile vs. 3rd tertile, 50.7 ± 12.4 vs. 52.6 ± 10.9, p = 0.04) for ARV, but not BPV-SD. However, the difference by ARV did not remain significant after adjustment for covariates on multivariate linear regression (β = −1.51, p = 0.07).
Visit-to-visit BPV - Analysis of the annual change in neurocognitive test performance
Subjects with systolic visit-to-visit BPV in the upper tertile had slightly different annual change in scores on the parent BRIEF GEC compared with subjects with BPV in the lower tertile. However, these differences did not remain significant after adjustment for covariates on multivariate linear regression (BPV-SD, β = −0.29, p = 0.35; ARV, β = −0.38, p = 0.16). There were no other differences on bivariate analysis of systolic or diastolic BPV for any neurocognitive measure using annual change in scores (data not shown). We were not able to analyze the annual change in D-KEFS Verbal Fluency scores as too few subjects had at least 3 study visits with that measure.
Ambulatory BPV
On analysis of the relation between ambulatory BPV and the most recent neurocognitive assessment, the only statistically significant difference was for WASI Digit Span Forward (systolic sleep, 1st vs. 3rd tertile, 8.0 ± 2.7 vs. 9.0 ± 3.0, p = 0.01; diastolic wake, 7.9 ± 2.8 vs. 9.0 ± 2.7, p = 0.005). However, these differences did not remain significant after adjustment for covariates on multivariate linear regression (systolic sleep, β = 0.48, p = 0.27; diastolic wake, β = 0.99, p = 0.11). On analysis of the relation between ambulatory BPV and the annual change in neurocognitive test performance, the only statistically significant difference was for the annual change in BRIEF GEC for systolic sleep (1st vs. 3rd tertile, −0.13 ± 2.21 vs. −1.30 ± 2.80, p = 0.01). However, this difference did not remain significant after adjustment for covariates on multivariate linear regression (β = −0.44, p = 0.34). There was no difference for any other neurocognitive measure for any ABPM parameter (full data for the ABPM bivariate and multivariate analyses are available in the Electronic Supplementary Tables 1a–d and 2a–d).
DISCUSSION
This study was conducted to determine whether children with CKD and increased BPV had poorer performance on selected tests of neurocognitive function when compared to the performance of children with CKD and lower BPV, and to examine the relative contribution of BPV to neurocognitive outcomes, taking into account mean BP and sociodemographic and disease-related variables. The results showed that increased visit-to-visit systolic BPV in children with mild-to-moderate CKD was independently associated with lower scores (worse performance) on the Category Switching Task of the D-KEFS Verbal Fluency Test by both BPV-SD and ARV.
In contrast, increased systolic visit-to-visit BPV was not associated with performance on any of the other measures. No relation was uncovered between neurocognitive test performance and diastolic visit-to-visit BPV in this sample. In contrast to the finding of an association between systolic visit-to-visit BPV and worse neurocognitive test performance, we did not find any independent relation between ambulatory BPV and any neurocognitive measure. However, these results were limited by the fact that the ABPM sessions in CKiD were obtained 6 – 12 months apart from the neurocognitive testing, by study design. In addition, it has been shown that the correlation between visit-to-visit BPV and ambulatory BPV is weak, suggesting that they are not interchangeable entities [21].
The D-KEFS Verbal Fluency Test was included in the current study because both CKD and primary hypertension in adults have been associated with performance deficits in neurocognitive measures of cognitive efficiency and processing speed [22, 23]. The Category Switching subtest of the D-KEFS Verbal Fluency Test requires the subject to alternate between saying words from two different semantic categories. Category switching falls within a group of executive function tasks called set shifting, the mental ability to adjust thinking or attention in response to changing expectations, goals, or environmental stimuli [24]. The finding that children with CKD and increased long term BPV had worse performance on the Category Switching Task suggests that children with CKD may have difficulties with this component of executive functioning that are related, in part, to increased BPV.
This study has several limitations. Because D-KEFS Verbal Fluency was added to the CKiD neurocognitive test battery only recently, we could not analyze the relationship between increased BPV and the longitudinal annual change of Category Switching over time. Therefore, the cross sectional nature of that part of the analysis limits inference about causality between increased BPV and lower Category Switching scores. Although the relation between increased BPV and lower Category Switching scores is intriguing, it is an isolated finding. Other measures of verbal speed/cognitive efficiency and set shifting will need to be examined in relation to elevated BPV in order to support the findings obtained from this study. Nonetheless, our previous work suggests that measures of executive function may be most sensitive to the effects of childhood CKD [25]. We excluded children less than 6 years old. While younger age is associated with lower systolic BPV in children with CKD [12], to our knowledge the effect of age on BPV in children with CKD who are less than six years old has not been reported. Lastly, because the effects of BP on neurocognitive test performance are known to be subtle, we did not adjust the bivariate analysis for multiple comparisons. Although this approach permits the detection of more subtle differences, it could increase the risk of a type 1 error.
In summary, we report the novel finding of an association between higher systolic visit-to-visit BPV and poorer neurocognitive function in children with CKD. This finding is particularly notable given the mild-to-moderate degree of CKD in the participants. Further studies are warranted to determine the significance of this isolated finding. The results are of potential clinical significance, in that some antihypertensive medications lower BPV as well as mean BP level [26]. Confirmation of these findings would raise the question as to whether treating hypertension with medications that ameliorate BPV as well as lower mean BP level would improve cognition, particularly cognitive flexibility, in children with CKD.
Supplementary Material
Supplementary Table 1a. Bivariate analyses comparing subjects with ABPM wake systolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 1b. Bivariate analyses comparing subjects with ABPM sleep systolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 1c. Bivariate analyses comparing subjects with ABPM wake diastolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 1d. Bivariate analyses comparing subjects with ABPM sleep diastolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 2a. The association of increased indexed ABPM wake systolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.
Supplementary Table 2b. The association of increased indexed ABPM sleep systolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.
Supplementary Table 2c. The association of increased indexed ABPM wake diastolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.
Supplementary Table 2d. The association of increased indexed ABPM sleep diastolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children's Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children's Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid
Footnotes
Conflict of Interest Statement: The authors report that they have no conflict of interest.
Compliance with ethical standards
The study protocol was approved by the Institutional Review Boards and informed consent was obtained at each participating center from all individual participants included in the study. The research activities being reported here adhere to the Declaration of Helsinki.
This work was presented in abstract form at the American Society of Nephrology Kidney Week 2015.
References
- 1.Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012;14:421–431. doi: 10.1007/s11906-012-0290-7. [DOI] [PubMed] [Google Scholar]
- 2.McMullan CJ, Lambers Heerspink HJ, Parving H, Dwyer JP, Forman JP, de Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: A post hoc analysis from the RENAAL study and the irbesartan diabetic nephropathy trial. Am J Kidney Dis. 2014;64:714–722. doi: 10.1053/j.ajkd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, Muntner P. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: A systematic review and meta-analysis. Hypertension. 2014;64:965–982. doi: 10.1161/HYPERTENSIONAHA.114.03903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu J, Cha R, Kim DK, Lee JH, Yoon SA, Ryu DR, Oh JE, Kim S, Han S, Lee EY, Kim YS APrODiTe investigators. The clinical association of the blood pressure variability with the target organ damage in hypertensive patients with chronic kidney disease. J Korean Med Sci. 2014;29:957–964. doi: 10.3346/jkms.2014.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 6.Hastie CE, Jeemon P, Coleman H, McCallum L, Patel R, Dawson J, Sloan W, Meredith P, Jones GC, Muir S, Walters M, Dominiczak AF, Morrison D, McInnes GT, Padmanabhan S. Long-term and ultra long-term blood pressure variability during follow-up and mortality in 14,522 patients with hypertension. Hypertension. 2013;62:698–705. doi: 10.1161/HYPERTENSIONAHA.113.01343. [DOI] [PubMed] [Google Scholar]
- 7.Sakakura K, Ishikawa J, Okuno M, Shimada K, Kario K. Exaggerated ambulatory blood pressure variability is associated with cognitive dysfunction in the very elderly and quality of life in the younger elderly. Am J Hypertens. 2007;20:720–727. doi: 10.1016/j.amjhyper.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Epstein NU, Lane KA, Farlow MR, Risacher SL, Saykin AJ, Gao S Alzheimer's Disease Neuroimaging Initiative. Cognitive dysfunction and greater visit-to-visit systolic blood pressure variability. J Am Geriatr Soc. 2013;61:2168–2173. doi: 10.1111/jgs.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crichton GE, Elias MF, Dore GA, Torres RV, Robbins MA. Measurement-to-measurement blood pressure variability is related to cognitive performance: The maine syracuse study. Hypertension. 2014;64:1094–1101. doi: 10.1161/HYPERTENSIONAHA.114.04282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: The honolulu-asia aging study. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 11.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: The coronary artery risk development in young adults (CARDIA) study. Hypertension. 2014;64:983–988. doi: 10.1161/HYPERTENSIONAHA.114.03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barletta GM, Flynn J, Mitsnefes M, Samuels J, Friedman LA, Ng D, Cox C, Poffenbarger T, Warady B, Furth S. Heart rate and blood pressure variability in children with chronic kidney disease: A report from the CKiD study. Pediatr Nephrol. 2014;29:1059–1065. doi: 10.1007/s00467-013-2737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, Shinnar S, Wentz A, Matheson M, Cox C, Furth SL, Warady BA. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1824–1830. doi: 10.2215/CJN.09751110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA. Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA Chronic Kidney Disease in Children Study Group. Blood pressure in children with chronic kidney disease: A report from the chronic kidney disease in children study. Hypertension. 2008;52:631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee. Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: A scientific statement from the american heart association atherosclerosis, hypertension, and obesity in youth committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 17.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 18.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM American Heart Association Atherosclerosis,Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the american heart association. Hypertension. 2014;63:1116–1135. doi: 10.1161/HYP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straus E, Sherman EMS, Spreen O, editors. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford University Press; New York: 2006. [Google Scholar]
- 20.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntner P, Shimbo D, Diaz KM, Newman J, Sloan RP, Schwartz JE. Low correlation between visit-to-visit variability and 24-h variability of blood pressure. Hypertens Res. 2013;36:940–946. doi: 10.1038/hr.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jassal SV, Roscoe J, LeBlanc D, Devins GM, Rourke S. Differential impairment of psychomotor efficiency and processing speed in patients with chronic kidney disease. Int Urol Nephrol. 2008;40:849–854. doi: 10.1007/s11255-008-9375-2. [DOI] [PubMed] [Google Scholar]
- 23.Waldstein SR. Hypertension and neuropsychological function: A lifespan perspective. Exp Aging Res. 1995;21:321–352. doi: 10.1080/03610739508253989. [DOI] [PubMed] [Google Scholar]
- 24.Scott WA. Cognitive complexity and cognitive flexibility. Sociometry. 1962;25:405–415. [Google Scholar]
- 25.Mendley SR, Matheson MB, Shinnar S, Lande MB, Gerson AC, Butler RW, Warady BA, Furth SL, Hooper SR. Duration of chronic kidney disease reduces attention and executive function in pediatric patients. Kidney Int. 2015;87:800–806. doi: 10.1038/ki.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1a. Bivariate analyses comparing subjects with ABPM wake systolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 1b. Bivariate analyses comparing subjects with ABPM sleep systolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 1c. Bivariate analyses comparing subjects with ABPM wake diastolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 1d. Bivariate analyses comparing subjects with ABPM sleep diastolic BP standard deviation in the 3rd tertile to subjects in the 1st tertile for the latest available neurocognitive assessment and for the annual change in neurocognitive test scores.
Supplementary Table 2a. The association of increased indexed ABPM wake systolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.
Supplementary Table 2b. The association of increased indexed ABPM sleep systolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.
Supplementary Table 2c. The association of increased indexed ABPM wake diastolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.
Supplementary Table 2d. The association of increased indexed ABPM sleep diastolic standard deviation with neurocognitive test scores in separate multivariate analyses for most recent Digit Span Forward and BRIEF GEC annual change.