In this issue Kimeswenger and colleagues (1) report that infrared A radiation (IRA) promotes survival of human melanocytes carrying UVR-induced DNA damage. The implications of this finding may exceed beyond proposed contribution to melanomagenesis (1) as discussed below.
Life on earth has been dependent on the electromagnetic energy of the sunlight for more than 3 billion years (s1). Sun-derived energy has also been efficiently exploited by living organisms to produce vitamin D3, which contributed to colonization of terrestrial area by marine creatures (2, 3). Skin, strategically located as the barrier between external environment and internal milieu, determines critical functions in the preservation of body homeostasis by regulation of immune, neuro-endocrine and pigmentary activities at the local and systemic levels (4).
The wavelength of ultraviolet radiation (UV) defines its penetration and strength of biological action. The shortest UV wavelengths, UVC, does not reach the earth surface, but when generated by artificial sources it is extremely hazardous because of their damaging effects on biomolecules (s1,2).
UVB (280 – 320 nm), absorbed primarily by the epidermis, induces specific DNA damages leading to cutaneous mutagenesis and carcinogenesis. It has also beneficial effects such as production of vitamin D (2) and can induce production of neurohormonal factors, cytokines and glucocorticoids, which may have either beneficial or pro-cancerogenic effects, depending on context (4–6). UVA (320 – 400 nm), although less energetic, penetrates skin more deeply and induces production of reactive oxygen species (ROS) that can damage cellular macromolecules, such as proteins, lipids and DNA. Light that is visible to human eyes (about 400 – 700 nm) is far less energetic than UVR, and exerts biological properties that have not been fully elucidated yet (s1).
Infrared radiation is an invisible electromagnetic energy with longer wavelengths (700 nm – 1 mm) than those of visible light, unable to induce ionization but capable of evoking significant biological effects (1)(s1,3,4). Infrared is absorbed by the mitochondrial respiratory chain, cytochrome c oxidase, and activates several pathways leading to increased production of ROS (s1,3). IRA enhances proliferation of keratinocytes and fibroblasts as a result of boosted energy availability and signal transduction induced by ROS formation and has been considered as beneficial in alleviating chemotherapy-induced oral ulcerations (s1,3–5). IRA-induced activation of mitogen-activated protein kinase signaling pathways upregulates matrix metalloproteinase-1 expression in dermal fibroblasts with the net of negative repercussions including photoaging and carcinogenesis (s5). Moreover, IRA may delay the onset of UVR-induced tumors, but might contribute to a worse outcome by shifting these tumors into a more aggressive phenotype (discussed in (1)). Thus, studies by Kimeswenger and colleagues (1) fill the gap in information concerning mutual interactions of UVR and IRA on skin homeostasis and melanomagenesis.
Although recent breakthroughs in MAPK pathway-targeted immune therapy of melanoma have prolonged the survival rate of melanoma patients (7), disseminated melanoma is still the most deadly skin type disease. The link between UV and melanoma has been intensively investigated for decades. UVB has simultaneous negative (melanomagenesis) and beneficial effects (vitamin D production). It must be noted that active forms of vitamin D3 also exert anti-inflammatory, anti-proliferative, anti-cancerogenic (including melanoma) effects and award some protection against UVR-induced damage (2, 3, 8).
In addition, to vitamin D production, UVB, but not UVA, radiation are known to activate the intracutaneous (5) and central (9) hypothalamic-pituitary-adrenal (HPA) axes. As a skin specific stressor, UVB triggers the network of neuro-endocrine pathways resulting in cytokine, neuropeptide, corticotropin releasing hormone, POMC-derived peptides, and glucocorticoid production on the local (skin) and central (adrenals) levels (4–6, 9) with mechanisms involving direct or indirect immunosuppressive effects as discussed in (s6–8). In addition, UVB can activate proopiomelanocortin (POMC) signaling in the arcuate nucleus of the hypothalamus and increases release of β-endorphin into the circulation (10). It is tempting to speculate that the above phenomena may explain the anorectic and addictive effects observed in frequently suntanned individuals (9, 10). Thus, UVR can regulate global homeostasis and brain functions through complex, vitamin D-dependent and -independent mechanisms.
A hypothesis proposed by Kimeswenger et al. (1), on an increased melanoma incidence after combined exposure of human epidermal melanocytes to IRA and UVB, deserves special attention, taking into consideration biological importance of IR proposed by others (s1,5). The authors report that the biological response of human melanocytes exposed to simultaneous UVB/IRA radiation consisted of a decline in apoptosis, similarly to what was seen in keratinocytes and fibroblasts (1). Pre- or co-treatment of UVB with IRA did not affect the rate of UV-induced DNA damage but partially reversed the apoptosis by modifying the activity of proteins from an extrinsic pathway (1). Apoptosis plays an essential role in survival of organisms by preventing the proliferation of cells with mutated DNA, normal embryonic development, removal of damaged cells and maintenance of cell homeostasis (s9). UV-induced cell death by apoptosis is considered as a natural protective mechanism that eliminates damaged melanocytes and reduces the risk of malignant transformation (s10,11). Kimeswegner at al. propose that IRA contribution to melanomagenesis may be due to inhibition of the extrinsic apoptotic pathway (1). It is known that UVB activates both the extrinsic and intrinsic apoptotic pathways with an enhanced expression of Caspase 8 and the cell-death receptor CD95 triggering the apoptotic executors (Caspase 3, 6, 9) and activating the mitochondrial Caspase 9-related intrinsic pathway (s9–11). IRA had no or minimal effect on Caspase 9, it inhibited expression of CD95 and partially restored the UVB-induced downregulated FLIPl, an enzyme preventing Caspase 8 activation with the following restoration of anti-apoptotic Bcl2 (1).
Available data concerning IRA biological action on melanomagenesis in the current study are limited to its attenuation of apoptosis (1) (Fig. 1). It would be very interesting to also test a possible influence of IRA on HPA axis activity, cutaneous steroidogenesis and immune status. These combined activities may have beneficial or negative effects (Fig. 1) depending on the context (4, 6)(s8). In order to better understand the mechanisms underlying cutaneous carcinogenesis including melanomagenesis it remains an important future challenge to define the complex interactions between UVR, visible light and IR in the skin (Fig. 1) in a manner that fully integrate the skin’s property as a major (neuro-)endocrine organ (4)(s7,12).
Figure 1.
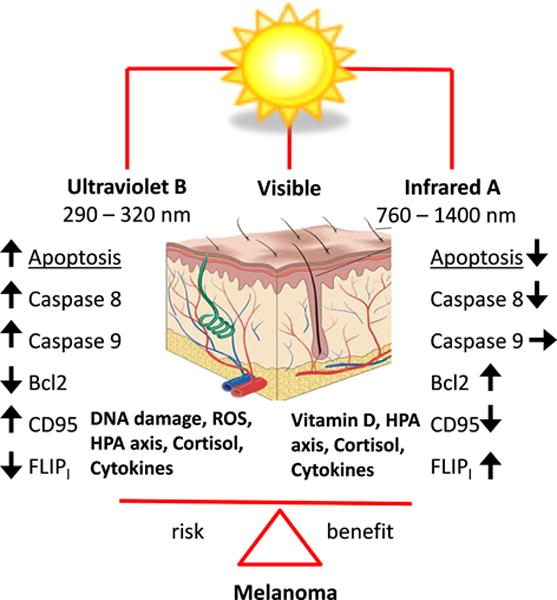
A possible influence of UVB and IRA radiation on melanomagenesis. Abbreviations: Bcl2, B-cell lymphoma 2; CD95, death receptor; CPD, cyclobutane pyrimidine dimer; FLIP1, antiapoptotic protein; HPA axis, hypothalamic-pituitary-adrenal axis.
Supplementary Material
Acknowledgments
Funding
Support from NIH grants R21AR066505-01A1 and 1R01AR056666-01A2 is acknowledged.
Footnotes
Conflict of interest
No conflict of interest to declare.
References
- 1.Kimeswenger S, Schwarz A, Fodinger D, et al. Exp Dermatol. 2016 doi: 10.1111/exd.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Anticancer Res. 2016;36:1345–1356. [PubMed] [Google Scholar]
- 3.Bikle DD. Exp Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 4.Slominski AT, Zmijewski MA, Skobowiat C, et al. Adv Anat Embryol Cell Biol. 2012;212:v–vii. 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skobowiat C, Dowdy JC, Sayre RM, et al. Am J Physiol Endocrinol Metab. 2011;301:E484–493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski AT, Manna PR, Tuckey RC. Exp Dermatol. 2014;23:369–374. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbe C, Peris K, Hauschild A, et al. Eur J Cancer. 2012;48:2375–2390. doi: 10.1016/j.ejca.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Slominski AT, Janjetovic Z, Kim TK, et al. J Steroid Biochem Mol Biol. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skobowiat C, Slominski AT. J Invest Dermatol. 2015;135:1638–1648. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skobowiat C, Slominski AT. Exp Dermatol. 2016;25:120–123. doi: 10.1111/exd.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


