Abstract
Based on studies that extend back to the early 1900s, regression and stabilization of atherosclerosis in humans has gone from a concept to one that is achievable. Successful attempts at regression generally applied robust measures to improve plasma lipoprotein profiles. Possible mechanisms responsible for lesion shrinkage include decreased retention of atherogenic apolipoprotein B within the arterial wall, efflux of cholesterol and other toxic lipids from plaques, emigration of lesional foam cells out of the arterial wall, and influx of healthy phagocytes that remove necrotic debris as well as other components of the plaque. Currently available clinical agents, though, still fail to stop most cardiovascular events. For years, HDL has been considered the “good cholesterol.” Clinical intervention studies to causally link plasma HDL-C levels to decreased progression or to the regression of atherosclerotic plaques, are relatively few because of the lack of therapeutic agents to selectively and potently raise HDL-C. The negative results of studies that were performed have casted uncertainty as to the role HDL possesses in terms of atherosclerosis. It is becoming clearer, though, that HDL function rather than quantity is most crucial and therefore, discovering agents that enhance the quality of HDL should be the goal.
Keywords: atherosclerosis, HDL, regression, macrophages, CCR7
PLAQUE REGRESSION-EVIDENCE FROM ANIMAL STUDIES
Regression of atherosclerosis-is it possible?
The idea that human atheromata can regress at all has met considerable resistance over the decades (1–3). Resistance to the idea of lesion regression has been due to the fact that advanced atheromata in humans and in animal models contain components that give an impression of permanence, such as necrosis, calcification and fibrosis. Furthermore, numerous theories have been proposed to explain atherogenesis that included processes thought to be difficult, if not impossible, to reverse including injury (4–5), oxidation (6), and cellular transformations resembling carcinogenesis (7). In this review, data will be presented that demonstrate that changes in the plaque environment can stabilize and regress even advanced lesions.
In the 1920s, Anichkov and colleagues reported that switching cholesterol-fed rabbits to low-fat chow over a 2–3 year period resulted in arterial lesions becoming more fibrous with a reduced lipid content (8), which from a modern perspective suggests plaque stabilization (9–10). The first prospective, interventional study demonstrating substantial shrinkage of atherosclerotic lesions was performed in cholesterol-fed rabbits over fifty years ago (11). The dietary regimen raised total plasma cholesterol to approximately 26 mmol/l (~1,000 mg/dl) and induced widespread lesions involving ~90% of the aorta. Animals received intravenous bolus injections of phosphatidylcholine (PC). Interestingly, after less than a week and a half of treatment, the remaining plaques were scattered and far less severe than initially, and three-quarters of arterial cholesterol stores had been removed. Williams and colleagues sought to determine the underlying mechanism of action (12–13). Initially, cholesterol-free PC liposomes remain intact in the circulation (14) and can mobilize cholesterol from tissues in vivo (14–17) by acting as high-capacity sinks into which endogenous HDL cholesterol shuttles lipid (12, 18–19).
The concept of regression gained support with a short-term study in squirrel monkeys by Maruffo and Portman (20), and more-extensive work by Armstrong and colleagues. The latter reported that advanced arterial lesions in cholesterol-fed Rhesus monkeys underwent shrinkage and remodeling during long-term follow-up when their diet was switched to low-fat or linoleate-rich diets (21–22). Further work by Wissler and Vesselinovich as well as Malinow confirmed and extended these findings (8, 23). Armstrong summarized the findings by writing, “In the primate the answer is clear: all grades of induced lesions studied to date improve … the primate lesion shows amazing metabolic responsiveness: some extracellular as well as intracellular lipid is depleted, there is resolution of necrotic lesions, crystalline lipid tends to diminish slowly, and fibroplasia is eventually contained” (22). Later, a series of studies achieved shrinkage of atheromata in rabbits with injections of HDL or HDL-like apolipoprotein A-I (apoA-I) and PC disks (24–25) supporting the concept that removal of cholesterol from the plaque can allow healing.
Unlike humans, mice have a naturally high plasma HDL:LDL ratio, providing a strong intrinsic resistance to atherosclerosis. Drastic manipulations of plasma lipoproteins are required, therefore, to induce arterial lipoprotein accumulation and sequelae. Most mouse models of atherosclerosis are derived from two basic models: the apolipoprotein E (apoE)-null (apoE−/−) mouse (26–27) and the LDL receptor-null (LDLR−/−) mouse (28). In these models, the normally low plasma apoB levels are increased to atherogenic levels by eliminating either a ligand (apoE−/−) or a receptor (LDLR−/−) for lipoprotein clearance. The ability of HDL-like particles to rapidly remodel plaques in mice was shown by infusion of apoA-IMilano/PC complexes, a variant of apolipoprotein A-I identified in individuals who exhibit very low HDL cholesterol levels. Infusion of this complex reduced foam cell content in arterial lesions in apoE−/− mice within 48 hours (29).
Transplantation murine model of atherosclerosis regression
To further explore cellular and molecular mechanisms of atherosclerosis regression in murine models, new approaches to rapidly induce robust improvements in the plaque environment and trigger lesion remodeling and regression was necessary. This culminated in the development of a model involving the transplantation of a plaque-containing aortic segment from a (WD-fed) hyperlipidemic apoE−/− mouse (i.e. an extremely pro-atherogenic milieu consisting of high plasma apoB levels and low HDL-cholesterol levels), into a wild-type recipient (i.e. rapidly normalizing the lipoprotein environment, which is sustainable indefinitely).
We found that transplanting early lesions (30–31) or advanced, complicated plaques into wild-type recipients substantially reduced foam cell content and increased the number of smooth muscle cells, particularly in the cap, which is consistent with plaque stabilization and regression (32–33). The loss of foam cells from early lesions was surprisingly rapid, with large decreases evident as early as three days post-transplantation (Figure 1) (30–31). With advanced lesions, all features regressed after nine weeks, including necrosis, cholesterol clefts and fibrosis (32–33). We found that the wild-type milieu provoked foam cells to display markers characteristic of both macrophages and, surprisingly, dendritic cells, which enabled emigration (30–31, 34).
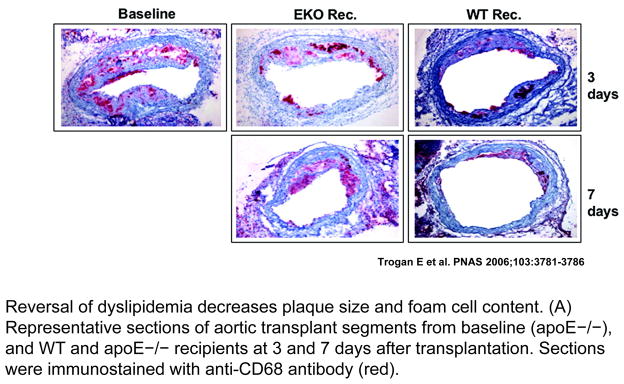
Figure 1. Regression of plaques in the mouse transplantation model.
ApoE−/− mice were fed a Western diet for 16 weeks to develop advanced atherosclerosis. Aortic arches from these mice were either harvested and analyzed by histochemical methods, or they were transplanted into apoE−/− (‘progression’) or wild-type (‘regression’) recipient mice. Three or seven days later, the same analyses were performed. Shown are the histochemical results for the foam-cell marker CD68 (red). The pictures show the immunostaining of representative aortic lesions in cross section. The virtual absence of foam cells can be seen in the ‘regression’ group. In contrast with the ‘regression’ results, the ‘progression’ group showed persistence of foam cells. (Adapted from Trogan E, Feig JE et al. PNAS 2006;103:3781–3786).
Using laser microdissection to remove foam cells from regressing and non-regressing plaques (35–36), analyses revealed the presence of mRNA for CCR7 (31), chemokine (C-C motif) receptor 7, which is required for dendritic cell emigration (37). Injection of wild-type recipient animals with antibodies against the two CCR7 ligands, CCL19 and CCL21, inhibited the majority of foam cells from emigrating from the aortic transplant lesions—establishing a functional role for CCR7 in regression (31). In addition, mRNA concentrations of several well-known proteins implicated in atherothrombosis, such as vascular cell adhesion protein-1 (VCAM-1), monocyte chemotactic protein 1 (MCP-1) and tissue factor, were determined to be decreased in foam cells during regression (31).
HDL AND PLAQUE REGRESSION
Epidemiologic studies have demonstrated that there exists a strong negative correlation between plasma HDL cholesterol (HDL-C) and the risk of cardiovascular disease (38–42). Recent insights have added to the potential mechanisms, which include the stimulation of reverse cholesterol transport (RCT) from foam cells in coronary plaques to the liver, protection of the endothelium (by activation of the eNOS pathway), and inhibition of LDL oxidation (3, 43–45).
HDL and Reverse Cholesterol Transport
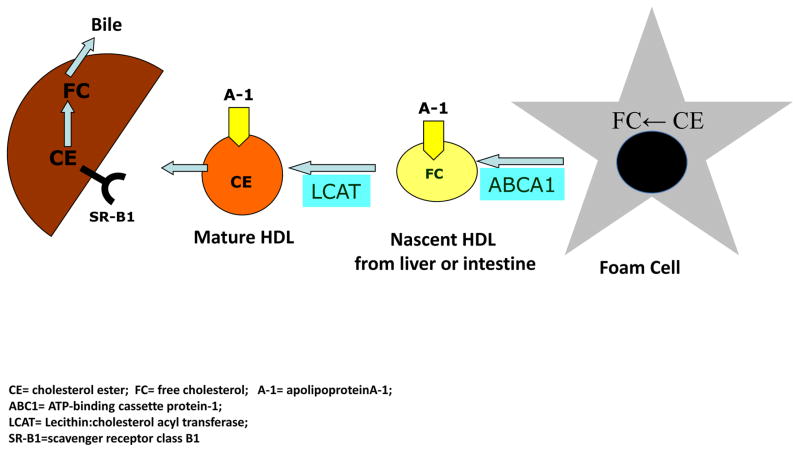
Lipid-free apoAI and lipid poor pre-β-HDL particles are produced in the liver and intestine. Cholesterol becomes associated with these HDL particles, and is then esterified by lecithin-cholesterol acyltransferase (LCAT). Cholesteryl ester (CE) moves to the developing core of the HDL particles, which converts them to spheres and also allows their surfaces to accept more free cholesterol. In human plasma, there is a reciprocal exchange of HDL-CE for triglycerides carried on apoB-containing lipoproteins, which is mediated by cholesteryl ester transfer protein (CETP). At the same time, the HDL that is becoming enriched in triglycerides is a substrate for hepatic lipase. The CE are subsequently cleared by the liver when the apoB-lipoproteins undergo hepatic uptake through LDL-receptor dependent and independent mechanisms. The activities of CETP and hepatic lipase help to remodel HDL particles to become a preferred binding partner for scavenger receptor type BI (SR-BI), the major HDL receptor on hepatocytes. Hence, RCT can be considered a cycle in which acceptors of cholesterol are continuously regenerated to undertake their function of promoting cholesterol efflux from the peripheral tissues to the liver (Figure 2) (45–48).
Figure 2. Reverse cholesterol transport and HDL metabolism.
Cholesterol from the periphery (macrophage) is effluxed and participates in the maturation of HDL ultimately returning to the liver and beginning the cycle again.
Traditionally, the anti-atherogenic role of HDL has been attributed to the presence of apoAI. For example, transgenic mice with high plasma human apolipoprotein AI and HDL plasma levels were protected from development of fatty streak lesions when fed an atherogenic diet (49). Furthermore, overexpression of human apoAI in apoE−/− mice resulted in greatly retarded progression of atherosclerosis (50–51). While it is clear that HDL is a key player in RCT, it has important cellular partners. The ATP-binding cassette transporters ABCA-1 and ABCG-1 are increased by liver X receptor transcription factors, key regulators of cholesterol homeostasis (52–54). A single deficiency of ABCA-1 in mice results in a moderate increase in lesion development, and deficiency of ABCG-1 has no effect; however, combined deficiency resulted in markedly accelerated atherosclerosis (55). Double-knockout macrophages showed markedly defective cholesterol efflux to HDL and apoAI as well as increased inflammatory responses when treated with lipopolysaccharide (56) emphasizing the anti-atherogenic effects of these lipoproteins.
Beyond Reverse Cholesterol Transport
Endothelial dysfunction is one of the early hallmarks in the pathogenesis of atherosclerosis (57). It was shown that oxidized LDL-induced displacement of endothelial nitric oxide synthase (eNOS) from caveolae and impairment of NO production was prevented in the presence of HDL (58). It has also been demonstrated that the apoAI mimetics, L-4F and D-4F, protect endothelial cell function in mice by inhibiting native and oxidized LDL’s uncoupling of eNOS activity, thereby preventing superoxide production from overtaking that of NO (59). Independent of the ability to counteract adverse effects of LDL and oxidized LDL, it also has been shown that HDL promotes eNOS activation and NO release, resulting in vasorelaxation (60–61). Experiments in vivo that support a positive role for HDL in promoting endothelial health include a study in which carotid artery re-endothelialization following perivascular electric injury was diminished in apoAI-null mice, but was normalized by the restoration of apoAI (62–63). Furthermore, Theilmeier and colleagues showed that overexpression of human apoAI in apoE−/− mice reduced endothelial adhesion molecule expression and macrophage homing to the endothelium (64).
In addition to the studies on endothelium, a growing body of research suggests that HDL counteracts a number of the adverse effects of LDL oxidation. Current thinking attributes some of this protection to anti-oxidant properties of HDL, particularly related to its content of α-tocopherol and other lipophilic anti-oxidants, as well as enzymes with antioxidant-like activities (platelet activating factor acetylhydrolase (PAF-AH) and paraoxonase (PON)). ApoAI, which possesses several methionine groups, may act directly as an anti-oxidant (45, 65–66). Anti-oxidant effects would be expected to prevent the formation of lipid hydroperoxides (LOOX), oxidized cholesteryl esters, and oxidized phospholipids. The oxidized lipid species are generated in a process that requires the presence of “seeding molecules,”(e.g., hydroperoxyoctadecadienoic acid [HPODE]) as catalysts, which are generated by 12-lipoxygenase. In fact, HDL was demonstrated to limit the levels of these seeding molecules and to degrade them in an enzymatic process catalyzed by PON and PAF-AH (65–69)
Pre-clinical Studies linking HDL and Atherosclerosis Regression
A series of studies achieved shrinkage of atheromata in hypercholesterolemic rabbits via injections of HDL (24) as well as demonstrating the anti-atherogenic effects of apoAI in cholesterol-fed rabbits (25). We used a model which allowed us to begin understanding the mechanism of atherosclerosis regression. At least three plasma parameters are changed in the transplantation model when regression was observed: (i) non-HDL levels decreased; (ii) HDL levels were restored from ~33% of normal to wild type levels; (iii) apoE was now present. For the purpose of this review, we will focus on the HDL change. To selectively test this as a regression factor, we adopted the transplant approach by using as recipients human apoAI transgenic/apoE−/− mice (hAI/EKO) (70) or apoAI−/− mice (71–72). Briefly, plaque-bearing aortic arches from apoE−/− mice (low HDL-C, high non-HDL-C) were transplanted into recipient mice with differing levels of HDL-C and non-HDL-C: C57BL/6 mice (normal HDL-C, low non-HDL-C), apoAI−/− mice (low HDL-C, low non-HDL-C), or hAI/EKO mice (normal HDL-C, high non-HDL-C). Remarkably, despite persistent elevated non-HDL-C in hAI/EKO recipients, plaque CD68+ cell content decreased by >50% by one week after transplantation, whereas there was little change in apoAI−/− recipient mice despite hypolipidemia. Interestingly, the decreased content of plaque CD68+ cells was associated with their emigration and induction of their chemokine receptor CCR7 (71). These data are consistent with a meta-analysis of clinical studies in which it was shown that atherosclerosis regression (assessed by intravascular ultrasound, IVUS) after LDL lowering was most likely to be achieved when HDL was also significantly increased (73).
The induction of CCR7 is likely related to changes in the sterol content of foam cells when they are placed in a regression environment, given that its promoter has a putative sterol regulatory element (SRE). This idea is in agreement with a report that demonstrated that loading THP-1 human monocytes with oxidized LDL suppresses the expression of this gene (74). Notably, we have found that statins, potent regulators of SRE-dependent transcription can induce CCR7 expression in vivo and promote regression via emigration of CD68+ cells in a CCR7 dependent manner (75).
Another aspect of interest has been the effect of HDL on the inflammatory state of CD68+ cells in plaques. A number of benefits from this can be envisioned such as a reduced production of monocyte attracting chemokines and plaque “healing” by macrophages prodded to become tissue re-modelers (M2 macrophages). There are multiple reasons for HDL to have anti-inflammatory effects on plaques, including the anti-oxidant properties of its enzymatic and non-enzymatic components, the ability to remove normal and toxic lipid species from cells, and the dampening of TLR signaling by regulating plasma membrane cholesterol content (45, 56, 76). It is important to note that in CD68+ cells laser-captured from the plaques, normalization of HDL-C led to decreased expression of inflammatory factors and enrichment of markers of the M2 macrophage state (71, 77–78).
Cholesterol homeostasis has also recently been investigated with microRNAs (miRNA), which are small endogenous non–protein-coding RNAs that are posttranscriptional regulators of genes involved in physiological processes. MiR-33, an intronic miRNA located within the gene encoding sterol-regulatory element binding protein-2, inhibits hepatic expression of both ABCA-1 and ABCG-1, reducing HDL-C concentrations, as well as ABCA-1 expression in macrophages, thus resulting in decreased cholesterol efflux (79). The treated mice exhibited plaque regression (fewer macrophages and smaller intimal area). The therapeutic potential of miR-33 antagamirs to cause similar benefits in people was suggested by plasma levels of HDL being raised in treated non-human primates (80). Thus, antagonism of miR-33 may represent a novel approach to enhancing macrophage cholesterol efflux and raising HDL-C levels in the future.
EVIDENCE FROM CLINICAL STUDIES
Statins, HDL Infusions, Niacin and CETP Inhibitors
The first prospective, interventional study to demonstrate plaque regression in humans was in the mid-1960s, in which approximately 10% of patients (n = 31) treated with niacin showed improved femoral angiograms (81). Larger trials of lipid lowering have since shown angiographic evidence of regression; however, though statistically significant, the effects were surprisingly small, particularly in light of large reductions in clinical events (1–3, 82). This ‘angiographic paradox’ was resolved with the realization that lipid-rich, vulnerable plaques have a central role in acute coronary syndromes. A vulnerable plaque is characterized by being small, causing less than 50% occlusion, and being full of intracellular and extracellular lipid, rich in macrophages and tissue factor, with low concentrations of smooth muscle cells, and with only a thin fibrous cap under an intact endothelial layer (9–10, 83–84). Rupture of a vulnerable plaque provokes the formation of a robust local clot, and hence vessel occlusion and acute infarction (57). Lipid lowering, which promoted measurable shrinkage of angiographically prominent but presumably stable lesions, probably had a greater impact on risk reduction by the remodeling and stabilization of small, rupture-prone lesions (82, 84). Regression studies in animal models strongly support this interpretation, given that macrophage content, a key hallmark of instability, can be rapidly corrected with robust improvements in the plaque lipoprotein environment.
In order to track potentially more important changes in plaque composition, to avoid the confounding effects of lesion remodeling on lumen size, arterial wall imaging is required. Recent human trials have switched from quantitative angiography, which images only the vascular lumen, to techniques that image plaque calcium (e.g. electron-beam CT) and plaque volume (e.g. intravascular ultrasonography; IVUS). A retrospective analysis found that aggressive LDL-cholesterol lowering with statins correlated significantly with reduction in coronary calcium-volume score by electron-beam CT, indicating that coronary artery calcifications can shrink (85). In the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) study (86) and A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID) (87), patients with acute coronary syndromes were treated for over a year with high-dose statins and evaluated by IVUS. The REVERSAL trial compared the high-dose statin therapy with a conventional, less-potent statin regimen. During 18 months of treatment, patients treated with the conventional regimen exhibited statistically significant progression of atheroma volume (+2.7%), despite achieving average LDL-cholesterol levels of 2.8 mmol/l (110 mg/dl) and, therefore, meeting the then-current Adult Treatment Panel III goal. By contrast, the high-dose statin group experienced no significant progression of atheroma volume (average LDL-cholesterol level, 2 mmol/l [79 mg/dl]). Importantly, analysis across the treatment groups found that LDL reduction exceeding approximately 50% was associated with a decrease in atheroma volume. In ASTEROID, all patients received the same high-dose therapy for 24 months, and IVUS findings pretreatment and posttreatment were compared. During treatment, LDL cholesterol dropped to 1.6 mmol/l (60.8 mg/dl), and atheroma volume shrank by a median of 6.8%. Thus, in both of these studies, extensive LDL-cholesterol lowering for extended periods caused established plaques to shrink. The greater efficacy seen in ASTEROID could be explained by the lower median LDL-cholesterol level, but also by the longer treatment period and higher HDL cholesterol levels achieved than those in REVERSAL. We believe that these reductions in plaque volume are accompanied by favorable alterations in plaque biology, a theory which is further supported by evidence that robust plasma LDL lowering to 1.0–1.6 mmol/l or below (≤40–60 mg/dl) is associated with further reductions in cardiovascular events (88).
In addition to the studies reviewed above, there are a limited number of human studies in which HDL levels have been manipulated by infusion, and the effects on plaques assessed. In the first (89), patients at high risk for cardiovascular disease were infused with either an artificial form of HDL (apoAI milano/phospholipid complexes) or saline (placebo) once a week for 5 weeks. By intravascular ultrasound (IVUS), there was a significant reduction in atheroma volume (−4.2%) in the combined (high and low dose) treatment group, though no dose response was observed of a higher vs. lower dose of the artificial HDL. There was no significant difference in atheroma volume compared to the placebo group, but the study was not powered for a direct comparison. In the second infusion study (90), high-risk patients received four weekly infusions with reconstituted HDL (rHDL containing wild type apoAI) or saline. Similar to the previous study, there was a significant decrease in atheroma volume (−3.4%) (assessed by IVUS) after treatment with rHDL compared to baseline, but not compared to placebo (which the study was not powered for). However, the rHDL group had statistically significant improvements in plaque characterization index and in a coronary stenosis score on quantitative coronary angiography compared to the placebo group. In the third infusion trial (91), a single dose of reconstituted human HDL was infused into patients undergoing femoral atherectomies, with the procedure performed 5–7 days later. Compared to the control group (receiving saline solution), in the excised plaque samples in the HDL infusion group, macrophage activation state (i.e., VCAM-1 expression) as well as cell size (i.e., lipid content) were diminished.
There are also a number of other drug studies in which effects on plaques were ascribed to the raising of HDL levels. This includes the VA-HIT study, in which coronary events were reduced by 11% with gemfibrozil for every 5-mg/dL increase in HDL-C (92). In another series of studies (“ARBITER”(93–96)), high-risk patients were placed on either statins or statins plus niacin. Over a 18–24 month observation periods, carotid intimal-medial thickness (cIMT) measurements were obtained as a surrogate for coronary artery plaque burden. When niacin was part of the treatment, HDL-C levels were increased (by 18.4%), and the authors attributed the improvement in cIMT particularly to this change. It is important to note that niacin does more than just raise HDL-C levels; it also decreases plasma triglyceride levels, makes LDL size increase, and possesses anti-inflammatory properties all of which have the potential to limit plaque progression (97–99). These pleiotropic effects obviously confound the interpretation of both the ARBITER and another statin-niacin clinical trial- the HATS study (100). In the latter study, the addition of niacin to statin treatment resulted not only in a reduction in coronary artery stenosis, but also in events. The encouraging results with niacin, however, were recently called into question by the early termination of the AIM-HIGH study, which failed to show a benefit in the treatment group (101). This study has been criticized due to the fact that both the treatment and the control groups received intense statin therapy, making additional benefits harder to detect, as well as for the fact that the patients received a rather low dose of niacin. The lack of efficacy was also observed in the Heart Protection Study 2- Treatment of HDL to Reduce the Incidence of Vascular Events [HPS2-Thrive] (102) which has brought into question the clinical value of niacin.
Recently, cholesteryl ester transfer protein (CETP) inhibitors have been investigated as pharmacological agents to raise HDL levels. Surprisingly, torcetrapib, the first CETP inhibitor tested in a clinical trial, increased the all-cause mortality and cardiovascular events, which led to the premature ending of the ILLUMINATE trial (103). Subsequent studies indicated that the observed off-target effects of torcetrapib (increased blood pressure and low serum potassium by stimulation of aldosterone production) were rather molecule specific, unrelated to CETP inhibition and thereby might have overshadowed the beneficial effects of the raised HDL-C levels. Interestingly, posthoc analysis of ILLUMINATE showed that subjects with greater increases of HDL-C or apoAI levels had a lower rate of major cardiovascular events within the torcetrapib group (104). Despite the general failure of torcetrapib, in the posthoc analysis of the ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) study, regression of coronary atherosclerosis (as assessed by IVUS) was observed in patients who achieved the highest HDL-C levels with torcetrapib treatment (105). In vitro studies showed an improved functionality of HDL-C particles under CETP inhibition, as HDL-C isolated from patients treated with torcetrapib and anacetrapib exhibited an increased ability to promote cholesterol efflux from macrophages (106–107). Indeed, the CETP inhibitors anacetrapib, dalcetrapib and evacetrapib increase HDL-C levels between 30–138%, and have not shown the off-target effects of torcetrapib in clinical phase II trials, confirming the premise of a non-class related toxicity of torcetrapib (108–111) Thus, raising HDL-C by CETP inhibition or modulation remains a potential therapeutic approach for atherosclerotic cardiovascular disease.
Large clinical outcome trials were initiated for dalcetrapib (dal-OUTCOMES) and anacetrapib (REVEAL) including a total of approximately 45,000 patients. Surprisingly, in May 2012, the sponsor stopped the dal-HEART program for dalcetrapib after an interim analysis of dal-OUTCOMES due to a lack of clinically meaningful efficacy. The failure of dal-OUTCOMES might have been a result of the rather moderate increases in HDL-C levels (30%) and minor impact on LDL-C levels induced by dalcetrapib, a fate that does not necessarily apply for anacetrapib which has been shown to increase HDL-C levels by 138% accompanied by more robust reductions in LDL-C levels (112). Whether the failure of dal-OUTCOMES challenges the benefits of raising HDL-C, in general, or rather the underlying mechanisms of how HDL-C is to be raised will be answered by the phase III study with anacetrapib which is expected over the next few years.
CHALLENGES TO THE HDL HYPOTHESIS
The HDL hypothesis states that a reduction of plasma HDL concentration may accelerate the development of atherosclerosis by impairing the clearance of cholesterol from the arterial wall. It implies that the efficiency of reverse cholesterol transport is partly dependent on the concentration of HDL and individuals with low HDL have a greater risk of CAD than individuals with high HDL levels. In a recent study, Voight and colleagues, using Mendelian randomization, tested the hypothesis that increased plasma HDL-C is protective for myocardial infarction (MI) by examining the relationship between genetic variations associated with elevated levels of plasma HDL-C and the risk of MI (113). The loss of function single nucleotide polymorphism (SNP) in the endothelial lipase gene (LIPG Asn396Ser) that is associated with an elevated mean plasma level of HDL-C (but no change in the plasma level of low-density lipoprotein cholesterol [LDL-C] or triglycerides) was evaluated in ~21,000 MI cases and ~95,000 controls. Interestingly, no significant effect on risk of MI was observed. The authors concluded that some genetic mechanisms that raise plasma HDL-C does not necessarily lower the risk of MI consistent with recent studies in which the plasma level of HDL-C was raised pharmacologically (i.e., AIM-HIGH (101), ILLUMINATE (103), dal-OUTCOMES (114–115), and HPS2-THRIVE (102)) without evidence that there were any reductions in cardiovascular events.
Although there are some who feel that the above data indicates that HDL should no longer be a therapeutic target, the above developments only emphasize that HDL biology is complex. In fact, they have led to a reshaping of the HDL hypothesis to focus on HDL function (i.e. efflux capacity). It should be mentioned that studies have indicated that HDL cholesterol efflux capacity is a significant inverse predictor of coronary heart disease even after adjusting for HDL-C concentrations (116). In other words, it is not HDL cholesterol itself that has a causal relation to atheroprotection, but rather HDL function, which cannot be reliably estimated through the simple measurement of HDL-C. This concept has recently been reinforced by a recent report that demonstrated that cholesterol efflux capacity was inversely associated with the incidence of cardiovascular events in a population-based cohort (117). That improvements in HDL function and HDL-C concentrations are dissociated has been demonstrated in the dal-ACUTE study where the effect of dalcetrapib on HDL efflux activity was disproportionately lower than on HDL-C levels (118).
FUTURE DIRECTIONS
It is quite clear that HDL-C does not provide all the pertinent information. Studies such as VA-HIT (119), MESA (120), HPS (121), and JUPITER (122) have shown that HDL particle number is a stronger, more independent predictor of CAD risk than HDL-C. In the Dallas Heart Study (117), though, cholesterol efflux capacity in macropages was an independent predictor of incident cardiovascular events, and unrelated to HDL particle number indicating that the measure of HDL function continues to have no true surrogate at this time. Although there are still many unanswered questions, one thing is clear-that the development of HDL targeted therapeutics should still be a goal. The key, however, is that the focus should be on promoting aspects of the reverse cholesterol transport pathway rather than on raising HDL-C (123). Infusion of apoAI containing recombinant HDL particles or of lipid-poor HDL particles is an approach that continues to progress in the clinic (89, 103, 124). There is substantial enthusiasm for this approach, which is the closest in concept to the HDL-targeted approaches that have been most successful in animal models. In addition, a therapeutic approach targeted towards upregulating efflux pathways in macrophages could be atheroprotective (125). For example, liver X receptor agonists (which can lead to upregulation of ABCA-1 and ABCG-1) have been shown to promote macrophage reverse cholesterol transport and reduce or even regress atherosclerosis in animals (126–130). The limitation to its use is increased lipogenesis which can be overcome by the use of selective agonists as there are two known isoforms, alpha and beta. Finally, another approach being explored is antagonism of miR-33 (which would lead to enhanced cholesterol efflux) (79–80), a target that was described earlier.
CONCLUSION
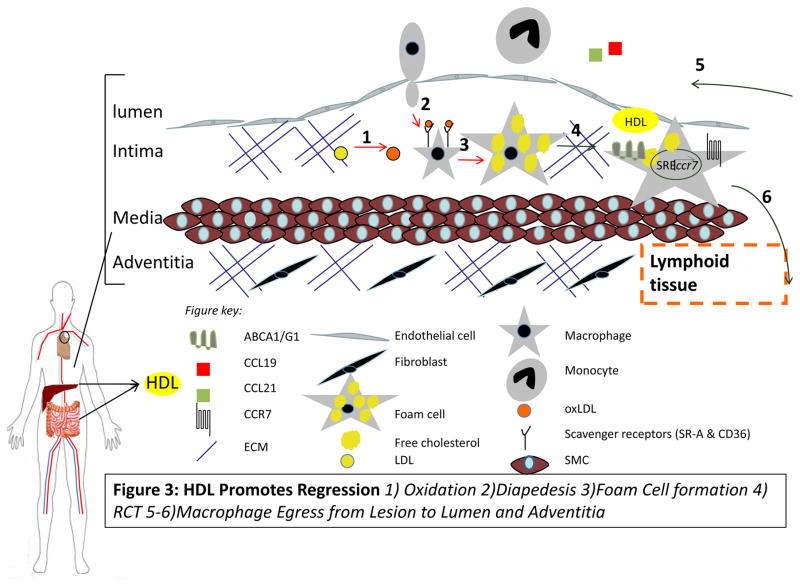
Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome with preferential expression of genes that reduce cellular adhesion, enhance cellular motility, and overall act to suppress inflammation (78). Plaque regression is actually a coordinated set of complex molecular events (Figure 3). To date, the animal and human studies that achieved plaque regression required reductions in plasma levels of apoB. Unfortunately, most patients who take statins, for example, will not achieve and sustain the dramatically low LDL-cholesterol levels seen in chow-fed nonhuman primates. The PCSK9 inhibitors, however, seem to be promising (131–132). In contrast to the intervention studies that have directly established LDL as a causative factor in atherosclerosis progression, several recent pharmacological and genetic studies in humans have failed to demonstrate that increased plasma levels of HDL-C resulted in decreased cardiovascular disease risk, giving rise to a controversy regarding whether HDL is even as protective as assumed. However, based on the pre-clinical and human HDL studies to date, a general pattern emerges that links HDL to beneficial effects on the plaque (Table 1, Figure 4). The evidence shows that HDL can indeed promote the regression when the levels of functional particles are increased suggesting that the recent negative clinical trials should not eliminate HDL from consideration as an atheroprotective agent. Indeed the plaque and its components are dynamic-atherosclerosis regression and the beneficial roles of HDL are not myths at all.
Figure 3. HDL promotes atherosclerosis regression.
HDL can inhibit processes 1–3 (red arrows) and promotes 4–6 (green arrows). Macrophage egress can occur through the upregulation of CCR7 via activation of the SREBP pathway.
Table 1.
Selected HDL and ApoAI regression studies
| Author | Species | Approach/drug | Dosage | Administration | Plaque site | Main findings in plaques |
|---|---|---|---|---|---|---|
| Badimon et al. 1990 (24) | Rabbit (New Zealand white rabbits) | HDL-VHDL | 50 mg | i.v., weekly over 4 weeks | Total aorta surface | Extent of fatty streaks ↓ Aortic lipid accumulation (TC, FC and PL) ↓ |
| Parolini et al. 2008 (133) | Rabbit (New Zealand white rabbits) | apoA-IMilano ( ETC-216) |
|
5 i.v. injections, every 4 days | Carotid arteries (assessed by IVUS and MRI) | Atheroma volume ↓ with 3 highest dosages Significant regression after 2nd administration of 150 mg/kg |
| Shah et al. 2001 (29) | Mouse (apoE−/−) | apoA-IMilano | 400 mg/kg | Single i.v. injection | Aortic root (48 hours post injection) | Lipid content ↓ Macrophage content ↓ |
| Cho et al. 2009 (134) | Mouse (apoE−/−) |
|
120 mg/kg | Single i.v. injection | Aortic root (24h and 48h post injection) | Lipid content: rHDL↓, V156K ↓↓, R173C ↓↓ Macrophage content: rHDL ↓, V156K ↓↓, R173C ↓↓ |
| Feig et al. 2011 (71) | Mouse (apoE−/−) | Aortic arch transplant into apoE−/− mice transgenic for human apoA-I | Aortic arch (7 days post transplant) | Plaque size ↓ Macrophage content ↓ M1 macrophages ↓ M2 macrophages ↑ CCR7 ↑ in plaque macrophages |
||
| Rayner et al. 2012 (79) | Mouse (LDLr−/−) | Anti-miR33 | 10 mg/kg | 1st week: 2 s.c. injections, followed by weekly injections | Aortic root (after 4 weeks of treatment) | Plaque size ↓ Macrophage content ↓ Lipid content ↓ Collagen content ↑ M1 macrophages ↓ M2 macrophages ↑ |
| Nissen et al. 2003 (89) | Human (ACS) | apoA-IMilano (ETC 216) |
|
i.v., weekly over 5 weeks | Coronary artery (assessed by IVUS) | Combined rHDL groups vs. baseline (Median):
|
| Tardif et al. 2007 (ERASE) (90) | Human (ACS) | rHDL (CSL-111) |
|
i.v., weekly over 4 weeks | Coronary artery (assessed by IVUS) | rHDL vs. placebo group (Median):
|
| Shaw et al. 2008 (91) | Human (PAD) | rHDL (CSL-111) | 80 mg/kg | i.v. | SFA (after atherectomy 5–7 days post infusion) | Lipid content ↓ Macrophages cell size ↓ VCAM-1 expression ↓ |
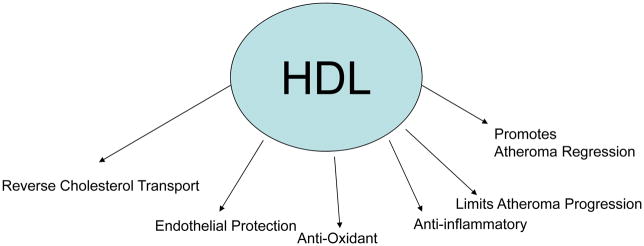
Figure 4. The Many Hats of HDL.
HDL has many functions including promoting reverse cholesterol transport, endothelial protection, possessing anti-oxidant as well as anti-inflammatory properties, limiting progression of atherosclerosis, and importantly can cause regression of lesions.
Acknowledgments
Some work cited was supported by NIH fellowship AG-029748 (J.E.F.).
Footnotes
Disclosures: The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Blankenhorn DH, Hodis HN. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb. 1994;14:177–192. doi: 10.1161/01.atv.14.2.177. [DOI] [PubMed] [Google Scholar]
- 2.Schell WD, Myers JN. Regression of atherosclerosis: a review. Prog Cardiovasc Dis. 1997;39:483–496. doi: 10.1016/s0033-0620(97)80041-2. [DOI] [PubMed] [Google Scholar]
- 3.Stein Y, Stein O. Does therapeutic intervention achieve slowing of progression or bona fide regression of atherosclerotic lesions? Arterioscler Thromb Vasc Biol. 2001;21:183–188. doi: 10.1161/01.atv.21.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977;86:675–684. [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 6.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 7.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wissler RW, Vesselinovitch D. Studies of regression of advanced atherosclerosis in experimental animals and man. Ann N Y Acad Sci. 1976;275:363–378. doi: 10.1111/j.1749-6632.1976.tb43368.x. [DOI] [PubMed] [Google Scholar]
- 9.Constantinides P. Coronary Thrombosis Linked to Fissure in Atherosclerotic Vessel Wall. Jama. 1964;188(SUPPL):35–37. doi: 10.1001/jama.1964.03060320157049. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman M, Byers SO, Rosenman RH. Resolution of aortic atherosclerotic infiltration in the rabbit by phosphatide infusion. Proc Soc Exp Biol Med. 1957;95:586–588. doi: 10.3181/00379727-95-23300. [DOI] [PubMed] [Google Scholar]
- 12.Williams KJ, Werth VP, Wolff JA. Intravenously administered lecithin liposomes: a synthetic antiatherogenic lipid particle. Perspect Biol Med. 1984;27:417–431. doi: 10.1353/pbm.1984.0031. [DOI] [PubMed] [Google Scholar]
- 13.Williams KJ, Tabas I. Lipoprotein retention--and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 14.Williams KJ, Scanu AM. Uptake of endogenous cholesterol by a synthetic lipoprotein. Biochim Biophys Acta. 1986;875:183–194. doi: 10.1016/0005-2760(86)90167-0. [DOI] [PubMed] [Google Scholar]
- 15.Williams KJ, Vallabhajosula S, Rahman IU, Donnelly TM, Parker TS, Weinrauch M, Goldsmith SJ. Low density lipoprotein receptor-independent hepatic uptake of a synthetic, cholesterol-scavenging lipoprotein: implications for the treatment of receptor-deficient atherosclerosis. Proc Natl Acad Sci U S A. 1988;85:242–246. doi: 10.1073/pnas.85.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigueza WV, Mazany KD, Essenburg AD, Pape ME, Rea TJ, Bisgaier CL, Williams KJ. Large versus small unilamellar vesicles mediate reverse cholesterol transport in vivo into two distinct hepatic metabolic pools. Implications for the treatment of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2132–2139. doi: 10.1161/01.atv.17.10.2132. [DOI] [PubMed] [Google Scholar]
- 17.Williams KJ, Scalia R, Mazany KD, Rodrigueza WV, Lefer AM. Rapid restoration of normal endothelial functions in genetically hyperlipidemic mice by a synthetic mediator of reverse lipid transport. Arterioscler Thromb Vasc Biol. 2000;20:1033–1039. doi: 10.1161/01.atv.20.4.1033. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigueza WV, Williams KJ, Rothblat GH, Phillips MC. Remodeling and shuttling. Mechanisms for the synergistic effects between different acceptor particles in the mobilization of cellular cholesterol. Arterioscler Thromb Vasc Biol. 1997;17:383–393. doi: 10.1161/01.atv.17.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams KJ, Phillips MC, Rodrigueza WV. Structural and metabolic consequences of liposome-lipoprotein interactions. Adv Drug Deliv Rev. 1998;32:31–43. doi: 10.1016/s0169-409x(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 20.Maruffo CA, Portman OW. Nutritional control of coronary artery atherosclerosis in the squirrel monkey. J Atheroscler Res. 1968;8:237–247. doi: 10.1016/s0368-1319(68)80060-2. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong ML, Warner ED, Connor WE. Regression of coronary atheromatosis in rhesus monkeys. Circ Res. 1970;27:59–67. doi: 10.1161/01.res.27.1.59. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong ML. Evidence of regression of atherosclerosis in primates and man. Postgrad Med J. 1976;52:456–461. doi: 10.1136/pgmj.52.609.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinow MR. Experimental models of atherosclerosis regression. Atherosclerosis. 1983;48:105–118. doi: 10.1016/0021-9150(83)90097-7. [DOI] [PubMed] [Google Scholar]
- 24.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki A, Sakuma S, Morikawa W, Takiue T, Miake F, Terano T, Sakai M, Hakamata H, Sakamoto Y, Natio M, et al. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1882–1888. doi: 10.1161/01.atv.15.11.1882. [DOI] [PubMed] [Google Scholar]
- 26.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 27.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 30.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo JG, Fallon JT, Fisher EA. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34:541–547. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- 33.Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, Chereshnev I, Fisher EA. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24:1714–1719. doi: 10.1161/01.ATV.0000139313.69015.1c. [DOI] [PubMed] [Google Scholar]
- 34.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–175. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trogan E, Choudhury RP, Dansky HM, Rong JX, Breslow JL, Fisher EA. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2002;99:2234–2239. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feig JE, Fisher EA. Laser capture microdissection for analysis of macrophage gene expression from atherosclerotic lesions. Methods Mol Biol. 2013;1027:123–135. doi: 10.1007/978-1-60327-369-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 38.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 39.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. Jama. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 40.Franceschini G. Epidemiologic evidence for high-density lipoprotein cholesterol as a risk factor for coronary artery disease. Am J Cardiol. 2001;88:9N–13N. doi: 10.1016/s0002-9149(01)02146-4. [DOI] [PubMed] [Google Scholar]
- 41.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 42.Miller NE, Miller GJ. Letter: High-density lipoprotein and atherosclerosis. Lancet. 1975;1:1033. doi: 10.1016/s0140-6736(75)91977-7. [DOI] [PubMed] [Google Scholar]
- 43.Banka CL. High density lipoprotein and lipoprotein oxidation. Curr Opin Lipidol. 1996;7:139–142. doi: 10.1097/00041433-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 45.Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- 46.Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 47.Nofer JR, Assmann G. Atheroprotective effects of high-density lipoprotein-associated lysosphingolipids. Trends Cardiovasc Med. 2005;15:265–271. doi: 10.1016/j.tcm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 49.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 50.Paszty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang N, Ranalletta M, Matsuura F, Peng F, Tall AR. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler Thromb Vasc Biol. 2006;26:1310–1316. doi: 10.1161/01.ATV.0000218998.75963.02. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 58.Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 59.Vergnani L, Hatrik S, Ricci F, Passaro A, Manzoli N, Zuliani G, Brovkovych V, Fellin R, Malinski T. Effect of native and oxidized low-density lipoprotein on endothelial nitric oxide and superoxide production : key role of L-arginine availability. Circulation. 2000;101:1261–1266. doi: 10.1161/01.cir.101.11.1261. [DOI] [PubMed] [Google Scholar]
- 60.Li XA, Titlow WB, Jackson BA, Giltiay N, Nikolova-Karakashian M, Uittenbogaard A, Smart EJ. High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J Biol Chem. 2002;277:11058–11063. doi: 10.1074/jbc.M110985200. [DOI] [PubMed] [Google Scholar]
- 61.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 62.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 63.Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 64.Theilmeier G, De Geest B, Van Veldhoven PP, Stengel D, Michiels C, Lox M, Landeloos M, Chapman MJ, Ninio E, Collen D, Himpens B, Holvoet P. HDL-associated PAF-AH reduces endothelial adhesiveness in apoE−/− mice. Faseb J. 2000;14:2032–2039. doi: 10.1096/fj.99-1029com. [DOI] [PubMed] [Google Scholar]
- 65.Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, Reddy S, Shih D, Shi W, Watson AD, Van Lenten BJ, Vora D, Fogelman AM. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–488. doi: 10.1161/01.atv.21.4.481. [DOI] [PubMed] [Google Scholar]
- 66.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 67.Ahmed Z, Ravandi A, Maguire GF, Emili A, Draganov D, La Du BN, Kuksis A, Connelly PW. Apolipoprotein A-I promotes the formation of phosphatidylcholine core aldehydes that are hydrolyzed by paraoxonase (PON-1) during high density lipoprotein oxidation with a peroxynitrite donor. J Biol Chem. 2001;276:24473–24481. doi: 10.1074/jbc.M010459200. [DOI] [PubMed] [Google Scholar]
- 68.Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garner B, Witting PK, Waldeck AR, Christison JK, Raftery M, Stocker R. Oxidation of high density lipoproteins. I. Formation of methionine sulfoxide in apolipoproteins AI and AII is an early event that accompanies lipid peroxidation and can be enhanced by alpha-tocopherol. J Biol Chem. 1998;273:6080–6087. doi: 10.1074/jbc.273.11.6080. [DOI] [PubMed] [Google Scholar]
- 70.Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 71.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feig JE, Shamir R, Joaquin V, Grauer L, Fisher EA. ApoAI is required for the regression of atherosclerosis and is a potent regulator of plaque monocyte-derived emigration and inflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:e13. [Google Scholar]
- 73.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. Jama. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 74.Damas JK, Smith C, Oie E, Fevang B, Halvorsen B, Waehre T, Boullier A, Breland U, Yndestad A, Ovchinnikova O, Robertson AK, Sandberg WJ, Kjekshus J, Tasken K, Froland SS, Gullestad L, Hansson GK, Quehenberger O, Aukrust P. Enhanced expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental atherosclerosis: possible pathogenic role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2007;27:614–620. doi: 10.1161/01.ATV.0000255581.38523.7c. [DOI] [PubMed] [Google Scholar]
- 75.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins Promote the Regression of Atherosclerosis via Activation of the CCR7-Dependent Emigration Pathway in Macrophages. PLoS One. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, Garabedian MJ, Fisher EA, Puig O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, Khatsenko OG, Kaimal V, Lees CJ, Fernandez-Hernando C, Fisher EA, Temel RE, Moore KJ. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ost CR, Stenson S. Regression of peripheral atherosclerosis during therapy with high doses of nicotinic acid. Scand J Clin Lab Invest Suppl. 1967;99:241–245. [PubMed] [Google Scholar]
- 82.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781–1791. doi: 10.1161/01.cir.87.6.1781. [DOI] [PubMed] [Google Scholar]
- 83.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Atherosclerosis regression, plaque disruption, and cardiovascular events: a rationale for lipid lowering in coronary artery disease. Annu Rev Med. 1993;44:365–376. doi: 10.1146/annurev.me.44.020193.002053. [DOI] [PubMed] [Google Scholar]
- 84.Farmer JA, Gotto AM., Jr Dyslipidemia and the vulnerable plaque. Prog Cardiovasc Dis. 2002;44:415–428. doi: 10.1053/pcad.2002.123474. [DOI] [PubMed] [Google Scholar]
- 85.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 86.Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. Jama. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 87.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. Jama. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 88.Wiviott SD, Cannon CP, Morrow DA, Ray KK, Pfeffer MA, Braunwald E. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol. 2005;46:1411–1416. doi: 10.1016/j.jacc.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 89.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. Jama. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 90.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. Jama. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 91.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 92.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. Jama. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 93.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–2060. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 94.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22:2243–2250. doi: 10.1185/030079906x148508. [DOI] [PubMed] [Google Scholar]
- 95.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 96.Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, Weissman NJ, Turco M. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 97.Yu BL, Zhao SP. Anti-inflammatory effect is an important property of niacin on atherosclerosis beyond its lipid-altering effects. Med Hypotheses. 2007;69:90–94. doi: 10.1016/j.mehy.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 98.McKenney JM, McCormick LS, Schaefer EJ, Black DM, Watkins ML. Effect of niacin and atorvastatin on lipoprotein subclasses in patients with atherogenic dyslipidemia. Am J Cardiol. 2001;88:270–274. doi: 10.1016/s0002-9149(01)01639-3. [DOI] [PubMed] [Google Scholar]
- 99.Superko HR, Krauss RM. Differential effects of nicotinic acid in subjects with different LDL subclass patterns. Atherosclerosis. 1992;95:69–76. doi: 10.1016/0021-9150(92)90177-i. [DOI] [PubMed] [Google Scholar]
- 100.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 101.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 102.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 103.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 104.Barter P. Lessons learned from the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Am J Cardiol. 2009;104:10E–15E. doi: 10.1016/j.amjcard.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 105.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118:2506–2514. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 106.Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK, Tall AR. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30:1430–1438. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yvan-Charvet L, Matsuura F, Wang N, Bamberger MJ, Nguyen T, Rinninger F, Jiang XC, Shear CL, Tall AR. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol. 2007;27:1132–1138. doi: 10.1161/ATVBAHA.106.138347. [DOI] [PubMed] [Google Scholar]
- 108.Luscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Munzel T, Kastelein JJ, Deanfield JE. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J. 2012;33:857–865. doi: 10.1093/eurheartj/ehs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cannon CP, Dansky HM, Davidson M, Gotto AM, Jr, Brinton EA, Gould AL, Stepanavage M, Liu SX, Shah S, Rubino J, Gibbons P, Hermanowski-Vosatka A, Binkowitz B, Mitchel Y, Barter P. Design of the DEFINE trial: determining the EFficacy and tolerability of CETP INhibition with AnacEtrapib. Am Heart J. 2009;158:513–519. e513. doi: 10.1016/j.ahj.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 110.Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Stepanavage M, Liu SX, Gibbons P, Ashraf TB, Zafarino J, Mitchel Y, Barter P. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 111.Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, Hu B, McErlean E, Nissen SE. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. Jama. 2011;306:2099–2109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 112.Hewing B, Fisher EA. Rationale for cholesteryl ester transfer protein inhibition. Curr Opin Lipidol. 2012 doi: 10.1097/MOL.0b013e328353ef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz GG, Olsson AG, Ballantyne CM, Barter PJ, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Shah PK, Tardif JC, Chaitman BR, Duttlinger-Maddux R, Mathieson J. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158:896–901. e893. doi: 10.1016/j.ahj.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 116.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N Engl J Med. 2014 doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ray KK, Ditmarsch M, Kallend D, Niesor EJ, Suchankova G, Upmanyu R, Anzures-Cabrera J, Lehnert V, Pauly-Evers M, Holme I, Stasek J, van Hessen MW, Jones P. The effect of cholesteryl ester transfer protein inhibition on lipids, lipoproteins, and markers of HDL function after an acute coronary syndrome: the dal-ACUTE randomized trial. Eur Heart J. 2014;35:1792–1800. doi: 10.1093/eurheartj/ehu105. [DOI] [PubMed] [Google Scholar]
- 119.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 120.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Le NA, Jin R, Tomassini JE, Tershakovec AM, Neff DR, Wilson PW. Changes in lipoprotein particle number with ezetimibe/simvastatin coadministered with extended-release niacin in hyperlipidemic patients. J Am Heart Assoc. 2013;2:e000037. doi: 10.1161/JAHA.113.000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. Circ Res. 2014;114:205–213. doi: 10.1161/CIRCRESAHA.114.300760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gille A, Easton R, D’Andrea D, Wright SD, Shear CL. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34:2106–2114. doi: 10.1161/ATVBAHA.114.303720. [DOI] [PubMed] [Google Scholar]
- 125.Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol. 2011;8:266–277. doi: 10.1038/nrcardio.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Verschuren L, de Vries-van der Weij J, Zadelaar S, Kleemann R, Kooistra T. LXR agonist suppresses atherosclerotic lesion growth and promotes lesion regression in ApoE*3Leiden mice: time course and potential mechanisms. J Lipid Res. 2008 doi: 10.1194/jlr.M800374-JLR200. [DOI] [PubMed] [Google Scholar]
- 127.Feig JE. Regression of Atherosclerosis: Insights from Animal and Clinical Studies. Ann Glob Health. 2014;80:13–23. doi: 10.1016/j.aogh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feig JE, Feig JL. Macrophages, dendritic cells, and regression of atherosclerosis. Front Physiol. 2012;3:286. doi: 10.3389/fphys.2012.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van der Stoep M, Li Z, Calpe-Berdiel L, van der Sluis RJ, Saleh P, McKinnon HJ, Smit MJ, Korporaal SJ, Van Berkel TJ, Van Eck M, Hoekstra M. Elimination of macrophages drives LXR-induced regression both in initial and advanced stages of atherosclerotic lesion development. Biochem Pharmacol. 2013;86:1594–1602. doi: 10.1016/j.bcp.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 131.Shapiro MD, Fazio S, Tavori H. Targeting PCSK9 for Therapeutic Gains. Curr Atheroscler Rep. 2015;17:499. doi: 10.1007/s11883-015-0499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tavori H, Giunzioni I, Fazio S. PCSK9 inhibition to reduce cardiovascular disease risk: recent findings from the biology of PCSK9. Curr Opin Endocrinol Diabetes Obes. 2015;22:126–132. doi: 10.1097/MED.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L, Chaabane L, Morisetti A, Lorusso V, Martin BJ, Bisgaier CL, Krause B, Newton RS, Sirtori CR, Chiesa G. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51:1098–1103. doi: 10.1016/j.jacc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 134.Cho KH. A reconstituted high density lipoprotein containing the V156E mutant of apolipoprotein A-I exhibits anti-atherosclerotic activity in Apo-E deficient mice. J Atheroscler Thromb. 2009;16:217–229. doi: 10.5551/jat.509. [DOI] [PubMed] [Google Scholar]